-
PDF
- Split View
-
Views
-
Cite
Cite
Richard Eastell, Clifford J Rosen, Dennis M Black, Angela M Cheung, M Hassan Murad, Dolores Shoback, Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society Clinical Practice Guideline, The Journal of Clinical Endocrinology & Metabolism, Volume 104, Issue 5, May 2019, Pages 1595–1622, https://doi.org/10.1210/jc.2019-00221
Close - Share Icon Share
Abstract
The objective is to formulate clinical practice guidelines for the pharmacological management of osteoporosis in postmenopausal women.
Evidence from clinical trials and insights from clinical experience with pharmacologic therapies for osteoporosis were critically evaluated in formulating this guideline for the management of postmenopausal osteoporosis. Patient preferences, data on adherence and persistence, and risks and benefits from the patient and provider perspectives were also considered in writing committee deliberations. A consensus by the Writing Committee members was achieved for four management principles: (i) The risk of future fractures in postmenopausal women should be determined using country-specific assessment tools to guide decision-making. (ii) Patient preferences should be incorporated into treatment planning. (iii) Nutritional and lifestyle interventions and fall prevention should accompany all pharmacologic regimens to reduce fracture risk. (iv) Multiple pharmacologic therapies are capable of reducing fracture rates in postmenopausal women at risk with acceptable risk-benefit and safety profiles.
List of Recommendations
Who to treat
1.1 We recommend treating postmenopausal women at high risk of fractures, especially those who have experienced a recent fracture, with pharmacological therapies, as the benefits outweigh the risks. (1|⊕⊕⊕⊕)
Bisphosphonates
2.1 In postmenopausal women at high risk of fractures, we recommend initial treatment with bisphosphonates (alendronate, risedronate, zoledronic acid, and ibandronate) to reduce fracture risk.(1|⊕⊕⊕⊕)
Technical remark: Ibandronate is not recommended to reduce nonvertebral or hip fracture risk.
2.2 In postmenopausal women with osteoporosis who are taking bisphosphonates, we recommend that fracture risk be reassessed after 3 to 5 years, and women who remain at high risk of fractures should continue therapy, whereas those who are at low-to-moderate risk of fractures should be considered for a “bisphosphonate holiday.” (1|⊕⊕OO)
Technical remark: A bisphosphonate holiday is operationally defined as a temporary discontinuation of bisphosphonate for up to 5 years. This period may be longer depending on the bone mineral density and clinical circumstances of the individual patient. The evidence is stronger for retention of benefits during a holiday for alendronate and zoledronic acid where there are randomized extension trials. A shorter reassessment period of 3 years is more appropriate for annual intravenous zoledronic acid (5 mg) based on evidence from research control trials showing residual effects after 3 years of annual use. Once a bisphosphonate holiday is initiated, reassess fracture risk at 2- to 4-year intervals and consider reinitiating osteoporosis therapy earlier than the 5-year suggested maximum if there is a significant decline in bone mineral density, an intervening fracture, or other factors that alter the clinical risk status.
Denosumab
3.1 In postmenopausal women with osteoporosis who are at high risk for osteoporotic fractures, we recommend using denosumab as an alternative initial treatment. (1|⊕⊕⊕⊕)
Technical remark: The recommended dosage is 60 mg subcutaneously every 6 months. The effects of denosumab on bone remodeling, reflected in bone turnover markers, reverse after 6 months if the drug is not taken on schedule. Thus, a drug holiday or treatment interruption is not recommended with this agent.
3.2 In postmenopausal women with osteoporosis who are taking denosumab, we suggest that the fracture risk be reassessed after 5 to 10 years and that women who remain at high risk of fractures should either continue denosumab or be treated with other osteoporosis therapies. (2|⊕OOO)
3.3 In postmenopausal women with osteoporosis taking denosumab, administration of denosumab should not be delayed or stopped without subsequent antiresorptive [e.g., bisphosphonates, hormone therapy, or selective estrogen receptor modulator] or other therapy administered to prevent a rebound in bone turnover and to decrease the risk of rapid bone mineral density loss and an increased risk of fracture. (Ungraded Good Practice Statement)
Teriparatide and abaloparatide (parathyroid hormone and parathyroid hormone–related protein analogs)
4.1 In postmenopausal women with osteoporosis at very high risk of fracture, such as those with severe or multiple vertebral fractures, we recommend teriparatide or abaloparatide treatment for up to 2 years for the reduction of vertebral and nonvertebral fractures. (1|⊕⊕⊕O)
4.2 In postmenopausal women with osteoporosis who have completed a course of teriparatide or abaloparatide, we recommend treatment with antiresorptive osteoporosis therapies to maintain bone density gains. (1|⊕⊕OO)
Selective estrogen receptor modulators
5.1. In postmenopausal women with osteoporosis at high risk of fracture and with the patient characteristics below, we recommend raloxifene or bazedoxifene to reduce the risk of vertebral fractures. (1|⊕⊕⊕⊕)
Patient characteristics: With a low risk of deep vein thrombosis and for whom bisphosphonates or denosumab are not appropriate, or with a high risk of breast cancer.
Menopausal hormone therapy and tibolone
6.1 In postmenopausal women at high risk of fracture and with the patient characteristics below, we suggest menopausal hormone therapy, using estrogen only in women with hysterectomy, to prevent all types of fractures. (2|⊕⊕⊕O)
Patient characteristics: Under 60 years of age or <10 years past menopause; at low risk of deep vein thrombosis; those in whom bisphosphonates or denosumab are not appropriate; with bothersome vasomotor symptoms; with additional climacteric symptoms; without contraindications; without prior myocardial infarction or stroke; without breast cancer; willing to take menopausal hormone therapy.
6.2 In postmenopausal women with osteoporosis at high risk of fracture and with the patient characteristics below, we suggest tibolone to prevent vertebral and nonvertebral fractures. (2|⊕⊕⊕O)
Patient characteristics: Under 60 years of age or <10 years past menopause; with a low risk of deep vein thrombosis; those in whom bisphosphonates or denosumab are not appropriate; with bothersome vasomotor symptoms; with additional climacteric symptoms; without contraindications; without prior myocardial infarction or stroke or high risk for cardiovascular disease; without breast cancer; willing to take tibolone.
Technical remark: Tibolone is not available in the United States or Canada.
Calcitonin
7.1 In postmenopausal women at high risk of fracture with osteoporosis, we suggest that nasal spray calcitonin be prescribed only in women who cannot tolerate raloxifene, bisphosphonates, estrogen, denosumab, tibolone, abaloparatide, or teriparatide or for whom these therapies are not considered appropriate. (2|⊕OOO)
Calcium and vitamin D
8.1 In postmenopausal women with low bone mineral density and at high risk of fractures with osteoporosis, we suggest that calcium and vitamin D be used as an adjunct to osteoporosis therapies. (2|⊕⊕OO)
8.2 In postmenopausal women at high risk of fracture with osteoporosis who cannot tolerate bisphosphonates, estrogen, selective estrogen response modulators, denosumab, tibolone, teriparatide, and abaloparatide, we recommend daily calcium and vitamin D supplementation to prevent hip fractures. (1|⊕⊕⊕O)
Monitoring
11.1 In postmenopausal women with a low bone mineral density and at high risk of fractures who are being treated for osteoporosis, we suggest monitoring the bone mineral density by dual-energy X-ray absorptiometry at the spine and hip every 1 to 3 years to assess the response to treatment. (2|⊕OOO)
Technical remark: Monitoring bone turnover markers (serum C-terminal crosslinking telopeptide for antiresorptive therapy or procollagen type 1 N-terminal propeptide for bone anabolic therapy) is an alternative way of identifying poor response or nonadherence to therapy.
Introduction
Postmenopausal osteoporosis is common, and fractures are injurious to patients and costly to the health care system; however, effective treatments are available. One in two postmenopausal women will have an osteoporotic fracture in her lifetime (1). Those who have had a fracture are at high risk of subsequent fractures (2). Fractures can cause pain, decreased mobility and function, and fear of falling and are associated with decreased quality of life and increased mortality (3–6). However, many postmenopausal women at highest risk do not receive treatment to prevent major osteoporotic fractures and their associated morbidity and mortality (7). With ongoing reports of atypical femoral fractures (AFFs) and osteonecrosis of the jaw (ONJ), there is uncertainty among postmenopausal women and their health care providers regarding the benefits and risks of different management strategies for osteoporosis, who to treat, when to monitor and what tests to do for monitoring, the appropriate duration of therapy, and when to consider a bisphosphonate holiday. In fact, there has been a decline in the use of bisphosphonates (8), and the recent hip fracture incidence among postmenopausal women is higher than projected in the United States, suggesting a leveling off and possible reversal in what had been a decade-and-a-half-long decline (9, 10). Recently, the American College of Physicians (ACP) published their guidelines for the treatment of low bone mineral density (BMD) or osteoporosis to prevent fractures in women and men (11), but certain recommendations in those guidelines have raised new questions and generated much discussion, especially with regard to the duration of therapy and monitoring. The ACP recommends that physicians should treat women with osteoporosis with drug therapy for 5 years and recommends against monitoring the BMD during that period. No differentiation between bisphosphonates and denosumab was made for duration of therapy even though the pharmacokinetics of the two classes of drugs are quite different. The ACP guidelines also do not include recommendations regarding the use of abaloparatide, a new bone-formation therapy, which was approved by the Food and Drug Administration just prior to the release of the guidelines. The ACP guidelines recommend against using menopausal hormone therapy (HT) or raloxifene, a selective estrogen receptor modulator, for osteoporosis treatment and do not consider teriparatide a potential treatment option for patients severely affected by the disease. The Endocrine Society’s international guideline Writing Committee has reviewed current evidence and has different recommendations regarding pharmacotherapies to treat osteoporosis in postmenopausal women.
Systematic Review and Meta-Analyses
The guideline Writing Committee commissioned two systematic reviews to support this guideline. The first review synthesized the evidence derived from randomized controlled trials (RCTs) enrolling postmenopausal women with primary osteoporosis (12). The review included 107 trials (193,987 postmenopausal women; mean age of 66 years; 55% white; median follow-up of 28 months). The maximum duration for most trials was 4 years. The meta-analyses were done in two ways: a direct comparison with placebo and a combination of direct and indirect comparisons, or network approach. We have focused on the results of the direct approach in this guideline except when there was a clear discrepancy. In that case, we took into account the quality of the trials of comparison with placebo and consistency within the class.
Significant reduction in vertebral fractures was observed with alendronate, risedronate, ibandronate, zoledronic acid, denosumab, teriparatide, abaloparatide, raloxifene, bazedoxifene, HT, tibolone, calcitonin, PTH(1-84), romosozumab, strontium ranelate, and lasofoxifene (Fig. 1). A significant reduction in hip fractures was observed with alendronate, risedronate, zoledronic acid, denosumab, menopausal HT (estrogen with or without progestogen), and a calcium with vitamin D combination (Fig. 1). A significant reduction in nonvertebral fractures was observed with alendronate, risedronate, zoledronic acid, denosumab, teriparatide, abaloparatide, HT, tibolone, calcium or vitamin D, romosozumab, and lasofoxifene (Fig. 1).
Relative risks of vertebral, hip, and nonvertebral fractures (and 95% CIs) in response to the treatments for postmenopausal osteoporosis, calculated directly and compared with placebo. Note that the evidence is based on a direct meta-analysis of 107 trials of drugs in postmenopausal women with primary osteoporosis in which the trial duration lasted for 3 to 120 mo. In this analysis, each agent was compared with placebo and so direct comparisons should not be made between treatments based on this figure. (12). [Adapted with permission from data presented in Moreno PB, Kapoor E, Asi N, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab. 2019;104(5):1623-1630].
