-
PDF
- Split View
-
Views
-
Cite
Cite
Paula Proença, Carla Monteiro, Carla Mustra, Alda Claro, João Franco, Francisco Corte-Real, Identification and Quantification of Antipsychotics in Blood Samples by LC–MS-MS: Case Reports and Data from Three Years of Routine Analysis , Journal of Analytical Toxicology, Volume 44, Issue 8, October 2020, Pages 915–922, https://doi.org/10.1093/jat/bkaa100
Close - Share Icon Share
Abstract
Antipsychotic drugs (AP) are widely prescribed for the treatment of schizophrenia and psychosis. The pharmacological treatment of schizophrenia is often performed with the simultaneous use of two or more antipsychotic agents to achieve the desired control of psychotic symptoms Available AP include both conventional (typical) and new (atypical) antipsychotic medications. Atypical AP, such as quetiapine, now account for the vast majority of AP prescriptions. In forensic toxicology, AP are of considerable interest because of their potential abuse and their involvement in intoxications and suicides. The authors retrospectively examined AP positive cases detected in samples collected during autopsies performed in the Forensic Clinical and Pathology Service of National Institute of Legal Medicine and Forensic Sciences Centre Branch or in other autopsies carried out in the central region of Portugal, between January 2016 and December 2018. A quantitative liquid chromatography–tandem mass spectrometry assay was developed for the simultaneous determination of 16 AP (amisulpride, aripiprazole, chlorpromazine, clozapine, cyamemazine, fluphenazine, haloperidol, levomepromazine, melperone, olanzapine, paliperidone, promethazine, quetiapine, risperidone, sulpiride and ziprasidone) in blood samples of postmortem cases. The Laboratory of Forensic Chemistry and Toxicology received 3,588 requests for toxicological analysis: 1,413 cases were positive for drugs from which 351 (24.8%) cases were positive for AP, 60.1% from male individuals and 39.9% from female. Quetiapine was the most prevalent AP (36.5%) followed by olanzapine (20.8%). During this period, there were 25 postmortem cases with AP blood concentrations above therapeutic range, in which 36% of those are in agreement with the information received (psychological history or acute intoxication suspicion) and the manner of death was suicide. Our results point that antipsychotics are an increasingly prevalent class of drugs. AP must be measured not only in toxic concentrations but also in therapeutic levels in postmortem cases; therefore, it is important to come up with a sensitive method to cover the low therapeutic range in which AP are usually present.
Introduction
Antipsychotic drugs (AP) are used to control a wide range of severe psychiatric disorders including schizophrenia and other psychoses, bipolar disorder, severe anxiety and depression, behavioral disorders and dementia (1).
The AP drugs are a heterogeneous group of compounds that can be divided into subgroups based on their chemical structure. The typical AP, also known as first-generation drugs, are represented mainly by phenothiazine derivatives, butyrophenones and thioxanthenes. Those drugs have a high affinity for D2 receptors (e.g., fluphenazine and haloperidol), and due to that pharmacological action, they carry relatively high risks of extrapyramidal symptoms, even at moderate doses (2–4).
The discovery of clozapine’s unique clinical features and binding profile has led to the development of second-generation AP that potently antagonize the 5HT2A receptor while possessing less affinity for D2 receptors than typical AP agents (5). Although atypical AP cause significantly milder extrapyramidal symptoms than typical AP, the former is associated with a higher incidence of other adverse effects, such as metabolic dysfunction, weight gain, sedation and an increase risk of mortality due to sudden death usually attributed to QT prolongation and increased risk of torsade de pointes and potentially lethal arrhythmias (1, 6, 7).
The pharmacokinetics of atypical AP are similar to that of conventional agents. AP are rapidly and completely absorbed after oral administration. Most antipsychotics are highly lipophilic and accumulate in the brain and in other organs with a rich blood supply. They are also highly protein bound and often undergo extensive first-pass hepatic metabolism. Time to peak plasma concentration ranges from 1 to 10 hours and have large volumes of distribution. Long elimination half-lives of AP allow for once to twice-daily dosing (2, 8, 9).
In Portugal, between 2008 and 2017, there has been a rise in the consumption of AP, which keeps up with the trend reported previously by INFARMED, I.P. (10), and the atypical antipsychotics are the most consumed group (11). In a forensic setting, the detection of AP is of considerable interest because of their potential abuse and their involvement in intoxications and suicides, since it is not infrequent to detect AP in postmortem samples (12). However, the diagnosis of fatal intoxication is a challenging task, mainly because the reference information about some substances is scarce or not available, and because the potential synergic effects of the different substances found in biological matrices still need to be clarified (13).
Liquid chromatography–tandem mass spectrometry (LC–MS-MS) has proven to be a powerful tool for determining drug concentrations in biological matrices, and several LC–MS-MS methods for the determination of AP have previously been published (13–19). Some authors (20–23) presented an overview of bioanalytical methods for the determination of AP.
In this study, we evaluate the detection of AP in postmortem blood samples in order to provide more details related to the toxicological analysis because these drugs play an important role in the cause of death.
Materials and Methods
Study population
This is a retrospective observational study based on case reports and data of 3 years of toxicological analysis from the Portuguese population-based registers. We have focused our study in the AP positive cases detected in blood samples collected during autopsies performed in the Forensic Clinical and Pathology Service of National Institute of Legal Medicine and Forensic Sciences Centre Branch or in other autopsies carried out in the central region of Portugal.
Between January 2016 and December 2018, the Laboratory of Forensic Chemistry and Toxicology received 3,588 requests for toxicological analysis: 1,413 cases were positive for drugs (including antipsychotics, cardiac drugs, anticonvulsants, antidepressants and antihypertensives) from which 351 cases were positive for AP.
The assessment of the examination protocols was conducted by means of a tabular database according to the following criteria: year, age, sex, manner of death and/or cause of intoxication information, and AP drugs detected.
Chemicals and materials
Aripiprazole, chlorpromazine, clozapine, fluphenazine, haloperidol, olanzapine, promethazine, quetiapine and risperidone were obtained from Cerilliant (Round Rock, TX, USA). Levomepromazine, melperone, paliperidone 9-hydroxyrisperidone, sulpiride and ziprasidone were provided by Lipomed (Arlesheim, Switzerland). Amisulpride was purchased from LGC Standards (Luckenwalde, Germany) and cyamemazine was from Sigma-Aldrich (Saint Louis, MO, USA). The deuterated internal standards (IS) clomipramina-d3, olanzapine-d8 and zolpidem-d6 were purchased from Cerilliant. Formic acid extra pure (98–100%) was from Merck (Darmstadt, Germany). Acetonitrile, methanol and water LC–MS grade were purchased from Honeywell Riedel-de-Haĕn (Seelze, Germany). Oasis HLB® SPE cartridges (60 mg, 3 mL), used for sample preparation, were from Waters® Corporation (Milford, MA, USA) and were used on a solid-phase system Vac Elut EPS 24 from Varian (Harbor City, CA, USA).
