-
PDF
- Split View
-
Views
-
Cite
Cite
Mika Shirasu, Kazushige Touhara, The scent of disease: volatile organic compounds of the human body related to disease and disorder, The Journal of Biochemistry, Volume 150, Issue 3, September 2011, Pages 257–266, https://doi.org/10.1093/jb/mvr090
Close - Share Icon Share
Abstract
Hundreds of volatile organic compounds (VOCs) are emitted from the human body, and the components of VOCs usually reflect the metabolic condition of an individual. Therefore, contracting an infectious or metabolic disease often results in a change in body odour. Recent progresses in analytical techniques allow rapid analyses of VOCs derived from breath, blood, skin and urine. Disease-specific VOCs can be used as diagnostic olfactory biomarkers of infectious diseases, metabolic diseases, genetic disorders and other kinds of diseases. Elucidation of pathophysiological mechanisms underlying production of disease-specific VOCs may provide novel insights into therapeutic approaches for treatments for various diseases. This review summarizes the current knowledge on chemical and clinical aspects of body-derived VOCs, and provides a brief outlook at the future of olfactory diagnosis.
In the animal kingdom, odours are used to detect food sources and environmental toxins and to distinguish kin and predators. In addition, odours emitted from a body often function as olfactory cues that convey information about the metabolic or psychological status of an individual. For example, studies on experimental animals like rodents suggested that stressed individuals excreted distinctive odours (1, 2). Human body odours also have this function; we emit a wide array of volatile organic compounds (VOCs), both odorous and non-odorous, from our bodies. The VOCs emitted from different areas of the human body vary with age, diet, sex, physiological status and possibly genetic background. Therefore, body odours can be considered as individual ‘odour-fingerprints’.
Pathological processes, such as infection and endogenous metabolic disorders, can influence our daily odour fingerprints by producing new VOCs or by changing the ratio of VOCs that are produced normally. Therefore, it is not surprising that physicians have used their olfactory senses to diagnose physical conditions of patients. Around 400 BD, Hippocrates recognized the diagnostic usefulness of body odours and reported on several disease-specific odours emanated from urine or sputum (3). Despite the potential clinical usefulness of VOCs and body odours, little work has been done to define these compounds as diagnostic criteria, either qualitatively or quantitatively.
During the second-half of the 20th century, gas chromatography (GC) and gas chromatography and mass spectrometry (GC-MS) have been used to separate and indentify VOCs. In the 1990s, a GC-MS-olfactometer (GC-MS-O) was developed, and it has enabled researchers to examine mass spectra and odour qualities of individual GC-separated odourants simultaneously. Using the GC-MS-O, researchers can identify characteristic odorous compounds that are in low abundance in a complex mixture of VOCs from various biological samples (4). These sophisticated analytical techniques have allowed studies to focus on searching for VOCs that represent specific odours from patients. The identified compounds may then have potential as biomarkers that could be useful for diagnosing diseases.
The origins of body odour
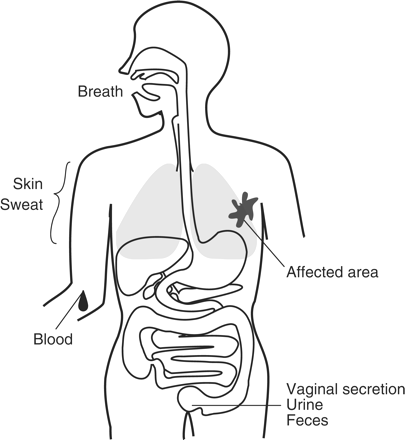
Body odours are a result of the combination of hundreds of emitted odorous VOCs that are originally secreted from various cells inside the body via metabolic pathways. The major sources of VOCs include breath, sweat, skin, urine, faeces and vaginal secretions (Fig. 1). Blood is also an important source of body odours because some metabolically produced VOCs are secreted into blood and eventually emitted to the external environment via breath and/or sweat.
Breath
Exhaled breath contains hundreds of VOCs that can be attributed to either exogenous or endogenous volatiles (5, 6). Exogenous volatiles include compounds inhaled from the external environment, compounds produced following the oral ingestion of food and compounds derived from smoking cigarettes. Endogenous volatiles consist of blood-borne compounds released to the environment via the lungs and/or compounds made from all classes of symbiotic bacteria. The concentrations of VOCs in breath are detected at nanomolar to picomolar levels; therefore, it is always challenging to distinguish contaminant environmental exogenous compounds from endogenously produced VOCs. Nonetheless, collecting breath samples is extremely simple, painless and non-invasive. Consequently, many GC-MS analyses of breath samples have been performed, and in some cases, researchers and clinicians have succeeded in identifying VOCs that are specific to certain diseases. For example, trimethylamine was found in the breath of patients with trimethylaminuria; acetone was found in the breath of patients with diabetes; and methylmercaptan was found in the breath of patients with fetor hepaticus (7, 8) (see the section on disease-specific odours below). Currently, these compounds are useful biomarkers for diagnosis of these diseases.
Skin and sweat
VOCs emitted from the skin surface are mainly derived from sweat, a fluid secreted by the sweat glands and sebum, an oily substance secreted by the sebaceous glands located in the axilla, the perinal region and the areola of the breasts. Although some of these VOCs result from internal hormonal or metabolic changes, many VOCs appear to be derived from symbiotic bacteria that live on the skin surface and metabolize and transform secreted compounds in sweat and sebum. Any alteration in homeostatic balance due, for instance, to some inherited metabolic disorder or bacterial infection of the diseased area can induce changes in both the quality and quantity of VOCs. For example, some infectious diseases or cancerous wounds develop characteristic, offensive odours. Samples are easily obtained by wiping the subject's skin with an organic solvent such as acetone or by collecting VOCs emitted from the affected area directly onto an absorbent solid-phase microextraction (SPME) fibre. However, care must be taken during sample collection to avoid contamination from the ambient air or from cosmetics.
