-
PDF
- Split View
-
Views
-
Cite
Cite
Marie Mardal, Mette Findal Andreasen, Christian Brinch Mollerup, Peter Stockham, Rasmus Telving, Nikolaos S Thomaidis, Konstantina S Diamanti, Kristian Linnet, Petur Weihe Dalsgaard, HighResNPS.com: An Online Crowd-Sourced HR-MS Database for Suspect and Non-targeted Screening of New Psychoactive Substances, Journal of Analytical Toxicology, Volume 43, Issue 7, September 2019, Pages 520–527, https://doi.org/10.1093/jat/bkz030
Close - Share Icon Share
Abstract
The number of new psychoactive substances (NPS) is constantly increasing. However, although the number might be large, most NPS have a low prevalence of use, so keeping screening libraries updated with the relevant analytical targets becomes a challenge. One way to ensure sufficient screening coverage is to use shared high resolution-mass spectrometry (HR-MS) databases, such as HighResNPS.com: a free, online, spreadsheet-format, crowd-sourced HR-MS database for NPS screening. The aims of this study were (i) to present the database to the scientific community and (ii) to verify that the HighResNPS database can be utilized in suspect screening workflows for LC–HR-MS instruments and software from four different instrument vendors. A sample was spiked with 10 NPS, and participating laboratories then analyzed the sample with their respective HR-MS vendor platforms and the HighResNPS database. The HighResNPS data were obtained via a spreadsheet converted to fit the import specifications of the different vendor platforms. Suspect screening was performed using LC–HR-MS vendor platforms from Thermo Fisher, Waters, Bruker and Agilent. All 10 NPS were identified in at least three workflows used for the four different vendor platforms. Multiple users have submitted data to HighResNPS for the same NPS, which resulted in multiple true-positive identifications for these NPS. Suspect screening with LC–HR-MS can be based on diagnostic fragment ions reported by users of different vendor platforms and can support NPS identification in biological samples and/or seizure analyses when no reference standard is available in-house. The present work clearly demonstrates that HighResNPS data is compatible with instruments and screening software from at least four different vendor platforms. The database can thus serve as a useful add-on in LC–HR-MS screening workflows.
Introduction
The emergence of new psychoactive substances (NPS) on the drug market is of great concern for forensic and clinical laboratories alike. Large numbers of NPS enter the market every year (1), and their time on the market is often short, as they can readily disappear when they are banned (2). Moreover, the prevalence of use can be quite low when compared with the traditional drugs of abuse (1). As of the end of 2017, the European monitoring center for drugs and drug addiction was monitoring 670 NPS. A group of particular concern at the moment is the fentanyl analog group, which has been associated with 250 deaths in Europe in 2016–2017 (1, 3). Screening of biological samples for NPS can require the use of multiple analytical targets, depending on the route of administration and the distribution, metabolism and excretion profile of the given NPS. Certain synthetic cannabinoids are not detected in unchanged form in urine, which means that the metabolites need to be added to the screening libraries (4, 5). Analytical reference materials are usually not immediately available after a NPS enters the market, although the reference material providers are focusing on reducing this time gap. On the other hand, purchasing costly reference material for around 100 NPS and their metabolites every year is hard to justify in a laboratory with limited funds and/or few NPS cases per year.
A common approach to address NPS in forensic laboratories is qualitative screening for this group of compounds, either using gas chromatography–mass spectrometry or liquid chromatography–high-resolution mass spectrometry (LC–HR-MS) (6–9). Gas chromatography–mass spectrometry and LC–HR-MS are suitable for screening of biological samples and/or seized materials, as analytical data can be compared with MS libraries covering thousands of analytical targets. Free HR-MS databases of relevance to forensic applications include MassBank (10), mzCloud1, HMDB (11) and orbitrap-based commercially available libraries (12). Also worth mentioning are the two online data repositories, NPS Data Hub (13) and the RESPONSE database2, which provide a wide range of analytical data on NPS. All these databases provide high-quality MS data, but the coverage of NPS varies and the data are not readily transferable for suspect screenings on HR-MS equipment from different vendors. Different HR-MS/MS instruments tend to generate comparable diagnostic fragment ions after collision-induced fragmentation (14), which is a prerequisite for using shared databases. HR-MS databases based on diagnostic fragment ions are less dependent on instrument- and method-specific variables, when compared with mass spectral databases. Furthermore, analytical equipment and data at modern forensic laboratories are occasionally operated offline to protect sensitive data, which reduces the suitability of data acquired from forensic HR-MS equipment for cloud-based data analyses. Therefore, these databases should be downloadable.
These obstacles led a group of researchers to initiate the HighResNPS3 database. Their intention was to help in keeping HR-MS screening libraries updated with analytical information from the moment a specific NPS becomes available to a given participating laboratory. The HighResNPS database currently has active users from at least 10 laboratories.
The aims of the present study were to illustrate how the HighResNPS database can be implemented as a part of HR-MS screening workflows using instrumental setups from four different HR-MS vendors. A mixture of 10 NPS was analyzed on four different instruments from different instrument vendors. We also explain the intention behind the database and describe its structure.
Methods and Experimental
Materials and reagents
Deschloro-ketamine, fluoropentedrone, 3-methylphenmetrazine, 2C-B-FLY, adinazolam, acrylfentanyl, bk-IVP, 25-NBP, CUMYL-4CN-BINACA and MDMB-CHMICA were obtained from seizures sent to the Section of Forensic Chemistry at the University of Copenhagen for analysis or kindly provided by the Slovenian National Forensic Laboratory. All solvents used in the preparation of mobile phases were of LC–MS grade.