The working standard solutions were prepared by dilution of stock solutions to appropriate concentrations. All solutions were stored at −20°C.
Blood samples
The antemortem blood samples, with whole blood stored in saline-adenine-glucose-mannitol, were obtained from the Portuguese Blood Institute (Coimbra, Portugal). The postmortem blood samples were obtained from autopsies performed at the National Institute of Legal Medicine and Forensic Sciences and were collected into plastic tubes containing 1% potassium fluoride. All the samples were stored at −20°C prior to analysis and were previously screened to verify that they were drug-free.
LC conditions
A Waters Acquity Ultra Performance LC system (Waters® Corporation) composed of a binary solvent delivery manager, a thermostatted autosampler and column oven compartment was used. Separation was performed with an Acquity UPLC® HSST3 column, 2.1 × 100 mm, packed with 1.8 μm particles, which was maintained at 35°C.
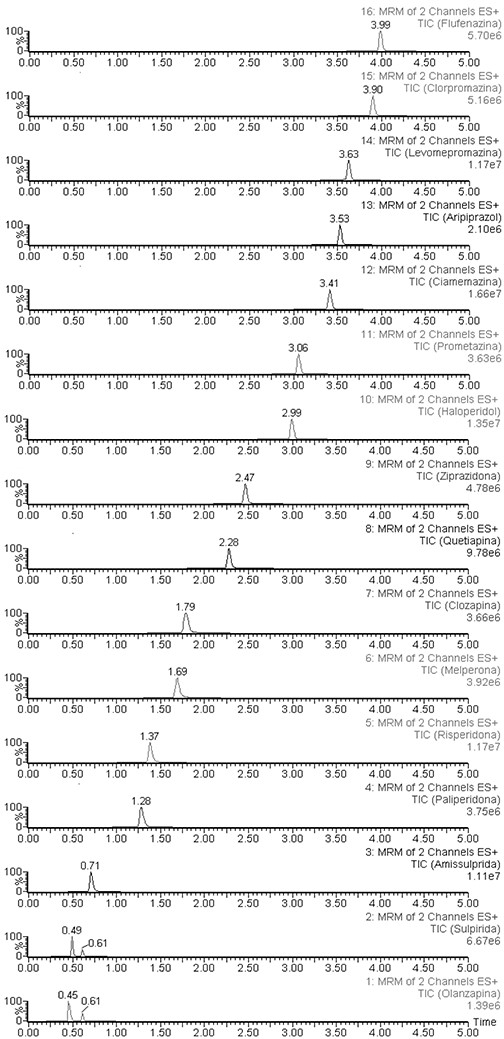
The mobile phase, consisting of acetonitrile (solvent A) and 0.1% formic acid in water (solvent B), was delivered at a flow rate of 0.50 mL/min. The gradient program was as follows: initial 75% B, during 1 min, then gradient elution was performed by changing the mobile phase from 75% to 40% B between 1 and 6 min, and after this time, reversion of the mobile phase to 75% B. At the end of this sequence, the column was equilibrated under initial conditions for 1 min. The autosampler temperature was set at 15°C, and the injection volume was 10 μL using partial loop injection with needle overfill flush. The system was equipped with strong and weak wash solution reservoirs. Figure 1 shows the chromatograms of the 16 AP.
MRM chromatograms of a blood sample with the 16 AP drugs fortified at 50 µg/L.
MS-MS conditions
Detection was carried out using an Acquity™ TQD tandem-quadrupole MS equipped with a Z-spray electrospray ionization source (Waters® Corporation).
Electrospray ionization-MS-MS detection was performed in the multiple reaction monitoring (MRM) mode using positive ionization. Parameters were as follows: capillary voltage was set to 3.0 kV, extractor voltage to 3.0 V and the source temperature to 150°C. Nitrogen obtained from a nitrogen generator (99.93%, Domnick hunter, N2MID 600, England) was used for desolvation, delivered at a temperature of 450°C and a gas flow rate of 900 L/h. The cone gas was set to 0 L/h; desolvation and collision gas (argon) flow was set to 0.21 mL/min in the collision cell.
The MRM transitions, cone voltages and collision energies for the different analytes were optimized by direct infusion into the MS. For each analyte, the most intense ion transition was used for quantitation and the second one to confirm its identification. Retention time, cone voltage, collision energy and MRM transitions for amisulpride, aripiprazole, chlorpromazine, clozapine, cyamemazine, fluphenazine, haloperidol, levomepromazine, melperone, olanzapine, paliperidone, promethazine, quetiapine, risperidone, sulpiride, ziprasidone and IS are listed in Table I.