The second review was aimed at evaluating values and preferences relevant to the management of osteoporosis in women and followed a qualitative approach (13). Women in general seemed to consider effectiveness and adverse events equally, followed by the convenience of taking the drug and the impact on daily routines (less frequent dosing was preferred, an oral route was preferred, but an injectable route was preferred over oral if given less frequently). Cost and duration of treatment were less important factors for decision-making. Fear of breast cancer and refusal to resume uterine bleeding were common reasons for not choosing menopausal HT. Calcium and vitamin D were viewed as safe and “natural.” Across studies, preference was not affected by age, previous drug exposure, or employment.
1. Who to Treat
1.1 We recommend treating postmenopausal women at high risk of fractures, especially those who have experienced a recent fracture, with pharmacological therapies, as the benefits outweigh the risks. (1|⊕⊕⊕⊕)
Evidence
The goal of using pharmacological therapies to treat low BMD or osteoporosis in postmenopausal women is to decrease the burden of major osteoporotic fractures. Various scientific bodies from different countries have determined treatment thresholds based on either a BMD T-score or various risk assessment tools such as the Fracture Risk Assessment Tool (FRAX), Canadian Association of Radiologists and Osteoporosis Canada calculator, Osteoporosis Self-Assessment Tool, and Garvan Institute fracture risk calculator, as well as the values, preferences, and costs for their populations. Currently there are 52 national guidelines in 36 countries. Some guidelines use fracture-risk thresholds, such as those used in the United States, Canada, and the United Kingdom (14–16), whereas other guidelines use T-score–based thresholds, such as those used in Austria, Belgium, India, and Brazil (17–22). Of the 52 guidelines, 30 include the FRAX-based 10-year absolute fracture risk in their treatment threshold (23). Some of these guidelines (such as those in the United States and Canada) have fixed fracture-risk thresholds across ages, whereas other guidelines, such as the United Kingdom National Osteoporosis Guideline Group guideline, the Lebanon osteoporosis guideline, and the Chilean guideline (14, 23, 24), are hybrid models, using age-dependent intervention thresholds for certain age groups and fracture-risk thresholds for other age groups (25). In the United States, pharmacological therapy is recommended for postmenopausal women with hip or vertebral fractures; those with T-scores of −2.5 or less in the femoral neck, total hip, or lumbar spine; and those with T-scores of −1 to −2.5 and a 10-year probability of ≥20% for major osteoporotic fractures or ≥3% for hip fractures based on the US-adapted FRAX tool (15). BMD T-score is defined as the number of SDs from the mean BMD of white females age 20 to 29 years in the Third National Health and Nutrition Examination Survey database. For the treatment of osteoporosis, only lumbar spine, total hip, and femoral neck BMD T-scores are usually considered.
Data suggest that a recent fracture (within the past 2 years) is a better predictor of imminent fracture risk (i.e., risk of fracture within the next 2 years) than is a distant fracture history (>5 years ago) (26, 27). This is true for recent vertebral fractures (28, 29) as well as nonvertebral fractures such as wrist and humerus fractures (30–33). Pharmacological therapies should be initiated without delay in patients with recent fractures to prevent more fractures, based on their fracture risk. Data on optimal timing of initiation of therapy after a fracture are scant. Based on the Horizon trial (34), we would suggest initiating therapy 2 weeks or more after a hip fracture. Women should also be counseled regarding adequate calcium and vitamin D intake, fall prevention strategies, smoking cessation, avoidance of excessive alcohol intake, and weight-bearing, muscle-strengthening exercises as well as balance training. Once osteoporosis is diagnosed, strategies to prevent subsequent fractures (pharmacologic and otherwise) need to be reinforced indefinitely, much like the management strategies for hypertension and hypercholesterolemia.
Patient values and preferences
When making decisions regarding who to treat, patient preferences and patient-specific clinical factors should be taken into account. Values and costs vary across countries depending on the needs and resources of the specific country. The Writing Committee is recommending pharmacologic therapies for postmenopausal women at high risk of fractures based on country-specific risk assessment guidelines, especially for women who have had a recent fracture.
2. Bisphosphonates
2.1 In postmenopausal women at high risk of fractures, we recommend initial treatment with bisphosphonates (alendronate, risedronate, zoledronic acid, and ibandronate) to reduce fracture risk.(1|⊕⊕⊕⊕)
Technical remark: Ibandronate is not recommended to reduce nonvertebral or hip fracture risk.
2.2 In postmenopausal women with osteoporosis who are taking bisphosphonates, we recommend that fracture risk be reassessed after 3 to 5 years, and women who remain at high risk of fractures should continue therapy, whereas those who are at low-to-moderate risk of fractures should be considered for a “bisphosphonate holiday.” (1|⊕⊕OO)
Technical remark: A bisphosphonate holiday is operationally defined as a temporary discontinuation of bisphosphonate for up to 5 years. This period may be longer depending on the BMD and clinical circumstances of the individual patient. The evidence is stronger for retention of benefits during a holiday for alendronate and zoledronic acid where there are randomized extension trials. A shorter reassessment period of 3 years is more appropriate for annual IV zoledronic acid (5 mg) based on evidence from RCTs showing residual effects after 3 years of annual use. Once a bisphosphonate holiday is initiated, reassess fracture risk at 2- to 4-year intervals and consider reinitiating osteoporosis therapy earlier than the 5-year suggested maximum if there is a significant decline in BMD, an intervening fracture, or other factors that alter the clinical risk status.
Evidence
Bisphosphonate treatment up to 5 years
Three oral bisphosphonates available in the United States and internationally include alendronate (weekly), ibandronate (monthly), and risedronate (weekly or monthly). Additionally, there are two IV agents: zoledronic acid, given annually, and ibandronate, given quarterly. Note that all results below are taken from the “direct” meta-analysis comparing each drug with placebo [see appendix in Moreno et al. (12)] unless otherwise specified.
Most of the evidence included in the meta-analysis reflects RCTs in older women (most >65 years of age) with high fracture risk, as defined by varying combinations of low BMD, prevalent vertebral fracture, or presence of other risk factors. The meta-analysis comparison of alendronate with placebo (Fig. 1) showed a 44% reduction in vertebral fracture risk [hazard ratio (HR), 0.56; 95% CI, 0.46 to 0.67], a 40% reduction in hip fracture risk (HR, 0.60; 95% CI, 0.39 to 0.92), and a 17% reduction in nonvertebral fracture risk (HR, 0.83; 95% CI, 0.74 to 0.93). The meta-analysis comparison of risedronate with placebo (Fig. 1) showed a 36% reduction in vertebral fracture risk (HR, 0.64; 95% CI, 0.53 to 0.77), a 26% reduction in hip fracture risk (HR, 0.74; 95% CI, 0.59 to 0.94), and a 20% reduction in nonvertebral fracture risk (HR, 0.80; 95% CI, 0.72 to 0.89). The meta-analysis comparison of ibandronate with placebo (Fig. 1) showed a 31% reduction in the vertebral fracture risk (HR, 0.69; 95% CI, 0.49 to 0.97). There was no evidence for a reduction in hip or nonvertebral fracture risk. The meta-analysis comparing zoledronic acid with placebo (Fig. 1) showed a 56% reduction in vertebral fracture risk (HR, 0.44; 95% CI, 0.23 to 0.84), a 42% reduction in hip fracture risk (HR, 0.58; 95% CI, 0.43 to 0.79), and an 18% reduction in nonvertebral fracture risk (HR, 0.82; 95% CI, 0.62 to 1.07). The absence of significance for the last result in the direct meta-analysis was one of the few that was inconsistent with the network meta-analysis, which showed a 21% (significant) reduction in nonvertebral fracture risk (HR, 0.79; 95% CI, 0.67 to 0.94) (12).
One large trial of zoledronic acid conducted among women and men after hip fracture found a 35% (significant) reduction (HR, 0.65; 95% CI, 0.50 to 0.84) in all clinical fractures, supporting the value of bisphosphonate treatment after a hip fracture (34). In this trial, there was also evidence of 28% (significant) reduction (HR, 0.72; 95% CI, 0.56 to 0.93) in mortality (34), although a reduction in mortality has not been shown in other trials with zoledronic acid.
Long-term bisphosphonate treatment beyond 5 years
Bisphosphonates are distinct from other osteoporosis therapies in that their positive effects persist for several years after discontinuation. For alendronate and zoledronic acid, two moderate-sized randomized, placebo-controlled trials (1099 and 1233 women, respectively) of long-term use (10 years vs 5 years for alendronate and 6 vs 3 years for zoledronic acid) (35, 36) form the primary evidence base. For the Fracture Intervention Trial Long-term Extension (FLEX) trial with alendronate, during the 5 years of the study, BMD (the primary endpoint) decreased gradually in the placebo compared with the continued alendronate group. Thus, at the end of 5 years, ∼50% to 75% of the BMD gains during the initial treatment period were lost in those who discontinued alendronate. Similarly, bone turnover gradually increased. Among those who continued alendronate compared with those who discontinued its use, vertebral fracture risk was significantly lower, but there were no significant reductions in nonvertebral or hip fractures. However, the CIs for nonvertebral and hip fracture rates were wide. In the extension study with zoledronic acid, BMD fell more slowly after discontinuation compared with the study with alendronate, and levels of bone turnover markers (BTMs) rose more slowly. Fracture results in this study were similar to the FLEX study with a reduction in vertebral fractures but no significant effects on nonvertebral fractures.
There are more limited data regarding discontinuation of risedronate and ibandronate. In the Tablets, Rings, and Injectables as Options for Women (TRIO) study, osteoporotic women were randomized to receive alendronate, risedronate, or ibandronate (37, 38). The effect of 2 years of use followed by 2 years of discontinuation was compared between the three groups (n = 57 women). Results showed no difference between groups in BTMs or change in BMD after discontinuation. These preliminary data did not show that the rate of offset of action after stopping ibandronate and risedronate on both BMD and BTMs differs from alendronate. A larger study with treatment longer than 2 years, however, is needed to obtain a more definitive comparison (39).
Bisphosphonate treatment holidays
The risk of AFFs and ONJ, particularly with long-term bisphosphonate use beyond 5 years, has prompted concerns about defining the treatment course (see “Optimal Duration of Treatment and Drug Holidays”). The American Society for Bone and Mineral Research (ASBMR) Task Force on Long-Term Bisphosphonates has proposed that AFF risk might be reduced, with little compromise in reduction in osteoporotic fractures, by taking a temporary holiday from oral bisphosphonates after 5 years and after 3 years of IV bisphosphonates, in patients who are not at high risk of fracture (40). That fracture efficacy might be maintained during a holiday is supported by several studies. First, results from the two long-term randomized trials with alendronate and zoledronic acid discussed above suggest that after stopping either of these treatments, BMD gains remain but are slowly lost during 3 to 5 years. Levels of BTMs remain decreased initially, but slowly increase, and the risk of nonvertebral fractures is not increased over 5 years after discontinuation. Second, a recent large observational study also showed no increase in nonvertebral or hip fracture risks for those discontinuing bisphosphonates compared with persistent users (41).
An important assumption about the value of a bisphosphonate holiday is that AFF risk would be reduced. There is limited evidence that AFF risk will be reduced during a bisphosphonate holiday: one large observational study showed that AFF is reduced by >80% in the 3 years after stopping bisphosphonates (42). Preliminary results from a study in Kaiser Permanente Southern California showed a similar reduction in AFF risk after stopping bisphosphonates (41).