Database concept
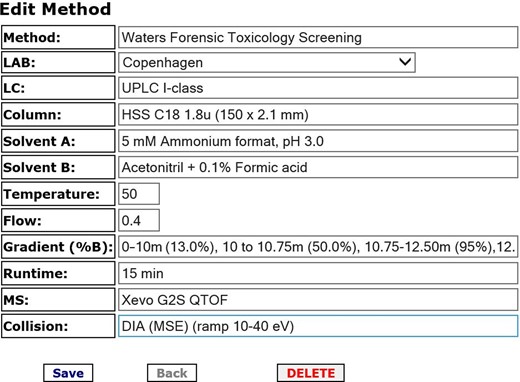
The initial HighResNPS format was a spreadsheet shared in a Dropbox folder among Nordic forensic laboratories that used HR-MS screening as a part of their routine toxicological analysis. The spreadsheet format allows data transfer to screening software from different vendors, and the data can easily be adjusted to the research question at hand. However, the shared-spreadsheet format quickly proved insufficient as the number of users increased. An online database was developed with the intention of having it serve as a free, crowd-sourced database of experimental HR-MS data that members can use in their respective HR-MS screening methods. Upon obtaining a login to the site, the user is encouraged to provide analytical data on at least five NPS per year. The user first defines the analytical instrumentation and method they use, based on the template shown in Figure 1. The database is intended to run with limited continuous data curation. When data on the same compound are shared from different laboratories with different analytical setups, as exemplified for MDMB-CHMICA in Table I, the multiple entries can serve to self-validate the analytical data. The users can also use the database to see which laboratories have a stock, seizure or knowledge of a certain NPS.
Selected data from HighResNPS.com on the synthetic cannabinoid MDMB-CHMICA with analytical data from five different participating laboratories. “0”: No value reported or not acquired
| ID . | Compound . | RT . | PMF . | Pmass . | F1MF . | F1mass . | F2MF . | F2mass . | F3MF . | F3mass . | originName . | LAB . | Country . | Method . | Owner . | Comment . | InChIKey . | IUPAC name . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 238 | MDMB-CHMICA | 8.15 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C23H32N2NaO3 | 407.2299 | 0 | RESPONSE | Aarhus | Denmark | Bruker 3 | M.F.A. | SRJKCVHWIDFUBO-HXUWFJFHSA-N | N-[[1-(cyclohexylmethyl)-1H-indol-3-yl]carbonyl]-3-methyl-valine, methyl ester | ||
| 175 | MDMB-CHMICA | 6.18 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | 0 | Seizure | Trondheim | Norway | Agilent 3 | Per Ole Gundersen | SRJKCVHWIDFUBO-HXUWFJFHSA-N | N-[[1-(cyclohexylmethyl)-1H-indol-3-yl]carbonyl]-3-methyl-valine, methyl ester | ||
| 354 | MDMB-CHMICA | 0 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | 0 | 0 | Theoretical | Copenhagen | Denmark | Thermo 2 | Petur Dalsgaard | Predicted fragments | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (2S)-2-{[1-(cyclohexylmethyl)-1H-indol-3-yl]formamido}-3,3-dimethylbutanoate | ||
| 637 | MDMB-CHMICA | 0 | C23H32N2O3 | 385.2486 | 0 | 0 | 0 | EMCDDA | Copenhagen | Denmark | No method | Petur Dalsgaard | From EMCDDA list | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate | |||
| 1190 | MDMB-CHMICA (MMB-CHMINACA) | 11.96 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | C8H6N | 116.0495 | Standard | Labor Krone | Germany | Waters Forensic Toxicology Screening | Ronald Agius | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate | |
| 1489 | MMB-CHMINACA | 12.43 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | C4H7 | 55.0542 | Standard | University of Athens | Greece | Brukers Pesticide Screener | Nikos Thomaidis | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate |
| ID . | Compound . | RT . | PMF . | Pmass . | F1MF . | F1mass . | F2MF . | F2mass . | F3MF . | F3mass . | originName . | LAB . | Country . | Method . | Owner . | Comment . | InChIKey . | IUPAC name . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 238 | MDMB-CHMICA | 8.15 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C23H32N2NaO3 | 407.2299 | 0 | RESPONSE | Aarhus | Denmark | Bruker 3 | M.F.A. | SRJKCVHWIDFUBO-HXUWFJFHSA-N | N-[[1-(cyclohexylmethyl)-1H-indol-3-yl]carbonyl]-3-methyl-valine, methyl ester | ||
| 175 | MDMB-CHMICA | 6.18 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | 0 | Seizure | Trondheim | Norway | Agilent 3 | Per Ole Gundersen | SRJKCVHWIDFUBO-HXUWFJFHSA-N | N-[[1-(cyclohexylmethyl)-1H-indol-3-yl]carbonyl]-3-methyl-valine, methyl ester | ||
| 354 | MDMB-CHMICA | 0 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | 0 | 0 | Theoretical | Copenhagen | Denmark | Thermo 2 | Petur Dalsgaard | Predicted fragments | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (2S)-2-{[1-(cyclohexylmethyl)-1H-indol-3-yl]formamido}-3,3-dimethylbutanoate | ||
| 637 | MDMB-CHMICA | 0 | C23H32N2O3 | 385.2486 | 0 | 0 | 0 | EMCDDA | Copenhagen | Denmark | No method | Petur Dalsgaard | From EMCDDA list | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate | |||
| 1190 | MDMB-CHMICA (MMB-CHMINACA) | 11.96 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | C8H6N | 116.0495 | Standard | Labor Krone | Germany | Waters Forensic Toxicology Screening | Ronald Agius | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate | |
| 1489 | MMB-CHMINACA | 12.43 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | C4H7 | 55.0542 | Standard | University of Athens | Greece | Brukers Pesticide Screener | Nikos Thomaidis | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate |
EMCDDA, European monitoring center for drugs and drug addiction; F1MF, fragment 1 molecular formula; F1mass, fragment ion 1 exact mass; ID, automatically generated unique item number; InChIKey, International chemical identified key; IUPAC name, “International union of pure and applied chemistry” name; LAB, laboratory; Pmass, parent exact mass; PMF, parent molecular formula; RT, retention time.