Retention Time (RT), Cone Voltage (CV), Optimized Ion Transitions, Collision Energy (CE) for Analytes and Internal Standards (IS)
| Analyte . | RT (min) . | CV (V) . | Target ion transition (m/z) . | CE (eV) . | Qualifier ion transition (m/z) . | CE (eV) . |
|---|---|---|---|---|---|---|
| Amisulpride | 0.71 | 60 | 370.1 > 242 | 30 | 370.1 > 195.9 | 41 |
| Aripiprazole | 3.53 | 40 | 448 > 285.2 | 20 | 448 > 176.1 | 20 |
| Chlorpromazine | 3.90 | 20 | 319.2 > 86.2 | 15 | 319.2 > 58.2 | 30 |
| Clomipramine-d3 (IS) | 3.86 | 30 | 318.1 > 88.8 | 20 | – | |
| Clozapine | 1.79 | 40 | 327.3 > 270.3 | 25 | 327.3 > 192.1 | 35 |
| Cyamemazine | 3.41 | 30 | 341.1 > 100.2 | 18 | 341.1 > 58.1 | 30 |
| Fluphenazine | 3.99 | 55 | 438 > 70 | 40 | 438 > 143.1 | 40 |
| Haloperidol | 2.99 | 40 | 376.3 > 165.2 | 26 | 376.3 > 122.9 | 30 |
| Levomepromazine | 3.63 | 41 | 329.1 > 100.2 | 25 | 329.1 > 58.1 | 59 |
| Melperone | 1.69 | 50 | 264 > 165.2 | 20 | 264 > 123.1 | 20 |
| Olanzapine | 0.45 | 20 | 313.1 > 256.3 | 25 | 313.1 > 84.4 | 20 |
| Olanzapine-d8 (IS) | 0.45 | 50 | 321.1 > 261 | 20 | – | |
| Paliperidone | 1.28 | 55 | 427.2 > 270.2 | 16 | 427.2 > 110 | 30 |
| Promethazine | 3.06 | 36 | 285.2 > 86.2 | 27 | 285.2 > 198.1 | 35 |
| Quetiapine | 2.28 | 46 | 384.3 > 253.2 | 20 | 384.3 > 221.2 | 30 |
| Risperidone | 1.37 | 46 | 411.2 > 191.2 | 26 | 411.2 > 110.2 | 52 |
| Sulpiride | 0.49 | 50 | 242.2 > 112.2 | 37 | 242.2 > 214.1 | 45 |
| Ziprasidone | 2.43 | 40 | 413 > 194 | 22 | 413 > 159.2 | 30 |
| Zolpidem-d6 (IS) | 1.38 | 56 | 314.5 > 235.3 | 38 | – |
| Analyte . | RT (min) . | CV (V) . | Target ion transition (m/z) . | CE (eV) . | Qualifier ion transition (m/z) . | CE (eV) . |
|---|---|---|---|---|---|---|
| Amisulpride | 0.71 | 60 | 370.1 > 242 | 30 | 370.1 > 195.9 | 41 |
| Aripiprazole | 3.53 | 40 | 448 > 285.2 | 20 | 448 > 176.1 | 20 |
| Chlorpromazine | 3.90 | 20 | 319.2 > 86.2 | 15 | 319.2 > 58.2 | 30 |
| Clomipramine-d3 (IS) | 3.86 | 30 | 318.1 > 88.8 | 20 | – | |
| Clozapine | 1.79 | 40 | 327.3 > 270.3 | 25 | 327.3 > 192.1 | 35 |
| Cyamemazine | 3.41 | 30 | 341.1 > 100.2 | 18 | 341.1 > 58.1 | 30 |
| Fluphenazine | 3.99 | 55 | 438 > 70 | 40 | 438 > 143.1 | 40 |
| Haloperidol | 2.99 | 40 | 376.3 > 165.2 | 26 | 376.3 > 122.9 | 30 |
| Levomepromazine | 3.63 | 41 | 329.1 > 100.2 | 25 | 329.1 > 58.1 | 59 |
| Melperone | 1.69 | 50 | 264 > 165.2 | 20 | 264 > 123.1 | 20 |
| Olanzapine | 0.45 | 20 | 313.1 > 256.3 | 25 | 313.1 > 84.4 | 20 |
| Olanzapine-d8 (IS) | 0.45 | 50 | 321.1 > 261 | 20 | – | |
| Paliperidone | 1.28 | 55 | 427.2 > 270.2 | 16 | 427.2 > 110 | 30 |
| Promethazine | 3.06 | 36 | 285.2 > 86.2 | 27 | 285.2 > 198.1 | 35 |
| Quetiapine | 2.28 | 46 | 384.3 > 253.2 | 20 | 384.3 > 221.2 | 30 |
| Risperidone | 1.37 | 46 | 411.2 > 191.2 | 26 | 411.2 > 110.2 | 52 |
| Sulpiride | 0.49 | 50 | 242.2 > 112.2 | 37 | 242.2 > 214.1 | 45 |
| Ziprasidone | 2.43 | 40 | 413 > 194 | 22 | 413 > 159.2 | 30 |
| Zolpidem-d6 (IS) | 1.38 | 56 | 314.5 > 235.3 | 38 | – |
Retention Time (RT), Cone Voltage (CV), Optimized Ion Transitions, Collision Energy (CE) for Analytes and Internal Standards (IS)
| Analyte . | RT (min) . | CV (V) . | Target ion transition (m/z) . | CE (eV) . | Qualifier ion transition (m/z) . | CE (eV) . |
|---|---|---|---|---|---|---|
| Amisulpride | 0.71 | 60 | 370.1 > 242 | 30 | 370.1 > 195.9 | 41 |
| Aripiprazole | 3.53 | 40 | 448 > 285.2 | 20 | 448 > 176.1 | 20 |
| Chlorpromazine | 3.90 | 20 | 319.2 > 86.2 | 15 | 319.2 > 58.2 | 30 |
| Clomipramine-d3 (IS) | 3.86 | 30 | 318.1 > 88.8 | 20 | – | |
| Clozapine | 1.79 | 40 | 327.3 > 270.3 | 25 | 327.3 > 192.1 | 35 |
| Cyamemazine | 3.41 | 30 | 341.1 > 100.2 | 18 | 341.1 > 58.1 | 30 |
| Fluphenazine | 3.99 | 55 | 438 > 70 | 40 | 438 > 143.1 | 40 |
| Haloperidol | 2.99 | 40 | 376.3 > 165.2 | 26 | 376.3 > 122.9 | 30 |
| Levomepromazine | 3.63 | 41 | 329.1 > 100.2 | 25 | 329.1 > 58.1 | 59 |
| Melperone | 1.69 | 50 | 264 > 165.2 | 20 | 264 > 123.1 | 20 |
| Olanzapine | 0.45 | 20 | 313.1 > 256.3 | 25 | 313.1 > 84.4 | 20 |
| Olanzapine-d8 (IS) | 0.45 | 50 | 321.1 > 261 | 20 | – | |
| Paliperidone | 1.28 | 55 | 427.2 > 270.2 | 16 | 427.2 > 110 | 30 |
| Promethazine | 3.06 | 36 | 285.2 > 86.2 | 27 | 285.2 > 198.1 | 35 |
| Quetiapine | 2.28 | 46 | 384.3 > 253.2 | 20 | 384.3 > 221.2 | 30 |
| Risperidone | 1.37 | 46 | 411.2 > 191.2 | 26 | 411.2 > 110.2 | 52 |
| Sulpiride | 0.49 | 50 | 242.2 > 112.2 | 37 | 242.2 > 214.1 | 45 |
| Ziprasidone | 2.43 | 40 | 413 > 194 | 22 | 413 > 159.2 | 30 |
| Zolpidem-d6 (IS) | 1.38 | 56 | 314.5 > 235.3 | 38 | – |
| Analyte . | RT (min) . | CV (V) . | Target ion transition (m/z) . | CE (eV) . | Qualifier ion transition (m/z) . | CE (eV) . |
|---|---|---|---|---|---|---|
| Amisulpride | 0.71 | 60 | 370.1 > 242 | 30 | 370.1 > 195.9 | 41 |
| Aripiprazole | 3.53 | 40 | 448 > 285.2 | 20 | 448 > 176.1 | 20 |
| Chlorpromazine | 3.90 | 20 | 319.2 > 86.2 | 15 | 319.2 > 58.2 | 30 |
| Clomipramine-d3 (IS) | 3.86 | 30 | 318.1 > 88.8 | 20 | – | |
| Clozapine | 1.79 | 40 | 327.3 > 270.3 | 25 | 327.3 > 192.1 | 35 |
| Cyamemazine | 3.41 | 30 | 341.1 > 100.2 | 18 | 341.1 > 58.1 | 30 |
| Fluphenazine | 3.99 | 55 | 438 > 70 | 40 | 438 > 143.1 | 40 |
| Haloperidol | 2.99 | 40 | 376.3 > 165.2 | 26 | 376.3 > 122.9 | 30 |
| Levomepromazine | 3.63 | 41 | 329.1 > 100.2 | 25 | 329.1 > 58.1 | 59 |
| Melperone | 1.69 | 50 | 264 > 165.2 | 20 | 264 > 123.1 | 20 |
| Olanzapine | 0.45 | 20 | 313.1 > 256.3 | 25 | 313.1 > 84.4 | 20 |
