-
PDF
- Split View
-
Views
-
Cite
Cite
Hertzel C Gerstein, Eric E Smith, Chinthanie Ramasundarahettige, Dipika Desai, Philip Awadalla, Philippe Broet, Sandra Black, Trevor J B Dummer, Jason Hicks, Alan Moody, Jean-Claude Tardif, Koon K Teo, Jennifer Vena, Salim Yusuf, Douglas S Lee, Matthias G Friedrich, Sonia S Anand, Diabetes, Brain Infarcts, Cognition, and Small Vessels in the Canadian Alliance for Healthy Hearts and Minds Study, The Journal of Clinical Endocrinology & Metabolism, Volume 106, Issue 2, February 2021, Pages e891–e898, https://doi.org/10.1210/clinem/dgaa815
Close - Share Icon Share
Abstract
Diabetes is a risk factor for cerebrovascular disease and cognitive impairment. The anatomical basis for this is uncertain.
The Canadian Alliance for Healthy Hearts and Minds collected brain and carotid magnetic resonance imaging (MRI) and 2 cognitive tests (the Digit Symbol Substitution Test and the Montreal Cognitive Assessment test) in a cross-sectional sample of men and women. Brain MRIs identified brain infarcts (BI), lacunar BI, high white matter hyperintensity (WMH), vascular brain injury (VBI; BI or high WMH), and small vessel VBI (lacunar BI or high WMH). Carotid MRIs estimated carotid wall volume, a measure of subclinical atherosclerosis. Cognitive scores were standardized to each site’s mean score, and cognitive impairment was identified by 1 or both test scores ≤1 standard deviation below the site’s mean score on that test.
The 7733 participants included 495 participants (6.4%) with diabetes, of whom 388 were taking diabetes drugs. After age and sex adjustment, diabetes was independently associated with BI (odds ratio [OR] 1.53, 95% confidence interval [CI] 1.05, 2.24), VBI (OR 1.64, 95% CI 1.26, 2.13), small vessel VBI (OR 1.67, 95% CI 1.28, 2.19), and cognitive impairment (OR 1.47, 95% CI 1.20, 1.80). The association between diabetes and small vessel VBI persisted after adjustment for cerebrovascular disease risk factors and nonlacunar infarcts (OR 1.52, 95% CI 1.15, 2.01), and the association with cognitive impairment persisted after adjustment for small vessel VBI (OR 1.27, 95% CI 1.03, 1.56).
Small vessel disease characterizes much of the relationship between diabetes and VBI. However, additional factors are required to disentangle the relationship between diabetes and cognitive impairment.
Diabetes is a common chronic disease currently affecting 1 in 11 adults globally, with a much higher prevalence in Indigenous peoples, South Asians, North Africans, and people of lower socioeconomic status (1). People with diabetes are at a high risk for a wide variety of serious health outcomes, including strokes, cognitive impairment, and dementia. Although the underlying mechanisms are uncertain, one possible mechanism is that the effects of diabetes on the brain may be due to diabetes-related abnormalities on small vessels throughout the body (2, 3), including the brain. This leads to hypoxia and secondary inflammation and its consequences, including large vessel disease and ischemia. Small vessel disease may manifest in the brain as lacunar strokes (4) and/or cognitive decline (5). It may also cause the white matter hyperintensities (WMHs) that are often observed in brain magnetic resonance images (MRIs) of people with diabetes and that are associated with both cerebrovascular disease and cognitive impairment (6).
The Canadian Alliance for Healthy Hearts and Minds (CAHHM) study (7) collected brain MRIs, phenotypic clinical information, and 2 measures of cognitive function (the Montreal Cognitive Assessment [MoCA] test and the Digit Symbol Substitution Test [DSST]) in a cross-sectional sample of men and women of mean age 57.9 (standard deviation [SD] 8.9) years living near 10 sites throughout Canada (8). These data provide a unique opportunity to assess the relationship between prevalent diabetes, small vessel disease, and cognitive impairment.
Materials and Methods
The CAHHM study is an alliance of 7 cohort studies comprising the Canadian Partnership for Tomorrow’s Health (which is a federation of 5 regional cohort studies from across Canada), the Canadian component of the Prospective Urban Rural Evaluation study, and the Montreal Heart Institute Biobank (7). Participants for each cohort were eligible for CAHHM if they were aged 35–69 years and willing to undergo an MRI scan and other study procedures. Selection of participants from each cohort was stratified to ensure that less than 20% had known cardiovascular disease (CVD), about 50% were women, and age was balanced across age strata of 35–45 years, 46–55 years, and 56–69 years.
Data from Canadian men and women with and without a history of diabetes who had an MRI scan using a 1.5 or 3T magnet, as well as other study procedures (7), were analyzed for this report. Magnetic resonance imaging scans included a 2-dimensional brain fluid-attenuated inversion recovery sequence and 3-dimensional carotid artery T1-weighted magnetization prepared rapid gradient-echo sequences. The brain MRI scans were read at 2 different reading labs, and the carotid MRI was read at 1 lab according to standard protocol, without knowledge of the participant’s clinical characteristics (7).
Measurements
Participants were classified as having diabetes, and as having type 1 diabetes, type 2 diabetes, or other types based on self-report. In ancillary analyses, diabetes was also defined on the basis of self-reported use of 1 or more drugs to treat diabetes.
Individuals with MRI evidence of 1 or more areas of brain infarction were classified as having a brain infarct (BI). A small subcortical BI, with an axial diameter ≤ 15 mm, was classified as a lacunar BI (8, 9). A cortical BI of any size or a subcortical BI > 15 mm was classified as a nonlacunar BI. White matter hyperintensities on the MRI scans were scored using the Fazekas scale (10), and individuals with a total score (summing subcortical and periventricular regions) ≥ 4 were classified as having a high burden of WMHs. Individuals who had ≥ 1 BI or a high WMH burden were classified as having evidence of vascular brain injury (VBI), and individuals with ≥ 1 lacunar BI or high WMH were classified as having evidence of small vessel VBI.