Urine
Urinary components and urine profiles have been well characterized because they are useful for diagnosis of disease (9). The substances in urine are intermediate products or end products of a number of metabolic pathways, and these substances contain a variety of structural motifs, such as ketone, alcohol, furan, pyrrole and sulphide, which often cause a particular odour. In some cases, characteristic urine odours have been directly linked to particular metabolic disorders, and the causes of the odours have been identified.
Urinary VOC patterns in cancer patients are often different from the patterns in urine samples from control subjects, although the differences depend on cancer types. Recently, Matsumura et al. (10) showed that urine from mice with artificially induced cancerous lung tumours could be clearly discriminated from control urine by a trained mouse and the differences in urine-derived VOCs could be analysed using GC-MS. These studies may provide clues for searching for volatile diagnostic biomarkers in the urine of cancer patients.
Since urine contains a complex mixture of components, VOC patterns are evident only after considerable computer processing of chromatographic data. In addition, considerable variation among individuals has been found in profiles of urine-derived volatiles. Notably, urinary components are affected not only by the metabolic status of the body, but also significantly by ingested foods and drinks. For example, the urine of people who have recently eaten asparagus has a sulphurous odour (11). Therefore, caution must be taken when determining whether or not a candidate VOC biomarker results from disease-related changes in metabolism.
Blood
Blood directly reflects the internal environment of the body, including nutritional, metabolic and immune status. Thus, the analysis of plasma-derived VOCs in blood has attracted the attention of many researchers. However, obtaining blood samples is not easy due to the onus on patients, and pre-treatment of blood samples is time-consuming. According to Horvath et al. (12), trained dogs can discriminate between blood samples from ovarian cancer patients and blood samples taken from patients with other gynaecological cancers or from healthy control subjects. This study suggests that specific odour(s) in the blood may be useful for screening and diagnosing different disorders such as lung cancer (13) and hepatic encephalopathy (14). Further studies are needed to evaluate these results and to apply these findings to clinical diagnoses.
Others
The investigation of faecal VOCs may be the best non-invasive way of diagnosing gastrointestinal diseases because human faecal samples represent dietary end-products resulting from digestive and excretory processes and intestinal bacterial metabolism. Distinct patterns of VOCs have been associated with faecal samples from patients with some types of bacterial infection, such as Vibrio cholerae (15), Clostridium difficile or Campylobacter jejuni infections (16).
Vaginal secretions contain many organic compounds that accurately reflect the stages of menstrual cycles. Normal vaginal secretions from every stage of the menstrual cycle are nearly odourless. Therefore, if vaginal secretions emit cheesy or fishy malodours, those odours could suggest vaginal and/or cervical bacterial infection in the genital organ.
In summarizing the origins of body odour, recognizing that the repertoire of VOCs found in different types of biological samples may be affected by various factors, such as age, sex, drug therapy, diet and smoking, care must be taken when investigating disease samples and when comparing them to control samples. At present, breath samples are mainly investigated because the sample collection methods are non-invasive and easy on patients, but other types of samples described in this section may be more useful in particular circumstances.
Disease-specific odours
In the following section, we discuss disease-specific odours, focusing on the chemical identity and the mechanism of production of odorous compounds resulting from diseases caused by infectious disease, metabolic disorders, toxins or poisoning, etc.
Infectious disease
The production of VOCs due to microbial interaction with organic media and biological fluids has been investigated since the beginning of the 19th century (17) (Table I). Microbial species produce various kinds of volatile compounds in the host body, and these volatiles are liberated in breath, urine, faeces and sweat. Using GC or GC-MS analysis of headspace volatiles of cultures, it has been shown that microorganisms produce various odorous compounds such as alcohols, aliphatic acids and terpenes, and that the ratio of these compounds differs depending on the infectious microorganism. Indeed, clinicians have long been aware of disease-specific odours due to infection, and profiles of volatiles can potentially be used as biomarkers of infectious diseases (18, 17).
Summary of key volatiles associated with infectious diseases.