Selected data from HighResNPS.com on the synthetic cannabinoid MDMB-CHMICA with analytical data from five different participating laboratories. “0”: No value reported or not acquired
| ID . | Compound . | RT . | PMF . | Pmass . | F1MF . | F1mass . | F2MF . | F2mass . | F3MF . | F3mass . | originName . | LAB . | Country . | Method . | Owner . | Comment . | InChIKey . | IUPAC name . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 238 | MDMB-CHMICA | 8.15 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C23H32N2NaO3 | 407.2299 | 0 | RESPONSE | Aarhus | Denmark | Bruker 3 | M.F.A. | SRJKCVHWIDFUBO-HXUWFJFHSA-N | N-[[1-(cyclohexylmethyl)-1H-indol-3-yl]carbonyl]-3-methyl-valine, methyl ester | ||
| 175 | MDMB-CHMICA | 6.18 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | 0 | Seizure | Trondheim | Norway | Agilent 3 | Per Ole Gundersen | SRJKCVHWIDFUBO-HXUWFJFHSA-N | N-[[1-(cyclohexylmethyl)-1H-indol-3-yl]carbonyl]-3-methyl-valine, methyl ester | ||
| 354 | MDMB-CHMICA | 0 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | 0 | 0 | Theoretical | Copenhagen | Denmark | Thermo 2 | Petur Dalsgaard | Predicted fragments | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (2S)-2-{[1-(cyclohexylmethyl)-1H-indol-3-yl]formamido}-3,3-dimethylbutanoate | ||
| 637 | MDMB-CHMICA | 0 | C23H32N2O3 | 385.2486 | 0 | 0 | 0 | EMCDDA | Copenhagen | Denmark | No method | Petur Dalsgaard | From EMCDDA list | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate | |||
| 1190 | MDMB-CHMICA (MMB-CHMINACA) | 11.96 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | C8H6N | 116.0495 | Standard | Labor Krone | Germany | Waters Forensic Toxicology Screening | Ronald Agius | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate | |
| 1489 | MMB-CHMINACA | 12.43 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | C4H7 | 55.0542 | Standard | University of Athens | Greece | Brukers Pesticide Screener | Nikos Thomaidis | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate |
| ID . | Compound . | RT . | PMF . | Pmass . | F1MF . | F1mass . | F2MF . | F2mass . | F3MF . | F3mass . | originName . | LAB . | Country . | Method . | Owner . | Comment . | InChIKey . | IUPAC name . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 238 | MDMB-CHMICA | 8.15 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C23H32N2NaO3 | 407.2299 | 0 | RESPONSE | Aarhus | Denmark | Bruker 3 | M.F.A. | SRJKCVHWIDFUBO-HXUWFJFHSA-N | N-[[1-(cyclohexylmethyl)-1H-indol-3-yl]carbonyl]-3-methyl-valine, methyl ester | ||
| 175 | MDMB-CHMICA | 6.18 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | 0 | Seizure | Trondheim | Norway | Agilent 3 | Per Ole Gundersen | SRJKCVHWIDFUBO-HXUWFJFHSA-N | N-[[1-(cyclohexylmethyl)-1H-indol-3-yl]carbonyl]-3-methyl-valine, methyl ester | ||
| 354 | MDMB-CHMICA | 0 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | 0 | 0 | Theoretical | Copenhagen | Denmark | Thermo 2 | Petur Dalsgaard | Predicted fragments | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (2S)-2-{[1-(cyclohexylmethyl)-1H-indol-3-yl]formamido}-3,3-dimethylbutanoate | ||
| 637 | MDMB-CHMICA | 0 | C23H32N2O3 | 385.2486 | 0 | 0 | 0 | EMCDDA | Copenhagen | Denmark | No method | Petur Dalsgaard | From EMCDDA list | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate | |||
| 1190 | MDMB-CHMICA (MMB-CHMINACA) | 11.96 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | C8H6N | 116.0495 | Standard | Labor Krone | Germany | Waters Forensic Toxicology Screening | Ronald Agius | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate | |
| 1489 | MMB-CHMINACA | 12.43 | C23H32N2O3 | 385.2486 | C16H18NO | 240.1383 | C9H6NO | 144.0444 | C4H7 | 55.0542 | Standard | University of Athens | Greece | Brukers Pesticide Screener | Nikos Thomaidis | SRJKCVHWIDFUBO-HXUWFJFHSA-N | methyl (S)-2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate |
EMCDDA, European monitoring center for drugs and drug addiction; F1MF, fragment 1 molecular formula; F1mass, fragment ion 1 exact mass; ID, automatically generated unique item number; InChIKey, International chemical identified key; IUPAC name, “International union of pure and applied chemistry” name; LAB, laboratory; Pmass, parent exact mass; PMF, parent molecular formula; RT, retention time.
The data analyzed in this study were obtained from www.HighResNPS.com. The following licenses/restrictions apply: The user needs a log-in, which is available upon request. Requests to access these datasets should be directed to P.W.D., petur.dalsgaard@sund.ku.dk.