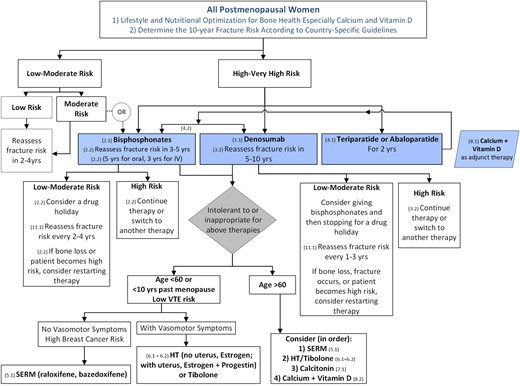
The ASBMR Task Force suggests that those at low to moderate fracture risk can initiate a bisphosphonate holiday, whereas those at high risk should continue the bisphosphonate or switch to another therapy (Fig. 2) (40). An algorithm based on the FLEX trial for identifying candidates for a drug holiday, based on vertebral fracture status and femoral neck BMD at the time of potential discontinuation, has been proposed (43). Once a holiday has begun, fracture risk and BMD should be re-evaluated every 2 to 4 years after discontinuation (Fig. 2). A significant drop in BMD (or a large increase in BTMs) may lead to reinitiation of osteoporosis therapy, depending on the individual’s fracture risk before the 5-year maximum holiday is completed.
Algorithm for the management of postmenopausal osteoporosis. Note that in this algorithm, we considered that a determination of fracture risk would include measurement of lumbar spine and hip BMD and inserting the total hip or femoral neck BMD value into the FRAX tool. Using that FRAX algorithm, we define the following risk categories: “low risk” includes no prior hip or spine fractures, a BMD T-score at the hip and spine both above −1.0, and 10-year hip fracture risk <3% and 10-year risk of major osteoporotic fractures <20%; “moderate risk” includes no prior hip or spine fractures, a BMD T-score at the hip and spine both above −2.5, or 10-year hip fracture risk <3% or risk of major osteoporotic fractures <20%; “high risk” includes a prior spine or hip fracture, or a BMD T-score at the hip or spine of −2.5 or below, or 10-year hip fracture risk ≥3%, or risk of major osteoporotic fracture risk ≥20%; and “very high risk” includes multiple spine fractures and a BMD T-score at the hip or spine of −2.5 or below.
Although there are some data suggesting that a lower dose of alendronate (5 mg/d) begun after 5 years of alendronate is equally effective in maintaining BMD and levels of BTMs, as is continuing the full dose (10 mg/d) (36), we do not know whether a dose reduction decreases AFF risk. Further study of this question might establish whether lowering the dose after 5 years might be an alternative to a bisphosphonate holiday.
Balance of benefits and harms
Original safety concerns for oral bisphosphonates focused on upper gastrointestinal irritation. However, in practice, these adverse effects can be minimized by careful adherence to correct dosing procedures even in patients with esophageal disease (12, 44). For IV zoledronic acid, an acute-phase reaction (flu-like symptoms, e.g., pyrexia and myalgia) is common (occurring in approximately one in four patients), but usually only after the first infusion, and lasts for 1 to 7 days. The frequency and severity can be reduced by pretreatment with agents such as acetaminophen or ibuprofen. Due to concerns about renal toxicity, bisphosphonates are indicated only for patients with estimated glomerular filtration rate (eGFR) >30 mL/min for risedronate and ibandronate and >35 mL/min for alendronate and zoledronic acid. Although there have been particular concerns about IV zoledronic acid and renal damage, as long as a minimum of a 15-minute infusion time is maintained and the patient is well hydrated, there has been no evidence of any loss of renal function with zoledronic acid treatment in randomized clinical trials (45) when only patients with an eGFR >35 mL/min are given the drug. A meta-analysis of the effect of bisphosphonate treatment on atrial fibrillation concluded that zoledronic acid may modestly increase the risk, but not the other bisphosphonates (46).
ONJ and AFFs (discussed in detail in “Optimal Duration of Treatment and Drug Holidays”) were first reported in case studies in 2003 and both are extremely rare (47). Epidemiologic studies suggest an association with long-term bisphosphonate use, and these complications have also been observed with other osteoporosis treatments (48). Despite these concerns, the benefits of bisphosphonate therapy for up to 5 years strongly outweigh any AFF risks in postmenopausal women at high risk for fracture. One analysis showed that treating 1000 osteoporotic women with bisphosphonates for 3 years was associated with 0.08 AFF while preventing 100 fractures, including 11 hip fractures (49). However, there are no comparable benefit/risk analyses for longer-term bisphosphonate treatment.
Patient values and preferences
Compliance with oral bisphosphonates, as with other medications used to lower chronic disease risks, is low (∼30% still continuing at 1 year) (50). In patients who may have difficulties with adherence to oral medications or who fail to respond, the use of zoledronic acid (given annually as an IV infusion) or denosumab (given by subcutaneous injection every 6 months, see “Denosumab”) may be advantageous for effectively lowering the fracture risk. Concerns of patients about risk of AFFs or ONJ should be taken into account when considering bisphosphonate holidays.
3. Denosumab
3.1 In postmenopausal women with osteoporosis who are at high risk for osteoporotic fractures, we recommend using denosumab as an alternative initial treatment. (1|⊕⊕⊕⊕)
Technical remark: The recommended dosage is 60 mg subcutaneously every 6 months. The effects of denosumab on bone remodeling, reflected in BTMs, reverse after 6 months if the drug is not taken on schedule. Thus, drug holiday or treatment interruption are not recommended with this agent.
3.2 In postmenopausal women with osteoporosis who are taking denosumab, we suggest that the fracture risk be reassessed after 5 to 10 years and that women who remain at high risk of fractures should either continue denosumab or be treated with other osteoporosis therapies. (2|⊕OOO)
3.3 In postmenopausal women with osteoporosis taking denosumab, administration of denosumab should not be delayed or stopped without subsequent antiresorptive (e.g., bisphosphonate, HT, or selective estrogen receptor modulator) or other therapy administered to prevent a rebound in bone turnover and to decrease the risk of rapid BMD loss and an increased risk of fracture. (Ungraded Good Practice Statement)
Evidence
A meta-analysis that compared denosumab with placebo (Fig. 1) showed a 68% reduction in the risk of vertebral fractures (HR, 0.32; 95% CI, 0.26 to 0.40), a 39% reduction in the risk of hip fractures (HR, 0.61; 95% CI, 0.37 to 0.98), and a 19% reduction in the risk of nonvertebral fractures (HR, 0.81; 95% CI, 0.69 to 0.95) (12).
The duration of the double-blind, placebo-controlled phase of the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial was 3 years. In the FREEDOM Extension study, all patients received denosumab during the 7-year extension. There was no control group during this extension. Continuing low rates of new radiographic vertebral fractures (0.9% to 1.86% per year), nonvertebral fractures (0.84% to 2.55% per year), and hip fractures (0% to 0.61% per year) were noted in years 4 to 10. These rates were comparable to those in the initial phase 3 study in subjects taking denosumab, supporting a stable level of fracture reduction up to 10 years (51). There are no published data on the use of denosumab beyond 10 years of treatment. Shorter courses of therapy with this agent may be considered depending on the BMD response and the ongoing fracture risk assessment done by the treating clinician. However, BMD gains are rapidly lost with cessation of denosumab and another therapy such as a bisphosphonate should be given after a course of denosumab is ended to maintain the BMD gains of the treatment course.
Balance of benefits and harms
One limitation in the use of denosumab is the risk of hypocalcemia due to concomitant medical conditions such as malabsorption or chronic kidney disease (CKD). In contrast to the bisphosphonates, denosumab may be administered to patients with CKD and those with eGFRs of ≤35 mL/min/1.73 m2. Denosumab has been shown to be effective at reducing fracture rates and increasing the BMD at all sites and (to a similar extent) in patients with CKD stage 1 (eGFR ≥90 mL/min), 2 (eGFR of 60 to 89 mL/min), or 3 (eGFR of 30 to 59 mL/min). In stage 4 CKD (eGFR of 15 to 29 mL/min), compared with placebo, denosumab increased the BMD at hip sites (P < 0.0002) but had no significant effects on fracture rates (52). No subjects with stage 5 CKD were enrolled in the FREEDOM trial. Denosumab should be administered with caution in patients with CKD, however, because the drug lowers bone turnover rapidly and substantially and blocks calcium mobilization from bone in defense of hypocalcemia. Two groups studied individuals with varying degrees of renal impairment, including those defined as severe (eGFR <30 mL/min/1.73 m2) or requiring dialysis (53, 54), and found that such individuals were at greater risk of posttreatment hypocalcemia than were those with normal renal function. The prescribing information approved by the US Food and Drug Administration states that clinical monitoring of the serum levels of calcium, magnesium, and phosphorus should be considered in patients predisposed to hypocalcemia and disturbances of mineral balance within 14 days of denosumab injection, as does the summary of product characteristics (SmPC) of the European Medicines Agency (55). Further recommendations from the SmPC emphasize the importance of identifying patients at risk for hypocalcemia and addressing this risk by assuring an adequate intake of calcium and vitamin D before initiating therapy. Serum calcium levels may be checked prior to each dose of denosumab. Individuals at risk for hypocalcemia should be educated about the signs and symptoms of hypocalcemia before administration of the agent.
Adverse events
Adverse events assessed in the phase 3 FREEDOM trial included infections, inflammatory disorders, and malignancies, as well as ONJ, AFFs, and hypocalcemia. In the first 3 years of the FREEDOM trial, there were no statistically greater risks of cancer, infection, delayed fracture union, hypocalcemia, or ONJ (56). In the FREEDOM Extension during 10 years, adverse events and serious adverse events did not increase with time (57, 58). There were seven and six cases, respectively, of ONJ in the long-term (10-year) vs crossover (7-year) denosumab treatment groups and two AFFs occurred during the 7-year extension study (one per treatment group) (51).
In a meta-analysis of safety incorporating data from 11 trials, compared with placebo, denosumab increased the risk of serious adverse events related to infection in postmenopausal women (relative risk, 1.23; 95% CI, 1.00 to 1.52; P = 0.05) (59). No increased risk of malignancy or of skeletal fragility (reflected by a greater rate of nonvertebral fractures) was noted. Infections reported as serious adverse events in the FREEDOM trial included several body sites, and the gastrointestinal and urinary tracts, heart, skin, and ear were numerically greater in the denosumab-treated group vs the placebo-treated group, but the differences were not statistically significant (60). Serious opportunistic and/or fatal infections were few in number and were not significantly different between the denosumab-treated and placebo-treated subjects (60). Serious adverse events involving the skin (among them, erysipelas and cellulitis) occurred in 1 placebo-treated subject (<0.1%) but in 15 denosumab-treated subjects (0.4%) (60). The eczema incidence was also higher (118 cases in denosumab-treated subjects vs 65 cases in placebo-treated subjects during 3 years) (56).
Several case reports and series of patients have emphasized that the cessation of denosumab treatment may be associated with a risk of multiple and/or severe vertebral fractures (61). In the FREEDOM and the FREEDOM Extension trials (62), there was an excess occurrence of multiple new vertebral fractures in patients who discontinued denosumab vs placebo, but those rates did not exceed the baseline fracture rate. BTM data (63, 64) show that the levels of serum C-terminal crosslinking telopeptide (CTX) and procollagen type 1 N-terminal propeptide (P1NP) increase above baseline values within 3 to 6 month of denosumab discontinuation. Concomitantly, levels of BMD at the spine and hip decline to pretreatment levels within 24 months. The vertebral fractures occurring after drug cessation have been ascribed to the rapid rebound in bone turnover as the medication effect quickly wears off. This situation has led to a cautionary note that doses of denosumab should not be delayed and should be administered on an every-6-month basis. The risk of hypocalcemia (decline in serum calcium to <1.88 mmol/L or 7.6 mg/dL) due to denosumab is estimated to be ∼0.05% in data compiled from two large clinical trials (2 of 4050 patients) (55) and in the range of 14% to 25% of subjects in small studies of subjects at risk for hypocalcemia (53, 65, 66). The key risk factor for that complication is underlying CKD, as noted above. Vitamin D deficiency at baseline and higher rates of baseline bone turnover, as assessed by turnover markers, may increase the risk of hypocalcemia (66).
Overall, the rates of these adverse events in patients with normal renal function are low, and there are significant antifracture benefits with the use of denosumab therapy. The agent acts rapidly, is straightforward to administer, and produces marked suppression of bone turnover (reduction in serum CTX levels) within the first week of administration (67). The weight of evidence supports the use of this agent for its strong antifracture efficacy.
