-
PDF
- Split View
-
Views
-
Cite
Cite
Appukuttannair Biju Kumar, Smrithy Raj, Peter K. L. Ng, Description of a new genus and new species of a fully arboreal crab (Decapoda: Brachyura: Gecarcinucidae) from the Western Ghats, India, with notes on the ecology of arboreal crabs, Journal of Crustacean Biology, Volume 37, Issue 2, 1 March 2017, Pages 157–167, https://doi.org/10.1093/jcbiol/rux012
Close - Share Icon Share
Abstract
A new genus and new species of tree crab is described from the Western Ghats biodiversity hotspot in Kerala, southern India. Kani maranjandun. gen., n. sp., is substantially different from all congeners in a suite of characters, notably the diagnostic carapace and male abdominal structure, as well as the conspicuously elongated ambulatory legs. The species is wholly arboreal, living in tree-hollows or the canopy.
INTRODUCTION
The biodiversity of the Western Ghats biodiversity hot spot in India has a high endemism of flora and fauna (Bossuyt et al., 2004; Gunawardene et al., 2007). The freshwater biodiversity, however, especially that of freshwater crabs, remains poorly documented (Molur et al., 2011; Raghavan et al., 2015, 2016). Despite the many recent studies on the Indian freshwater crab fauna (Potamidae and Gecarcinucidae) (Bahir & Yeo, 2005, 2007; Klaus, et al., 2014; Raghavan et al., 2015; Pati & Sharma, 2013, 2014; Pati & Sudha Devi, 2015a, b; Pati et al., 2016), many new genera and new species still await description, and there as yet no records of completely arboreal or cavernicolous crabs
We describe a new genus and new species of tree crab from the Western Ghats and compare it with congeners in India.
MATERIAL AND METHODS
The specimens were obtained during surveys of the freshwater crab fauna in the Western Ghats in Kerala, India. Accompanying tribesmen, notably the Kani tribes along the southern part of the Ghats, told the survey team about the presence of ‘long-legged’ tree crabs in their area (Figs. 1–3). With their help, these tree crabs were sighted on many occasions during the two-year survey. Early attempts to capture them proved futile but finally, one female specimen was collected on 5 September 2016. With the help of Kani tribes from Kottoor Reserve Forest region of the Agasthyamala Biological Park, southern Western Ghats, we later managed to obtain more material, including a large adult male.
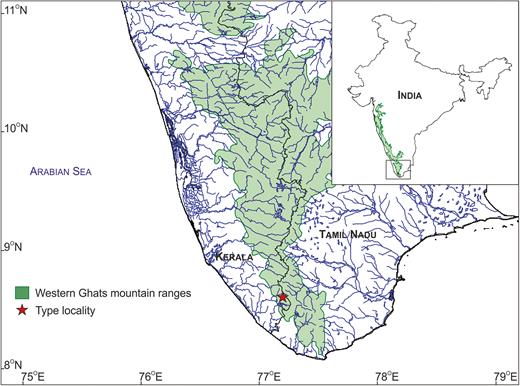
Type locality of Kani maranjandun. gen., n. sp., southern Western Ghats, southern India.
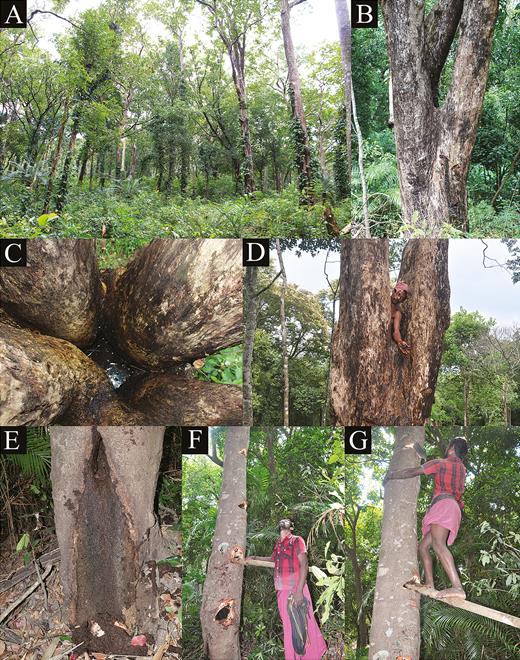
A, Degraded forest area near the tribal settlement at Agathyamala Biological Park, Kottoor Reserve Forest, Kottoor, Kerala, India, where specimens of Kani maranjandun. gen., n. sp. were found; B, tree fork of Terminalia paniculata; C, phytotelm in tree fork of Terminalia paniculata; D, paratype male (33.1 × 24.3 mm) (DABFUK/AR-BR-57) of Kani maranjandun. gen., n. sp. collected from inside a phytotelm in a tree fork of Terminalia paniculata; E, tree hollow of Cinnamomum verum, where individuals of the new species may live; F, G, when approached by members of the Kani tribe, the crabs climb up the trunk of Cinnamomum verum, the tribesmen then cut a series of holes in trees to climb up after them. This figure is available in colour at Journal of Crustacean Biology online.