Data format
The HR-MS data in HighResNPS are described via the molecular formulas and exact masses for the precursor ion and 0–3 diagnostic fragment ions. The simplicity of the database format makes it compatible with a wide range of screening software. The nomenclature for NPS is not always rational and consistent, as illustrated with MDMB-CHMICA, which is also known as MMC-CHMINACA. To solve the problem with inconsistent naming conventions and type errors, users are encouraged to include the InChIKey as a structure-specific identifier, which allows the automatic generation of hyperlinks for a Google search, thereby enabling further information retrieval. Users are also encouraged to include the IUPAC name in the submission, which can be converted into a molecular structure and used for validation of the InChIKey. Data on the laboratory and analysis method link to unique entries, as defined below. Additional metadata include the owner, origin of the compound, and drug class based on the classification used on EDND: the European information system and database on new drugs.4 NPS are not a well-defined chemical group; therefore, no exclusion criteria have been defined for entries in the database.
The database contains two search boxes: one for text queries and one for mass queries. Compounds can be searched by full or partial (e.g., “fenta”) names, and the mass search field can search across precursor and fragment ions. Alternatively, the database content can be exported to a spreadsheet and further manipulated. The exported file can then either be handled in Microsoft Excel or converted to text-based formats for further handling in other programs.
Adding data
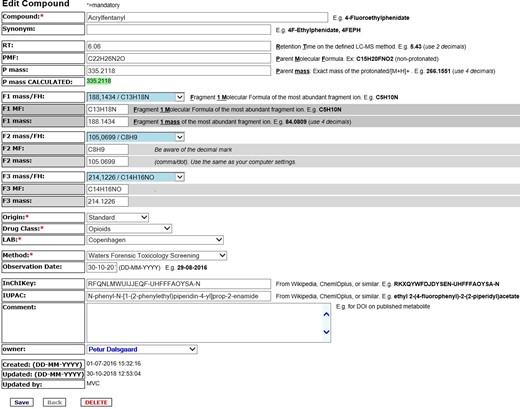
The contributor adds data using the template in Figure 2, which consists of the name, retention time, and molecular formulas, as well as the exact mass calculated from the molecular formula and compared with the measured accurate mass. Fragment ions can be selected from a drop-down list of commonly observed diagnostic fragment ions. If the experimentally determined fragment ions are not on the drop-down list, the data should be typed in manually. The origin of the injected analyte should be stated, as well as the contributing laboratory and which of the defined methods were used. To whatever extent possible, the drug class, InChIKey and IUPAC should be added; however, this is not always possible for tentatively identified metabolites. Following the data addition, the contributor is encouraged to upload pictures of the mass spectrum.
Data entry for acrylfentanyl with analytical mass spectral data and compound identifiers.
Application in forensic workflows
The direct applicability of HighResNPS in forensic screening was verified by conducting a test using four instruments to provide an example of how the database can be utilized with different screening software. A selection of 10 NPS from seized materials, which covered some of the main NPS groups, were mixed and then screened on four LC–HR-MS instruments. The NPS mixture was shipped out as a methanolic extract of around 1 μg/mL, and the sample was analyzed without dilutions or other sample preparation for all instruments. A brief overview of each instrument setting is given in Table II, and the data analysis workflow is given in Thermo Fisher TraceFinder, Waters UNIFI, Bruker data analysis and Agilent MassHunter. The participants were requested to abstain from using additional information beyond the diagnostic fragment ions in HighResNPS, to use only one diagnostic fragment ion as the identification criterion, and to allow multiple identifications per peak if relevant.
Experimental setups used in the participating laboratories for the HighResNPS data analysis
| . | Thermo Fisher TraceFinder . | Waters UNIFI . | Bruker Data analysis . | Agilent MassHunter . |
|---|---|---|---|---|
| LC system | Dionex Ultimate 3000 HPLC system (Thermo) | Acquity UPLC I-Class (Waters) | Acquity I-Class UPLC system (Waters) | 1290 Infinity II LC system (Agilent) |
| LC column | Acquity UPLC 1.8 μm HSS C18 (150 mm × 2.1 mm) (Waters) | Acquity UPLC 1.8 μm HSS C18 (150 mm × 2.1 mm) (Waters) | Acquity UPLC 1.7 μm BEH C18, (100 mm × 2.1 mm) (Waters) | Acquity 1.7 μm BEH C18 (50 × 3.0 mm2) (Waters) |
