-
PDF
- Split View
-
Views
-
Cite
Cite
Chien-Yi Hsu, Yung-Tai Chen, Yu-Wen Su, Chun-Chin Chang, Po-Hsun Huang, Shing-Jong Lin, Statin Therapy Reduces Future Risk of Lower-Limb Amputation in Patients With Diabetes and Peripheral Artery Disease, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 7, 1 July 2017, Pages 2373–2381, https://doi.org/10.1210/jc.2016-3717
Close - Share Icon Share
Abstract
Although there is evidence to support the beneficial effects of statins on major cardiovascular events, few studies address the protective effect of statins on limb outcome.
To investigate whether the use of statin is associated with a risk reduction in lower-extremity amputation in type 2 diabetes mellitus (DM) patients with peripheral arterial disease (PAD).
Observational cohort study.
A nationwide DM database in Taiwan from 2000 to 2011.
A total of 69,332 patients aged ≥20 years with DM and PAD were identified.
Patients were divided into three groups: 11,409 patients were statin users, 4430 patients used nonstatin lipid-lowering agents, and 53,493 patients were nonusers.
The primary outcome was lower-extremity amputation. Secondary outcomes were in-hospital cardiovascular death and all-cause mortality.
Compared with nonusers, statin users were associated with lower risks of lower-extremity amputation [adjusted hazard ration (aHR), 0.75; 95% confidence interval (CI), 0.62 to 0.90], in-hospital cardiovascular death (aHR, 0.78; 95% CI, 0.69 to 0.87), and all-cause mortality (aHR, 0.73; 95% CI, 0.69 to 0.77). In the propensity score matching analysis, the effect of statin on the risk of lower-extremity amputation was consistent. Only statin users were associated with the risk reduction of lower-extremities amputation (HR, 0.77; 95% CI, 0.61 to 0.97) and cardiovascular death (HR, 0.78; 95% CI, 0.68 to 0.89) when taking competing risk of death into consideration.
Compared with statin nonusers who were never treated with lipid-lowering drugs, this study found that statin users had a lower risk of lower-extremity amputation and cardiovascular death in patients with DM and PAD.
Diabetes mellitus (DM) is responsible for large health care costs worldwide owing to its frequent microvascular and macrovascular complications (1). It is intimately correlated with development of atherosclerotic diseases (2), including coronary artery disease (CAD), stroke, and peripheral arterial disease (PAD). According to previous epidemiologic studies, patients with DM not only suffer from a higher prevalence of PAD (3), but they also have an increased risk of critical limb ischemia (4), contralateral leg disease (5), and poorer outcome after revascularization (6).
PAD serves as an indicator for the severity of systemic atherosclerosis and is associated with a greater prevalence of cardiovascular and cerebrovascular diseases. When not managed properly, PAD may lead to amputation, resulting in disability, hospitalization, and death. Current guidelines recommend statin treatment of PAD patients (7–10). Although there is evidence supporting its beneficial effects on functional reserve (11–13) and major cardiovascular events (14, 15), few studies address the protective effect of statins in terms of the risk of amputation. Limited amounts of clinical data support the advantage of statins over other lipid-lowering agents on PAD outcome. Moreover, death is a potential competing risk for amputation (16). Because many DM patients with PAD may die before the initial amputation (17), this issue has not been fully clarified. By using a nationwide DM cohort database in Taiwan, we sought to investigate whether the use of statins is associated with a lower-extremity amputation rate in a high-risk population with known PAD as compared with two propensity score-matched cohorts without statin use (including a nonstatin lipid-lowering agents group and a nonuser group) while taking into consideration the competing risk of death.