Kani maranjandun. gen., n. sp. in its natural habitat. A, B, specimens on a tree trunk (not preserved) (courtesy of Sali Palode); C–F, paratype male (40.5 × 30.0 mm) (DABFUK). Kerala, India. This figure is available in colour at Journal of Crustacean Biology online.
Specimens examined are deposited in the Zoological Survey of India (ZSI) and in the museum collections of the Department of Aquatic Biology and Fisheries, University of Kerala (DABFUK), India. Measurements provided (in millimetres) indicate the maximum carapace width and length, respectively. The abbreviations G1 and G2 are used for the male first and second pleopods (gonopods), respectively. The terminology essentially follows that in Ng (1988) and Davie et al. (2015).
SYSTEMATICS
Superfamily GecarcinucoideaRathbun, 1904
Family GecarcinucidaeRathbun, 1904
Kani n. gen.
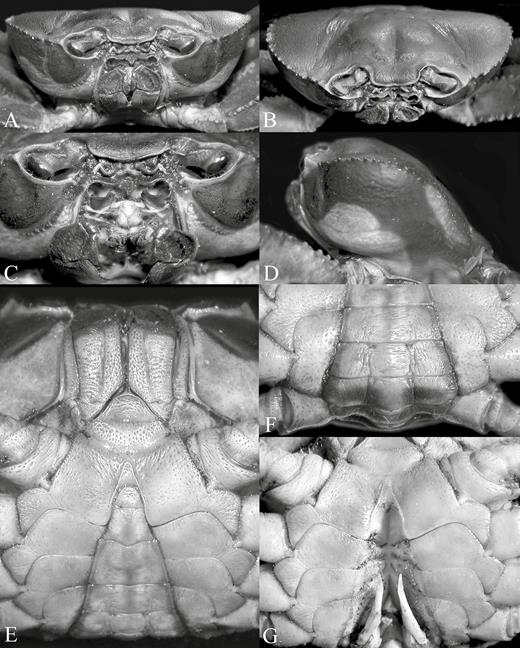
Diagnosis: Carapace transversely ovate, high from frontal view, swollen; dorsal surface unevenly convex (Figs. 5, 6A, B, D); suborbital, pterygostomial regions smooth, glabrous, separated from suborbital region by low granulated ridge (Figs. 5, 6A–D); epigastric cristae distinct but low; postorbital cristae low, confluent with epigastric cristae (Figs. 5, 6A, B); cervical grooves deep, broad; external orbital tooth prominent, triangular, tip extending anteriorly slightly beyond orbit, front, clearly demarcated from anterolateral margin by deep V-shaped notch; anterolateral margins strongly convex, subcristate, lined with prominent small unevenly arranged teeth, short spines (Figs. 5, 6B); posterolateral margin sharply converging towards posterior carapace margin (Fig. 5); posterior margin of epistome with distinct median triangle, sunken below margin of epistome; anteroexternal angle of merus of third maxilliped convex, exopod with distinct flagellum reaching to just across width of merus (Fig. 7A, B); ambulatory legs long, dactylus long with tip strongly curved (Figs. 5A, 7G–J); male anterior thoracic sternum (sternites 3, 4) relatively narrow transversely (Fig. 6E), sternites 3, 4 separated by prominent oblique depression; male pleon narrowly triangular, telson acutely triangular, somite 6 trapezoidal (Figs. 6E, F); G1 with terminal and subterminal segments clearly demarcated, terminal segment slender, conical (Fig. 8A, B, D-F); G2 longer than G1, basal segment long, distal segment slender, long, about a third length of basal segment (Fig. 8C).