The volume of each carotid artery wall was calculated by subtracting its MRI-measured lumen volume from total vessel volume within a 32-mm vessel length centered on each carotid bifurcation (that included the distal common and proximal internal carotid arteries). The largest of the 2 volumes was used for analyses (8).
Two cognitive tests were administered at the time of assessment. The MoCA test is a cognitive test comprising 30 questions that, together, assess 7 cognitive domains, including short-term memory, visuospatial abilities, executive function, attention, concentration, working memory, and language (11). Validation studies report that cognitively intact individuals have a mean MoCA score of 27.4 (SD 2.2), whereas population-based studies of presumably cognitive intact people report lower mean scores (12). The DSST assesses visual motor speed and coordination, capacity for learning, attention, concentration, and short-term memory (13). It has been extensively used in people with and without type 2 diabetes, and its score is correlated with future cognitive decline (14). Previous studies in cognitively intact people with diabetes have reported mean scores between 36 (15) and 52 (16).
To account for possible between-site differences in the administration of these 2 tests, each of the cognitive scores for each person were standardized to the mean value at each site. This created a site-standardized mean cognitive score for each individual, with a mean value at each site of 0 (SD 1) (17). As suggested in recent papers (17, 18), an individual was classified as having cognitive impairment if his or her site-standardized score was ≤ -1 (ie, 1 or more SDs below the site-standardized mean).
Statistical analysis
Continuous data were summarized as means and SDs and were compared using the Student’s t-test; categorical data were summarized as the number and percentage and were compared using the chi-square test. Univariable and multivariable logistic regression models were used to estimate the odds of BI, VBI, and cognitive impairment (ie, dependent variables) in people with self-reported diabetes and in people with self-reported diabetes who were also using glucose-lowering medications. A P-value < 0.05 was considered nominally significant with no adjustment for multiple testing. All analyses were done using SAS (version 9.4). The protocol was approved by local ethics committees associated with each site and all participants provided written informed consent.
Results
Participants who had an interpreted MRI scan, completed at least 1 cognitive test, and recorded information regarding risk factors were included in this analysis. As noted in the supplement, Figure S1; all supplementary material and figures are located in a digital research materials repository (19), 7733/8580 (90.1%) consenting participants (54.4% women) of mean age 57.9 years (SD 8.9) were included, of whom 495 (6.4%) reported a history of diabetes.
Clinical characteristics of the participants according to self-reported diabetes status are summarized in Table 1. Compared to the 7238 participants without a history of diabetes, the 495 with diabetes (comprising 407 with self-reported type 2 diabetes) included more males, were older, less educated, had lower cognitive scores, and included more people who were currently smoking, had hypertension, a previous stroke, a BI, or a lacunar BI. Diabetes duration was available in 71% of participants and was 12.2 years (SD 8.7) overall, 22.1 (SD 12.2) years in people with type 1 diabetes, 11.9 (SD 8.1) years in people with type 2 diabetes, and 7.4 (SD 6.5) years in people whose diabetes type was unknown.
Baseline characteristics of people with or without self-reported diabetes
| Characteristic . | Overall . | Diabetesa . | No Diabetes . | P-value . |
|---|---|---|---|---|
| N | 7733 | 495 | 7238 | |
| Diabetes drugs | 388 (5.1%) | 388 (78.4%) | 0 | – |
| Age (years) | 57.9 (8.9) | 61.1 (7.9) | 57.7 (8.9) | < 0.001 |
| Femalesb | 4207 (54.0%) | 205 (41.4%) | 4002 (55.3%) | < 0.001 |
| High school education or less | 1023 (13.2%) | 89 (18.0%) | 934 (12.9%) | 0.001 |
| White | 6279 (81.2%) | 372 (75.2%) | 5907 (81.6%) | < 0.001 |
| Asian Chinese | 861 (11.1%) | 69 (13.9%) | 792 (10.9%) | – |
| Other | 593 (7.7%) | 54 (10.9%) | 539 (7.4%) | – |
| Current smoking | 422 (5.5%) | 31 (6.3%) | 391 (5.4%) | 0.001 |
| Hypertension | 3035 (39.3%) | 350 (70.7%) | 2685 (37.1%) | < 0.001 |
| Carotid wall volume (mm3) | 902.7 (168.2) | 911.4 (163.2) | 902.2 (168.5) | 0.080 |