| LC gradient | Linear gradient of 5–95% B within 12.5 min.Mobile phase A: 2 mM ammonium formate buffer with 0.1% formic acid (v/v). mobile phase B: 0.1% formic acid in methanol (v/v) | Linear gradient of 13–50% B from 0.5 to 10 min, then 50–95% B from 10 to 10.75 min. Mobile phase A: 5 mM aqueous ammonium formate buffer (pH 3), mobile phase B: 0.1% formic acid in acetonitrile (v/v) | Linear gradient of 0–20% B from 0 to 4 min, then 20–80% B to 8 min, constant 80% B to 8.5 min, 80–100% B to 9 min, constant 100% B to 10 min, and reequilibration at 0% B to 13.5 min. Mobile phase A: 0.1% aqueous formic acid, mobile phase B: acetonitrile | Linear gradient of 10–50 from 0.5 to 8 min, then 50–5% B from 8 to 10 min, constant for 0.1 min (with concurrent increase in flow rate to 400 μL/min), 0% B over 0.1 min and maintained for 1.8 min. Mobile phase A: 0.1% aqueous formic acid, mobile phase B: acetonitrile |
| Injection volume | 1 μL | 3 μL | 10 μL | 0.3 μL |
| Flow rate | 200 μL/min | 400 μL/min | 600 μL/min | 350–400 μL/min |
| Mass spectrometer type | Qq-Orbitrap | QqTOF | QqTOF | QqTOF |
| Trade name | Q-Exactive (Thermo) | Xevo G2-S QTof (Waters) | maXis Impact QTOF (Bruker) | 6545 QTOF (Agilent) |
| Acquisition control | DDA (full-scan followed by MS/MS scan of top-5 signals with a dynamic exlusion of 5 s. Isolation width: 1 m/z) | DIA (MS scan at low and high collision energy; intensity threshold of 200 and 20 counts; deconvolution based on chromatographic coelution) | DIA (MS scan at low and high collision energy) | DDA (full-scan followed by MS/MS, based on a preferred precursor mass list then abundance with two precursors selected per full MS. Exclusion for 0.05 min. Isolation width: 1.3 m/z) |
| Collision energy | NCE: 30 eV |
|
| 20 eV |
| Scan range(s) | MS 130–600, MS/MS dynamic first mass | 50–950 | 50–1,000 | MS: 100–1,000, MS/MS: 40–700 |
| Data analysis software | TraceFinder Forensic 4.1 | UNIFI 1.8.2 | Target Analysis (1.3) and Data Analysis (4.1) | MassHunter Quantitative Analysis (B.09) and MassHunter PCDL version B07 |
| . | Thermo Fisher TraceFinder . | Waters UNIFI . | Bruker Data analysis . | Agilent MassHunter . |
|---|---|---|---|---|
| LC system | Dionex Ultimate 3000 HPLC system (Thermo) | Acquity UPLC I-Class (Waters) | Acquity I-Class UPLC system (Waters) | 1290 Infinity II LC system (Agilent) |
| LC column | Acquity UPLC 1.8 μm HSS C18 (150 mm × 2.1 mm) (Waters) | Acquity UPLC 1.8 μm HSS C18 (150 mm × 2.1 mm) (Waters) | Acquity UPLC 1.7 μm BEH C18, (100 mm × 2.1 mm) (Waters) | Acquity 1.7 μm BEH C18 (50 × 3.0 mm2) (Waters) |
| LC gradient | Linear gradient of 5–95% B within 12.5 min.Mobile phase A: 2 mM ammonium formate buffer with 0.1% formic acid (v/v). mobile phase B: 0.1% formic acid in methanol (v/v) | Linear gradient of 13–50% B from 0.5 to 10 min, then 50–95% B from 10 to 10.75 min. Mobile phase A: 5 mM aqueous ammonium formate buffer (pH 3), mobile phase B: 0.1% formic acid in acetonitrile (v/v) | Linear gradient of 0–20% B from 0 to 4 min, then 20–80% B to 8 min, constant 80% B to 8.5 min, 80–100% B to 9 min, constant 100% B to 10 min, and reequilibration at 0% B to 13.5 min. Mobile phase A: 0.1% aqueous formic acid, mobile phase B: acetonitrile | Linear gradient of 10–50 from 0.5 to 8 min, then 50–5% B from 8 to 10 min, constant for 0.1 min (with concurrent increase in flow rate to 400 μL/min), 0% B over 0.1 min and maintained for 1.8 min. Mobile phase A: 0.1% aqueous formic acid, mobile phase B: acetonitrile |
| Injection volume | 1 μL | 3 μL | 10 μL | 0.3 μL |
| Flow rate | 200 μL/min | 400 μL/min | 600 μL/min | 350–400 μL/min |
| Mass spectrometer type | Qq-Orbitrap | QqTOF | QqTOF | QqTOF |
| Trade name | Q-Exactive (Thermo) | Xevo G2-S QTof (Waters) | maXis Impact QTOF (Bruker) | 6545 QTOF (Agilent) |
| Acquisition control | DDA (full-scan followed by MS/MS scan of top-5 signals with a dynamic exlusion of 5 s. Isolation width: 1 m/z) | DIA (MS scan at low and high collision energy; intensity threshold of 200 and 20 counts; deconvolution based on chromatographic coelution) | DIA (MS scan at low and high collision energy) | DDA (full-scan followed by MS/MS, based on a preferred precursor mass list then abundance with two precursors selected per full MS. Exclusion for 0.05 min. Isolation width: 1.3 m/z) |
| Collision energy | NCE: 30 eV |
|
| 20 eV |
| Scan range(s) | MS 130–600, MS/MS dynamic first mass | 50–950 | 50–1,000 | MS: 100–1,000, MS/MS: 40–700 |
| Data analysis software | TraceFinder Forensic 4.1 | UNIFI 1.8.2 | Target Analysis (1.3) and Data Analysis (4.1) | MassHunter Quantitative Analysis (B.09) and MassHunter PCDL version B07 |
bbCID, Broad-band collision induced dissociation; DDA, data-dependent acquisition; DIA, data-independent acquisition; HE, high energy; LC, liquid chromatography; LE, low energy; MS, mass spectrometry; MS/MS, tandem mass spectrometry; NCE, normalized collision energy.