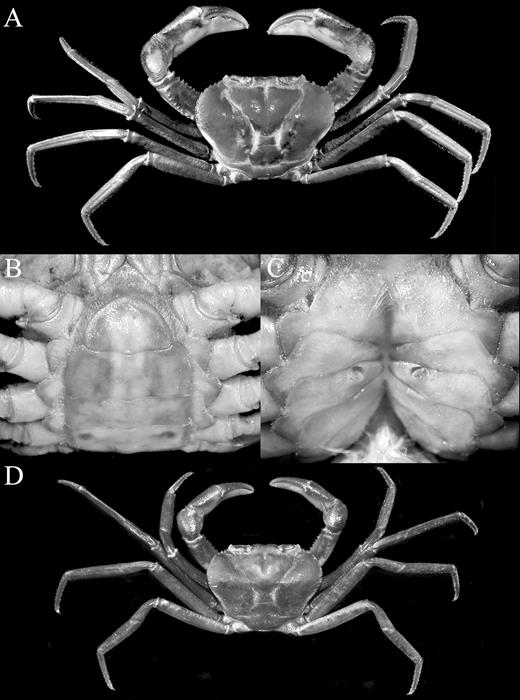
Kani maranjandun. gen., n. sp.A–D, holotype male (42.6 × 30.8 mm) (ZSI/WGRC/IR-INV 8234), Kerala, India; E, F, paratype female (40.6 × 30.4 mm) (DABFUK), Kerala, India.
Kani maranjandun. gen., n. sp. holotype male (42.6 × 30.8 mm) (ZSI/WGRC/IR-INV 8234), Kerala, India. A, overall habitus; B, dorsal view of carapace.
Kani maranjandun. gen., n. sp., holotype male (42.6 × 30.8 mm) (ZSI/WGRC/IR-INV 8234), Kerala, India. A, B, frontal view of cephalothorax; C, frontal view showing epistome, antennules, antennae and orbits; D, lateral view of cephalothorax; E, third maxillipeds, anterior thoracic sternum, pleonal somites 4–6 and telson; F, posterior thoracic sternum and pleonal somites 1–4; G, sternopleonal cavity and gonopods.
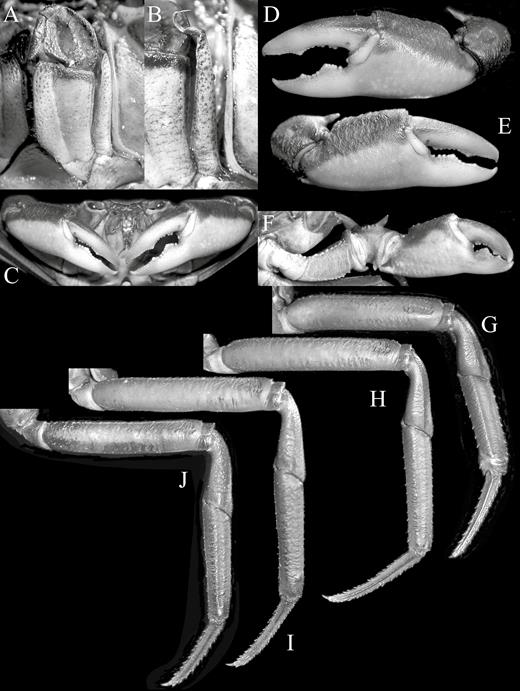
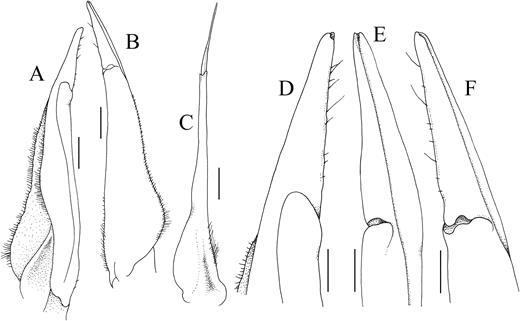
Kani maranjandun. gen., n. sp., holotype male (42.6 × 30.8 mm) (ZSI/WGRC/IR-INV 8234), Kerala, India. A, left third maxilliped; B, exopod of left third maxilliped showing flagellum; C, chelipeds; D, outer view of left chela; E, outer view of right chela; F, inner surface of left cheliped; G–J, first to fourth right ambulatory legs, respectively.