| Disease(s)/disorder(s) . | Source . | Odour quality . | Pathogenic microbe . | Volatile compound(s) . | References . |
|---|---|---|---|---|---|
| Cholera | Faeces | Sweetish | Vibrio cholerae | Dimethyl disulphide, p-menth-1-en-8-ol | (15, 20) |
| Advanced breast cancer | Affected area | Rotting | Not determined | Dimethyl trisulphide, fatty acids | (4, 18) |
| Advanced head-and-neck cancer | Affected area | Rotting | Not determined | Dimethyl trisulphide, fatty acids | (4) |
| Gynaecological tumours | Tumour | Rotting | Not determined | Volatile fatty acids | (23) |
| Diphtheria | Body odour | Sweetish and putrid | Corynebacterium diphtheria | (17–19) | |
| Scarlet fever | Skin, breath | Foul | Streptococcus bacteria | (26) | |
| Smallpox | Skin | Sweetish and pungent | Variola virus | (17, 18, 27) | |
| Pneumonia | Breath | Foul | Bacteria, viruses, fungi, etc. | (18) | |
| Tuberculosis | Breath | Foul | Mycobacterium tuberculosis | (28) | |
| Tuberculous lymphadentis, Scrofula | Skin | Stale-beer | Mycobacterium tuberculosis | (17–19) | |
| Typhoid fever | Body odour | Musty or baked bread | Salmonella typhi | (17, 18) | |
| Yellow fever | Skin | Bucher's shop | Yellow fever virus | (18) |
| Disease(s)/disorder(s) . | Source . | Odour quality . | Pathogenic microbe . | Volatile compound(s) . | References . |
|---|---|---|---|---|---|
| Cholera | Faeces | Sweetish | Vibrio cholerae | Dimethyl disulphide, p-menth-1-en-8-ol | (15, 20) |
| Advanced breast cancer | Affected area | Rotting | Not determined | Dimethyl trisulphide, fatty acids | (4, 18) |
| Advanced head-and-neck cancer | Affected area | Rotting | Not determined | Dimethyl trisulphide, fatty acids | (4) |
| Gynaecological tumours | Tumour | Rotting | Not determined | Volatile fatty acids | (23) |
| Diphtheria | Body odour | Sweetish and putrid | Corynebacterium diphtheria | (17–19) | |
| Scarlet fever | Skin, breath | Foul | Streptococcus bacteria | (26) | |
| Smallpox | Skin | Sweetish and pungent | Variola virus | (17, 18, 27) | |
| Pneumonia | Breath | Foul | Bacteria, viruses, fungi, etc. | (18) | |
| Tuberculosis | Breath | Foul | Mycobacterium tuberculosis | (28) | |
| Tuberculous lymphadentis, Scrofula | Skin | Stale-beer | Mycobacterium tuberculosis | (17–19) | |
| Typhoid fever | Body odour | Musty or baked bread | Salmonella typhi | (17, 18) | |
| Yellow fever | Skin | Bucher's shop | Yellow fever virus | (18) |
Summary of key volatiles associated with infectious diseases.
| Disease(s)/disorder(s) . | Source . | Odour quality . | Pathogenic microbe . | Volatile compound(s) . | References . |
|---|---|---|---|---|---|
| Cholera | Faeces | Sweetish | Vibrio cholerae | Dimethyl disulphide, p-menth-1-en-8-ol | (15, 20) |
| Advanced breast cancer | Affected area | Rotting | Not determined | Dimethyl trisulphide, fatty acids | (4, 18) |
| Advanced head-and-neck cancer | Affected area | Rotting | Not determined | Dimethyl trisulphide, fatty acids | (4) |
| Gynaecological tumours | Tumour | Rotting | Not determined | Volatile fatty acids | (23) |
| Diphtheria | Body odour | Sweetish and putrid | Corynebacterium diphtheria | (17–19) | |
| Scarlet fever | Skin, breath | Foul | Streptococcus bacteria | (26) | |
| Smallpox | Skin | Sweetish and pungent | Variola virus | (17, 18, 27) | |
| Pneumonia | Breath | Foul | Bacteria, viruses, fungi, etc. | (18) | |
| Tuberculosis | Breath | Foul | Mycobacterium tuberculosis | (28) | |
| Tuberculous lymphadentis, Scrofula | Skin | Stale-beer | Mycobacterium tuberculosis | (17–19) | |
| Typhoid fever | Body odour | Musty or baked bread | Salmonella typhi | (17, 18) | |
| Yellow fever | Skin | Bucher's shop | Yellow fever virus | (18) |
| Disease(s)/disorder(s) . | Source . | Odour quality . | Pathogenic microbe . | Volatile compound(s) . | References . |
|---|---|---|---|---|---|
| Cholera | Faeces | Sweetish | Vibrio cholerae | Dimethyl disulphide, p-menth-1-en-8-ol | (15, 20) |
| Advanced breast cancer | Affected area | Rotting | Not determined | Dimethyl trisulphide, fatty acids | (4, 18) |
| Advanced head-and-neck cancer | Affected area | Rotting | Not determined | Dimethyl trisulphide, fatty acids | (4) |
| Gynaecological tumours | Tumour | Rotting | Not determined | Volatile fatty acids | (23) |
| Diphtheria | Body odour | Sweetish and putrid | Corynebacterium diphtheria | (17–19) | |
| Scarlet fever | Skin, breath | Foul | Streptococcus bacteria | (26) | |
| Smallpox | Skin | Sweetish and pungent | Variola virus | (17, 18, 27) | |
| Pneumonia | Breath | Foul | Bacteria, viruses, fungi, etc. | (18) | |
| Tuberculosis | Breath | Foul | Mycobacterium tuberculosis | (28) | |
| Tuberculous lymphadentis, Scrofula | Skin | Stale-beer | Mycobacterium tuberculosis | (17–19) | |
| Typhoid fever | Body odour | Musty or baked bread | Salmonella typhi | (17, 18) | |
| Yellow fever | Skin | Bucher's shop | Yellow fever virus | (18) |
Cholera
Cholera is caused by infectious bacteria, Vibrio cholerae. This acute intestinal infection causes profuse watery diarrhoea, vomiting and rapid dehydration. The faeces of patients with cholera are referred to as ‘rice-water stools’ and have a characteristic sweetish odour. Recently, dimethyl disulphide and p-menth-1-en-8-ol were identified as candidate biomarkers based on analyses of VOCs in faecal samples from patients with cholera (15, 20).
Infection in advanced cancer
Some patients with advanced cancer are distressed with unpleasant odours emanating from fungating wounds (21). Fungating wounds are referred to as ‘masses’ or ‘ulcerative lesions’, and are defined as the condition of ulceration and proliferation that occurs when malignant tumour cells infiltrate and erode through the skin. Fungating wounds reportedly occur in ∼5% of patients with cancer. These ulcers are usually superinfected with bacteria (22); and therefore, the infected area tends to emit malodour (23, 24). Indeed, anti-microbial preparations have been used to reduce the malodour. Recently, GC-MS-O-based methods were used to identify dimethyl trisulphide (DMTS) as the main odourant that causes severe malodour in some patients with advanced breast or head-and-neck cancer (4). DMTS is also produced by aerobes such as Pseudomonas aeruginosa that reside in leg ulcers (25). While DMTS may be a product of infectious bacteria in fungating wounds, the source of DMTS found in the fungating cancer wounds remains to be elucidated.