| History of clinical stroke | 83 (1.1%) | 24 (4.8%) | 59 (0.8%) | < 0.0001 |
| History of myocardial infarction | 82 (1.16%) | 17 (3.4%) | 65 (0.9%) | <0.001 |
| Depression | 1640 (21.2%) | 116 (23.4%) | 1524 (21.1%) | 0.21 |
| Cancer | 537 (6.9%) | 33 (6.7%) | 504 (7.0%) | 0.79 |
| Aspirin use | 1114 (14.4%) | 197 (39.8%) | 917 (12.7%) | <0.001 |
| Statin use | 1504 (19.5%) | 317 (64.0%) | 1187 (16.4%) | <0.001 |
| Brain infarcts | 296 (3.8%) | 34 (6.9%) | 262 (3.6%) | < 0.001 |
| Number of brain infarcts | ||||
| 0 | 7437 (96.2%) | 461 (93.1%) | 6976 (96.4%) | < 0.001 |
| 1 | 216 (2.8%) | 22 (4.4%) | 194 (2.7%) | – |
| ≥ 2 | 80 (1.0%) | 12 (2.4%) | 68 (0.94%) | – |
| Lacunar brain infarcts | 213 (2.8%) | 26 (5.3%) | 187 (2.6%) | < 0.001 |
| Number of lacunar brain infarcts | ||||
| 0 | 7520 (97.3%) | 469 (94.8%) | 7051 (97.4%) | 0.003 |
| 1 | 172 (2.2%) | 21 (4.2%) | 151 (2.1%) | – |
| ≥ 2 | 41 (0.53%) | 5 (1.0%) | 36 (0.50%) | – |
| Nonlacunar brain infarcts | 108 (1.4%) | 11 (2.2%) | 97 (1.3%) | 0.11 |
| Number of nonlacunar brain infarcts | ||||
| 0 | 7625 (98.6%) | 484 (97.8%) | 7141 (98.7%) | 0.092 |
| 1 | 59 (0.76%) | 4 (0.81%) | 55 (0.76%) | – |
| ≥ 2 | 49 (0.63%) | 7 (1.4%) | 42 (0.58%) | – |
| High white matter hyperintensityc | 470 (6.1%) | 56 (11.3%) | 414 (5.7%) | <0.001 |
| Vascular brain injuryd | 715 (9.3%) | 82 (16.6%) | 633 (8.8%) | <0.001 |
| Site-standardized MoCA | 0.01 (0.99) | -0.22 (1.11) | 0.02 (0.98) | <0.001 |
| Site-standardized DSST | 0.01 (1.00) | -0.39 (0.98) | 0.03 (0.99) | <0.001 |
| Cognitive impairmente | 1927 (24.9%) | 186 (37.6%) | 1741 (24.1%) | <0.001 |
| Characteristic . | Overall . | Diabetesa . | No Diabetes . | P-value . |
|---|---|---|---|---|
| N | 7733 | 495 | 7238 | |
| Diabetes drugs | 388 (5.1%) | 388 (78.4%) | 0 | – |
| Age (years) | 57.9 (8.9) | 61.1 (7.9) | 57.7 (8.9) | < 0.001 |
| Femalesb | 4207 (54.0%) | 205 (41.4%) | 4002 (55.3%) | < 0.001 |
| High school education or less | 1023 (13.2%) | 89 (18.0%) | 934 (12.9%) | 0.001 |
| White | 6279 (81.2%) | 372 (75.2%) | 5907 (81.6%) | < 0.001 |
| Asian Chinese | 861 (11.1%) | 69 (13.9%) | 792 (10.9%) | – |
| Other | 593 (7.7%) | 54 (10.9%) | 539 (7.4%) | – |
| Current smoking | 422 (5.5%) | 31 (6.3%) | 391 (5.4%) | 0.001 |
| Hypertension | 3035 (39.3%) | 350 (70.7%) | 2685 (37.1%) | < 0.001 |
| Carotid wall volume (mm3) | 902.7 (168.2) | 911.4 (163.2) | 902.2 (168.5) | 0.080 |
| History of clinical stroke | 83 (1.1%) | 24 (4.8%) | 59 (0.8%) | < 0.0001 |
| History of myocardial infarction | 82 (1.16%) | 17 (3.4%) | 65 (0.9%) | <0.001 |
| Depression | 1640 (21.2%) | 116 (23.4%) | 1524 (21.1%) | 0.21 |
| Cancer | 537 (6.9%) | 33 (6.7%) | 504 (7.0%) | 0.79 |
| Aspirin use | 1114 (14.4%) | 197 (39.8%) | 917 (12.7%) | <0.001 |
| Statin use | 1504 (19.5%) | 317 (64.0%) | 1187 (16.4%) | <0.001 |
| Brain infarcts | 296 (3.8%) | 34 (6.9%) | 262 (3.6%) | < 0.001 |
| Number of brain infarcts | ||||
| 0 | 7437 (96.2%) | 461 (93.1%) | 6976 (96.4%) | < 0.001 |
| 1 | 216 (2.8%) | 22 (4.4%) | 194 (2.7%) | – |
| ≥ 2 | 80 (1.0%) | 12 (2.4%) | 68 (0.94%) | – |
| Lacunar brain infarcts | 213 (2.8%) | 26 (5.3%) | 187 (2.6%) | < 0.001 |
| Number of lacunar brain infarcts | ||||
| 0 | 7520 (97.3%) | 469 (94.8%) | 7051 (97.4%) | 0.003 |
| 1 | 172 (2.2%) | 21 (4.2%) | 151 (2.1%) | – |
| ≥ 2 | 41 (0.53%) | 5 (1.0%) | 36 (0.50%) | – |
| Nonlacunar brain infarcts | 108 (1.4%) | 11 (2.2%) | 97 (1.3%) | 0.11 |
| Number of nonlacunar brain infarcts | ||||
| 0 | 7625 (98.6%) | 484 (97.8%) | 7141 (98.7%) | 0.092 |
| 1 | 59 (0.76%) | 4 (0.81%) | 55 (0.76%) | – |
| ≥ 2 | 49 (0.63%) | 7 (1.4%) | 42 (0.58%) | – |
| High white matter hyperintensityc | 470 (6.1%) | 56 (11.3%) | 414 (5.7%) | <0.001 |
| Vascular brain injuryd | 715 (9.3%) | 82 (16.6%) | 633 (8.8%) | <0.001 |
| Site-standardized MoCA | 0.01 (0.99) | -0.22 (1.11) | 0.02 (0.98) | <0.001 |
| Site-standardized DSST | 0.01 (1.00) | -0.39 (0.98) | 0.03 (0.99) | <0.001 |
| Cognitive impairmente | 1927 (24.9%) | 186 (37.6%) | 1741 (24.1%) | <0.001 |
Data are shown as either N (%) or mean (standard devitation); P values were calculated with a 2-sample t-test, Mann-Whitney U test, or chi-square test
Abbreviations: DSST, Digit Symbol Substitution Test; MoCA, Montreal Cognitive Assessment.
aA total of 162 (79%) participants with diabetes and 2758 (69%) without diabetes were postmenopausal.
bSelf-reported type 1 diabetes = 28; type 2 diabetes = 407; and unreported type = 60.
cFazekas score ≥ 4.
d≥1 brain infarct or high white matter hyperintensity.
eMoCA or DSST score ≤ -1.