Kani maranjandun. gen., n. sp. holotype male (42.6 × 30.8 mm) (ZSI/WGRC/IR-INV 8234), Kerala, India. A, left G1 (ventral view); B, left G1 (dorsal view); C, left G2 (ventral view); D, terminal segment of left G1 (ventral view); E, terminal segment of left G1 (dorsomedial view); F, terminal segment of left G1 (dorsal view). Scales: A–C = 1.0 mm; D–F = 0.5 mm.
Type species: Kani maranjandu n. sp., by present designation.
Etymology: The genus is named after the people of the Kanikkaran (also known as Kani) tribe in Kerala, who helped in the collection of tree crab from near their hamlet. India’s Agasthyamala Biosphere Reserve, southern Western Ghats is home to the largest population of this ancient Indian tribe. The gender of the genus name is neuter.
Remarks: Kanin. gen. has a suite of characters that differentiate it from all gecarcinucid genera in India. Bahir & Yeo (2007) discussed the characters distinguishing the various genera in Kerala and the rest of India using the structures of the carapace, male anterior thoracic sternum (sternites 1–4), exopod of the third maxilliped, male abdomen, G1 and G2. The carapace of the new genus is transversely ovate and swollen (Figs. 5, 6A, B), the exopod of the third maxilliped has a relatively long flagellum (Fig. 7B), the male thoracic sternites 3 and 4 are clearly separated by a deep groove (Fig. 6E); the male abdomen is distinctly triangular, not T-shaped, with somite 6 broad and the telson elongated (Fig. 6E, F), and the G2 has a long distal segment (Fig. 8C). This combination of characters is unique among the Indian crabs. Most importantly, no other Indian gecarcinucid genus has ambulatory legs as long as those on Kanin. gen. (Figs. 5A, 9A, D).
Kani maranjandun. gen., n. sp. A–C, paratype female (40.6 × 30.4 mm) (DABFUK/AR-BR-56), Kerala, India; D, young female (26.7 × 20.4 mm) (DABFUK/AR-BR-59), Kerala, India. A, D, overall habitus; B, pleonal somites 4–6 and telson; C, thoracic sternum showing vulvae.
The long distal segment of the G2 (Fig. 8C) allies Kanin. gen. with genera like VanniBahir & Yeo, 2007, VelaBahir & Yeo, 2007, BarathaBahir & Yeo, 2007, and TravancorianaBott, 1969. The carapaces of Vanni and Travancoriana, however, are relatively less inflated (from frontal view), male thoracic sternites 3 and 4 are not clearly separated by a groove, and their male abdomens are still somewhat T-shaped, with somite 6 longer than broad (e.g., see Bahir & Yeo, 2007: figs. 7D, 8B, 10C, 31B, 32B, C). The carapaces of Vela and Baratha are more inflated from frontal view (Bahir & Yeo, 2007: figs. 19B, 46B) and the thoracic sternites 3 and 4 of the males are separated by a deep groove (Bahir & Yeo, 2007: figs. 19C, 46C), but the abdomens of the males are even more distinctly T-shaped, with somite 6 long (Bahir & Yeo, 2007: figs. 18B, 31B) and the G1 terminal segments short (Bahir & Yeo, 2007: figs. 20A–D, 31C–F). The relatively broader and more triangular male abdomen of Kanin. gen. (Fig. 6E, F) most closely resembles the condition in species of BarytelphusaAlcock, 1909 (e.g., Bahir & Yeo, 2007: fig. 3C) but its members have a conspicuously short G2 terminal segment (e.g., Bahir & Yeo, 2007: fig. 2D). The G1 structure of the new genus (Fig. 8A, B) most closely resembles that of species of Vela (e.g., Bahir & Yeo, 2007: fig. 45C–E), but the male abdominal somite 6 in species of Vela is distinctly longer than broad (Bahir & Yeo, 2007: fig. 45B). The carapace of Kanin. gen. (Fig. 5) superficially resembles that of Vanni ashiniBahir & Yeo, 2007 (cf. Bahir & Yeo, 2007: fig. 40A), but differs in all other carapace and abdominal and gonopod characters of the male.