Some patients with gynaecological tumours also complain of heavy vaginal discharge with an offensive odour that results from production of acetic acids, isovaleric acids and/or butyric acids (23). Use of metronidazole, an antibiotic effective against anaerobes, reportedly reduces the discharge and putrid odour associated with these tumours (24). Identification of disease-specific odours may lead to improvements in the quality of life of patients distressed by cancer-derived malodour.
Diphtheria
Diphtheria is caused by bacterial infection with Corynebacterium diphtheria. Diphtheria usually affects the larynx or the lower and upper respiratory tracts and causes a sore throat. Some patients with diphtheria have a sickening, sweetish or putrid odour in their breath (19).
Scarlet fever
Scarlet fever is caused by bacterial infection with Streptococcus pyogenes. The characteristic symptoms are a red-coloured rash on the body, sore throat and fever. A distinctive foul odour is emitted from the patient's skin and breath (26).
Smallpox
Smallpox is caused by viral infection with Variola virus. After an incubation period of ∼2 weeks, patients experience high fevers, headaches, backaches and vomiting, and a pus-filled rash then appears over the entire body. When infected lesions are opened, a sweetish, pungent odour emanates from the sore (27).
Pneumonia
Pneumonia is caused by a lung infection due to bacteria, viruses, fungi or parasites. The characteristic symptoms are inflammation of the alveoli, fluid filled lungs, cough, fever and difficulty in breathing. Some people with pneumonia may have foul-smelling breath (18).
Tuberculosis
Tuberculosis is caused by a bacterial infection with Mycobacterium tuberculosis. The bacteria usually attack the lungs, but they can also damage other parts of the body. Reportedly, tuberculosis can cause breath to have a foul odour (28). Recently, two groups identified a specific mixture of VOCs from cultured Mycobacterium tuberculosis, and another group showed that sputum samples from the patients can be distinguished from control samples by an electronic nose sensor (29–31). These studies are potentially useful in the development of non-invasive diagnostic tests for tuberculosis.
Scrofula, or tuberculosis of the neck, results from bacterial infection of the lymph nodes and skin of the neck, and sometimes results in localized swelling. Some patients have ulcerated lymph nodes that produce a stale beer odour.
Typhoid fever
Typhoid fever is caused by Salmonella Typhi infection of the intestinal tract. The characteristic symptoms are a high fever, drenching sweat and gastroenteritis. Patients with Typhoid fever emit a musty or baked-bread odour (17, 18).
Yellow fever
Yellow fever is a viral disease caused by the yellow fever virus, which is transmitted to humans by female mosquitoes. Yellow fever causes fever and vomiting, and it sometimes causes severe bleeding from the mouth and intestinal tract. Patients with yellow fever often have body odour that smells like a butcher's shop (18).
Inherited disorder of metabolism
Metabolic disorders derived from enzyme deficiencies or transport defects often result in the accumulation of particular metabolites in body fluids due to impairment in normal biochemical pathways. Thus, these disorders are usually diagnosed by the detection of increased concentrations of these metabolites. In some cases, these disorders lead to irreparable damage or death unless they are discovered at a very early age and treated appropriately. Neonatal screening tests, such as measuring the amount of particular metabolites or analysing genotypes, have been developed and are generally available. Some of these disorders are associated with a characteristic odour due to the accumulation of certain odorous metabolites in the body, and these odours are sometimes distinctive enough to be diagnosed by clinicians (32) (Table II).
Profiles of volatile compounds from inherited disorder of metabolism.
| Disorder(s) . | Source . | Odour quality . | Volatile compound(s) . | Reference . |
|---|---|---|---|---|
| Phenylketonuria | Sweat, urine, infant skin | Musty, sweaty locker room towels | Phenylacetic acid | (33) |
| Isovaleric acidemia | Skin, sweat | Cheesy, acrid, sweaty feet | Isovaleric acid | (32, 34–36) |
| Maple syrup urine disease | Sweat, urine, ear wax | Maple syrup, caramerized sugar | Short-chain fatty acids | (32, 39) |
| Methionine malabsorption syndrome | Skin, urine | Malt, hop | α-Hydroxybutyric acid | (40) |
| Hypermethioninemia | Breath, sweat, urine | Cabbage-like | Dimethyl sulphide | (41–43) |
| Trimethylaminuria | Breath, sweat, urine | Foul, rotten fish-like | Trimethylamine | (44, 48) |
| Tyrosinaemia | Breath | Cabbage or rancid butter | p-Hydroxyphenylpyruvic acid | (81) |
| 3-Methylcrotonylglycinuria | Urine | Male cat urine | 3-Hydroxyisovaleric acid | (82) |
| Cystinuria | Urine | Rotten egg | Cadaverine, piperidine, putrescine, pyrrolidine | (81) |
| Disorder(s) . | Source . | Odour quality . | Volatile compound(s) . | Reference . |
|---|---|---|---|---|
| Phenylketonuria | Sweat, urine, infant skin | Musty, sweaty locker room towels | Phenylacetic acid | (33) |
| Isovaleric acidemia | Skin, sweat | Cheesy, acrid, sweaty feet | Isovaleric acid | (32, 34–36) |
| Maple syrup urine disease | Sweat, urine, ear wax | Maple syrup, caramerized sugar | Short-chain fatty acids | (32, 39) |
| Methionine malabsorption syndrome | Skin, urine | Malt, hop | α-Hydroxybutyric acid | (40) |
| Hypermethioninemia | Breath, sweat, urine | Cabbage-like | Dimethyl sulphide | (41–43) |
| Trimethylaminuria | Breath, sweat, urine | Foul, rotten fish-like | Trimethylamine | (44, 48) |
| Tyrosinaemia | Breath | Cabbage or rancid butter | p-Hydroxyphenylpyruvic acid | (81) |
| 3-Methylcrotonylglycinuria | Urine | Male cat urine | 3-Hydroxyisovaleric acid | (82) |
| Cystinuria | Urine | Rotten egg | Cadaverine, piperidine, putrescine, pyrrolidine | (81) |
Profiles of volatile compounds from inherited disorder of metabolism.