Baseline characteristics of people with or without self-reported diabetes
| Characteristic . | Overall . | Diabetesa . | No Diabetes . | P-value . |
|---|---|---|---|---|
| N | 7733 | 495 | 7238 | |
| Diabetes drugs | 388 (5.1%) | 388 (78.4%) | 0 | – |
| Age (years) | 57.9 (8.9) | 61.1 (7.9) | 57.7 (8.9) | < 0.001 |
| Femalesb | 4207 (54.0%) | 205 (41.4%) | 4002 (55.3%) | < 0.001 |
| High school education or less | 1023 (13.2%) | 89 (18.0%) | 934 (12.9%) | 0.001 |
| White | 6279 (81.2%) | 372 (75.2%) | 5907 (81.6%) | < 0.001 |
| Asian Chinese | 861 (11.1%) | 69 (13.9%) | 792 (10.9%) | – |
| Other | 593 (7.7%) | 54 (10.9%) | 539 (7.4%) | – |
| Current smoking | 422 (5.5%) | 31 (6.3%) | 391 (5.4%) | 0.001 |
| Hypertension | 3035 (39.3%) | 350 (70.7%) | 2685 (37.1%) | < 0.001 |
| Carotid wall volume (mm3) | 902.7 (168.2) | 911.4 (163.2) | 902.2 (168.5) | 0.080 |
| History of clinical stroke | 83 (1.1%) | 24 (4.8%) | 59 (0.8%) | < 0.0001 |
| History of myocardial infarction | 82 (1.16%) | 17 (3.4%) | 65 (0.9%) | <0.001 |
| Depression | 1640 (21.2%) | 116 (23.4%) | 1524 (21.1%) | 0.21 |
| Cancer | 537 (6.9%) | 33 (6.7%) | 504 (7.0%) | 0.79 |
| Aspirin use | 1114 (14.4%) | 197 (39.8%) | 917 (12.7%) | <0.001 |
| Statin use | 1504 (19.5%) | 317 (64.0%) | 1187 (16.4%) | <0.001 |
| Brain infarcts | 296 (3.8%) | 34 (6.9%) | 262 (3.6%) | < 0.001 |
| Number of brain infarcts | ||||
| 0 | 7437 (96.2%) | 461 (93.1%) | 6976 (96.4%) | < 0.001 |
| 1 | 216 (2.8%) | 22 (4.4%) | 194 (2.7%) | – |
| ≥ 2 | 80 (1.0%) | 12 (2.4%) | 68 (0.94%) | – |
| Lacunar brain infarcts | 213 (2.8%) | 26 (5.3%) | 187 (2.6%) | < 0.001 |
| Number of lacunar brain infarcts | ||||
| 0 | 7520 (97.3%) | 469 (94.8%) | 7051 (97.4%) | 0.003 |
| 1 | 172 (2.2%) | 21 (4.2%) | 151 (2.1%) | – |
| ≥ 2 | 41 (0.53%) | 5 (1.0%) | 36 (0.50%) | – |
| Nonlacunar brain infarcts | 108 (1.4%) | 11 (2.2%) | 97 (1.3%) | 0.11 |
| Number of nonlacunar brain infarcts | ||||
| 0 | 7625 (98.6%) | 484 (97.8%) | 7141 (98.7%) | 0.092 |
| 1 | 59 (0.76%) | 4 (0.81%) | 55 (0.76%) | – |
| ≥ 2 | 49 (0.63%) | 7 (1.4%) | 42 (0.58%) | – |
| High white matter hyperintensityc | 470 (6.1%) | 56 (11.3%) | 414 (5.7%) | <0.001 |
| Vascular brain injuryd | 715 (9.3%) | 82 (16.6%) | 633 (8.8%) | <0.001 |
| Site-standardized MoCA | 0.01 (0.99) | -0.22 (1.11) | 0.02 (0.98) | <0.001 |
| Site-standardized DSST | 0.01 (1.00) | -0.39 (0.98) | 0.03 (0.99) | <0.001 |
| Cognitive impairmente | 1927 (24.9%) | 186 (37.6%) | 1741 (24.1%) | <0.001 |
| Characteristic . | Overall . | Diabetesa . | No Diabetes . | P-value . |
|---|---|---|---|---|
| N | 7733 | 495 | 7238 | |
| Diabetes drugs | 388 (5.1%) | 388 (78.4%) | 0 | – |
| Age (years) | 57.9 (8.9) | 61.1 (7.9) | 57.7 (8.9) | < 0.001 |
| Femalesb | 4207 (54.0%) | 205 (41.4%) | 4002 (55.3%) | < 0.001 |
| High school education or less | 1023 (13.2%) | 89 (18.0%) | 934 (12.9%) | 0.001 |
| White | 6279 (81.2%) | 372 (75.2%) | 5907 (81.6%) | < 0.001 |
| Asian Chinese | 861 (11.1%) | 69 (13.9%) | 792 (10.9%) | – |
| Other | 593 (7.7%) | 54 (10.9%) | 539 (7.4%) | – |
| Current smoking | 422 (5.5%) | 31 (6.3%) | 391 (5.4%) | 0.001 |
| Hypertension | 3035 (39.3%) | 350 (70.7%) | 2685 (37.1%) | < 0.001 |
| Carotid wall volume (mm3) | 902.7 (168.2) | 911.4 (163.2) | 902.2 (168.5) | 0.080 |
| History of clinical stroke | 83 (1.1%) | 24 (4.8%) | 59 (0.8%) | < 0.0001 |
| History of myocardial infarction | 82 (1.16%) | 17 (3.4%) | 65 (0.9%) | <0.001 |
| Depression | 1640 (21.2%) | 116 (23.4%) | 1524 (21.1%) | 0.21 |
| Cancer | 537 (6.9%) | 33 (6.7%) | 504 (7.0%) | 0.79 |
| Aspirin use | 1114 (14.4%) | 197 (39.8%) | 917 (12.7%) | <0.001 |
| Statin use | 1504 (19.5%) | 317 (64.0%) | 1187 (16.4%) | <0.001 |
| Brain infarcts | 296 (3.8%) | 34 (6.9%) | 262 (3.6%) | < 0.001 |
| Number of brain infarcts | ||||
| 0 | 7437 (96.2%) | 461 (93.1%) | 6976 (96.4%) | < 0.001 |
| 1 | 216 (2.8%) | 22 (4.4%) | 194 (2.7%) | – |
| ≥ 2 | 80 (1.0%) | 12 (2.4%) | 68 (0.94%) | – |