Methods
Data source
The Taiwan National Health Insurance program is a social insurance program organized by the government of Taiwan. The program was launched in 1995 and provides comprehensive medical care, including outpatient care, emergency department care, hospital care, dental services, medical examinations, laboratory tests, medication prescriptions, and interventional procedures. It is compulsory for all citizens from birth, and therefore it covers nearly all (98.4%) of Taiwan’s population (∼22.96 million citizens were enrolled in 2007). Data for this study were obtained from the National Health Insurance Research Database (NHIRD), which was released for research purposes by Taiwan’s National Health Research Institutes. For the present study, we used the Longitudinal Cohort of Diabetes Patients dataset, which is validated by the National Health Research Institutes for research purposes. This database, which represents most of the population of Taiwan, consists of de-identified secondary data from a random sample of 120,000 patients diagnosed with DM each year since 1999. This database was released for research purposes after encryption and de-identification, wherein patient’s personal information is removed to protect individual privacy. Diseases are classified using the International Classification of Disease, ninth revision, clinical modification diagnosis codes (2001 edition). Numerous high-quality scientific research papers have been published using data from NHIRD (18–20). This study was approved by the institutional review board of Taipei City Hospital (TCHIRB-10404107-W), and written informed consent of patients was waived.
Study cohort
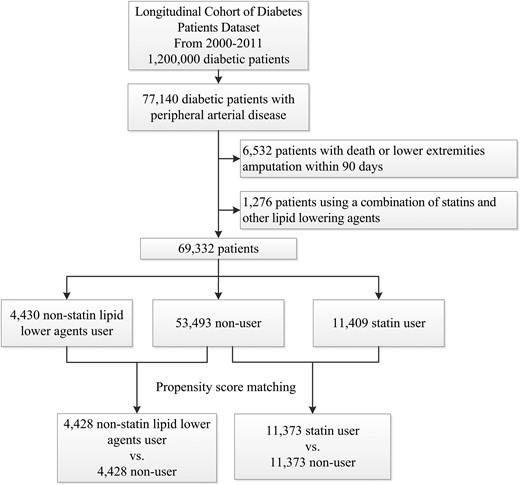
In this nationwide population-based study, we aimed to identify the association between statin use and limb outcome in patients with DM and PAD. We identified all patients aged ≥20 years with DM and PAD for the period from 1 January 2000 to 31 December 2011. Patients were categorized into three groups: statin user group, nonstatin lipid-lowering agents group, and nonuser group. Patients who received treatment with statins or nonstatin lipid-lowering agents within 90 days after PAD diagnosis were allocated to the statin group and the nonstatin lipid-lowering agents group. Other patients who did not receive any lipid-lowering agents were included in the nonuser group. Patients using a combination of statins and other lipid-lowering agents were excluded. The patient selection algorithm is shown in Fig. 1. To avoid immortal time bias, the index date was set at 91 days after PAD diagnosis.
For each subject in the study groups, we extracted data on demographic variables, diagnosis and procedure codes, and drug prescriptions for the period extending from January 1995 to December 2011 and ensured that all individuals had available data for at least 5 years before study inclusion. In the present study, sociodemographic data (including age, sex, and monthly income), index year, urbanization level (four levels, with one1 referring to most urbanized and 4 referring to least urbanized), Charlson Comorbidity Index (CCI) score, and adapted Diabetes Complications Severity Index (aDCSI) scores and other comorbidities known to be associated with vascular disease were analyzed. Concomitant use of other medications (including α-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β-blockers, calcium channel blockers, diuretics, other antihypertensive drugs, antiplatelet agents, steroids, nitrates, nonsteroidal anti-inflammatory drugs, proton pump inhibitors, selective serotonin reuptake inhibitors, and antidiabetic drugs) that could be a confounding factor were also taken into consideration.
For the propensity score–matched study, we calculated propensity scores, which predicted the probability of receiving lipid lower agent conditional on the observed baseline covariates by multivariable logistic regression (see Supplemental Tables 1 and 2). Each patient in the control group was matched to a subject in either the statin user group or nonstatin lipid-lowering agents group with a similar propensity score based on nearest neighbor matching without replacement using a caliper width equal to 0.1 of the standard deviation of the logit of the propensity score. Subjects without matched pairs were excluded in the propensity score–matched analyses.