| Disorder(s) . | Source . | Odour quality . | Volatile compound(s) . | Reference . |
|---|---|---|---|---|
| Phenylketonuria | Sweat, urine, infant skin | Musty, sweaty locker room towels | Phenylacetic acid | (33) |
| Isovaleric acidemia | Skin, sweat | Cheesy, acrid, sweaty feet | Isovaleric acid | (32, 34–36) |
| Maple syrup urine disease | Sweat, urine, ear wax | Maple syrup, caramerized sugar | Short-chain fatty acids | (32, 39) |
| Methionine malabsorption syndrome | Skin, urine | Malt, hop | α-Hydroxybutyric acid | (40) |
| Hypermethioninemia | Breath, sweat, urine | Cabbage-like | Dimethyl sulphide | (41–43) |
| Trimethylaminuria | Breath, sweat, urine | Foul, rotten fish-like | Trimethylamine | (44, 48) |
| Tyrosinaemia | Breath | Cabbage or rancid butter | p-Hydroxyphenylpyruvic acid | (81) |
| 3-Methylcrotonylglycinuria | Urine | Male cat urine | 3-Hydroxyisovaleric acid | (82) |
| Cystinuria | Urine | Rotten egg | Cadaverine, piperidine, putrescine, pyrrolidine | (81) |
| Disorder(s) . | Source . | Odour quality . | Volatile compound(s) . | Reference . |
|---|---|---|---|---|
| Phenylketonuria | Sweat, urine, infant skin | Musty, sweaty locker room towels | Phenylacetic acid | (33) |
| Isovaleric acidemia | Skin, sweat | Cheesy, acrid, sweaty feet | Isovaleric acid | (32, 34–36) |
| Maple syrup urine disease | Sweat, urine, ear wax | Maple syrup, caramerized sugar | Short-chain fatty acids | (32, 39) |
| Methionine malabsorption syndrome | Skin, urine | Malt, hop | α-Hydroxybutyric acid | (40) |
| Hypermethioninemia | Breath, sweat, urine | Cabbage-like | Dimethyl sulphide | (41–43) |
| Trimethylaminuria | Breath, sweat, urine | Foul, rotten fish-like | Trimethylamine | (44, 48) |
| Tyrosinaemia | Breath | Cabbage or rancid butter | p-Hydroxyphenylpyruvic acid | (81) |
| 3-Methylcrotonylglycinuria | Urine | Male cat urine | 3-Hydroxyisovaleric acid | (82) |
| Cystinuria | Urine | Rotten egg | Cadaverine, piperidine, putrescine, pyrrolidine | (81) |
Phenylketonuria
Phenylketonuria (PKU) is an inherited autosomal recessive disorder characterized by hyperphenylalaninemia resulting from deficiency of hepatic phenylalanine hydroxylase (PAH), which converts phenylalanine into tyrosine. PAH deficiencies result in the accumulation of phenylalanine, which is abnormally metabolized to phenylpyruvic acid and phenylacetate. The first PKU children were recognized because their mothers complained of a musty odour, which was due to the presence of phenylacetate in the urine (33). Phenylacetate in sweat and urine of the patients appears to be the cause of a musty odour that can smell like sweaty locker room towels.
Isovaleric acidemia
Isovaleric acidemia (IVA) is an autosomal recessive inherited leucine metabolism disorder caused by a deficiency of the mitochondrial enzyme isovaleryl-CoA dehydrogenase. Derivatives of isovaleryl-CoA accumulate in patients with IVA (34). These patients possess a specific body odour that is variably described as cheesy, acrid or similar to sweaty feet. The cause of this unusual odour was identified as isovaleric acid based on GC-MS analyses of blood samples from patients with IVA (35). Moreover, patients’ urine samples contain isovalerylglycine and 3-hydroxyisovaleric acid (36–38).
Maple syrup urine disease
Maple syrup urine disease is a defect in the metabolism of the branched-chain amino acids (BCAAs), valine, leucine and isoleucine. This disease leads to mental retardation and cerebral degeneration, and is caused by the deficiency of an enzyme activity that catalyses oxidative decarboxylation of 2-oxocarboxylic acids in the degradation of the BCAAs. As a result of this enzyme deficiency, 2-oxocarboxylic acids and their corresponding reduced products accumulate in tissues, blood, cerebro-spinal fluid and urine. Consequently, the characteristic maple syrup or caramelized sugar-like odour is found in sweat and urine and even cerumen (39). This symptomatic odour is evident during the first week after birth, and therefore, the correct diagnosis and dietary management (e.g. consumption of BCAA-free foods) must be started immediately upon diagnosis and soon after birth.
Methionine malabsorption syndrome
Methionine malabsorption syndrome is an autosomal recessive inherited disease caused by malabsorption of methionine. Some portion of unabsorbed methionine is converted to α-hydroxybutyric acid by intestinal bacteria, and the acid causes a yeast-, malt-, or hop-like odour in the patient's urine (40).