Experimental setups used in the participating laboratories for the HighResNPS data analysis
| . | Thermo Fisher TraceFinder . | Waters UNIFI . | Bruker Data analysis . | Agilent MassHunter . |
|---|---|---|---|---|
| LC system | Dionex Ultimate 3000 HPLC system (Thermo) | Acquity UPLC I-Class (Waters) | Acquity I-Class UPLC system (Waters) | 1290 Infinity II LC system (Agilent) |
| LC column | Acquity UPLC 1.8 μm HSS C18 (150 mm × 2.1 mm) (Waters) | Acquity UPLC 1.8 μm HSS C18 (150 mm × 2.1 mm) (Waters) | Acquity UPLC 1.7 μm BEH C18, (100 mm × 2.1 mm) (Waters) | Acquity 1.7 μm BEH C18 (50 × 3.0 mm2) (Waters) |
| LC gradient | Linear gradient of 5–95% B within 12.5 min.Mobile phase A: 2 mM ammonium formate buffer with 0.1% formic acid (v/v). mobile phase B: 0.1% formic acid in methanol (v/v) | Linear gradient of 13–50% B from 0.5 to 10 min, then 50–95% B from 10 to 10.75 min. Mobile phase A: 5 mM aqueous ammonium formate buffer (pH 3), mobile phase B: 0.1% formic acid in acetonitrile (v/v) | Linear gradient of 0–20% B from 0 to 4 min, then 20–80% B to 8 min, constant 80% B to 8.5 min, 80–100% B to 9 min, constant 100% B to 10 min, and reequilibration at 0% B to 13.5 min. Mobile phase A: 0.1% aqueous formic acid, mobile phase B: acetonitrile | Linear gradient of 10–50 from 0.5 to 8 min, then 50–5% B from 8 to 10 min, constant for 0.1 min (with concurrent increase in flow rate to 400 μL/min), 0% B over 0.1 min and maintained for 1.8 min. Mobile phase A: 0.1% aqueous formic acid, mobile phase B: acetonitrile |
| Injection volume | 1 μL | 3 μL | 10 μL | 0.3 μL |
| Flow rate | 200 μL/min | 400 μL/min | 600 μL/min | 350–400 μL/min |
| Mass spectrometer type | Qq-Orbitrap | QqTOF | QqTOF | QqTOF |
| Trade name | Q-Exactive (Thermo) | Xevo G2-S QTof (Waters) | maXis Impact QTOF (Bruker) | 6545 QTOF (Agilent) |
| Acquisition control | DDA (full-scan followed by MS/MS scan of top-5 signals with a dynamic exlusion of 5 s. Isolation width: 1 m/z) | DIA (MS scan at low and high collision energy; intensity threshold of 200 and 20 counts; deconvolution based on chromatographic coelution) | DIA (MS scan at low and high collision energy) | DDA (full-scan followed by MS/MS, based on a preferred precursor mass list then abundance with two precursors selected per full MS. Exclusion for 0.05 min. Isolation width: 1.3 m/z) |
| Collision energy | NCE: 30 eV |
|
| 20 eV |
| Scan range(s) | MS 130–600, MS/MS dynamic first mass | 50–950 | 50–1,000 | MS: 100–1,000, MS/MS: 40–700 |
| Data analysis software | TraceFinder Forensic 4.1 | UNIFI 1.8.2 | Target Analysis (1.3) and Data Analysis (4.1) | MassHunter Quantitative Analysis (B.09) and MassHunter PCDL version B07 |
| . | Thermo Fisher TraceFinder . | Waters UNIFI . | Bruker Data analysis . | Agilent MassHunter . |
|---|---|---|---|---|
| LC system | Dionex Ultimate 3000 HPLC system (Thermo) | Acquity UPLC I-Class (Waters) | Acquity I-Class UPLC system (Waters) | 1290 Infinity II LC system (Agilent) |
| LC column | Acquity UPLC 1.8 μm HSS C18 (150 mm × 2.1 mm) (Waters) | Acquity UPLC 1.8 μm HSS C18 (150 mm × 2.1 mm) (Waters) | Acquity UPLC 1.7 μm BEH C18, (100 mm × 2.1 mm) (Waters) | Acquity 1.7 μm BEH C18 (50 × 3.0 mm2) (Waters) |
| LC gradient | Linear gradient of 5–95% B within 12.5 min.Mobile phase A: 2 mM ammonium formate buffer with 0.1% formic acid (v/v). mobile phase B: 0.1% formic acid in methanol (v/v) | Linear gradient of 13–50% B from 0.5 to 10 min, then 50–95% B from 10 to 10.75 min. Mobile phase A: 5 mM aqueous ammonium formate buffer (pH 3), mobile phase B: 0.1% formic acid in acetonitrile (v/v) | Linear gradient of 0–20% B from 0 to 4 min, then 20–80% B to 8 min, constant 80% B to 8.5 min, 80–100% B to 9 min, constant 100% B to 10 min, and reequilibration at 0% B to 13.5 min. Mobile phase A: 0.1% aqueous formic acid, mobile phase B: acetonitrile | Linear gradient of 10–50 from 0.5 to 8 min, then 50–5% B from 8 to 10 min, constant for 0.1 min (with concurrent increase in flow rate to 400 μL/min), 0% B over 0.1 min and maintained for 1.8 min. Mobile phase A: 0.1% aqueous formic acid, mobile phase B: acetonitrile |
| Injection volume | 1 μL | 3 μL | 10 μL | 0.3 μL |
| Flow rate | 200 μL/min | 400 μL/min | 600 μL/min | 350–400 μL/min |
| Mass spectrometer type | Qq-Orbitrap | QqTOF | QqTOF | QqTOF |
| Trade name | Q-Exactive (Thermo) | Xevo G2-S QTof (Waters) | maXis Impact QTOF (Bruker) | 6545 QTOF (Agilent) |
| Acquisition control | DDA (full-scan followed by MS/MS scan of top-5 signals with a dynamic exlusion of 5 s. Isolation width: 1 m/z) | DIA (MS scan at low and high collision energy; intensity threshold of 200 and 20 counts; deconvolution based on chromatographic coelution) | DIA (MS scan at low and high collision energy) | DDA (full-scan followed by MS/MS, based on a preferred precursor mass list then abundance with two precursors selected per full MS. Exclusion for 0.05 min. Isolation width: 1.3 m/z) |
| Collision energy | NCE: 30 eV |
|
| 20 eV |
| Scan range(s) | MS 130–600, MS/MS dynamic first mass | 50–950 | 50–1,000 | MS: 100–1,000, MS/MS: 40–700 |
| Data analysis software | TraceFinder Forensic 4.1 | UNIFI 1.8.2 | Target Analysis (1.3) and Data Analysis (4.1) | MassHunter Quantitative Analysis (B.09) and MassHunter PCDL version B07 |
bbCID, Broad-band collision induced dissociation; DDA, data-dependent acquisition; DIA, data-independent acquisition; HE, high energy; LC, liquid chromatography; LE, low energy; MS, mass spectrometry; MS/MS, tandem mass spectrometry; NCE, normalized collision energy.