| Olanzapine-d8 (IS) | 0.45 | 50 | 321.1 > 261 | 20 | – | |
| Paliperidone | 1.28 | 55 | 427.2 > 270.2 | 16 | 427.2 > 110 | 30 |
| Promethazine | 3.06 | 36 | 285.2 > 86.2 | 27 | 285.2 > 198.1 | 35 |
| Quetiapine | 2.28 | 46 | 384.3 > 253.2 | 20 | 384.3 > 221.2 | 30 |
| Risperidone | 1.37 | 46 | 411.2 > 191.2 | 26 | 411.2 > 110.2 | 52 |
| Sulpiride | 0.49 | 50 | 242.2 > 112.2 | 37 | 242.2 > 214.1 | 45 |
| Ziprasidone | 2.43 | 40 | 413 > 194 | 22 | 413 > 159.2 | 30 |
| Zolpidem-d6 (IS) | 1.38 | 56 | 314.5 > 235.3 | 38 | – |
System operation and data acquisition were controlled using Masslynx™ v4.2 SNC 989 software (Waters® Corporation) with automated data processing using the TargetLynx™ Application Manager (Waters® Corporation). IntelliStart™ software (Waters® Corporation) was used to control the fluidics device to infuse solutions for tuning the MS.
Sample preparation
The quality control (QC) and the calibration samples were prepared by spiking drug-free blood samples with methanolic working standard solutions.
Qualitative analysis was performed in central blood sample (clomipramine-d3 as IS), and peripheral blood was selected for the corresponding quantitative analysis (zolpidem-d6 used as IS and olanzapine-d8).
A 0.5 mL aliquot of blood sample was spiked with 50 µL of IS at a concentration of 100 µg/L, and 2 mL of water was added. The mixture was vortexed for 30 s following centrifugation at 1670 × g for 5 min and loaded into HLB cartridge, which has been conditioned with 2 mL methanol followed by 2 mL water. The washing of the cartridges was carried out with 5% (v/v) aqueous methanol (2 mL). Vacuum was applied on the solid-phase system for 10 min until dryness of solvent. The analytes were eluted with 2 mL methanol. The eluate was evaporated at 40°C until dryness under a gentle stream of nitrogen using a Caliper Turbo Vap LV evaporator (Caliper, Hopkinton, MA, USA). The dried extract was then reconstituted with 150 μL of mobile phase [25:75, (v/v) acetonitrile/0.1% formic acid in water], and 10 μL was injected into the LC–MS-MS system.
Method validation
The described procedure was validated according to internationally accepted recommendations (24–26).
The selectivity and capacity of identification were ensured by a combination of relative retention time and ion ratio criteria (27) that guaranteed the substance identification for the positive samples and by the analysis of the negative samples that showed no peaks that could interfere with the detection of the compounds of interest. Ten pools of drug-free blood samples, nine consisting of a mixture of four postmortem blood samples and one prepared with three antemortem blood samples, were extracted as described previously without the addition of IS. In addition, a blank sample with IS was analyzed for peaks deriving from IS and interfering with the detection of the analytes. The assay was found to be selective and specific for all tested compounds; no interfering peaks were observed.
There were no distinct differences in ion-suppression or enhancement effects for antemortem and postmortem blood samples. Therefore, it was possible to conclude that the method did not present significant matrix effects.
Calibration curves were linear in a concentration range of 5–500 µg/L and a linear regression (1/x2 weighting) was used for all drugs. The calibration fit showed a coefficient of determination of r2 > 0.99 for all AP.
The limit of quantification was defined as the lowest point of calibration curve.
QC samples were prepared at the concentration of 50 and 200 µg/L. Three samples of each QC concentration were measured over a period of 5 consecutive days. Precision data for within-day (repeatability) and time-different intermediate precision (combination of within and between-day effect) were calculated using one-way analysis of variance with the grouping-variable day. Repeatability was estimated in a single assay by extraction and analysis of QC samples with five replicates at each concentration. Repeatability and intermediate precision values expressed as relative standard deviation (RSD) % were lower than 15%, which is within the acceptance criteria for validating analytical methods.
Accuracy data were within the acceptance interval of ±15% of the nominal values for all drugs.
As previously described (14, 28–30), olanzapine showed instability in several validation experiments due to its degradation rate. To prevent degradation of olanzapine, all samples including extracted standards were protected from light and olanzapine was analyzed by LC–MS-MS quickly after extraction.
Statistical analyses
Statistical analyses were conducted using the IBM Statistical Package for Social Sciences, version 25 for Windows (IBM, Armonk, NY, USA). Levene test was performed to compare continuous and normally distributed variable and categorical variable. Statistical significance was accepted at the P < 0.05.
Results and Discussion
A total of 1,413 positive drug cases (including antipsychotics, cardiac drugs, anticonvulsants, antidepressants and antihypertensives) from central Portugal were obtained between January 2016 and December 2018. Of these, 351 cases yielded positive results for antipsychotics (24.8%) (Table II). Over these 3 years, there was a rise in the detection of AP drugs (Table II) as it was identified in the nationwide trend of the consumption of AP drugs from 2008 and 2017 (11).