| Lacunar brain infarcts | 213 (2.8%) | 26 (5.3%) | 187 (2.6%) | < 0.001 |
| Number of lacunar brain infarcts | ||||
| 0 | 7520 (97.3%) | 469 (94.8%) | 7051 (97.4%) | 0.003 |
| 1 | 172 (2.2%) | 21 (4.2%) | 151 (2.1%) | – |
| ≥ 2 | 41 (0.53%) | 5 (1.0%) | 36 (0.50%) | – |
| Nonlacunar brain infarcts | 108 (1.4%) | 11 (2.2%) | 97 (1.3%) | 0.11 |
| Number of nonlacunar brain infarcts | ||||
| 0 | 7625 (98.6%) | 484 (97.8%) | 7141 (98.7%) | 0.092 |
| 1 | 59 (0.76%) | 4 (0.81%) | 55 (0.76%) | – |
| ≥ 2 | 49 (0.63%) | 7 (1.4%) | 42 (0.58%) | – |
| High white matter hyperintensityc | 470 (6.1%) | 56 (11.3%) | 414 (5.7%) | <0.001 |
| Vascular brain injuryd | 715 (9.3%) | 82 (16.6%) | 633 (8.8%) | <0.001 |
| Site-standardized MoCA | 0.01 (0.99) | -0.22 (1.11) | 0.02 (0.98) | <0.001 |
| Site-standardized DSST | 0.01 (1.00) | -0.39 (0.98) | 0.03 (0.99) | <0.001 |
| Cognitive impairmente | 1927 (24.9%) | 186 (37.6%) | 1741 (24.1%) | <0.001 |
Data are shown as either N (%) or mean (standard devitation); P values were calculated with a 2-sample t-test, Mann-Whitney U test, or chi-square test
Abbreviations: DSST, Digit Symbol Substitution Test; MoCA, Montreal Cognitive Assessment.
aA total of 162 (79%) participants with diabetes and 2758 (69%) without diabetes were postmenopausal.
bSelf-reported type 1 diabetes = 28; type 2 diabetes = 407; and unreported type = 60.
cFazekas score ≥ 4.
d≥1 brain infarct or high white matter hyperintensity.
eMoCA or DSST score ≤ -1.
As noted in Table 2, self-reported diabetes was significantly associated with higher odds of having 1 or more BIs after adjusting for age and sex (odds ratio [OR] = 1.53, 95% CI [confidence interval] 1.05, 2.24; P = 0.027). Further adjustment for differences in education, ethnicity, smoking, and hypertension attenuated the OR to 1.40 (95% CI 0.96, 2.06; P = 0.082). Similar relationships were noted for evidence of ≥ 2 BIs (nonlacunar or lacunar) and when diabetes was diagnosed on the basis of self-report as well as use of diabetes drugs (Table 2).
Relationship between diabetes and brain infarctions
| . | Self-reported Diabetes . | . | . | . | Self-reported Diabetes Plus Diabetes Drugs . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| Adjustment For . | ≥1 BI OR (95% CI) . | P-value . | ≥2 BI OR (95% CI) . | P-value . | ≥1 BI OR (95% CI) . | P-value . | ≥2 BI OR (95% CI) . | P-value . |
| Age, sex | 1.53 (1.05, 2.24) | 0.027 | 1.96 (1.04, 3.67) | 0.037 | 1.54 (1.01, 2.34) | 0.044 | 2.29 (1.19, 4.41) | 0.013 |
| Age, sex, 4 RFa | 1.40 (0.96, 2.06) | 0.082 | 1.84 (0.96, 3.50) | 0.064 | 1.38 (0.91, 2.11) | 0.13 | 2.09 (1.07, 4.09) | 0.032 |
| Age, sex, 4 RF, carotid wall | 1.46 (0.99, 2.13) | 0.054 | 1.95 (1.03, 3.72) | 0.042 | 1.44 (0.94, 2.20) | 0.093 | 2.21 (1.13, 4.34) | 0.021 |
| Age, sex, 4 RF, stroke | 1.48 (1.01, 2.17) | 0.045 | 1.94 (1.02, 3.69) | 0.044 | 1.46 (0.95, 2.23) | 0.081 | 2.21 (1.13, 4.32) | 0.021 |
| . | Self-reported Diabetes . | . | . | . | Self-reported Diabetes Plus Diabetes Drugs . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| Adjustment For . | ≥1 BI OR (95% CI) . | P-value . | ≥2 BI OR (95% CI) . | P-value . | ≥1 BI OR (95% CI) . | P-value . | ≥2 BI OR (95% CI) . | P-value . |
| Age, sex | 1.53 (1.05, 2.24) | 0.027 | 1.96 (1.04, 3.67) | 0.037 | 1.54 (1.01, 2.34) | 0.044 | 2.29 (1.19, 4.41) | 0.013 |
| Age, sex, 4 RFa | 1.40 (0.96, 2.06) | 0.082 | 1.84 (0.96, 3.50) | 0.064 | 1.38 (0.91, 2.11) | 0.13 | 2.09 (1.07, 4.09) | 0.032 |
| Age, sex, 4 RF, carotid wall | 1.46 (0.99, 2.13) | 0.054 | 1.95 (1.03, 3.72) | 0.042 | 1.44 (0.94, 2.20) | 0.093 | 2.21 (1.13, 4.34) | 0.021 |
| Age, sex, 4 RF, stroke | 1.48 (1.01, 2.17) | 0.045 | 1.94 (1.02, 3.69) | 0.044 | 1.46 (0.95, 2.23) | 0.081 | 2.21 (1.13, 4.32) | 0.021 |
Odds ratios are from logistic regression models. Abbreviations: BI, brain infarcts; CI, confidence interval; OR, odds ratio; MRI, magnetic resonance imaging; RF, risk factor.