The exported data from the HighResNPS spreadsheet was first reduced by filtering out entries without fragment ions. The resulting data were then applied for suspect screening on the respective LC–HR-MS(/MS) equipment and screening software.
Thermo Fisher TraceFinder
A TraceFinder database was developed based on the HighResNPS spreadsheet. All entries were renamed to [name@item number] to allow identifications based on analytical data from multiple entries. Retention time was set to 9 min ± 540 s (the entire run) to allow identification from precursor and fragment ions alone. A master method was then created with the following processing parameters for identification: mass error MS1, 5 ppm; mass error fragment ions, 10 ppm; and at least one fragment ion. Retention time and isotopic match were not used as identification criteria. The acquired data files were then processed with the developed master method.
Waters UNIFI
A UNIFI database (Scientific Library) was created based on the exported HighResNPS spreadsheet. All entries were renamed to [name@item number] to allow identifications based on analytical data from multiple entries. All renamed entries were also converted to.mol files from their IUPAC name. Retention time was set to 0–13 min (the entire run) to allow identification from precursor and fragment ions alone. A master method was then created with the following processing parameters for identification: mass error MS1, 3 mDa; mass error fragment ions, 3 mDa; and at least one fragment ion. Retention time and isotopic match were not used as identification criteria. The acquired data files were then processed with the developed master method.
Bruker data analysis
A library (.csv file) was created based on the HighResNPS spreadsheet. All entries were renamed to [name@item number] to allow identifications based on analytical data from multiple entries. Retention time was set to 0–10 min (the entire run). Identification was based only on precursor and fragment ions; no retention time or isotopic parameters were used in the identification. A master method was created with the following processing parameters for identification: mass error precursor set to 10 ppm and at least one fragment ion. The acquired data files were then processed with the developed master method.
Agilent MassHunter
HighResNPS data, including drug names, formulae, accurate parent masses and measured fragment ion masses, were imported into a Quant method (see Supplementary material for the Python script and methodology). Nominal retention times were set to 6 ± 6 min (the entire run) and the extracted ion chromatogram width was set to ±0.02 Thomsons. The method outlier settings were applied as follows: mass accuracy ±0.005 Thomsons; mass match score (isotopic pattern) minimum 50%; and qualifier coelution score 50%. After applying the method to the data file, the Compounds-At-A-Glance functionality was used to sort the data by showing only hits that met the method outlier criteria.
Results and Discussion
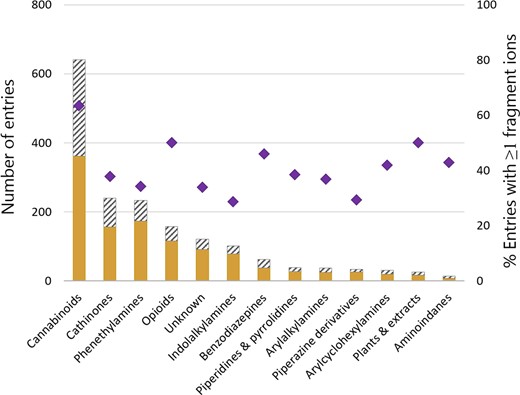
A visual representation of the entries in HighResNPS is given in Figure 3, where the entries are divided into drug classes with information on total entries, unique entries and percentage with a minimum of one fragment ion. Synthetic cannabinoids has been the largest group, as measured by new entries on the European drug market in the last few years; this pattern is also reflected in the HighResNPS database, where synthetic cannabinoids account for 37% of all classified entries.
Entries in the HighResNPS database divided into drug categories. Unshaded bars: Entries with unique InChIKeys, unshaded plus shaded bars: total entries. Diamonds: % entries with a minimum of one fragment ion within the group.
The HighResNPS database currently contains more than 1,700 entries (1,149 unique entries), and 827 have a minimum of one recorded fragment ion (401 unique entries). Within the group of entries with a minimum of one fragment ion, 30% have two fragment ions and 59% have three fragment ions.
All 10 NPS in the tested samples were identified by at least three of the four instruments as presented in Table III. The researcher responsible for screening the spiked samples decided the dilution level and screening settings based on their knowledge of the dynamic range of their instrument.