Kani maranjandu n. gen., n. sp.
Material examined: Holotype: male (42.6 × 30.8 mm) (ZSI/WGRC/IR-INV 8234), Cherumankal, Agathyamala Biological Park, Kottoor Reserve Forest, Kottoor, Kerala, 8°60’ N 77°20’E, Western Ghats, India, coll. S. Raj, 5 September 2016. Paratypes: 1 juvenile male (17.6 × 14.1 mm, carapace damaged), 1 young female (26.7 × 20.4 mm), 1 female (40.6 × 30.4 mm) (DABFUK/AR-BR-56), same data as holotype; 2 males (40.5 × 30.0 mm, 33.1 × 24.3 mm) (DABFUK/AR-BR-57, 58), Cherumankal, Agathyamala Biological Park, Kottoor Reserve Forest, Kottoor, Kerala, 8°36’00.8”N 77°11’57.9”E, Western Ghats, India, coll. A. B. Kumar, 1 November 2016.
Description of male holotype: Carapace transversely ovate, 1.4 times wider than long; high in frontal view, swollen; dorsal surface unevenly convex (Figs. 4, 5, 6A, B, D). Frontal region longitudinally narrow, surface uneven but not granular, relatively sunken, distinctly lower than epigastric, postorbital cristae; lateral parts of anterolateral, branchial regions covered with low, short oblique striae; mesogastric, urogastric, cardiac, branchial, intestinal regions finely pitted or uneven, otherwise smooth; orbital regions finely pitted; suborbital region smooth, glabrous; pterygostomial region smooth, glabrous, separated from suborbital region by low granular ridge; sub-branchial region covered with low striae, pits (Figs. 5, 6A–E). Epigastric cristae distinct but low, not cristate, separated by broad, shallow, Y-shaped furrow; postorbital cristae low, not conspicuously sharp, confluent with epigastric cristae, at approximately same level (Figs. 5, 6A, B). Cervical grooves deep, broad; H-shaped median gastric groove distinct; posterior part of carapace at intestinal region with shallow groove that transverses width, reaching lateral margin (Figs. 5, 6A, B). Frontal margin divided into 2 broad lobes, separated by shallow concavity; margin of each lobe gently convex, joining supraorbital margin at slight angle (Figs. 5, 6B). External orbital tooth prominent, triangular, tip extending anteriorly slightly beyond orbit, front, clearly demarcated from anterolateral margin by deep V-shaped notch, outer margin convex or straight, inner margin gently sloping to join supraorbital margin; epibranchial tooth low but distinct (Figs. 5, 6B). Anterolateral margins strongly convex, subcristate, lined with prominent, small, unevenly arranged teeth, short spines (Figs. 5, 6B). Posterolateral margin gently concave, sharply converging towards gently convex posterior carapace margin (Fig. 5). Orbits subovate, inner surface with short setae; eye filling up most of orbital space, angled obliquely when retracted and viewed frontally; eye peduncle moderately long, stout; cornea large, pigmented (Figs. 5, 6A–C). Supraorbital margin gently sinuous, entire (Figs. 5, 6B). Suborbital margin concave, complete, lined with very low, rounded granules (Fig. 6A, C). Antennae distinctly short, not reaching cornea of eyes; basal antennal article quadrate, not fused but not fully mobile; first article (urinary article) large, lodged between edge of epistome, pterygostomial margin; antennules short, folding transversely in narrow fossa (Fig. 6A, C). Posterior margin of epistome with distinct median triangle, sunken below margin of epistome; each lateral margin distinctly sinuous (Fig. 6A-C). Endostome smooth, without ridges. Mandibular palp distinctly 2-segmented; terminal article distinctly bilobed.