a 4 risk factors that include high school or less education, white ethnicity, current smoking, and hypertension.
b MRI-measured carotid wall volume.
c History of clinical stroke.
Relationship between diabetes and brain infarctions
| . | Self-reported Diabetes . | . | . | . | Self-reported Diabetes Plus Diabetes Drugs . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| Adjustment For . | ≥1 BI OR (95% CI) . | P-value . | ≥2 BI OR (95% CI) . | P-value . | ≥1 BI OR (95% CI) . | P-value . | ≥2 BI OR (95% CI) . | P-value . |
| Age, sex | 1.53 (1.05, 2.24) | 0.027 | 1.96 (1.04, 3.67) | 0.037 | 1.54 (1.01, 2.34) | 0.044 | 2.29 (1.19, 4.41) | 0.013 |
| Age, sex, 4 RFa | 1.40 (0.96, 2.06) | 0.082 | 1.84 (0.96, 3.50) | 0.064 | 1.38 (0.91, 2.11) | 0.13 | 2.09 (1.07, 4.09) | 0.032 |
| Age, sex, 4 RF, carotid wall | 1.46 (0.99, 2.13) | 0.054 | 1.95 (1.03, 3.72) | 0.042 | 1.44 (0.94, 2.20) | 0.093 | 2.21 (1.13, 4.34) | 0.021 |
| Age, sex, 4 RF, stroke | 1.48 (1.01, 2.17) | 0.045 | 1.94 (1.02, 3.69) | 0.044 | 1.46 (0.95, 2.23) | 0.081 | 2.21 (1.13, 4.32) | 0.021 |
| . | Self-reported Diabetes . | . | . | . | Self-reported Diabetes Plus Diabetes Drugs . | . | . | . |
|---|---|---|---|---|---|---|---|---|
| Adjustment For . | ≥1 BI OR (95% CI) . | P-value . | ≥2 BI OR (95% CI) . | P-value . | ≥1 BI OR (95% CI) . | P-value . | ≥2 BI OR (95% CI) . | P-value . |
| Age, sex | 1.53 (1.05, 2.24) | 0.027 | 1.96 (1.04, 3.67) | 0.037 | 1.54 (1.01, 2.34) | 0.044 | 2.29 (1.19, 4.41) | 0.013 |
| Age, sex, 4 RFa | 1.40 (0.96, 2.06) | 0.082 | 1.84 (0.96, 3.50) | 0.064 | 1.38 (0.91, 2.11) | 0.13 | 2.09 (1.07, 4.09) | 0.032 |
| Age, sex, 4 RF, carotid wall | 1.46 (0.99, 2.13) | 0.054 | 1.95 (1.03, 3.72) | 0.042 | 1.44 (0.94, 2.20) | 0.093 | 2.21 (1.13, 4.34) | 0.021 |
| Age, sex, 4 RF, stroke | 1.48 (1.01, 2.17) | 0.045 | 1.94 (1.02, 3.69) | 0.044 | 1.46 (0.95, 2.23) | 0.081 | 2.21 (1.13, 4.32) | 0.021 |
Odds ratios are from logistic regression models. Abbreviations: BI, brain infarcts; CI, confidence interval; OR, odds ratio; MRI, magnetic resonance imaging; RF, risk factor.
a 4 risk factors that include high school or less education, white ethnicity, current smoking, and hypertension.
b MRI-measured carotid wall volume.
c History of clinical stroke.
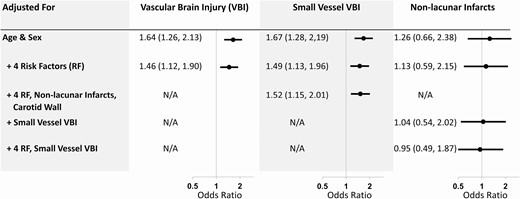
As seen in Fig. 1, self-reported diabetes was also significantly associated with a higher age- and sex-adjusted odds of VBI (OR 1.64, 95% CI 1.26, 2.13; P = 0.0002) and small vessel VBI (OR 1.67, 95% CI 1.28, 2.19; P = 0.0002), but not nonlacunar BI. The relationship with VBI and small vessel VBI remained significant after additionally accounting for differences in education, ethnicity, smoking and hypertension, nonlacunar BI, and carotid wall volume. Similar findings were noted when diabetes was diagnosed on the basis of self-report as well as use of diabetes drugs (Supplement, Table S1) (19).