List of positive identifications with the four different screening workflows
| NPS . | Identified . |
|---|---|
| Deschloro-ketamin | 3 |
| Fluoropentedrone | 4 |
| 3-Methylphenmetrazine | 4 |
| 2C-B-FLY | 3 |
| Adinazolam | 3 |
| Acrylfentanyl | 4 |
| bk-IVP | 4 |
| 25C-NBF | 3 |
| CUMYL-4CN-BINACA | 4 |
| MDMB-CHMICA (MMB-CHMINACA) | 4 |
| NPS . | Identified . |
|---|---|
| Deschloro-ketamin | 3 |
| Fluoropentedrone | 4 |
| 3-Methylphenmetrazine | 4 |
| 2C-B-FLY | 3 |
| Adinazolam | 3 |
| Acrylfentanyl | 4 |
| bk-IVP | 4 |
| 25C-NBF | 3 |
| CUMYL-4CN-BINACA | 4 |
| MDMB-CHMICA (MMB-CHMINACA) | 4 |
List of positive identifications with the four different screening workflows
| NPS . | Identified . |
|---|---|
| Deschloro-ketamin | 3 |
| Fluoropentedrone | 4 |
| 3-Methylphenmetrazine | 4 |
| 2C-B-FLY | 3 |
| Adinazolam | 3 |
| Acrylfentanyl | 4 |
| bk-IVP | 4 |
| 25C-NBF | 3 |
| CUMYL-4CN-BINACA | 4 |
| MDMB-CHMICA (MMB-CHMINACA) | 4 |
| NPS . | Identified . |
|---|---|
| Deschloro-ketamin | 3 |
| Fluoropentedrone | 4 |
| 3-Methylphenmetrazine | 4 |
| 2C-B-FLY | 3 |
| Adinazolam | 3 |
| Acrylfentanyl | 4 |
| bk-IVP | 4 |
| 25C-NBF | 3 |
| CUMYL-4CN-BINACA | 4 |
| MDMB-CHMICA (MMB-CHMINACA) | 4 |
Acrylfentanyl and MDMB-CHMICA had multiple entries, with diagnostic fragment ions in the HighResNPS database and consequently multiple identifications across all instrument platforms. The NPS mixture was prepared from drug seizures, so some of the drugs contained impurities. N-ethylhexedrone was identified across all four instrument platforms, and the analytical spectra corresponded to reference spectra for that compound. However, as N-ethylhexedrone was not spiked into the sample, it was considered an impurity rather than a false positive. The participating laboratories were requested to use only one diagnostic fragment ion as an identification criterion, even when more diagnostic fragment ions were available to rank positive hits. For this reason, false positive hits were reported, mainly corresponding to isomers of phenethylamine 3-methylphenmetrazine. The positional isomer 4-methylphenmetrazine was also identified across all four instrument platforms from the same analytical data as 3-methylphenmetrazine. Each compound has three common fragment ions based on the analytical data available in HighResNPS. As such, distinguishing them is impossible without the analytical standards to test chromatographic behavior. False positives as a result of isomers are an acceptable finding in the context of this approach to screening, since, in practice, no compounds would be reported without further investigation and confirmation. When structurally related isomers (but not positional isomers) produce identical precursor ions together with overlapping fragment ions, additional diagnostic fragment ions must be used. The isomers 3- and 4-methylphenmetrazine share molecular formulas and one fragment ion with the isomer 3-methylethcathinone. However, the use of the fragment ions 131.0855 and 119.0491, for 3- and 4-methylphenmetrazine and 3-methylcathinone, respectively, allows the two isomers to be distinguished. Note, however, that the most abundant fragment ions might not be the most suitable diagnostic fragment ions in the HR-MS–MS spectrum for differentiating among isomers (15). Most post-targeted screening workflows would be aided by either a higher number of fragment ions or additional identification parameters to circumvent this problem. The identification confidence was increased with the observation of multiple identifications for the same target, corresponding to multiple HighResNPS entries.
Notably, a set of prerequisites were made for the screening in the present study; consequently, the applications presented in Methods and Experimental are merely illustrative examples of how to use HighResNPS in a forensic setting. In-silico fragmentation and isotope matching are often embedded features in modern screening software aimed at supporting suspect screening. These features are not fully utilized in the present study, as the aim here was to provide a comparable screening workflow using only HighResNPS rather than providing a comprehensive report on four different HR-MS screening workflows. Additional applications of the database could include examining the retention times from other laboratories using identical chromatographic conditions, or additionally screening for entries without fragment ions with additional filtering tools to reduce false positive hits, such as predicted retention times or collision cross section values and/or in silico fragmentation (16–19).
The strength of using external MS databases in comprehensive screening strategies has been shown in a number of studies, either as a stand-alone strategy (16) or in conjunction with a targeted screening (7). The in-silico filtering tools offer great advantages and prospects for compound identification, but the use of diagnostic fragment ions in MS screening is a great tool to improve identification accuracy (16, 18, 20).
Two limitations of HighResNPS.com are the manual data curation and the lack of peer reviewing. Continuous curation is an advantage of the commercial databases, which have designated funds and personnel for this. When a given NPS has multiple analytical entries in the database, the data self-validate, since different users with different standards are unlikely to report the same faulty data. Thus, if a positive hit is obtained after screening with HighResNPS, the user can further support identification by retrieving full HR-MS spectra on mzcloud5 and/or additional information on NPS Data Hub (13), and ultimately perform an unambiguous identification with an analytical reference standard.
Conclusion
The present work clearly demonstrates that HighResNPS is fully compatible with screening software from Waters, Thermo Fisher, Bruker and Agilent, as verified in this publication. The database can serve as a useful add-on in LC–HR-MS screening workflows, to help in keeping the screening libraries up-to-date and thereby facilitate a more rapid and global response to emerging NPS.
Acknowledgments
P.S. acknowledges staff from Agilent Technologies, particularly James Pyke (Melbourne, Australia) and Li Sun (Santa Clara, USA), for their support in development of successful strategies to apply HighResNPS to Agilent HRMS data. Furthermore, all co-authors would like to thank our colleagues throughout the world who are adding their analytical data to HighResNPS.com.
Footnotes
https://www.mzcloud.org/ (accessed December 19, 2018).
https://www.policija.si/apps/nfl_response_web/seznam.php (accessed December 19, 2018).
URL: www.HighResNPS.com (accessed January 3, 2019).
URL: https://ednd.emcdda.europa.eu (accessed December 19, 2018).
https://www.mzcloud.org/ (accessed December 19, 2018).