Number of Positive Analysis for Drugs and for AP Between January 2016 and December 2018
| Year . | Positive cases for drugs | Positive cases for AP . | ||
|---|---|---|---|---|
| Total, n (%) . | Male, n (%) . | Female, n (%) . | ||
| 2016 | 509 | 112 (20.0) | 70 (62.5) | 42 (37.5) |
| 2017 | 454 | 113 (24.9) | 68 (60.2) | 45 (39.8) |
| 2018 | 450 | 126 (28.0) | 74 (58.7) | 52 (41.3) |
| Total | 1,413 | 351 (24.8) | 211 (60.1) | 140 (39.9) |
| Year . | Positive cases for drugs | Positive cases for AP . | ||
|---|---|---|---|---|
| Total, n (%) . | Male, n (%) . | Female, n (%) . | ||
| 2016 | 509 | 112 (20.0) | 70 (62.5) | 42 (37.5) |
| 2017 | 454 | 113 (24.9) | 68 (60.2) | 45 (39.8) |
| 2018 | 450 | 126 (28.0) | 74 (58.7) | 52 (41.3) |
| Total | 1,413 | 351 (24.8) | 211 (60.1) | 140 (39.9) |
Number of Positive Analysis for Drugs and for AP Between January 2016 and December 2018
| Year . | Positive cases for drugs | Positive cases for AP . | ||
|---|---|---|---|---|
| Total, n (%) . | Male, n (%) . | Female, n (%) . | ||
| 2016 | 509 | 112 (20.0) | 70 (62.5) | 42 (37.5) |
| 2017 | 454 | 113 (24.9) | 68 (60.2) | 45 (39.8) |
| 2018 | 450 | 126 (28.0) | 74 (58.7) | 52 (41.3) |
| Total | 1,413 | 351 (24.8) | 211 (60.1) | 140 (39.9) |
| Year . | Positive cases for drugs | Positive cases for AP . | ||
|---|---|---|---|---|
| Total, n (%) . | Male, n (%) . | Female, n (%) . | ||
| 2016 | 509 | 112 (20.0) | 70 (62.5) | 42 (37.5) |
| 2017 | 454 | 113 (24.9) | 68 (60.2) | 45 (39.8) |
| 2018 | 450 | 126 (28.0) | 74 (58.7) | 52 (41.3) |
| Total | 1,413 | 351 (24.8) | 211 (60.1) | 140 (39.9) |
Among the postmortem cases, males accounted for 60.1% (n = 211) of the positive cases for AP (Table II). The mean age was 59.74 years old (SD = 16.94) and was not statistically significant in both genders (56.63 ± 16.63 in males vs 64.42 ± 16.36 in females, P > 0.05), ensuring equality of variables.
Table III presents the total number of positive AP cases subdivided according to the manner of death. Suicide was the most common cause of death (n = 99, 28.2%) followed by natural death (n = 52, 14.8%), in agreement with several studies which reveal that suicide is considered to be the more prevalent cause of death among individuals suffering from psychiatric disorders (31, 32). There was only one homicide reported among the cases. In the majority of the cases, we were unable to collect the information about the type of death (n = 174, 49.6%).
Manner of Death and Intoxication Information of Positive AP Cases
| Year . | Manner of death . | |||||
|---|---|---|---|---|---|---|
| Suicide, n (%) . | Accident, n (%) . | Homicide, n (%) . | Natural, n (%) . | Unknown, n (%) . | Positive cases for AP . | |
| 2016 | 34 (30.4) | 11 (9.8) | - | 16 (12.3) | 51 (45.5) | 112 |
| 2017 | 31 (27.4) | 10 (8.8) | 1 (0.9) | 14 (12.4) | 57 (50.4) | 113 |
| 2018 | 34 (26.9) | 4 (3.2) | - | 22 (17.5) | 66 (52.4) | 126 |
| Total | 99 (28.2) | 25 (7.1) | 1 (0.3) | 52 (14.8) | 174 (49.6) | 351 |
| Year . | Manner of death . | |||||
|---|---|---|---|---|---|---|
| Suicide, n (%) . | Accident, n (%) . | Homicide, n (%) . | Natural, n (%) . | Unknown, n (%) . | Positive cases for AP . | |
| 2016 | 34 (30.4) | 11 (9.8) | - | 16 (12.3) | 51 (45.5) | 112 |
| 2017 | 31 (27.4) | 10 (8.8) | 1 (0.9) | 14 (12.4) | 57 (50.4) | 113 |
| 2018 | 34 (26.9) | 4 (3.2) | - | 22 (17.5) | 66 (52.4) | 126 |
| Total | 99 (28.2) | 25 (7.1) | 1 (0.3) | 52 (14.8) | 174 (49.6) | 351 |
Manner of Death and Intoxication Information of Positive AP Cases
| Year . | Manner of death . | |||||
|---|---|---|---|---|---|---|
| Suicide, n (%) . | Accident, n (%) . | Homicide, n (%) . | Natural, n (%) . | Unknown, n (%) . | Positive cases for AP . | |
| 2016 | 34 (30.4) | 11 (9.8) | - | 16 (12.3) | 51 (45.5) | 112 |
| 2017 | 31 (27.4) | 10 (8.8) | 1 (0.9) | 14 (12.4) | 57 (50.4) | 113 |
| 2018 | 34 (26.9) | 4 (3.2) | - | 22 (17.5) | 66 (52.4) | 126 |
| Total | 99 (28.2) | 25 (7.1) | 1 (0.3) | 52 (14.8) | 174 (49.6) | 351 |
| Year . | Manner of death . | |||||
|---|---|---|---|---|---|---|
| Suicide, n (%) . | Accident, n (%) . | Homicide, n (%) . | Natural, n (%) . | Unknown, n (%) . | Positive cases for AP . | |
| 2016 | 34 (30.4) | 11 (9.8) | - | 16 (12.3) | 51 (45.5) | 112 |
| 2017 | 31 (27.4) | 10 (8.8) | 1 (0.9) | 14 (12.4) | 57 (50.4) | 113 |
| 2018 | 34 (26.9) | 4 (3.2) | - | 22 (17.5) | 66 (52.4) | 126 |
| Total | 99 (28.2) | 25 (7.1) | 1 (0.3) | 52 (14.8) | 174 (49.6) | 351 |
The treatment of psychiatric patients often consists in the use of two or more antipsychotic agents to achieve the desired control of psychotic symptoms. The frequency of each antipsychotic detected in the blood samples is presented in Table IV. Quetiapine was the most prevalent AP (n = 128, 36.5%) followed by olanzapine (n = 73, 20.8%).