Hypermethioninemia (Methionine adenosyltransferase deficiency)
Methionine adenosyltransferase is a key enzyme in transmethylation, transsulphuration and the biosynthesis of polyamines. Genetic deficiency of α/β-methionine adenosyltransferase causes hypermethioninemia and neural demyelination. In some cases, patients exhibit breath, sweat and/or urine with a cabbage-like odour that is due to the presence of unusually large amounts of dimethylsulphide, a metabolite of methionine (41–43).
Trimethylaminuria
Trimethylaminuria (TMAU) is a metabolic disorder characterized by the inability of individuals to oxidize trimethylamine (TMA) to trimethylamine N-oxide (TMAO) (44, 45). This disorder results from an autosomal recessive mutation in the gene encoding flavin-containing monooxygenase enzyme 3 (FMO3) (46, 47). TMA is produced in the gut by bacterial metabolism of choline-rich foods, such as eggs, certain legumes and organ meat. In healthy individuals, TMA is oxidized to TMAO at high efficiency by FMO3 in the liver. Individuals suffering from TMAU have a reduced capacity to oxidize TMA to TMAO. TMAO is non-odorous compound. However, TMA is a gas at normal body temperature and has a strong rotten-fish odour. The unmetabolized TMA is excreted in urine, sweat, breath, saliva and reproductive fluid. Therefore, the inability to efficiently oxidize TMA results in a foul, unpleasant and fish-like body odour (48, 49).
Toxin/poisoning
Poisoning with certain toxins affects body odours (50, 51). For example, individuals that have ingested cyanide have breath with a bitter-almond odour. A garlic-like body odour may suggest ingestion of arsenic, thallium or organic phosphate insecticides. Turpentine poisoning is indicated by urine with the odour of violets. These characteristic odours are indicative of the ingested toxic substances and may provide diagnostic clues that facilitate rapid treatments to prevent clinical deterioration while waiting for laboratory confirmation.
Other diseases
Diabetes/diabetic ketoacidosis
Glucose is the main source of energy for cells of the body and insulin, a hormone made by the pancreas, signals cells to take up glucose. Since patients with type 1 diabetes cannot synthesize insulin, glucose accumulates in the blood, and is excreted in the urine. Under these conditions, cells use fatty acids rather than glucose as an energy source. However, fatty acid catabolism creates ketone compounds, such as acetoacetate, 3-hydroxybutyrate and acetone, as by-products and these ketone bodies cause blood acidity, leading to ketoacidosis of the blood. These excess ketones are secreted in the urine and breath. As a result, the patient's urine and breath have a fruity odour due to the presence of acetone (5, 52, 53) (Table III).
Summary of key volatiles associated with different disease types.
| Disease(s)/Disorder(s) . | Source . | Odour quality . | Volatile compound(s) . | Reference . |
|---|---|---|---|---|
| Diabetes/diabetic ketoacidosis | Breath | Rotten apple, acetone-like | Acetone, other ketones | (5, 39, 53) |
| Uraemia/kidney failure | Breath | Urine | Dimethylamine, trimethylamine | (54) |
| Ozena | Body odour | Rotting | (18) | |
| Scurvy | Sweat | Putrid | (18, 83) | |
| Ileus/intestinal obstruction | Breath | Faecal | (18) | |
| Breast cancer | Breath | Not perceived | 2-Propanol, 2,3-dihydro-1-phenyl-4 (1H)-quinazolinone, 1-phenyl-ethanone, heptanal, isopropyl myristate | (56, 57) |
| Lung cancer | Breath | Not perceived | Alkanes | (58) |
| Schizophrenia | Body odour | Musty, skunk, characteristic | (72–74) | |
| Breath | Not perceived | Carbon disulphide, pentane | (78) | |
| Acromegaly | Skin | Sweaty | (84) | |
| Asthma | Breath | Not perceived | Pentane, ethane, 8-isoprostane | (85–87) |
| Hepatic encephalopathy | Blood | Amine | 3-Methylbutanol | (14) |
| Leukaemia | Breath | Sweet | (88) | |
| Liver disease, fetor hepaticus | Breath | Malodour | C2–C5 Aliphatic acids, methylmercaptan | (7, 8) |
| Disease(s)/Disorder(s) . | Source . | Odour quality . | Volatile compound(s) . | Reference . |
|---|---|---|---|---|
| Diabetes/diabetic ketoacidosis | Breath | Rotten apple, acetone-like | Acetone, other ketones | (5, 39, 53) |
| Uraemia/kidney failure | Breath | Urine | Dimethylamine, trimethylamine | (54) |
| Ozena | Body odour | Rotting | (18) | |
| Scurvy | Sweat | Putrid | (18, 83) | |
| Ileus/intestinal obstruction | Breath | Faecal | (18) | |
| Breast cancer | Breath | Not perceived | 2-Propanol, 2,3-dihydro-1-phenyl-4 (1H)-quinazolinone, 1-phenyl-ethanone, heptanal, isopropyl myristate | (56, 57) |
| Lung cancer | Breath | Not perceived | Alkanes | (58) |
| Schizophrenia | Body odour | Musty, skunk, characteristic | (72–74) | |
| Breath | Not perceived | Carbon disulphide, pentane | (78) | |
| Acromegaly | Skin | Sweaty | (84) | |
| Asthma | Breath | Not perceived | Pentane, ethane, 8-isoprostane | (85–87) |
| Hepatic encephalopathy | Blood | Amine | 3-Methylbutanol | (14) |
| Leukaemia | Breath | Sweet | (88) | |
| Liver disease, fetor hepaticus | Breath | Malodour | C2–C5 Aliphatic acids, methylmercaptan | (7, 8) |
Summary of key volatiles associated with different disease types.