Third maxillipeds covering most of buccal cavity when closed; ischium subrectangular, surface pitted, with distinct median oblique groove; merus subovate, as wide as long, surface with median depression, anteroexternal angle evenly convex; exopod relatively slender, reaching lower third of merus, with distinct flagellum reaching to just across width of merus (Fig. 7A, B).
Chelipeds asymmetrical (Figs. 5, 7C). Anterior margin of basis-ischium lined with small granules; dorsal, ventral margins of merus lined with low granules, weakly serrated (Fig. 5, 7F). Outer surface of carpus rugose, inner margins granular, distal angle with prominent sharp tooth (Figs. 5A, 7D–F). Outer surfaces of chelae rugose; major chela stouter than minor chela (Fig. 7C, E). Fingers of major chela short, stout, gently curved, shorter than palm; cutting edges of both fingers with variously sized teeth on proximal half; dorsal margin of dactylus, ventral margin of propodal finger with scattered small, fine granules (Fig. 7C, D). Fingers of minor chela similar to that of major chela but palm more slender (Fig. 7C, E).
Ambulatory legs slender, long; second pair longest, last pair shortest (Figs. 5, 9A, D). Outer surface of merus slightly rugose, dorsal margin weakly serrated to entire, without obvious subdistal spine or tooth, very short if present; carpus outer surface with submedian crista on first to third legs, that on fourth leg almost smooth; outer surface of propodus with low, submedian crista; dactylus long, longest in second leg, gently curved, quadrate in cross section, margins with short, sharp pectinate spines, tip strongly curved (Figs. 5A, 7G-J).
Thoracic sternum (notably sternites 3, 4) relatively narrow transversely, surface conspicuously pitted (Fig. 6E). Sternites 1, 2 completely fused to form broadly triangular plate; separated from sternite 3 by shallow but distinct gently concave suture; sternites 3, 4 completely separated by prominent oblique depression marking suture; sutures between sternites 4/5, 5/6, 6/7 medially interrupted; suture between sternites 7/8 complete; deep longitudinal groove on sternite 8, extending on posterior four-fifths of length of sternite 7 (Fig. 6E, G). Penis coxal, on condyle of coxa of fourth ambulatory leg. Sternoabdominal cavity deep, reaching to imaginary line connecting submedian points of edges of cheliped coxae (Fig. 6E). Male abdominal-locking tubercle low, round, positioned on submedian part of sternite 5 (Fig. 6G).
Pleon narrowly triangular, all somites (including telson) free; telson acutely triangular with rounded tip, surface pitted, crenulated, with distal third possessing distinct transverse crease lateral margins gently concave; somite 6 trapezoidal, much wider than long; somites 3–5 trapezoidal, gradually decreasing in width; somites 1, 2 subrectangular, conspicuously wide, reaching to bases of coxae of fourth ambulatory legs, thoracic sternite 8 not visible when pleon closed (Figs. 6E, F).
G1 with terminal, subterminal segments clearly demarcated by distinct membranous suture; subterminal segment relatively slender throughout length; basal part broader, gradually tapering; terminal segment slender, conical, gradually tapering to rounded tip (Fig. 8A, B, D–F). G2 longer than G1, basal segment long, distal segment slender, long, about a third length of basal segment (Fig. 8C).
Females: The largest adult paratype female specimen (40.6 × 30.4 mm, DABFUK) resembles the holotype in most non-sexual characters, with the chelipeds not as prominently asymmetrical or swollen (Fig. 9A). Its pleon is ovate, with all the somites and telson free, covering most of the thoracic sternum except the lateral edges and the distal part of thoracic sternite 3 when closed (Fig. 9B). The vulvae, on somite 6, are moderately large, subovate, positioned just adjacent to margin of sternite 5, with outer rim slightly raised; membranous operculum covering the opening (Fig. 9C). The smaller paratype female (26.7 × 20.4 mm, DABFUK/AR-BR-56) is immature.
Variation: The length of the flagellum on the exopod of the third maxilliped varies slightly, from about two-thirds to the full width of the merus. The ambulatory legs of smaller specimens (Fig. 9D) are slightly shorter when compared to larger ones (Fig. 9A), but the difference is not substantial.