Overview of Single Substance Findings in Positive AP Cases
| Antipsychotic . | Number of cases in which the antipsychotic was detected (n = 351) n (%) . |
|---|---|
| Quetiapine | 128 (36.5) |
| Olanzapine | 73 (20.8) |
| Haloperidol | 53 (15.0) |
| Cyamemazine | 37 (10.5) |
| Clozapine | 29 (8.3) |
| Risperidone | 23 (6.5) |
| Levomepromazine | 18 (5.1) |
| Chlorpromazine | 17 (4.8) |
| Others AP | 46 (13.1) |
| Antipsychotic . | Number of cases in which the antipsychotic was detected (n = 351) n (%) . |
|---|---|
| Quetiapine | 128 (36.5) |
| Olanzapine | 73 (20.8) |
| Haloperidol | 53 (15.0) |
| Cyamemazine | 37 (10.5) |
| Clozapine | 29 (8.3) |
| Risperidone | 23 (6.5) |
| Levomepromazine | 18 (5.1) |
| Chlorpromazine | 17 (4.8) |
| Others AP | 46 (13.1) |
Overview of Single Substance Findings in Positive AP Cases
| Antipsychotic . | Number of cases in which the antipsychotic was detected (n = 351) n (%) . |
|---|---|
| Quetiapine | 128 (36.5) |
| Olanzapine | 73 (20.8) |
| Haloperidol | 53 (15.0) |
| Cyamemazine | 37 (10.5) |
| Clozapine | 29 (8.3) |
| Risperidone | 23 (6.5) |
| Levomepromazine | 18 (5.1) |
| Chlorpromazine | 17 (4.8) |
| Others AP | 46 (13.1) |
| Antipsychotic . | Number of cases in which the antipsychotic was detected (n = 351) n (%) . |
|---|---|
| Quetiapine | 128 (36.5) |
| Olanzapine | 73 (20.8) |
| Haloperidol | 53 (15.0) |
| Cyamemazine | 37 (10.5) |
| Clozapine | 29 (8.3) |
| Risperidone | 23 (6.5) |
| Levomepromazine | 18 (5.1) |
| Chlorpromazine | 17 (4.8) |
| Others AP | 46 (13.1) |
With regard to suicide, quetiapine was detected in 49 cases (49.5%) and olanzapine in 24 cases (24.2%). Both data are in concordance with AP consumption in Portugal, where quetiapine was the most consumed AP in 10-year period (2008–2017), followed by olanzapine, risperidone and haloperidol (11). Söderberg et al. present a similar study with AP in a Sweden population (12). According to that study, quetiapine is more associated with accidental intoxication cases, while clozapine is related to suicidal cases.
During this period, there were 25 postmortem cases with AP blood concentrations above therapeutic range (33, 34). The results of antipsychotic blood concentrations, the manner of death and other relevant findings of each of the cases are given in Table V. Both typical AP (cyamemazine and levomepromazine) and atypical AP (clozapine, olanzapine and quetiapine) were present in high concentrations. The femoral blood was used in order to confirm and quantify the AP present in these postmortem cases. As already known in the literature, heart blood concentrations are significantly higher than those measured in femoral blood; for some AP, it is 3.4-fold on average (35, 36).
Positive Findings in 25 Postmortem Cases with Blood AP Concentrations above Therapeutic Range
| Case . | AP found . | Conc.a (µg/L) . | Age (sex) . | Information received . | Manner of death . | Other substances . |
|---|---|---|---|---|---|---|
| 1 | Olanzapine | 1,382 | 53 (F) | Intoxication | Suicide | Quetiapine; risperidone; BZD |
| 2 | Olanzapine | 886 | 59 (F) | Intoxication | Suicide | Paroxetine; trazodone; BZD |
| 3 | Olanzapine | 950 | 54 (M) | Unknown | Natural | Fluvoxamine; risperidone; tramadol; trazodone; BZD |
| 4 | Olanzapine | 563 | 62 (M) | Bipolar disorder | Natural | Clozapine; BZD |
| 5 | Olanzapine | 816 | 61 (F) | Bipolar disorder | Suicide | Carbamazepine; BZD |
| 6 | Olanzapine | 1,245 | 57 (M) | Unknown | Unknown | Cyamemazine; haloperidol |
| 7 | Olanzapine | 891 | 53 (M) | Hanging | Suicide | Fluvoxamine; BZD |
| 8 | Olanzapine | 843 | 62 (M) | Hanging | Suicide | Mirtazapine; trazodone; BZD |
| 9 | Olanzapine | 2,183 | 46 (M) | Intoxication | Suicide | Fluoxetine; BZD |
| 10 | Quetiapine | 1,104 | 46 (F) | Unknown | Unknown | Paroxetine; trazodone; BZD |
| 11 | Quetiapine | 10,384 | 47 (F) | Unknown | Suicide | Mirtazapine; sertraline; tramadol |
| 12 | Quetiapine | 3,829 | 52 (F) | Intoxication | Suicide | Levetiracetam; BZD |
| 13 | Quetiapine | 2,648 | 49 (M) | Intoxication | Suicide | BZD |
| 14 | Quetiapine | 2,383 | 30 (M) | Manic depression Epilepsy | Natural | Carbamazepine; trazodone |
| 15 | Quetiapine | 4,518 | 46 (F) | Unknown | Unknown | Fluoxetine |
| 16 | Quetiapine | 1,216 | 75 (M) | Intoxication Depression | Suicide | BZD |
| 17 | Quetiapine | 2,266 | 42 (M) | Schizophrenia Manic depression | Unknown | - |
| 18 | Quetiapine | 963 | 26 (M) | Intoxication | Natural | Mirtazapine; BZD |
| 19 | Clozapine | 1,049 | 57 (F) | Intoxication | Suicide | Olanzapine |
| 20 | Clozapine | 1,084 | 66 (F) | Schizophrenia Manic depression | Unknown | Citalopram; BZD |
| 21 | Clozapine | 1,754 | 61 (M) | Unknown | Unknown | Mirtazapine; tramadol; phenobarbital; primidone |
| 22 | Cyamemazine | 804 | 50 (M) | Intoxication Unspecified psych. disorder | Unknown | Olanzapine; sertraline; BZD |
| 23 | Cyamemazine | 2,615 | 48 (M) | Intoxication | Unknown | BZD |
| 24 | Cyamemazine | 1,428 | 49 (F) | Intoxication | Suicide | Venlafaxine |
| 25 | Levomepromazine | 1,152 | 42 (F) | Unspecified psych. disorder | Accident | Fluoxetine; haloperidol; BZD |
| Case . | AP found . | Conc.a (µg/L) . | Age (sex) . | Information received . | Manner of death . | Other substances . |
|---|---|---|---|---|---|---|
| 1 | Olanzapine | 1,382 | 53 (F) | Intoxication | Suicide | Quetiapine; risperidone; BZD |
| 2 | Olanzapine | 886 | 59 (F) | Intoxication | Suicide | Paroxetine; trazodone; BZD |