Relationship between self-reported diabetes and type of vascular brain injury. Odds ratios and 95% confidence intervals for each model are shown in the forest plot. Abbreviations: carotid wall, MRI-measured carotid wall volume; RF, risk factors; small vessel VBI, lacunar brain infarct or high white matter hyperintensity score; VBI, vascular brain injury.
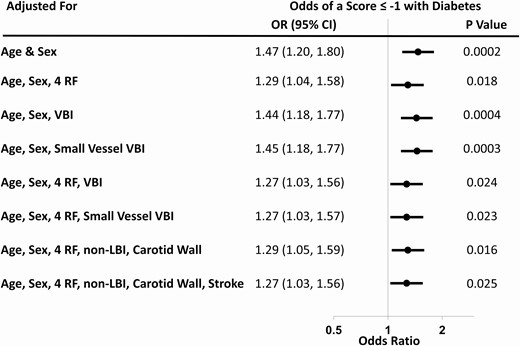
Diabetes also was significantly associated with a higher age- and sex-adjusted odds of cognitive impairment (OR 1.47, 95% CI 1.20, 1.80; P = 0.0002), defined as a site-standardized MoCA or DSST score ≤ -1 (Fig. 2 and Supplement, Table S2) (19). This relationship was attenuated but remained significant after also accounting for various combinations of education, ethnicity, smoking, hypertension, VBI, small vessel VBI, nonlacunar BI, high WMH, carotid wall volume, and a history of a clinically evident stroke. For example, after accounting for age, sex, education, ethnicity, smoking, hypertension, nonlacunar BI, carotid wall volume, and stroke, diabetes was associated with an OR of 1.27 (95% CI 1.03, 1.56). Similar relationships were observed when diabetes was defined based on self-report plus the use of at least 1 glucose-lowering drug (Supplement, Table S3) (19).
Relationship between self-reported diabetes and cognitive impairment (defined as a site-standardized MoCA or DSST score that is ≤ 1 standard deviation below the site’s mean value). Odds ratios and 95% confidence intervals for each model are shown in the forest plot. Abbreviations: carotid wall, MRI-measured carotid wall volume; non-LBI—nonlacunar brain infarct; RF, risk factors (high school or less education, white ethnicity, current smoking, and hypertension); small vessel VBI, lacunar brain infarct or high white matter hyperintensity score; stroke, history of clinical stroke; VBI, vascular brain injury.
Discussion
This large cross-sectional analysis of Canadians demonstrates that diabetes is independently associated with VBI and cognitive impairment. It shows a robust relationship between diabetes and small vessel VBI but not nonlacunar BI. It also shows that brain infarcts of any time are insufficient to disentangle the relationship between diabetes and cognitive impairment.
These findings are consistent with accumulating evidence implicating small vessel disease in the brain as an important pathophysiologic link between diabetes and cerebrovascular disease (20–22). They are also consistent with the hypothesis that diabetes’ effects on the brain are similar to its adverse effects elsewhere. For example, small vessel disease is implicated in diabetic retinopathy and other long-term consequences of diabetes (2, 23), and there is a graded relationship between the degree of retinopathy and both WMH burden (24) and incident cerebrovascular and cardiovascular events (25). This, and the incremental relationship between the degree of glucose elevation and retinal disease (26), incident strokes (27), and cognitive impairment (28) all support the hypothesis that diabetes’ adverse effect on cerebral vessels may be due to a selective effect on small vessels, in addition to a more widespread adverse effect on all vessels due to concomitant hypertension, dyslipidemia, renal disease, coagulation abnormalities, and cardiovascular disease (29).
People with diabetes are also at risk of cognitive impairment (30), which may occur as a result of cerebrovascular disease or other processes. Adjustment for VBI, which included both small and large vessel disease as well as clinical strokes, did not eliminate the relationship between diabetes and cognitive impairment in this study. This suggests that comorbidities, metabolic factors, lifestyle, or other unmeasured factors, in addition to vascular changes, may be required to disentangle the relationship between diabetes and cognitive impairment.
The strengths of this study include a sampling methodology that assembled a population that is representative of developed countries, the blinded independent reading of the MRI scans, the large sample size, and the minimization of systematic variation across sites by standardizing the individual cognitive score to each person’s study site. Limitations include its cross-sectional design, the absence of metabolic data, and the fact that diabetes status was self-reported. The absence of data regarding cerebral microbleeds, comprehensive data regarding medication use, blood test results (including known risk factors for cognitive dysfunction, such as Apo E status), or complete information related to diabetes type and duration are further limitations.
Small vessel disease involving the retina, kidney, and nerves is clearly an important consequence of diabetes. Indeed, the glucose criteria that are used to diagnose diabetes were established by identifying glucose thresholds that differentiate people at high versus low risk of small vessel disease in one vascular bed—the retina (23). These findings demonstrate that microvascular disease is also present in the brain of people with diabetes and may be related to the high burden of strokes and cognitive impairment suffered by affected individuals.
Acknowledgments
This work was funded by grants from the Canadian Partnership Against Cancer (CPAC), Heart and Stroke Foundation of Canada (HSF-Canada), and the Canadian Institutes of Health Research (CIHR).
Steering Committee: S. Anand (Chair), M.G. Friedrich (Co-Chair), Douglas Lee (Co-Chair), J. Tu (former Co-Chair), P. Awadalla (OHS), T. Dummer (BCGP), J. Vena (ATP), P. Broet (CaG), J. Hicks (APATH), J-C. Tardif (MHI Biobank), K. Teo (PURE-Central), B-M. Knoppers (ELSI).