Patient values and preferences
Studies have compared adherence (combination of persistence and compliance) and patient preference for denosumab injections every 6 months to oral bisphosphonates (weekly or monthly) (68). The Denosumab Adherence Preference Satisfaction (DAPS) study reported that regardless of the treatment sequence during 24 months (alendronate for 12 months, followed by denosumab for 12 months or vice versa), trial participants significantly preferred denosumab to alendronate based on questionnaires (69, 70). Subject scores for denosumab showed greater preference and satisfaction than with alendronate (69). In an observational study of routine clinical practice, persistence with treatment was estimated at ∼87% to 95% and adherence at ∼83% to 89% after 12 months (71). High rates of persistence and adherence help to ensure that the fracture reduction benefits reported in clinical trials are attained in clinical practice.
Remarks
At the doses used to treat osteoporosis and with the lack of incorporation of this monoclonal antibody into bone, the drug’s actions reverse after 6 months. Injections should be given every 6 (±1) months. If longer intervals between doses occur, then the drug’s effect wears off and bone resorption rates rise promptly. Bone turnover increases to pretreatment levels or higher, and eventually BMD declines by 18 to 24 months after treatment discontinuation (62, 63). In the period after treatment discontinuation, patients may be more vulnerable to sustaining vertebral fractures, and this vulnerability may underlie the “rebound” vertebral fractures that have been reported with denosumab discontinuation or missed dosing, which is to be strictly avoided (see “Impact of Stopping Non-Bisphosphonate Therapies”).
4. Teriparatide and Abaloparatide (PTH and PTH-Related Protein Analogs)
4.1 In postmenopausal women with osteoporosis at very high risk of fracture, such as those with severe or multiple vertebral fractures, we recommend teriparatide or abaloparatide treatment of up to 2 years for the reduction of vertebral and nonvertebral fractures. (1|⊕⊕⊕O)
4.2 In postmenopausal women with osteoporosis who have completed a course of teriparatide or abaloparatide, we recommend treatment with antiresorptive osteoporosis therapies to maintain bone density gains. (1|⊕⊕OO)
Evidence
Anabolic agents increase BMD by increasing bone formation when administered intermittently (i.e., daily). There are now two licensed peptides that are anabolic for bone: PTH(1–34) (teriparatide) and a PTH-related protein analog (abaloparatide). Compared with other agents, the evidence base for teriparatide and abaloparatide fracture reduction is more limited both in terms of number of trials and number of patients who have participated in the trials.
The meta-analysis comparison of teriparatide with placebo (Fig. 1) showed a 74% reduction in the risk of vertebral fractures (HR, 0.26; 95% CI, 0.18 to 0.39) and a 39% reduction in the risk of nonvertebral fractures (HR, 0.61; 95% CI, 0.44 to 0.85) (12). There is evidence that teriparatide reduces fractures more than risedronate based on the VERtebral Fracture Treatment Comparisons in Osteoporotic Women (VERO) trial. In this study of women at very high risk of fracture, teriparatide reduced vertebral and clinical (nonvertebral plus clinical vertebral) fractures compared with risedronate (72).
The meta-analysis comparison of abaloparatide with placebo (Fig. 1) showed an 87% reduction in the risk of vertebral fractures (HR, 0.13; 95% CI, 0.05 to 0.38) and a 46% reduction in the risk of nonvertebral fractures (HR, 0.54; 95% CI, 0.31 to 0.96) (12). In the meta-analysis, hip fracture reductions for both agents were not statistically significant, despite trends toward reductions for both. However, the numbers of hip fractures in these studies were small and the studies were inadequately powered for this endpoint.
A significant increase in osteosarcoma in rats given lifelong treatment with teriparatide or abaloparatide led to black box warnings for both of these agents with limits for lifetime therapy to a maximum of 24 months. However, since the introduction of teriparatide in 2002, with >1 million human users, the rate of osteosarcoma has not been greater than expected, with only one case reported as of 2016 (49). Side-effects of teriparatide (20 mg dose) vs placebo included greater rates of dizziness and leg cramps (73), while side-effects of abaloparatide that led to study discontinuation were nausea, postural hypotension, dizziness, headache, and palpitations (74). Teriparatide and abaloparatide have been shown to increase serum calcium slightly and can result in cases of hypercalcemia. Therefore, it is recommended that serum calcium should be assessed prior to use and that neither agent be used in patients with elevated serum calcium.
The durability of the effect of anabolic drugs after they are stopped has been tested for several anabolic agents. For example, a randomized trial compared 1 year of PTH(1–84) followed by a second year of placebo vs a second year of alendronate. As assessed by decreases in dual-energy X-ray absorptiometry BMD and trabecular BMD by quantitative CT as well as finite element modeling of bone strength, most of the effect of the drug had worn off within 1 year of stopping use (75, 76). Studies of antiresorptive agents used after anabolic drugs are stopped have shown that antiresorptive agents can maintain and possibly slightly enhance their anabolic effects (74, 75, 77–79). Since the benefits of anabolic therapy are quickly lost after discontinuation, we concur with most clinical guidelines, which recommend that a course of teriparatide or abaloparatide (up to 2 years) be followed by a bisphosphonate, raloxifene, denosumab, or menopausal HT.
Bisphosphonates are generally the initial therapy for osteoporosis for most women. However, in cases where a patient on bisphosphonates continues to lose bone mass or sustains a fracture, a clinician may want to consider switching to an anabolic treatment. Since bisphosphonate effects as measured by BTMs and BMD persist after stopping, there has been some controversy about the efficacy of anabolic agents following bisphosphonate therapy. Several studies have examined the effects of teriparatide on BTMs and BMD following bisphosphonates. Those studies have suggested that teriparatide retains its anabolic effect, although the timing of onset may be somewhat delayed and the magnitude of the effect somewhat blunted (80). A randomized trial of 24 months of teriparatide vs risedronate (VERO trial) recently published a subgroup analysis comparing fracture efficacy in those with and without bisphosphonate use prior to study entry (81). This analysis suggested similar fracture reductions for vertebral and clinical fractures in prior bisphosphonate users compared to treatment-naive patients. There was a suggestion (not significant) that fracture reductions were slightly delayed for prior bisphosphonate users compared with treatment-naive patients. These results provide support that anabolic therapy remains efficacious in reducing fracture risk even after a prior course of bisphosphonates.
Teriparatide and abaloparatide are the only anabolic agents currently approved for osteoporosis. However, other anabolic agents (e.g., romosozumab) have been or are being tested and may be available in the future. PTH(1–84) (82) was approved and used for several years in Europe but is no longer available for this indication.
Patient values and preferences
The BMD increases with either teriparatide or abaloparatide are substantial, as are reductions in vertebral fracture. However, there are two important limitations of these medications. First, they require a daily injection, which some patients may not be willing to do or to which many patients may find adherence challenging. Second, both teriparatide and abaloparatide are much more expensive than other therapies, and this may be an important barrier for many patients, particularly when insurance coverage may be limited.
5. Selective Estrogen Receptor Modulators
5.1. In postmenopausal women with osteoporosis at high risk of fracture and with the patient characteristics below, we recommend raloxifene or bazedoxifene to reduce the risk of vertebral fractures. (1|⊕⊕⊕⊕)
Patient characteristics: With a low risk of deep vein thrombosis (DVT) and for whom bisphosphonates or denosumab are not appropriate, or with a high risk of breast cancer.
Evidence
The meta-analysis that compared raloxifene with placebo (Fig. 1) showed a 40% reduction in the risk of vertebral fractures (HR, 0.60; 95% CI, 0.52 to 0.69), but no significant effect on reduction in the risk of hip or nonvertebral fractures (12). Several side effects limit use, including venous thromboembolism, hot flushes, and leg cramps (83).
The effect of raloxifene (60 mg daily) on vertebral fractures was present in women with osteoporosis (BMD T-score of −2.5 or less) with or without a prior vertebral fracture (84). This effect was also present in women not selected on the basis of fracture risk (85). The effect of raloxifene on BMD is less than that of menopausal HT (86), but there are no comparative fracture data.
The meta-analysis that compared bazedoxifene with placebo (Fig. 1) showed a 39% reduction in the risk of vertebral fractures (HR, 0.61; 95% CI, 0.48 to 0.77), but no significant effect on the reduction in the risk of hip or nonvertebral fractures (12). Several side effects limit use, including venous thromboembolism, hot flushes, and leg cramps (87). There is no evidence regarding the effects on breast cancer. The effects of 3 years of treatment with bazedoxifene (20 mg or 40 mg daily) on the risk of vertebral fracture are similar to those of raloxifene (60 mg daily) (87). The effect of bazedoxifene on vertebral fracture risk after 5 years of treatment is similar to that after 3 years of treatment (88).
Bazedoxifene is licensed in Germany, Lithuania, Sweden, Croatia, Japan, and Israel for the prevention of osteoporosis, but not in the United States or Canada (89). Bazedoxifene is only licensed in the United States and Canada as a combination with conjugated estrogens for the treatment of hot flushes or prevention of osteoporosis in patients for whom other treatments for osteoporosis are not suitable. The combination of conjugated estrogens and bazedoxifene results in less increase in spinal BMD at 1 year compared with conjugated estrogens and a progestin, but less breast tenderness and more amenorrhea (90). Conjugated estrogens/bazedoxifene have not been shown to reduce the risk of fracture (11).
Balance of benefits and harms
Raloxifene has the added benefit of a reduced incidence of invasive estrogen receptor–positive breast cancer both during treatment and for at least 5 years after completion (91). This benefit of treatment should be taken into account when counseling patients.
Raloxifene may be well suited to younger women with osteoporosis and no vasomotor symptoms, as we have insufficient data to link this drug to long-term harm such as AFFs. Furthermore, it may be particularly suitable in women who are at increased risk of breast cancer. The more minor adverse events (hot flushes, leg cramps) tend to be worse in the first 6 months of administration. Therefore, it is common practice to encourage perseverance with the drug during the first few months of treatment.
The risk of thromboembolic events is similar to that with the current use of HT. The SmPC (92) recommends the following: “The risk-benefit balance should be considered in patients at risk of venous thromboembolic events of any etiology. Raloxifene should be discontinued in the event of an illness or a condition leading to a prolonged period of immobilization. Discontinuation should happen as soon as possible in case of illness, or from 3 days before the immobilization occurs. Therapy should not be restarted until the initiating condition has resolved and the patient is fully mobile.”
6. Menopausal Hormone Therapy and Tibolone
6.1 In postmenopausal women at high risk of fracture and with the patient characteristics below, we suggest menopausal HT, using estrogen only in women with hysterectomy, to prevent all types of fractures. (2|⊕⊕⊕O)
Patient characteristics: Under 60 years of age or <10 years past menopause; at low risk of DVT; those in whom bisphosphonates or denosumab are not appropriate; with bothersome vasomotor symptoms; with additional climacteric symptoms; without contraindications; without prior myocardial infarction or stroke; without breast cancer; willing to take menopausal HT.
6.2 In postmenopausal women with osteoporosis at high risk of fracture and with the patient characteristics below, we suggest tibolone to prevent vertebral and nonvertebral fractures. (2|⊕⊕⊕O)
Patient characteristics: Under 60 years of age or <10 years past menopause; with a low risk of DVT; those in whom bisphosphonates or denosumab are not appropriate; with bothersome vasomotor symptoms; with additional climacteric symptoms; without contraindications; without prior myocardial infarction or stroke or high risk for cardiovascular disease; without breast cancer; willing to take tibolone.
Technical remark: Tibolone is not available in the United States or Canada.