Lipid-lowering agent exposure
From the Longitudinal Cohort of Diabetes Patients dataset, we extracted information on all lipid-lowering agent prescriptions, including drug name, quantity, dose, and starting and discontinuation dates for the study period. We collected information on prescribed drug types according to the Anatomical Therapeutic Chemical classification system code for statins, including atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin. Nonstatin lipid-lowering agents included fibrates (bezafibrate, ciprofibrate, clofibrate, gemfibrozil, and fenofibrate), nicotinic acid (niacin), bile acid sequestrants (cholestyramine, colesevelam, and colestipol), and cholesterol absorption inhibitors (ezetimibe).
Outcomes
The primary outcome of interest was new lower-extremity amputation (any lower-extremity amputation). Secondary outcomes of interest were in-hospital cardiovascular death and all-cause mortality. To increase the validity of our findings, we also performed sensitivity analysis by using specific surgical procedure code 64022B (amputation above knee) and 64026B (amputated stump revision) to determine the condition of total lower-extremity amputation. All subjects were followed until death or 31 December 2012.
Statistical analysis
Descriptive statistics were used to characterize baseline demographic and clinical variables of the study cohort. Standardized differences were used to check for balance between groups after matching. The incidence rates of interest outcome in the groups were calculated using the Poisson distribution. Cox regression models with a conditional approach and stratification were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk outcomes of each group. Our results are also presented prior to matching using the Cox regression model adjusted for baseline covariates. After propensity score matching, we used the crude results from the propensity-matched cohort without further adjustments. Owing to the high mortality rate in DM patients with PAD, competing-risk regression using Fine and Gray’s model was also performed. Finally, the likelihood ratio test was used to examine the interaction between the occurrence of lower-extremities amputation and the following variables: age, sex, CCI score, hypertension, chronic kidney disease (CKD), heart failure, CAD, use of an antiplatelet agent, and the potency of statins. Subgroup analyses were performed accordingly. SQL Server 2012 (Microsoft, Redmond, WA) was used for data linkage, processing, and sampling. Propensity scores were calculated using SAS version 9.3 (SAS Institute, Cary, NC). All other statistical analyses were conducted using STATA statistical software (version 12.0; StataCorp, College Station, TX). A P value of <0.05 was considered statistically significant.
Results
Characteristics of the study population
A total of 69,332 DM patients with newly diagnosed PAD between January 2000 and December 2011 who met the inclusion criteria were identified. Among them, 11,409 patients were statin users, 4430 patients were nonstatin lipid-lowering agent users, and 53,493 patients were nonusers. Overall, the mean age of the PAD cohort was 62.6 ± 13.0 years. Sex was almost equally distributed (male 49%). The mean aDCSI was 3.3 (standard deviation, 2.2). Hypertension, present in 73.6% of PAD patients, was the most common comorbidity in the study cohort. The prevalence of other comorbid conditions was as follows: CAD, 44.9%; cerebrovascular disease, 30.8%; heart failure, 14.6%; CKD, 19.0%; atrial fibrillation, 3.5%; and cancer, 11.0%. The statin user group exhibited more comorbidities with hypertension, CAD, and cerebrovascular disease than did the nonuser group. More patients with statin therapy were also taking antidiabetic drugs (including insulin, metformin, sulfonylurea, thiazolidinedione, meglitinides, dipeptidyl peptidase-4 inhibitor, and acarbose), antiplatelet drugs, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, calcium channel blockers, diuretics, and nitrate as compared with patients in the nonuser group. The characteristics of the study population are shown in Table 1.