| 3 | Olanzapine | 950 | 54 (M) | Unknown | Natural | Fluvoxamine; risperidone; tramadol; trazodone; BZD |
| 4 | Olanzapine | 563 | 62 (M) | Bipolar disorder | Natural | Clozapine; BZD |
| 5 | Olanzapine | 816 | 61 (F) | Bipolar disorder | Suicide | Carbamazepine; BZD |
| 6 | Olanzapine | 1,245 | 57 (M) | Unknown | Unknown | Cyamemazine; haloperidol |
| 7 | Olanzapine | 891 | 53 (M) | Hanging | Suicide | Fluvoxamine; BZD |
| 8 | Olanzapine | 843 | 62 (M) | Hanging | Suicide | Mirtazapine; trazodone; BZD |
| 9 | Olanzapine | 2,183 | 46 (M) | Intoxication | Suicide | Fluoxetine; BZD |
| 10 | Quetiapine | 1,104 | 46 (F) | Unknown | Unknown | Paroxetine; trazodone; BZD |
| 11 | Quetiapine | 10,384 | 47 (F) | Unknown | Suicide | Mirtazapine; sertraline; tramadol |
| 12 | Quetiapine | 3,829 | 52 (F) | Intoxication | Suicide | Levetiracetam; BZD |
| 13 | Quetiapine | 2,648 | 49 (M) | Intoxication | Suicide | BZD |
| 14 | Quetiapine | 2,383 | 30 (M) | Manic depression Epilepsy | Natural | Carbamazepine; trazodone |
| 15 | Quetiapine | 4,518 | 46 (F) | Unknown | Unknown | Fluoxetine |
| 16 | Quetiapine | 1,216 | 75 (M) | Intoxication Depression | Suicide | BZD |
| 17 | Quetiapine | 2,266 | 42 (M) | Schizophrenia Manic depression | Unknown | - |
| 18 | Quetiapine | 963 | 26 (M) | Intoxication | Natural | Mirtazapine; BZD |
| 19 | Clozapine | 1,049 | 57 (F) | Intoxication | Suicide | Olanzapine |
| 20 | Clozapine | 1,084 | 66 (F) | Schizophrenia Manic depression | Unknown | Citalopram; BZD |
| 21 | Clozapine | 1,754 | 61 (M) | Unknown | Unknown | Mirtazapine; tramadol; phenobarbital; primidone |
| 22 | Cyamemazine | 804 | 50 (M) | Intoxication Unspecified psych. disorder | Unknown | Olanzapine; sertraline; BZD |
| 23 | Cyamemazine | 2,615 | 48 (M) | Intoxication | Unknown | BZD |
| 24 | Cyamemazine | 1,428 | 49 (F) | Intoxication | Suicide | Venlafaxine |
| 25 | Levomepromazine | 1,152 | 42 (F) | Unspecified psych. disorder | Accident | Fluoxetine; haloperidol; BZD |
Samples with concentrations above the calibration range were diluted in blank blood before analysis.
Positive Findings in 25 Postmortem Cases with Blood AP Concentrations above Therapeutic Range
| Case . | AP found . | Conc.a (µg/L) . | Age (sex) . | Information received . | Manner of death . | Other substances . |
|---|---|---|---|---|---|---|
| 1 | Olanzapine | 1,382 | 53 (F) | Intoxication | Suicide | Quetiapine; risperidone; BZD |
| 2 | Olanzapine | 886 | 59 (F) | Intoxication | Suicide | Paroxetine; trazodone; BZD |
| 3 | Olanzapine | 950 | 54 (M) | Unknown | Natural | Fluvoxamine; risperidone; tramadol; trazodone; BZD |
| 4 | Olanzapine | 563 | 62 (M) | Bipolar disorder | Natural | Clozapine; BZD |
| 5 | Olanzapine | 816 | 61 (F) | Bipolar disorder | Suicide | Carbamazepine; BZD |
| 6 | Olanzapine | 1,245 | 57 (M) | Unknown | Unknown | Cyamemazine; haloperidol |
| 7 | Olanzapine | 891 | 53 (M) | Hanging | Suicide | Fluvoxamine; BZD |
| 8 | Olanzapine | 843 | 62 (M) | Hanging | Suicide | Mirtazapine; trazodone; BZD |
| 9 | Olanzapine | 2,183 | 46 (M) | Intoxication | Suicide | Fluoxetine; BZD |
| 10 | Quetiapine | 1,104 | 46 (F) | Unknown | Unknown | Paroxetine; trazodone; BZD |
| 11 | Quetiapine | 10,384 | 47 (F) | Unknown | Suicide | Mirtazapine; sertraline; tramadol |
| 12 | Quetiapine | 3,829 | 52 (F) | Intoxication | Suicide | Levetiracetam; BZD |
| 13 | Quetiapine | 2,648 | 49 (M) | Intoxication | Suicide | BZD |
| 14 | Quetiapine | 2,383 | 30 (M) | Manic depression Epilepsy | Natural | Carbamazepine; trazodone |
| 15 | Quetiapine | 4,518 | 46 (F) | Unknown | Unknown | Fluoxetine |
| 16 | Quetiapine | 1,216 | 75 (M) | Intoxication Depression | Suicide | BZD |
| 17 | Quetiapine | 2,266 | 42 (M) | Schizophrenia Manic depression | Unknown | - |
| 18 | Quetiapine | 963 | 26 (M) | Intoxication | Natural | Mirtazapine; BZD |
| 19 | Clozapine | 1,049 | 57 (F) | Intoxication | Suicide | Olanzapine |
| 20 | Clozapine | 1,084 | 66 (F) | Schizophrenia Manic depression | Unknown | Citalopram; BZD |
| 21 | Clozapine | 1,754 | 61 (M) | Unknown | Unknown | Mirtazapine; tramadol; phenobarbital; primidone |
| 22 | Cyamemazine | 804 | 50 (M) | Intoxication Unspecified psych. disorder | Unknown | Olanzapine; sertraline; BZD |
| 23 | Cyamemazine | 2,615 | 48 (M) | Intoxication | Unknown | BZD |
| 24 | Cyamemazine | 1,428 | 49 (F) | Intoxication | Suicide | Venlafaxine |
| 25 | Levomepromazine | 1,152 | 42 (F) | Unspecified psych. disorder | Accident | Fluoxetine; haloperidol; BZD |
| Case . | AP found . | Conc.a (µg/L) . | Age (sex) . | Information received . | Manner of death . | Other substances . |
|---|---|---|---|---|---|---|
| 1 | Olanzapine | 1,382 | 53 (F) | Intoxication | Suicide | Quetiapine; risperidone; BZD |
| 2 | Olanzapine | 886 | 59 (F) | Intoxication | Suicide | Paroxetine; trazodone; BZD |
| 3 | Olanzapine | 950 | 54 (M) | Unknown | Natural | Fluvoxamine; risperidone; tramadol; trazodone; BZD |
| 4 | Olanzapine | 563 | 62 (M) | Bipolar disorder | Natural | Clozapine; BZD |
| 5 | Olanzapine | 816 | 61 (F) | Bipolar disorder | Suicide | Carbamazepine; BZD |
| 6 | Olanzapine | 1,245 | 57 (M) | Unknown | Unknown | Cyamemazine; haloperidol |
| 7 | Olanzapine | 891 | 53 (M) | Hanging | Suicide | Fluvoxamine; BZD |
| 8 | Olanzapine | 843 | 62 (M) | Hanging | Suicide | Mirtazapine; trazodone; BZD |
| 9 | Olanzapine | 2,183 | 46 (M) | Intoxication | Suicide | Fluoxetine; BZD |