| Disease(s)/Disorder(s) . | Source . | Odour quality . | Volatile compound(s) . | Reference . |
|---|---|---|---|---|
| Diabetes/diabetic ketoacidosis | Breath | Rotten apple, acetone-like | Acetone, other ketones | (5, 39, 53) |
| Uraemia/kidney failure | Breath | Urine | Dimethylamine, trimethylamine | (54) |
| Ozena | Body odour | Rotting | (18) | |
| Scurvy | Sweat | Putrid | (18, 83) | |
| Ileus/intestinal obstruction | Breath | Faecal | (18) | |
| Breast cancer | Breath | Not perceived | 2-Propanol, 2,3-dihydro-1-phenyl-4 (1H)-quinazolinone, 1-phenyl-ethanone, heptanal, isopropyl myristate | (56, 57) |
| Lung cancer | Breath | Not perceived | Alkanes | (58) |
| Schizophrenia | Body odour | Musty, skunk, characteristic | (72–74) | |
| Breath | Not perceived | Carbon disulphide, pentane | (78) | |
| Acromegaly | Skin | Sweaty | (84) | |
| Asthma | Breath | Not perceived | Pentane, ethane, 8-isoprostane | (85–87) |
| Hepatic encephalopathy | Blood | Amine | 3-Methylbutanol | (14) |
| Leukaemia | Breath | Sweet | (88) | |
| Liver disease, fetor hepaticus | Breath | Malodour | C2–C5 Aliphatic acids, methylmercaptan | (7, 8) |
| Disease(s)/Disorder(s) . | Source . | Odour quality . | Volatile compound(s) . | Reference . |
|---|---|---|---|---|
| Diabetes/diabetic ketoacidosis | Breath | Rotten apple, acetone-like | Acetone, other ketones | (5, 39, 53) |
| Uraemia/kidney failure | Breath | Urine | Dimethylamine, trimethylamine | (54) |
| Ozena | Body odour | Rotting | (18) | |
| Scurvy | Sweat | Putrid | (18, 83) | |
| Ileus/intestinal obstruction | Breath | Faecal | (18) | |
| Breast cancer | Breath | Not perceived | 2-Propanol, 2,3-dihydro-1-phenyl-4 (1H)-quinazolinone, 1-phenyl-ethanone, heptanal, isopropyl myristate | (56, 57) |
| Lung cancer | Breath | Not perceived | Alkanes | (58) |
| Schizophrenia | Body odour | Musty, skunk, characteristic | (72–74) | |
| Breath | Not perceived | Carbon disulphide, pentane | (78) | |
| Acromegaly | Skin | Sweaty | (84) | |
| Asthma | Breath | Not perceived | Pentane, ethane, 8-isoprostane | (85–87) |
| Hepatic encephalopathy | Blood | Amine | 3-Methylbutanol | (14) |
| Leukaemia | Breath | Sweet | (88) | |
| Liver disease, fetor hepaticus | Breath | Malodour | C2–C5 Aliphatic acids, methylmercaptan | (7, 8) |
Uraemia/kidney failure
Uraemia is a type of kidney failure defined by the presence of excessive nitrogenous waste products, such as urea, in the blood stream. The dysfunctional kidneys fail to filter the blood correctly, causing an imbalance of electrolytes. Uraemia produces an ammonia or urine-like odour in breath, which is caused by the breakdown of urea to ammonia and trimethylamine in the saliva (54).
Ozena
Ozena is a disease caused by the breakdown of the bony ridges and mucous membranes in the nose which, in turn, produces a foul odour.
Scurvy
Scurvy is a disease caused by vitamin C deficiency, which is required for the synthesis of collagen. Scurvy patients produce sweat with a putrid odour.
Ileus/intestinal obstruction
Ileus is a disruption of the normal peristaltic movement of the bowel. If the obstructed, intestine regurgitates bowel contents backward into the stomach, patients may vomit stomach contents and have breath with a faecal odour.
Cancer
Early detection of cancer is often important in successful treatment of the disease. Many DNA, RNA and protein molecules are used as or are candidates for biomarkers of specific diseases (55). Moreover, medical imaging techniques, such as computed tomography, magnetic resonance imaging and positron emission tomography, have been developed. However, the quality of these tests is still uncertain, and some between-study inconsistencies have been noted. Based on GC-MS analyses, some VOCs were recently identified as candidate cancer-specific substances in breath, urine, tissue and/or blood samples from cancer patients.
For example, breath samples from breast cancer patients have been examined. Hydrocarbons, such as alkanes and monomethylated alkanes, were originally identified as markers for oxidative stress, and a unique combination of these hydrocarbon VOCs was found in breath samples from breast cancer patients. However, the biological and clinical significance of this combination of VOCs is unclear (56, 57).
Breath samples from lung cancer patients were also examined (58). However, the findings on lung cancer-specific VOCs differ among research groups, and reportedly, there are only quantitative differences in VOCs. A recent study indicates that some VOCs emitted from cultured lung cancer cells were the same as those in breath samples of lung cancer patients (59–62). These findings show the existence of lung cancer-specific VOCs and VOC biomarkers will become effective tools in cancer screening.