Evidence
The meta-analysis that compared menopausal HT (estrogen with or without progestogen) with placebo (Fig. 1) showed a 34% reduction in the risk of vertebral fractures (HR, 0.66; 95% CI, 0.49 to 0.89), a 29% reduction in the risk of hip fractures (HR, 0.71; 95% CI, 0.52 to 0.98), and a 21% reduction in the risk of nonvertebral fractures (HR, 0.79; 95% CI, 0.70 to 0.90) (12). The fracture benefits are present in women at lower risk of fractures (93–95) and at high risk of fractures (96, 97) and with oral conjugated equine estrogen (0.625 mg daily) or with estradiol (100-mg patch or 2 mg daily oral) use. However, the evidence of fracture benefit in women is based mainly on clinical trials in women not at high risk of fracture.
Several potential side effects limit use, including venous thromboembolism, stroke, myocardial infarction, cancer (breast, endometrial, ovary), dementia, gallbladder disease, and urinary incontinence (98). Recent data from the Women’s Health Initiative Study covering 13 years showed reversal of most of these risks after stopping therapy; furthermore, the risks with estrogen alone (e.g., breast cancer) are less than those with the combination (99). Benefits include relief of menopausal symptoms (e.g., hot flushes), less diabetes, and a lower risk of colon cancer.
The meta-analysis that compared tibolone with placebo (Fig. 1) showed a 44% reduction in the risk of vertebral fractures (HR, 0.56; 95% CI, 0.42 to 0.74), no significant effect on reduction in the risk of hip fractures, and a 27% reduction in the risk of nonvertebral fractures (HR, 0.73; 95% CI, 0.59 to 0.92) (12). Several side effects limit use, including stroke (HR, 2.19; 95% CI, 1.14 to 4.23), vaginal discharge, and bleeding. There are benefits for menopausal symptoms such as hot flushes and for the risks of breast cancer (HR, 0.32; 95% CI, 0.13 to 0.80) and colon cancer (HR, 0.31; 95% CI, 0.10 to 0.96), and patients taking tibolone have fewer falls (100). Even though the risk of breast cancer was reduced in the Long-Term Intervention on Fractures with Tibolone (LIFT) study, there was an increased recurrence of breast cancer in women with previous breast cancer in the Livial Intervention Following Breast Cancer: Efficacy, Recurrence and Tolerability Endpoints (LIBERATE) study (101).
Balance of benefits and harms
The Writing Committee has no preference between estrogen and tibolone; most of the evidence for HT is based on women at low risk of fracture, whereas the evidence for tibolone is based on women at high risk of fracture. The benefits and risks of estrogen and tibolone need to be evaluated on an individual basis (102). These risks depend on the duration of treatment, the woman’s age, and her underlying health and are lower in healthy younger women, hence the recommendation to select women <60 years of age or within 10 years of menopause (102). The risk of venous thromboembolic disease may be lower with transdermal rather than oral estrogen (103). The balance of risks and benefits differs between individual women according to their needs for treatment. If menopausal HT is prescribed for osteoporosis and it is stopped, then alternative treatments for osteoporosis should be given.
7. Calcitonin
7.1 In postmenopausal women at high risk of fracture with osteoporosis, we suggest that nasal spray calcitonin be prescribed only in women who cannot tolerate raloxifene, bisphosphonates, estrogen, denosumab, tibolone, abaloparatide, or teriparatide or for whom these therapies are not considered appropriate. (2|⊕OOO)
Evidence
The meta-analysis that compared calcitonin with placebo (Fig. 1) showed a 46% reduction in the risk of vertebral fractures (HR, 0.54; 95% CI, 0.36 to 0.82), but no significant effect on reduction in the risk of hip or nonvertebral fractures (12). The trials with nasal spray calcitonin were never powered to show fracture risk reduction; however, the Prevent Recurrence of Osteoporotic Fractures (PROOF) trial did show efficacy for vertebral fracture reduction (at the dose of 200 IU but not 100 or 400 IU of nasal spray calcitonin per day) (104). Similarly, there is weak evidence for vertebral fracture pain relief from calcitonin, with one randomized, placebo-controlled trial of 68 postmenopausal women showing efficacy (105).
Patient values and preferences
In patients who cannot tolerate denosumab, bisphosphonates, hormone-based therapies, selective estrogen response modulators, tibolone, or anabolic treatments, nasal spray calcitonin may be used and is well tolerated. However, there is considerable doubt about its benefit in reducing fractures, particularly nonvertebral fractures.
Balance of benefits and harms
Recent studies have raised doubt about the long-term safety of nasal spray calcitonin due to an increased risk (from cross-sectional and cohort studies and a meta-analysis) of prostate and liver cancer and other malignancies, although the pathophysiologic basis is unclear (106, 107). The European Medicines Agency and Health Canada have both withdrawn nasal spray calcitonin from the market.
8. Calcium and Vitamin D
8.1 In postmenopausal women with low BMD and at high risk of fractures with osteoporosis, we suggest that calcium and vitamin D be used as an adjunct to osteoporosis therapies. (2|⊕⊕OO)
8.2 In postmenopausal women at high risk of fracture with osteoporosis who cannot tolerate bisphosphonates, estrogen, selective estrogen response modulators, denosumab, tibolone, teriparatide, and abaloparatide, we recommend daily calcium and vitamin D supplementation to prevent hip fractures. (1|⊕⊕⊕O)
Evidence
The meta-analysis that compared calcium with placebo (Fig. 1) showed no significant effect on reduction in the risk of vertebral or hip fractures, but a 37% reduction in the risk of nonvertebral fractures (HR, 0.63; 95% CI, 0.45 to 0.90). The meta-analysis that compared vitamin D with placebo (Fig. 1) showed no significant effect on reduction in the risk of vertebral or hip fractures, but a 56% reduction in the risk of nonvertebral fractures (HR, 0.44; 95% CI, 0.22 to 0.88). The meta-analysis that compared the combination of calcium and vitamin D with placebo (Fig. 1) showed no significant effect on reduction in the risk of vertebral fractures, but a 19% reduction in the risk of hip fractures (HR, 0.81; 95% CI, 0.71 to 0.93), and a 5% reduction in the risk of nonvertebral fractures (HR, 0.95; 95% CI, 0.90 to 1.00) (12).
The level of evidence for hip fracture prevention with the combination of calcium and vitamin D supplementation is strong but only in selected circumstances, and with the following caveats. First, the greatest risk reduction (33%) from calcium and vitamin D supplementation is in elderly individuals living in residential care (108). (The data are based on studies predominantly of women 70 years and older in residential care.) Second, the largest trial to date in postmenopausal women, the Women’s Health Initiative, demonstrated risk reduction, but the original analysis was per protocol and was not an intention-to-treat analysis (109). Third, women in that trial were also randomized to either HT or placebo. Only those receiving calcium and vitamin D plus HT showed significant hip fracture reduction. For the prevention of vertebral fractures, calcium and vitamin D have no impact on risk, but the level of evidence is weak. Expert opinion suggests that increasing dietary calcium intake is the most appropriate and safest way to enhance bone mineralization.
As mentioned above, the strength of the evidence for hip fracture risk reduction with calcium and vitamin D is driven principally by one large randomized, placebo-controlled trial in elderly women who were nursing home residents in which there was a significant (i.e., 33%) risk reduction in hip fractures (108). However, the mean 25-hydroxyvitamin D levels in that cohort were low (<16 ng/mL). In addition to fracture risk reduction, with adequate vitamin D repletion, the mean PTH levels decreased, almost certainly as a result of vitamin D and calcium supplementation (108, 110, 111). No other studies have shown this degree of hip fracture risk reduction. It has also been thought that calcium and vitamin D could prevent falls, and that in turn could result in a reduction in hip fractures (112). This led to the use of high-dose supplementation with vitamin D administered less frequently. However, a recent trial showed the opposite effect, with an increased risk of fracture (113). Additionally, a new meta-analysis revealed inconsistent or no effects from vitamin D supplementation on falls, fractures, or BMD (114).
Note that virtually all the recent trials of drugs to treat osteoporosis use a study design of drug plus calcium and vitamin D supplementation vs calcium and vitamin D alone. Hence, the proven antifracture efficacy for all osteoporosis drugs includes the addition of calcium and vitamin D supplementation. For example, in the Women’s Health Initiative trial of calcium and vitamin D, there was a 2×2 design in which some women who received calcium (1000 mg/d) plus vitamin D supplementation (400 IU/d) were also randomized to HT (estrogen or estrogen plus progesterone) or no treatment. In that arm, women who received both active treatments (calcium plus vitamin D plus HT) had a 42% reduction in hip fractures (0.37 to 0.93) vs calcium plus vitamin D alone. Similar results have been noted with other antiresorptive therapies (34, 36, 56).
The amount of calcium supplementation in randomized trials ranges from 500 to 1500 mg/d. Expert opinion currently recommends ≤1000 mg/d in the form of supplements, whereas the overall recommendation from the National Osteoporosis Foundation and Institute of Medicine (for women >50 years and men >70 years of age) is a total calcium intake of 1200 mg/d. The total calcium intake per day should include both dietary and supplemental calcium. We prefer that this be achieved through dietary intake, but this is often difficult, especially in older individuals. In the largest randomized, placebo-controlled trial for calcium and vitamin D in postmenopausal women, the Women’s Health Initiative, the daily dietary intake in the active arm was ∼1100 mg/d in addition to the 1000 mg of calcium supplementation, resulting in a total intake of ∼2100 mg/d (109). This intake led to a 17% increase in the development of renal stones (109).
In the current meta-analysis, calcium plus vitamin D supplementation reduced the risk of hip fractures but not vertebral or nonhip fractures. The magnitude of risk reduction is consistent with that reported by a previous meta-analysis (115), although not the most recent meta-analysis (116), and is driven principally by trials in older individuals (108). The highest absolute risk of hip fracture occurs in the frail elderly, owing to both low bone mass and a higher rate of falls. Notwithstanding, among all women in the Women’s Health Initiative trial (mean age, 61 years) who were randomized to calcium (1000 mg/d) and vitamin D (400 IU/d), and who were adherent after 6 months, there was a 29% reduction in hip fractures, but no effect on other fractures. As noted, however, those subjects included women who were also randomized to HT (116). Although there are no clinical trial data, most experts would agree that dietary intake with calcium is the safest and most appropriate approach for postmenopausal women undergoing treatment for osteoporosis.
Balance of benefits and harms
With respect to cardiovascular safety, some meta-analyses analyzing the effects of calcium supplementation alone (without vitamin D) on cardiovascular events show a weak association with increased risk of myocardial infarction and stroke, whereas others show no association (117–119).
There is no evidence that supplementation with vitamin D of up to 4000 IU/d (i.e., the tolerable upper limit set by the Institute of Medicine) is associated with any safety issues beyond hypercalciuria (118). However, when combined with high amounts of supplemental calcium, there is the potential for a greater risk of kidney stones (109). Additionally, there is evidence from two randomized trials that high-dose intermittent vitamin D (500,000 IU/y or 20,000 IU/wk) can lead to a greater risk of falls and fractures (113, 120).
Thus, most postmenopausal women in the United States now consume close to 1000 mg of calcium per day from their diet, which is an increase of >200 mg/d from the late 1990s (109, 121). The Institute of Medicine recommends 1000 to 1200 mg of calcium per day in diet and/or supplements (121). Writing Committee members prefer encouraging women to increase their dietary intake of calcium and to keep calcium supplement intake <1000 mg/d because of potential safety concerns with supplements, particularly renal calculi. There is no consensus concerning a threshold level of vitamin D that should be reached when supplementing postmenopausal women. However, all postmenopausal women with a confirmed diagnosis of osteoporosis should be screened with a serum level of 25-hydroxyvitamin D. The preference of the Writing Committee is that adequate serum 25-hydroxyvitamin D levels in women with osteoporosis should be at least 20 ng/mL (50 nmol/L) as noted by European guidelines, although Endocrine Society guideline recommends a threshold of 30 ng/mL (75nmol/L), either of which can often be met by ingesting 1000 IU of vitamin D per day.