Project Office Staff at the Population Health Research Institute (PHRI): D. Desai, S. Nandakumar (Ex), M. Thomas (Ex), S. Zafar.
Statistics/Biometrics Programmers Team at PHRI: K. Schulze, L. Dyal, S. Bangdiwala, C. Ramasundarahettige, X Yang.
Central Operations Leads: D. Desai (PHRI), H. Truchon (Montreal Heart Institute), N. Tusevljak (Institute for Clinical Evaluative Sciences).
Cohort Operations Research Personnel: K. McDonald (OHS), N. Noisel (CaG), J. Chu (BCGP), J. Hicks (APATH), H. Whelan (ATP), S. Rangarajan (PURE), D. Busseuil (MHI Biobank).
Site Investigators and Staff: (112) J. Leipsic, S. Lear, V. de Jong; (306) M. Noseworthy, K. Teo, E. Ramezani, N. Konyer; (402) P. Poirier, A-S. Bourlaud, E. Larose, K. Bibeau; (512) J. Leipsic, S. Lear, V. de Jong; (609) E. Smith, R. Frayne, A. Charlton, R Sekhon; (703) A. Moody, V. Thayalasuthan; (704) A. Kripalani, G Leung; (706) M. Noseworthy, S. Anand, R. de Souza, N. Konyer, S. Zafar; (707) G. Paraga, L. Reid; (714) A. Dick, F. Ahmad; (799) D. Kelton, H. Shah; (801) F. Marcotte, H. Poiffaut; (802) M. Friedrich, J. Lebel; (817) E. Larose, K. Bibeau; (913) R. Miller, L. Parker, D. Thompson, J. Hicks; (1001) J-C. Tardif, H. Poiffaut; (1103) J. Tu, K. Chan, A. Moody, V. Thayalasuthan.
MRI Working Group and Core Lab Investigators/Staff: Chair: M. Friedrich; Brain Core Lab: E. Smith, C. McCreary, S. E. Black, C. Scott, S. Batool, F. Gao; Carotid Core Lab: A. Moody, V. Thayalasuthan; Abdomen: E. Larose, K. Bibeau; Cardiac: F. Marcotte, F. Henriques.
Contextual Working Group: R. de Souza, S. Anand, G. Booth, J. Brook, D. Corsi, L. Gauvin, S. Lear, F. Razak, S.V. Subramanian, J. Tu.
CAHHM Founding Advisory Group: Jean Rouleau, Pierre Boyle, Caroline Wong, Eldon Smith.
CAHHM Scientific Review Board: Bob Reid, Ian Janssen, Amy Subar, Rhian Touyz
Financial Support: CAHHM was funded by the Canadian Partnership Against Cancer (CPAC), Heart and Stroke Foundation of Canada (HSF-Canada), and the Canadian Institutes of Health Research (CIHR). Financial contributions were also received from the Population Health Research Institute and CIHR Foundation Grant no. FDN-143255 to S.S.A.; FDN-143313 to J.V.T.; and FDN 154317 to E.E.S. In-kind contributions from A.R.M. and S.E.B. from Sunnybrook Hospital, Toronto, for MRI reading costs, and Bayer AG for provision of IV contrast. The Canadian Partnership for Tomorrow’s Health (CanPath) is funded by the Canadian Partnership Against Cancer, BC Cancer Foundation, Genome Quebec, Ontario Institute for Cancer Research and Alberta Health and the Alberta Cancer Foundation. The PURE Study was funded by multiple sources. The Montreal Heart Institute Biobank is funded by Mr André Desmarais and Mrs France Chrétien-Desmarais and the Montreal Heart Institute Foundation. S.S.A. was supported by a Tier 1 Canada Research Chair in Ethnicity and Cardiovascular Disease and Heart and Stroke Foundation Chair in Population Health. P.A. was supported by a Ministry of Research and Innovation of Ontario Investigator Award. S.E.B. was supported by the Hurvitz Brain Sciences Research Program, Sunnybrook Research Institute, and the Department of Medicine, Sunnybrook Health Sciences Centre, University of Toronto. J.-C.T. holds the Tier 1 Canada Research Chair in translational and personalized medicine and the Université de Montréal Pfizer endowed research chair in atherosclerosis. Some of the data used in this research were made available by the Canadian Partnership for Tomorrow’s Health (CanPath) along with the BC Generations Project (BCGP) hosted by BC Cancer, Alberta’s Tomorrow Project (ATP), Ontario Health Study (OHS), CARTaGENE (CaG), and the Atlantic PATH (APATH). Data were harmonized by Maelstrom Research and access policies and procedures were developed by the Centre of Genomics and Policy in collaboration with the Cohorts.
Author Contributions: H.C.G., E.S., and S.A. designed the analytic plan; H.C.G. wrote the first draft; C.R. performed the statistical analyses; all of the authors reviewed and edited the manuscript and contributed to interpretation of the data. H.C.G. and S.S.A. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Information
Disclosure Summary: H.C.G. holds the McMaster-Sanofi Population Health Institute Chair in Diabetes Research and Care. He reports research grants from Eli Lilly, AstraZeneca, Merck, Novo Nordisk and Sanofi; honoraria for speaking from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi; and consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, Janssen, Sanofi, and Kowa. E.E.S. reports consulting fees from Biogen and Alnylam, and royalties from UpToDate. S.S.A. is supported by a Tier 1 Canada Research Chair in Ethnicity and CVD and the Heart and Stroke Foundation Chair in Population Health; grant from the Canadian Partnership Against Cancer, Heart and Stroke Foundation of Canada, and the Canadian Institutes of Health Research; and personal fees from Bayer AG and in-kind support from the Population Health Research Institute.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References