Colour: In life, most of the carapace and legs is a distinct bluish-black, with the pterygostomial region, lower part of the branchiostegal surfaces, posterolateral margins, orbital margins, and external orbital teeth yellowish-orange (Figs. 3A, B, 4A, C, E). The ventral surfaces of the chelipeds and the lower two-thirds of the chelae are yellowish-orange (Figs. 3A, B, 4C, D). In smaller males, the ventral surfaces of the chelipeds and the lower two-thirds of the chelae are pale yellow (Fig. 3C–F). The ventral surfaces of the thoracic sterna and pleons are dirty-orange (Fig. 4B, F). The joints of the ambulatory legs are orange (Fig. 4A, B, E, F).
Etymology: The species name is derived from the Malayalam word mara for “tree” and njandu for “crab”. The name is used as a noun in apposition.
Ecology: Kani maranjandun. gen., n. sp. is the first tree-climbing crab reported from India. Morphological features such as distinctly long legs with hooked dactyli are clearly arboreal adaptations. The type series was collected from the tree hollows of a Bedda nut tree, Terminalia bellirica (Gaertn.) Roxb. (Combretaceae). Other crabs have been observed on trees or in the tree hollows of the Flowering Murdah Terminalia paniculata Roth. (Combretaceae), Malabar Ironwood Hopea parviflora Bedd. (Dipterocarpaceae), true Cinnamon tree Cinnamomum verum J. Presl (Lauraceae), and evergreen tropical blackboard, or devil, tree Alstonia scholaris (L.) R. Br. (Apocynaceae) (Fig. 2E–G). The crab is a completely arboreal species, using phytotelms for water and food (Fig. 2B–D). We found that many individuals can share some of the larger phytotelms. The tribesmen mentioned that they locate the presence of crabs inside the hollows by looking at the debris they push out of the hollows and by observing the air bubbles that come out of water in the phytotelms. When approached, the crabs move swiftly and retreat deep inside the hollow or other cavities insides the hollow, making them very difficult to collect. They are found only in tree holes that contain water. When outside of the hollows, crabs move very fast along tree trunks (Fig. 2D, E), often with the help of their stout chelipeds. Young individuals have been observed taking shelter in the canopy. The trees which have crabs are always some distance from streams. According to the tribesmen, the primary predators of these tree crabs are mongooses and owls, which prey on the crabs at night. The crabs have been observed to feed on leaves, seeds, slugs, worms, and insects on the trees. They were observed in tree hollows close to the ground (Fig. 2E) and up to 10 m up in the trees.
While many semiterrestrial freshwater crabs will climb the base of trees or low shrubs while foraging for food, they do not normally go higher than a few metres above the ground and are more common and/or forage on the forest floor. The West African tree crab, Globonautes macropus (Rathbun, 1898) lives in tree holes 1.5 to 3 m above the ground but is known to forage on the forest floor (Cumberlidge & Sachs, 1991). The same is true for the Kenyan and Tanzanian Potamonautes raybouldiCumberlidge & Vannini, 2004, which occurs in tree holes in closed forests 1 to 2 or more metres above the ground (Bayliss, 2002; Cumberlidge & Vannini, 2004). The gecarcinucid Ghatiana atropurpureaPati, Thackeray & Khaire, 2016, has been reported living in tree holes near the ground in Maharashtra, southern India. This species is nevertheless not a true arboreal crab as it is also found near streams and among boulders (Pati et al., 2016).
True arboreal crabs on the other hand, spend most or all of their time on the trees themselves (see Sivasothi et al., 1993; Sivasothi, 2000; Cumberlidge et al., 2005; Fratini et al., 2005). From what is so far known, the new species is an obligate tree crab. In this respect, it is probably more like fully arboreal inland species like the Bornean potamids ArachnothelphusaNg, 1991 (see Ng, 1991; Grinang et al., 2015) and southeast and east Asian sesarmids LabuaniumSerène & Soh, 1970, and ScandarmaSchubart, Liu & Cuesta, 2003 (see Ng & Liu, 2003; Schubart et al., 2003; Naruse & Ng, 2007; Ng, 2012; Ng et al., 2015), the Madagascan potamonautid Gecarcinautes goodmaniCumberlidge, Boyko & Harvey, 2002 (see Cumberlidge et al., 2002, 2005), and the Caribbean pseudothelphusids Epilobocera spp. (see Mendez, 1983; Wehrtmann et al., 2016).