Patient values and preferences
There is consensus that calcium and vitamin D should be added to all osteoporosis treatment regimens to enhance mineralization and maintenance of bone mass in high-risk postmenopausal women, many of whom also consume diets low in calcium. There is no direct evidence that the calcium and vitamin D added to other treatments for osteoporosis contribute to the reduction in fracture risk from clinical trials testing those agents, because calcium and vitamin D supplements are the standard baseline intervention at randomization. There may be a small additional BMD benefit of calcium plus vitamin D for individuals in addition to a prescribed osteoporosis medication, particularly because calcium plus vitamin D are thought to be important for mineralization (122). Supplemental intake of calcium and vitamin D has increased during the last two decades, but dietary calcium intake, with the advent of more supplemented food choices, has also increased.
9. Approach to Treatment or Choosing Among Approved Therapies
The goal of treatment is to decrease fractures associated with osteoporosis; thus, the overall approach is to recommend good bone health maintenance efforts. These include adequate calcium and vitamin D intake, resistance and balance exercises, smoking cessation, limited alcohol use, decreased use of drugs, and optimization of comorbid conditions that can harm bone for all postmenopausal women. For those at high fracture risk, we recommend treatment with approved medications. For those at low-to-moderate fracture risk, we recommend following the country-specific guidelines for treatment, as the fracture risks, values, and costs of therapies vary across populations.
Decisions regarding the choice of therapies must take into account the country-specific availability of various drugs, local guidelines, values, and preferences of the patient, costs, and drug coverage (e.g., insurance, government coverage). Because of the lower costs and longer experience with bisphosphonates, they are often used as initial therapies for postmenopausal osteoporosis in most countries. However, it is important to weigh risks, benefits, and preferences on an individual basis, and there may be individual patient characteristics that help to determine which drug is optimal. For example, a woman in her late 50s with osteoporosis and at high risk of breast cancer may consider raloxifene as an initial therapy, whereas another woman with gastroesophageal reflux disease at high risk of fracture may prefer to start with denosumab or zoledronic acid. Theoretically, one may want to use bone formation therapy as the initial therapy, if the patient has sustained recent vertebral fractures. In general, calcium and vitamin D aside, we recommend using one drug therapy at a time, and not combining them.
The decision to switch therapy from one agent to another is often based on availability, tolerability, costs, and preferences. Health care providers may also want to consider switching therapy because of adherence issues or “failure” of therapy. As osteoporosis drug therapies do not totally eliminate fracture risk, it is often unclear whether sustaining a fracture while on therapy is considered a failure of therapy. In general, we consider loss of BMD greater than the least significant change (usually 5% in the lumbar spine, 4% in the total hip, and 5% in the femoral neck) over 2 years and BTM decrease on antiresorptive drugs less than the least significant change as “failure” of therapy. We would consider having two or more fractures while on therapy as treatment failure, especially vertebral fractures (123). In clinical practice, the occurrence of one fracture while on effective therapy and in a compliant patient will raise the consideration for changing therapy. In such cases, we suggest switching to one of the alternative therapies discussed in this guideline. It is important to rule out secondary osteoporosis when a patient “fails” therapy, as intervening medical conditions (such as multiple myeloma) or medications (such as tenofovir or oversupplementation of thyroid hormone in hypothyroidism) may be the root cause of BMD loss or fracture, rather than failure of osteoporosis drug therapy. What is less clear is when to switch from an antiresorptive therapy to a bone formation therapy. Again, there is a lack of evidence to guide such decisions. Patients for whom we would consider switching from an antiresorptive to a bone formation therapy include the following: a woman with recurrent vertebral fractures due to osteoporosis, a woman at high fracture risk who has been on long-term potent antiresorptive therapy and is sustaining fractures, and a woman with ONJ or an AFF on antiresorptive therapy.
There is an alternative to decision-making about the choice of treatment and when to stop treatment, namely “treat to target” BMD (124, 125). The idea of “treat to target” is to choose therapy that will most likely achieve the target BMD, change to a more potent agent if the initial therapy is not achieving the BMD goal, and stop when fracture risk is at an acceptable low level. Existing therapies, however, may not be potent enough to achieve the target or maintain the target BMD once it is stopped.
We propose that the algorithm in Fig. 2 be applied to an individual postmenopausal woman when considering the management of her osteoporosis. We considered those women at high risk as being eligible for drug therapy and defined this high-risk group as having a prior spine or hip fracture, or a BMD T-score of −2.5 or below at either the hip or spine, or a 10-year hip fracture risk ≥3%, or a risk of major osteoporotic fracture ≥20% (Fig. 2).
10. Optimal Duration of Treatment and Drug Holidays
There is an abundance of evidence that treatment of 3 to 5 years with osteoporosis therapies described in earlier sections is highly beneficial with only minimal risk. However, recent concerns about AFFs and ONJ have led to reconsideration of the optimal length of therapy. Considerations for longer-term treatment are more complex and depend on the individual woman’s fracture risk, risk factors for AFF, ONJ, and vertebral, nonvertebral, and hip fractures, as well as the type of therapy being used. The ASBMR established a task force that reviewed the current evidence and published guidelines for long-term treatment in 2016 (40). These recommendations are incorporated into Fig. 2. However, there are important evidence gaps that, when filled, may help to establish more precise and stronger evidence-based guidelines.
Considerations for continuation of therapy depend strongly on the type of medication being used. The evidence on which to base long-term therapy decisions is most robust for bisphosphonates, which represent the vast majority of treatment in the United States and internationally. This evidence is described in detail in the “Bisphosphonate” section and includes two randomized trials (one of alendronate and one of zoledronic acid) that inform decisions about long-term continuation or temporary discontinuations for this class of drugs (see “Long-term bisphosphonate treatment beyond 5 years”). These studies support a residual effect of bisphosphonates after stopping, which support bisphosphonate holidays. However, for all other therapies, as described below, after discontinuation, benefits are quickly lost. Thus, these therapies must be continued indefinitely or followed with bisphosphonates or another type of therapy to retain the gains achieved.
In terms of continued efficacy for fracture reduction with long-term continued therapy, there are some data for bisphosphonates, denosumab, and HT that are described in the respective earlier sections of this report for each of those types of medications.
Impact of stopping nonbisphosphonate therapies
There are data showing that the effects of all nonbisphosphonate therapies (denosumab, abaloparatide, teriparatide, selective estrogen response modulator, HT, tibolone, and calcitonin) disappear with the discontinuation of therapy. When these therapies are discontinued, the gains in BMD observed with these therapies are lost rapidly. Discontinuation of denosumab is associated with a BMD decrease of 6.6% in the lumbar spine and 5.3% in the total hip within the first 12 months of treatment discontinuation (126, 127). In fact, there are data to suggest that the discontinuation of denosumab, a potent antiresorptive therapy, can result in increased vertebral fractures (128–131). Whether this increased risk is the return to the baseline risk of the individual if that individual were not on therapy or whether there is a “rebound” effect (an increased risk beyond the individual’s baseline risk) is unclear. Recent data from the FREEDOM trial suggest that the increase in risk is likely secondary to the absence of drug protection, rather than to a rebound phenomenon (132). One study of alendronate following 1 year of denosumab showed retention of BMD gains for at least 12 months (133). Two small case series examining zoledronic acid after denosumab suggested that it was most effective in BMD retention when administered 8 months, rather than 6 months, after the last dose of denosumab (134, 135). The second study (134, 135) also suggested that a second annual infusion was needed to continue retention of benefits. This study also showed only a partial retention of benefits for risedronate after denosumab. Several studies have shown that use of alendronate after anabolic treatment will retain and perhaps increase BMD gains (36, 136), and thus alendronate after anabolic therapy is usually given.
Osteonecrosis of the jaw
ONJ is a nonhealing wound in the oral mucosa with exposed bone that lasts >8 weeks, usually associated with an invasive dental procedure such as dental extraction or implantation but can occur de novo as well (47). An international task force on ONJ reported on the association of bisphosphonate therapy and ONJ; the absolute risk of ONJ in osteoporosis patients was estimated to range from 1 in 10,000 to 1 in 100,000 (or 0.001% to 0.01%) (137). Higher doses and more frequent use of bisphosphonate and denosumab have been associated with greater ONJ risks in the oncology setting (138), but these patients have other risk factors such as cancer, chemotherapy, radiation therapy, and antiangiogenic therapies. In osteoporosis patients on long-term oral bisphosphonate therapy, the risk of ONJ has been reported to be as high as 21 in 10,000 (or 0.21%) for patients on >4 years of therapy (139). Tooth extraction in a patient exposed to bisphosphonate therapy carries a 0.5% risk of developing ONJ (140). Currently, the American Dental Association does not recommend stopping bisphosphonates for dental procedures; however, if a tooth extraction or implant is planned or ongoing, initiation of potent antiresorptive therapy could be deferred until the area healed (141). In contrast, the American Association of Oral and Maxillofacial Surgeons recommends a 2-month drug holiday for those who have taken >4 years of bisphosphonates (140). Routine dental care is also important for the prevention of ONJ in patients treated with potent antiresorptive therapy (142, 143).
Conservative management such as antibacterial mouth rinse is recommended as initial therapy for stage 0 to 2 ONJ, whereas surgical debridement and resection is recommended for stage 3 ONJ (140). Although there have been case reports of using teriparatide as well as other therapies (such as platelet-rich plasma, low-level laser irradiation, and bone morphogenic protein) in the successful treatment of ONJ, controlled studies are needed to establish efficacy of these therapies (140).
Atypical femoral fractures
AFFs are insufficiency stress fractures of the femoral shaft first noted in case reports in about 2007 (144, 145) that suggested a possible association with bisphosphonate use. These fractures have specific radiological characteristics that have been formalized in a case definition by the ASBMR (146, 147) based on radiographic criteria and low trauma. AFFs have been most studied in relationship to bisphosphonate use but have been noted with other osteoporosis medications including denosumab, odanacatib, and romosozumab (48). Patients often present with pain or aching in the thigh or groin with or after weight-bearing activities. The pathogenesis of these fractures is not understood, although a number of hypotheses including factors related to femur shape, genetics, and ethnicity have been advanced (48).
A large number of epidemiologic studies focusing on bisphosphonate usage and duration have been published. However, many have not had assessments of individual radiographs and instead relied on femoral shaft fractures as defined by ICD codes from population registries [e.g., see Refs (148) and (149)]. Unfortunately, this endpoint is problematic because <5% of these femoral shaft fractures would have met AFF criteria if radiographs had been evaluated (42, 150). This nonspecific endpoint could have masked AFF increases in these studies, as well as in a meta-analysis of AFFs published in 2013 (151), which included many such studies.
Calculation of AFF incidence restricted to studies with radiographic evaluation shows incidence to be very low. For example, a study in Sweden using national data for women and men >55 years of age for 2008 to 2010 showed that among a total of 50,325 femur (hip or femoral shaft) fractures, only 172 (about 3 AFFs per 1000 hip fractures) met ASBMR criteria. A similarly low ratio of AFFs to hip fractures was shown in a study performed in Kaiser Permanente Northwest (152). This study showed very low AFF incidence in their population (about 5 per 100,000 person-years) compared with an almost 100-fold higher incidence of hip fractures (300 to 400 per 100,000 person-years). Despite low incidence rates, studies with radiographic assessment have shown strong increases in AFFs with longer duration of bisphosphonate use. For example, one widely cited study from a large health maintenance organization in California of people >45 years of age (∼1.8 million) showed AFF risk (unadjusted) for 2 years of use to be ∼3 per 100,000 person-years, increasing to ∼20 per 100,000 person-years with 5 years of use and ∼50 per 100,000 with >8 years of use (153). Despite this increase, risks of hip and other osteoporotic fractures that can be prevented by treatment are much higher, suggesting a positive benefit-risk ratio even for long-term treatment, particularly in older women at highest risk of hip and other osteoporotic fractures. Whereas one analysis of benefit vs risk for bisphosphonate treatment of 3 years showed that treating 1000 osteoporotic women would prevent 100 fractures, including 11 hip fractures, while causing about 0.1 AFF (49), similar data for longer-term treatment are not available, and the consistent increase shown for treatment beyond 5 years suggests consideration of AFF risk in treating patients for >5 years. As discussed in section 2, the ASBMR long-term has proposed that the risk could be reduced by taking a “holiday” from oral bisphosphonates after 5 years and from IV bisphosphonates after 3 years in patients who are at low to moderate fracture risk (40). Specific recommendations for bisphosphonate holidays are discussed in “Bisphosphonates”. There are insufficient data about the relationship of long-term denosumab to AFF risk to make a recommendation about duration of use.