| 10 | Quetiapine | 1,104 | 46 (F) | Unknown | Unknown | Paroxetine; trazodone; BZD |
| 11 | Quetiapine | 10,384 | 47 (F) | Unknown | Suicide | Mirtazapine; sertraline; tramadol |
| 12 | Quetiapine | 3,829 | 52 (F) | Intoxication | Suicide | Levetiracetam; BZD |
| 13 | Quetiapine | 2,648 | 49 (M) | Intoxication | Suicide | BZD |
| 14 | Quetiapine | 2,383 | 30 (M) | Manic depression Epilepsy | Natural | Carbamazepine; trazodone |
| 15 | Quetiapine | 4,518 | 46 (F) | Unknown | Unknown | Fluoxetine |
| 16 | Quetiapine | 1,216 | 75 (M) | Intoxication Depression | Suicide | BZD |
| 17 | Quetiapine | 2,266 | 42 (M) | Schizophrenia Manic depression | Unknown | - |
| 18 | Quetiapine | 963 | 26 (M) | Intoxication | Natural | Mirtazapine; BZD |
| 19 | Clozapine | 1,049 | 57 (F) | Intoxication | Suicide | Olanzapine |
| 20 | Clozapine | 1,084 | 66 (F) | Schizophrenia Manic depression | Unknown | Citalopram; BZD |
| 21 | Clozapine | 1,754 | 61 (M) | Unknown | Unknown | Mirtazapine; tramadol; phenobarbital; primidone |
| 22 | Cyamemazine | 804 | 50 (M) | Intoxication Unspecified psych. disorder | Unknown | Olanzapine; sertraline; BZD |
| 23 | Cyamemazine | 2,615 | 48 (M) | Intoxication | Unknown | BZD |
| 24 | Cyamemazine | 1,428 | 49 (F) | Intoxication | Suicide | Venlafaxine |
| 25 | Levomepromazine | 1,152 | 42 (F) | Unspecified psych. disorder | Accident | Fluoxetine; haloperidol; BZD |
Samples with concentrations above the calibration range were diluted in blank blood before analysis.
The mean age of these 25 postmortem cases was 51.72 years (SD = 10.65) and 56.0% (n = 14) of the cases were males. Only two cases had history of psychiatric that disclosed schizophrenia, two cases noted medical history of depression, two cases had information of bipolar disorder and two cases had psychiatric disorders that were unknown or unspecified. In the remaining cases, the information provided indicate intoxication, hanging or was missing.
Taking into account these 25 cases, 36% (n = 9) of those are in agreement with the information received (psychological history or acute intoxication suspicion) and the manner of death was suicide.
Furthermore, in seven cases, there is a concomitant intake of other AP, even though they were all within therapeutic ranges. The simultaneous ingestion of drugs that induce or inhibit cytochrome P450 enzyme systems can affect normal metabolism of drugs and lead to adverse events and potential accidental overdose (8). In two cases (5 and 14), carbamazepine was identified as one of the concomitant medications and it may increase the rate at which the other drug is metabolized (8, 37). It is unlikely that the small concentration of carbamazepine (therapeutic values) would have had a significant impact on the large concentration of olanzapine and quetiapine found in these cases.
In addition, nine of the cases mentioned in Table V had quetiapine concentrations that were considered to be contributing to the cause of death. Of those, only one case had quetiapine as the only drug detected. Some studies found in the literature that evaluated the detection, quantification and analysis of this AP in postmortem blood samples reveal that this drug overdose is the potential cause of death (36–39). Recently, a systematic review on quetiapine abuse and dependence in psychiatric patients (40) indicated that psychiatrists and physicians should be aware of the addictive potential of quetiapine and the phenomenon of its misure and abuse (41–43). In fact, in cases with information on psychiatric treatment, the phenomenon of quetiapine abuse may have been verified, and this may have been one of the causes verified in the case represented with the number 14, being a male person, 30 years old, with a history of epilepsy, depressive syndrome and drug addiction, whose toxicological results revealed a high quetiapine blood concentration, and positive results for carbamazepine, trazodone and cannabinoids, with a blood alcohol concentration of 1.28 g/L.
Olanzapine was also found in nine cases in high blood concentration and, in all of them, other drugs were presented in sufficient concentrations that might have had a synergistic effect for the cause of death. In four cases (1, 3, 4 and 6), olanzapine and other AP were detected, but only olanzapine was in toxic values. In other multidrug-related olanzapine, case 7, fluvoxamine and diazepam were found in high blood concentrations. In this suicide case, the cause of death was hanging. Toxic levels achieved in these postmortem cases (Table V) were similar to previous papers published on olanzapine blood samples results (44, 45).
Finally, the remaining cases mentioned in Table V values of high blood concentrations similar to published papers for these drugs: clozapine (46–48), cyamemazine (49) and levomepromazine (50). Ethanol and other volatile substances were also tested, but only the case 14 was positive to ethanol.
Conclusion
Our results reveal that antipsychotics are an increasingly prevalent class of drugs. AP must be measured not only in toxic concentrations but also in therapeutic levels in postmortem cases; therefore, it is important to come up with a sensitive method to cover the low therapeutic range in which AP are usually present.
This retrospective study has provided significant data on postmortem blood results of AP in both therapeutic and toxic levels in order to better understand this group of drugs.
Conflict of Interest
None.