The only other primarily arboreal freshwater crab known from the Indian subcontinent is Perbrinckia scansor (Ng, 1995) from the highlands of Sri Lanka (Ng, 1995). Like Kani maranjandun. gen., n. sp., Perbrinckia scansor also has elongated ambulatory legs and has phytotelmic tendencies but has also been occasionally found on the forest floor (Ng, 1995; Ng & Tay, 2001). Its biology, however, is poorly known.
The swollen carapace of arboreal crabs like Kani maranjandun. gen., n. sp., Perbrinckia scansor, Globonautes macropus, Arachnothelphusa spp., and Labuanium spp. are probably associated with the low levels of dissolved oxygen in phytotelms, as also observed in other freshwater crabs which live in similar habitats (see Ng, 1988; Cumberlidge & Ng, 2009). Vannini & Chelazi (1985) and Vannini et al. (1997), in discussing the morphological adaptations of arboreal crabs, noted that most tree-climbers have relatively short ambulatory dactyli (see also Ng et al., 2015). The ambulatory dactyli of Kanin. gen. are distinctive in that they are long, with the distal part slender, strongly curved and very sharp. These structures hook onto surfaces very well (observed even in preserved specimens) and make the crabs very effective climbers.
Additional remarks: The tribesmen occasionally catch the tree crabs to prepare an oil which is used against skin diseases and ear ache. They believe the post-monsoon (September–October) to be the breeding season of the crab and they will not catch females which bear eggs or carry juveniles.
The Western Ghats is one the major global biodiversity sites (Myers et al., 2000), and the freshwater crab fauna there is no exception. The number of new taxa of freshwater gecarcinucid crabs discovered from the Western Ghats has been gradually increasing over the last decade with dozens of taxa described (Bahir & Yeo, 2005, 2007; Pati & Sharma, 2013, 2014; Klaus et al., 2014; Pati & Sudha Devi, 2015a, b; Pati et al., 2016), and many more still being studied (A.B. Kumar, unpublished data). As has been discussed by various authors, the fauna of the Western Ghats is quite distinct, with many taxa endemic to the area (Bossuyt et al., 2004; Beenaerts et al., 2010; Klaus et al., 2014). The gecarcinucid fauna of Kerala is still poorly understood, with the current known number of species still a substantial under-estimation (see Klaus et al., 2014; Raghavan et al., 2015, 2016). Increasing areas of the southern Western Ghats are ecologically debilitated and there is clear urgency to better understand this fauna as the conservation challenges grow (see Molur et al., 2011).
Kani maranjandun. gen., n. sp. was found in degraded forest areas near the tribal settlements in the Kottoor Reserve Forests, Agasthyamala Biological Park (Figs. 1, 2A), and the presence of this key species suggests that this site should also be included as part of the Agasthyamala Biosphere Reserve. This discovery further stresses the urgent need to conserve larger trees in the degraded forest ecosystems of the Western Ghats.
ACKNOWLEDGEMENTS
The authors thank the Kerala Forests and Wildlife Department for the sanction (WL10-8404-2014 dated 23.06.2014) to collect freshwater crabs from the forests of Kerala. The authors thank the support of University Grants Commission Special Assistance Programme for the fieldwork. We thank the tribesmen Mathan Kani and Rajan Kani for their assistance in collection. The map was kindly prepared by Neelesh Dahanukar. We thank wildlife photographer Sali Palode for providing us the in situ photographs of the tree crabs. The third author’s visit to the University of Kerala to help conduct a crustacean taxonomy workshop coincided with the first author’s work on this crab, and the joint study resulted in the discovery of the present new taxon. Thank are due to Célio Magalhães and an anonymous referee for their helpful comments on the manuscript.
REFERENCES
Author notes
Correspondence: Peter K. L. Ng; email: peterng@nus.edu.sg