11. Monitoring
11.1 In postmenopausal women with a low BMD and at high risk of fractures who are being treated for osteoporosis, we suggest monitoring the BMD by dual-energy X-ray absorptiometry at the spine and hip every 1 to 3 years to assess the response to treatment. (2|⊕OOO)
Technical remark: Monitoring BTMs (serum CTX for antiresorptive therapy or P1NP for bone anabolic therapy) is an alternative way of identifying poor response or nonadherence to therapy.
Evidence
Treatments for osteoporosis increase the BMD, but only modestly. The usual time point for monitoring is after 2 years of treatment. The expected (mean) changes in lumbar spine BMD after alendronate at 70 mg weekly, risedronate at 35 mg weekly, ibandronate at 150 mg monthly, zoledronate at 5 mg annually, denosumab at 60 mg 6-monthly, and raloxifene at 60 mg daily are 7%, 3%, 7%, 7%, 8%, and 3%, respectively (37, 56, 84, 154). The expected (mean) changes in total hip BMD after alendronate at 70 mg weekly, risedronate at 35 mg weekly, ibandronate at 150 mg monthly, zoledronate at 5 mg annually, denosumab at 60 mg 6-monthly, and raloxifene at 60 mg daily are 5%, 2%, 3%, 4%, 5%, and 1%, respectively (37, 56, 84, 154). Teriparatide (20 μg/d) increased the BMD of the spine and total hip by ∼13% and 4%, respectively, after 24 months (73). Evidence to support the use of BMD for monitoring the treatment response is weak but suggests that BMD can be used for this purpose (155). It has been suggested that serial BMD measurements in treated subjects may identify patients who are not adhering to treatment or patients who have a secondary cause for bone loss. Although there is evidence that total hip BMD changes reflect medication compliance (156), use of serial BMD measurements to identify subjects with secondary osteoporosis is anecdotal. It has also been suggested that serial BMD measurements may identify subjects who fail therapy. A retrospective study showed that BMD monitoring was associated with improved compliance (157, 158).
There is uncertainty over what constitutes an adequate BMD response to treatment. Stable or increasing BMD appears to indicate a good response (155). One approach is to consider whether any BMD change exceeds that expected due to normal intermeasurement variation (the least significant change approach); this requires information about the variability of BMD measurements. In women with osteopenia, estimates of the least significant changes at the spine and hip made in research settings are between 4% and 5% in the short term (123). In all of the studies mentioned above, the changes in the spine BMD were greater than the least significant change in most women treated for 2 years, whereas the changes in the hip BMD were generally within the expected reproducibility error. It has been estimated that the BMD response to treatment accounts for a substantial proportion of the fracture risk reduction with treatments for osteoporosis (159). The least significant change approach can also be used to identify significant bone loss in women who are untreated or to identify the offset of effects after stopping treatment of osteoporosis. Because the expected rate of bone loss is slower in these situations than the rate of gain during treatment, it may be better to wait longer between measurements (e.g., 2 to 4 years) in untreated women. Assessing changes in BMD on serial measurements requires careful attention to detail. Using the same machine and a trained technologist aware of the pitfalls of bone densitometry can mitigate these problems. The provider responsible for reporting the results also needs to be aware of these limitations. Degenerative changes in the spine or a new fracture in the region of the scan may falsely give the impression of a gain in BMD.
Treatments for osteoporosis in women produce significant changes in BTMs. In postmenopausal women, alendronate reduces serum CTX and serum P1NP by 80% and 60%, respectively (38). Reductions in BTMs become maximal within several months and remain stable throughout therapy. Bone formation and resorption markers increase dramatically during the first 6 to 12 months of teriparatide therapy in women, after which they gradually decline toward baseline levels (160).
There is uncertainty over what constitutes an optimal BTM response to treatment. Decreasing bone resorption markers (for antiresorptive agents) or increasing bone formation markers (for anabolic agents) indicates a good response to treatment. There is evidence that an inadequate response may be due to the presence of secondary osteoporosis or noncompliance with treatment (161). A change in BTMs relates to fracture risk reduction with antiresorptive treatments (162, 163).
Monitoring treatment with BTMs requires attention to detail. Because of diurnal variations (a higher turnover in the morning) and the effects of food (bone resorption markers decrease after eating), samples for bone resorption markers (urinary N-terminal telopeptide and serum CTX) should be collected with the patient in the fasting state, in the morning. Because manual and automated assays give different results for the same analysis, changes can be compared only if the laboratory continues to use the same assay (164).
As with changes in BMD, changes in BTMs can be compared with the least significant change to determine whether the observed changes exceed those likely to occur as a result of normal variation. Least significant change estimates are ∼56% for serum CTX and 38% for serum P1NP. With oral bisphosphonates, by 12 weeks on treatment, between 70% and 90% of women are expected to respond (38). The BTM response to treatment may account for 30% to 75% of the fracture risk reduction with standard treatments for osteoporosis (163). Additionally, the magnitude of the BTM response has been shown to be associated with the level of compliance (156).
Some experts recommend measuring BTMs before and 3 to 6 months after starting treatment (165). If the change in markers exceeds the least significant change (∼40%), then one goal has been met. In women, a low risk of fractures while on treatment is associated with BTMs that are below the median of the reference interval for young women (166). If the markers do not change, there are several options, including questioning the patient about her compliance with medication, considering causes of secondary osteoporosis, or changing the medication or the route of administration.
Method of Development of Evidence-Based Clinical Practice Guidelines
Participants
The Writing Committee consisted of five content experts representing endocrinology and epidemiology. Two of the committee members brought an international perspective to this guideline topic. The Writing Committee also included a clinical practice guideline methodologist who led the team of comparative effectiveness researchers that conducted the systematic reviews and meta-analyses. The methodologist also supervised application of the GRADE methodological framework for each recommendation, including quality of evidence assessments and strength of recommendation designations.
Guideline development process
The Endocrine Society’s guideline development process combines elements of the GRADE framework (167) with, when relevant, an approach thought to be appropriate for rare endocrine diseases where scientific evidence is limited or nonexistent. The Society applies the steps in the GRADE framework to research questions for which there is an ample body of knowledge of low quality or higher (see Table 1). In these situations, GRADE provides the methodological and statistical rigor that results in robust recommendations that are classified using quality of evidence and strength of recommendation as described by Guyatt et al. (168) and also represented graphically in Table 1.
Where evidence is extremely limited and/or not systematically analyzed, we provide recommendations based on an expert review of the limited data. This process is less systematic than the GRADE methodological framework; however, these recommendations are also clearly classified using the GRADE classification system.
Some of the Society’s clinical practice guidelines also include ungraded good practice statements (169). This unclassified clinical guidance can include expert opinion statements on good practice, references to recommendations made in other guidelines, and observations on preventive care and shared decision-making.
Guideline recommendations include the relevant population, intervention, comparator, and outcome. When further clarification on implementation is needed, we include technical remarks. These provide supplemental information such as timing, setting, dosing regimens, and necessary expertise. All recommendations are followed by a synopsis of the evidence on which they are based. Authors may also include short statements on patients’ values and preferences, the balance of benefits and harms, and minority opinions, where relevant.
Internal and external review
Approximately 18 months into the development process, Endocrine Society clinical practice guidelines undergo a comment review period of 1 month when there is an opportunity for internal and external stakeholders to review the guideline draft and provide comments. These stakeholders include Endocrine Society members, the Society’s Clinical Guidelines Subcommittee, representatives of any cosponsoring organizations, a representative of Council, and an expert reviewer. Following revisions to the guideline manuscript in response to comment review period comments, it is returned to Clinical Guidelines Subcommittee, the Council reviewer, and the expert reviewer for a second review and ballot. Finally, the guideline manuscript is subject to the Journal of Clinical Endocrinology & Metabolism publisher’s review prior to publication. This review is undertaken by an individual with expertise in the topic, without relevant conflicts of interest, and external to the guideline Writing Committee, Clinical Guidelines Subcommittee, and Council.
Conflict of interest
The Endocrine Society’s conflict of interest (COI) policy and procedures for the development of clinical practice guidelines can be found on the Endocrine Society Web site. In summary, the rules are as follows:
To be considered for membership of a Writing Committee, nominees are required to disclose all relationships with industry for the 12-month period prior to guideline Writing Committee initiation. This is consistent with the reporting timeframe for the National Institutes of Health and the Food and Drug Administration.
Potential COIs that should be declared include all relationships with commercial, noncommercial, institutional, and patient/public organizations that are (or may be) pertinent to the scope of the guideline.
The Chair of the Clinical Guidelines Subcommittee reviews all disclosed relationships and determines whether they are relevant to the topic of the guideline and present a potentially relevant COI.
The Chair of the Clinical Guidelines Subcommittee selects Writing Committee Chairs and Co-Chairs based on COI information, as well as the individuals’ clinical expertise and other skills. The Endocrine Society Council reviews and endorses the nominees or makes appropriate changes. The three Chairs then select and appoint Writing Committee members.
The Chair and Co-Chair of the Writing Committee must be free of any COI or other biases that could undermine the integrity or credibility of the work.
A majority (≥50%) of the Writing Committee members must be free of relevant COIs.
Writing Committee members with relevant COIs are required to declare the situation and recuse themselves from any relevant discussions, votes, and from drafting recommendations.
All Writing Committee members must refrain from adding new relevant industry relationships throughout the guideline development process.
If a member is aware of another person who might have a conflict and has not declared it for some reason, they are obliged to bring this to the Writing Committee Chair’s attention.
Staff, Writing Committee Chairs, and members must be alert for situations that might present a potential or perceived COI.
Footnotes
Cosponsoring Association: European Society of Endocrinology.