Dogs can be used as cancer detectors because they have an extraordinary sense of smell; with odour detection thresholds as low as parts per trillion (63). In cases of melanoma, bladder cancer, ovarian cancer and colorectal cancer, dogs can be trained to distinguish patients based on VOCs from patients’ urine, tumours or breath samples, more successfully than would be expected by chance alone (64–67). However, it is possible that the dogs in these studies responded to odours indirectly associated with cancer, such as inflammation, necrosis or metabolic products that often exist in patients with advanced disease, rather than specifically to odours derived from cancer itself. Thus, it is necessary to ascertain whether the positive response by a canine is directly induced by cancer-specific odours. Recently, the application of an electronic nose is also recommended. For example, melanomas have reportedly been identified by measuring VOCs from the lesions using a chemical sensor array (68).
Schizophrenia
Schizophrenia is a severe mental disorder with a lifetime risk of ∼1% that is characterized by hallucinations, delusions and cognitive deficits. The disorder is caused by multiple factors, including genetic and environmental factors and their interaction. Recent studies indicate that specific genetic variants are associated with some cases of schizophrenia (69, 70), but the genes and biological mechanisms that underlie susceptibility to schizophrenia are not known entirely. In the absence of clear biological markers, diagnosis of schizophrenia has historically been based on behavioural signs, symptoms and neuropsychological testing (71). Therefore, identification of novel biomarkers that could assist in early diagnosis and prevention and treatment protocols is desired.
It has been claimed that ‘a peculiar smell’ has been detected in psychiatric hospitals since the 19th century (72–74). Patients with schizophrenia have long been incriminated as the cause of an above-mentioned odour, but it was not until 1960 that Smith and Sines demonstrated that sweat from patients with schizophrenia could be distinguished from sweat from control subjects by the odour alone, using trained rats (75). In 1969, trans-3-methyl-2-hexenoic acid (TMHA) was found to be the only component that consistently differed in sweat from patients with schizophrenia and sweat from control subjects (76). However in 1973, Gordon et al. (77) showed that TMHA was detected in sweat from both normal subjects and patients with schizophrenia, indicating that there was no special relationship between TMHA and schizophrenia. In the 1990s, Phillips et al. (78) found that breath from patients with schizophrenia high levels of CS2, a known neurotoxin and pentane, a marker of lipid peroxidation. However, the origin of the endogenous CS2 is still unknown, and further studies are required to determine if these observed differences are due to schizophrenia or some factors other than the disease itself. Phillips et al. (79) also suggested that they can discriminate VOCs between the patients with schizophrenia and non-schizophrenic controls by analysing patterns of VOCs in breath. Currently, the identity of the ‘peculiar smell in the psychiatric hospital’ that had been reported since the end of the 19th century is still a mystery.
Conclusions
Progresses in analytical methods such as GC, GC-MS and GC-MS-O have provided an opportunity to identify VOCs related to diseases and disease-specific VOCs in research laboratories. However, there are some problems when VOCs are used as diagnostic tools because the analytical equipment is expensive and the techniques are time-consuming and not readily adapted to high-throughput processing. Moreover, if clinicians want to employ the identification of body odours in practical diagnosis, they first require both training on many kinds of disease-specific odours, and odour recognition skills beforehand.
Since the 1980s, devices that electronically mimic the human olfactory system, called the ‘electronic nose’, have been developed and repeatedly improved (80). Electronic noses employ several gas sensors that are combined with a pattern recognition system to analyse and characterize sample-derived complex VOCs without separation of the mixture into individual components. At present, it is necessary to improve the accuracy of the sensors and their sensitivity to marker VOCs. Once these improvements are made, disease-specific ‘volatile biomarkers’ will be used more regularly in clinical practice.
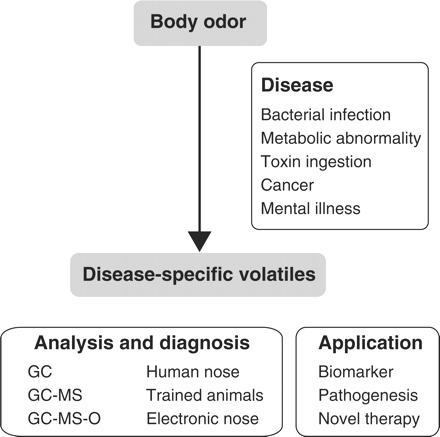
Finally, it should be pointed out, again, that odour information is helpful in elucidating the cause of disorders (Fig. 2). The therapeutic targets for some infectious or metabolic diseases could be identified if we elucidate the mechanisms underlying the production of specific odours. Microanalyses of VOCs from biological samples and investigation of the biosynthetic pathways that produce the relevant VOCs from patients may lead to a better understanding of pathophysiological mechanisms that cause a particular disease. While it is necessary to pay close attention in ethical respect when researchers deal with a delicate issue of human body odours, this type of information would enable alternative ways for diagnosing and treating diseases. For example, we may be able to classify a disease into new subtypes, reclassify already known diseases for better treatment, or even develop novel therapies, including tailor-made treatments.
Overview and future outlook of the field of studies on volatiles and diseases.
Funding
Strategic Research Program for Brain Sciences and Grant-in-Aid for Scientific Research on Priority Areas from MEXT, Japan.
Conflict of interest
None declared.
Abbreviations
- BCAAs, branched-chain amino acids
DMTS
- dimethyl trisulphide
FMO3
- flavin-containing monooxygenase enzyme 3
GC
- gas chromatography
GC-MS
- gas chromatography and mass spectrometry
GC-MS-O
- GC-MS-olfactometer
IVA
- isovaleric acidemia
PAH
- hepatic phenylalanine hydroxylase
PKU
- phenylketonuria
SPME
- solid-phase microextraction
TMA
- trimethylamine
TMAO
- trimethylamine N-oxide
TMAU
- trimethylaminuria
TMHA
- trans-3-methyl-2-hexenoic acid
VOCs
- volatile organic compounds