Appendix
Relevant COIs of the Osteoporosis Guideline Writing Committee
| Writing Committee Member . | Employment . | Uncompensated Memberships . | Uncompensated Leadership . | Personal Financial . | Organizational Financial . | Spousal/ Family Info. . |
|---|---|---|---|---|---|---|
| Clifford Rosen, Chair | Senior Scientist, Director, Maine Medical Center Research Institute | None | None | None | None | None |
| Dennis Black | Professor in Residence, Division of Clinical Trials and Multicenter Studies, University of California San Francisco | None | None | • Asahi-Kasei, consultant • EffRx, consultant • Merck, CME presentations • Zuellig, speaker | None | None |
| Angela Cheung | Director, Osteoporosis Program, University of Toronto Professor of Medicine, University of Toronto Director of Osteoporosis Program, University Health Network/Sinai Health System | • International Osteoporosis Foundation Committee of Scientific Advisors, member • International Society for Clinical Densitometry Position Development Conference Monitoring Task Force member • Osteoporosis Canada, Guidelines Committee member • 2019 Osteoporosis Canada Clinical Practice Guidelines Fracture Risk Assessment Task Force member | • International Society for Clinical Densitometry Canadian, Panel Chair • International Society for Clinical Densitometry, Board Member • Quantitative Musculoskeletal Imaging, 2019 Meeting Co-Chair | • Amgen, consultant and speaker for accredited CME programs • Eli Lilly, consultant and speaker for accredited CME programs | • Amgen, educational grant awardee | None |
| Richard Eastell | Professor and Head of the Academic Unit of Bone Metabolism, University of Sheffield–Sheffield Teaching Hospitals NHS Trust Director of the Mellanby Centre for Bone Research | • International Osteoporosis Foundation Committee of Scientific Advisors, member • National Institute for Health Research, Senior Investigator Emeritus | • European Calcified Tissue Society, Program Co-Chair • American Society for Bone and Mineral Research, grant review • IBMS, Treasurer • Endocrine Reviews, IAB • Lancet Diabetes and Endocrinology, IAB | • Immunodiagnostic Systems, Nittobo, and Roche Diagnostics, consultant • D-Star, consultant • GlaxoSmithKline Nutrition, consultant • Foundation for the National Institutes of Health, consultant • Bone, Senior Editor • Sandoz, consultant | • Amgen, grant awardee • Alexion, grant awardee • Immunodiagnostic Systems, Nittobo, and Roche Diagnostics, grant awardee • D-Star, consultant • GlaxoSmithKline Nutrition, consultant • Medical Research Council, grant awardee • National Osteoporosis Society, grant awardee • Arthritis Research UK, grant awardee | None |
| M. Hassan Murad | Professor of Medicine, Mayo Clinic | None | None | None | None | None |
| Dolores Shoback | Professor, University of California San Francisco | None | None | None | None | None |
| Writing Committee Member . | Employment . | Uncompensated Memberships . | Uncompensated Leadership . | Personal Financial . | Organizational Financial . | Spousal/ Family Info. . |
|---|---|---|---|---|---|---|
| Clifford Rosen, Chair | Senior Scientist, Director, Maine Medical Center Research Institute | None | None | None | None | None |
| Dennis Black | Professor in Residence, Division of Clinical Trials and Multicenter Studies, University of California San Francisco | None | None | • Asahi-Kasei, consultant • EffRx, consultant • Merck, CME presentations • Zuellig, speaker | None | None |
| Angela Cheung | Director, Osteoporosis Program, University of Toronto Professor of Medicine, University of Toronto Director of Osteoporosis Program, University Health Network/Sinai Health System | • International Osteoporosis Foundation Committee of Scientific Advisors, member • International Society for Clinical Densitometry Position Development Conference Monitoring Task Force member • Osteoporosis Canada, Guidelines Committee member • 2019 Osteoporosis Canada Clinical Practice Guidelines Fracture Risk Assessment Task Force member | • International Society for Clinical Densitometry Canadian, Panel Chair • International Society for Clinical Densitometry, Board Member • Quantitative Musculoskeletal Imaging, 2019 Meeting Co-Chair | • Amgen, consultant and speaker for accredited CME programs • Eli Lilly, consultant and speaker for accredited CME programs | • Amgen, educational grant awardee | None |
| Richard Eastell | Professor and Head of the Academic Unit of Bone Metabolism, University of Sheffield–Sheffield Teaching Hospitals NHS Trust Director of the Mellanby Centre for Bone Research | • International Osteoporosis Foundation Committee of Scientific Advisors, member • National Institute for Health Research, Senior Investigator Emeritus | • European Calcified Tissue Society, Program Co-Chair • American Society for Bone and Mineral Research, grant review • IBMS, Treasurer • Endocrine Reviews, IAB • Lancet Diabetes and Endocrinology, IAB | • Immunodiagnostic Systems, Nittobo, and Roche Diagnostics, consultant • D-Star, consultant • GlaxoSmithKline Nutrition, consultant • Foundation for the National Institutes of Health, consultant • Bone, Senior Editor • Sandoz, consultant | • Amgen, grant awardee • Alexion, grant awardee • Immunodiagnostic Systems, Nittobo, and Roche Diagnostics, grant awardee • D-Star, consultant • GlaxoSmithKline Nutrition, consultant • Medical Research Council, grant awardee • National Osteoporosis Society, grant awardee • Arthritis Research UK, grant awardee | None |
| M. Hassan Murad | Professor of Medicine, Mayo Clinic | None | None | None | None | None |
| Dolores Shoback | Professor, University of California San Francisco | None | None | None | None | None |
Relevant COIs of the Osteoporosis Guideline Writing Committee
| Writing Committee Member . | Employment . | Uncompensated Memberships . | Uncompensated Leadership . | Personal Financial . | Organizational Financial . | Spousal/ Family Info. . |
|---|---|---|---|---|---|---|
| Clifford Rosen, Chair | Senior Scientist, Director, Maine Medical Center Research Institute | None | None | None | None | None |
| Dennis Black | Professor in Residence, Division of Clinical Trials and Multicenter Studies, University of California San Francisco | None | None | • Asahi-Kasei, consultant • EffRx, consultant • Merck, CME presentations • Zuellig, speaker | None | None |
| Angela Cheung | Director, Osteoporosis Program, University of Toronto Professor of Medicine, University of Toronto Director of Osteoporosis Program, University Health Network/Sinai Health System | • International Osteoporosis Foundation Committee of Scientific Advisors, member • International Society for Clinical Densitometry Position Development Conference Monitoring Task Force member • Osteoporosis Canada, Guidelines Committee member • 2019 Osteoporosis Canada Clinical Practice Guidelines Fracture Risk Assessment Task Force member | • International Society for Clinical Densitometry Canadian, Panel Chair • International Society for Clinical Densitometry, Board Member • Quantitative Musculoskeletal Imaging, 2019 Meeting Co-Chair | • Amgen, consultant and speaker for accredited CME programs • Eli Lilly, consultant and speaker for accredited CME programs | • Amgen, educational grant awardee | None |
| Richard Eastell | Professor and Head of the Academic Unit of Bone Metabolism, University of Sheffield–Sheffield Teaching Hospitals NHS Trust Director of the Mellanby Centre for Bone Research | • International Osteoporosis Foundation Committee of Scientific Advisors, member • National Institute for Health Research, Senior Investigator Emeritus | • European Calcified Tissue Society, Program Co-Chair • American Society for Bone and Mineral Research, grant review • IBMS, Treasurer • Endocrine Reviews, IAB • Lancet Diabetes and Endocrinology, IAB | • Immunodiagnostic Systems, Nittobo, and Roche Diagnostics, consultant • D-Star, consultant • GlaxoSmithKline Nutrition, consultant • Foundation for the National Institutes of Health, consultant • Bone, Senior Editor • Sandoz, consultant | • Amgen, grant awardee • Alexion, grant awardee • Immunodiagnostic Systems, Nittobo, and Roche Diagnostics, grant awardee • D-Star, consultant • GlaxoSmithKline Nutrition, consultant • Medical Research Council, grant awardee • National Osteoporosis Society, grant awardee • Arthritis Research UK, grant awardee | None |
| M. Hassan Murad | Professor of Medicine, Mayo Clinic | None | None | None | None | None |
| Dolores Shoback | Professor, University of California San Francisco | None | None | None | None | None |
| Writing Committee Member . | Employment . | Uncompensated Memberships . | Uncompensated Leadership . | Personal Financial . | Organizational Financial . | Spousal/ Family Info. . |
|---|---|---|---|---|---|---|
| Clifford Rosen, Chair | Senior Scientist, Director, Maine Medical Center Research Institute | None | None | None | None | None |
| Dennis Black | Professor in Residence, Division of Clinical Trials and Multicenter Studies, University of California San Francisco | None | None | • Asahi-Kasei, consultant • EffRx, consultant • Merck, CME presentations • Zuellig, speaker | None | None |
| Angela Cheung | Director, Osteoporosis Program, University of Toronto Professor of Medicine, University of Toronto Director of Osteoporosis Program, University Health Network/Sinai Health System | • International Osteoporosis Foundation Committee of Scientific Advisors, member • International Society for Clinical Densitometry Position Development Conference Monitoring Task Force member • Osteoporosis Canada, Guidelines Committee member • 2019 Osteoporosis Canada Clinical Practice Guidelines Fracture Risk Assessment Task Force member | • International Society for Clinical Densitometry Canadian, Panel Chair • International Society for Clinical Densitometry, Board Member • Quantitative Musculoskeletal Imaging, 2019 Meeting Co-Chair | • Amgen, consultant and speaker for accredited CME programs • Eli Lilly, consultant and speaker for accredited CME programs | • Amgen, educational grant awardee | None |
| Richard Eastell | Professor and Head of the Academic Unit of Bone Metabolism, University of Sheffield–Sheffield Teaching Hospitals NHS Trust Director of the Mellanby Centre for Bone Research | • International Osteoporosis Foundation Committee of Scientific Advisors, member • National Institute for Health Research, Senior Investigator Emeritus | • European Calcified Tissue Society, Program Co-Chair • American Society for Bone and Mineral Research, grant review • IBMS, Treasurer • Endocrine Reviews, IAB • Lancet Diabetes and Endocrinology, IAB | • Immunodiagnostic Systems, Nittobo, and Roche Diagnostics, consultant • D-Star, consultant • GlaxoSmithKline Nutrition, consultant • Foundation for the National Institutes of Health, consultant • Bone, Senior Editor • Sandoz, consultant | • Amgen, grant awardee • Alexion, grant awardee • Immunodiagnostic Systems, Nittobo, and Roche Diagnostics, grant awardee • D-Star, consultant • GlaxoSmithKline Nutrition, consultant • Medical Research Council, grant awardee • National Osteoporosis Society, grant awardee • Arthritis Research UK, grant awardee | None |
| M. Hassan Murad | Professor of Medicine, Mayo Clinic | None | None | None | None | None |
| Dolores Shoback | Professor, University of California San Francisco | None | None | None | None | None |
Abbreviations:
- ACP
American College of Physicians
- AFF
atypical femoral fracture
- ASBMR
American Society for Bone and Mineral Research
- BMD
bone mineral density
- BTM
bone turnover marker
- CKD
chronic kidney disease
- CTX
C-terminal crosslinking telopeptide
- DVT
deep venous thrombosis
- eGFR
estimated glomerular filtration rate
- FLEX
Fracture Intervention Trial Long-term Extension
- FRAX
Fracture Risk Assessment Tool
- FREEDOM
Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months
- HR
hazard ratio
- HT
hormone therapy
- ONJ
osteonecrosis of the jaw
- P1NP
procollagen type 1 N-terminal propeptide
- RCT
randomized control trial
- SmPC
summary of product characteristics
Acknowledgments
Financial Support: This guideline was supported by the Endocrine Society. No other entity provided financial support.
Disclosure Summary: See Appendix.
References



![Relative risks of vertebral, hip, and nonvertebral fractures (and 95% CIs) in response to the treatments for postmenopausal osteoporosis, calculated directly and compared with placebo. Note that the evidence is based on a direct meta-analysis of 107 trials of drugs in postmenopausal women with primary osteoporosis in which the trial duration lasted for 3 to 120 mo. In this analysis, each agent was compared with placebo and so direct comparisons should not be made between treatments based on this figure. (12). [Adapted with permission from data presented in Moreno PB, Kapoor E, Asi N, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab. 2019;104(5):1623-1630].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/104/5/10.1210_jc.2019-00221/2/m_jcem_104_5_1595_f1.jpeg?Expires=1716563918&Signature=uabTTQLRcyLInfL1cj~EreHquy9ZIpPWaz7IgB4NDoCCVbO-kCYsZMpQpdg7s0LyRG0G3mjvpetNTdJroZsUR89FX36fErJD5guNizNB52uB~XCpKvDMAeJ~jXysWBtKnGmlpqZr2O3W9AWjTXNHM4jUMcoRox590Uwx~w4a4AGmOVo3qT9G1evP~tL9Pt4lOT8OiVjjzeMHE8GsdNeONL858B0wzebKQDkJcwhh02Y9BimH9YgZCk6DNre5JYbpuX6fF~kkijlBBbP1MsVtwChmqP0YX5~91Emx2R01ttaUsqh5~HFulRLKlvBBNAlKT3u6jkJibVSEUDdgq80K~Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)