Baseline Characteristics of Patients With Diabetes and PAD
| Characteristics . | Statin User . | Other Lipid-Lowering Agent User . | Nonuser . | P Value . |
|---|---|---|---|---|
| Patients, no. | 11,409 | 4430 | 53,493 | |
| Mean age (standard deviation), y | 62.2 (11.5) | 57.9 (12.0) | 63.0 (13.3) | <0.001 |
| Male | 5019 (44.0) | 2402 (54.2) | 26,731 (50.0) | <0.001 |
| Outpatient visits related to metabolism and endocrinology in the last year | <0.001 | |||
| 0 visits | 9563 (83.8) | 3962 (89.4) | 47,253 (88.3) | |
| 1−5 visits | 1146 (10.0) | 323 (7.3) | 4387 (8.2) | |
| 6−10 visits | 451 (4.0) | 96 (2.2) | 1192 (2.2) | |
| >10 visits | 249 (2.2) | 49 (1.1) | 661 (1.2) | |
| Outpatient visits to cardiologist in the last year | <0.001 | |||
| 0 visits | 7470 (65.5) | 3340 (75.4) | 38,941 (72.8) | |
| 1−5 visits | 2254 (19.8) | 684 (15.4) | 9535 (17.8) | |
| 6−10 visits | 1044 (9.2) | 232 (5.2) | 2964 (5.5) | |
| >10 visits | 641 (5.6) | 174 (3.9) | 2053 (3.8) | |
| Duration of DM, mo (IQR) | 22 (3–58) | 14 (3–47) | 12 (3–45) | <0.001 |
| aDCSI scorea | 3.5 (2.2) | 2.9 (2.0) | 3.3 (2.2) | <0.001 |
| Comorbidities | ||||
| Hypertension | 9138 (80.1) | 3331 (75.2) | 38,532 (72.0) | <0.001 |
| CAD | 5761 (50.5) | 1912 (43.2) | 23,430 (43.8) | <0.001 |
| Cerebrovascular disease | 3753 (32.9) | 1106 (25.0) | 16,477 (30.8) | <0.001 |
| Heart failure | 1664 (14.6) | 468 (10.6) | 7986 (14.9) | <0.001 |
| CKD | 2172 (19.0) | 773 (17.4) | 10,204 (19.1) | 0.029 |
| Liver disease | 4700 (41.2) | 2045 (46.2) | 21,713 (40.6) | <0.001 |
| Atrial fibrillation | 336 (2.9) | 78 (1.8) | 2006 (3.8) | <0.001 |
| Valvular heart disease | 1244 (10.9) | 330 (7.4) | 5329 (10.0) | <0.001 |
| Cancer | 1134 (9.9) | 374 (8.4) | 6111 (11.4) | <0.001 |
| Peptic ulcer disease | 6075 (53.2) | 2230 (50.3) | 28,071 (52.5) | 0.004 |
| Physically limiting conditions | 1466 (12.8) | 449 (10.1) | 6613 (12.4) | <0.001 |
| Autoimmune disease | 577 (5.1) | 155 (3.5) | 2466 (4.6) | <0.001 |
| Antidiabetic drug | ||||
| Insulin | 297 (2.6) | 76 (1.7) | 1058 (2.0) | <0.001 |
| Metformin | 4732 (41.5) | 1747 (39.4) | 14,895 (27.8) | <0.001 |
| Sulfonylurea | 4208 (36.9) | 1594 (36.0) | 13,942 (26.1) | <0.001 |
| Thiazolidinedione | 929 (8.1) | 229 (5.2) | 1733 (3.2) | <0.001 |
| Meglitinide | 483 (4.2) | 156 (3.5) | 1583 (3.0) | <0.001 |
| DPP-4 inhibitor | 165 (1.4) | 44 (1.0) | 267 (0.5) | <0.001 |
| Acarbose | 773 (6.8) | 288 (6.5) | 1962 (3.7) | <0.001 |
| CCI scoreb | 4.8 (2.4) | 4.4 (2.3) | 4.8 (2.6) | <0.001 |
| Antihypertensive drug | ||||
| α-Blocker | 404 (3.5) | 126 (2.8) | 1752 (3.3) | 0.080 |
| ACE inhibitor or ARB | 4021 (35.2) | 1165 (26.3) | 11,596 (21.7) | <0.001 |
| β-Blocker | 2193 (19.2) | 905 (20.4) | 7863 (14.7) | <0.001 |
| Calcium channel blocker | 3845 (33.7) | 1297 (29.3) | 14,039 (26.2) | <0.001 |
| Diuretics | 1806 (15.8) | 575 (13.0) | 6984 (13.1) | <0.001 |
| Other antihypertensive drug | 186 (1.6) | 134 (3.0) | 1079 (2.0) | <0.001 |
| Other concomitant medications | ||||
| Antiplatelet agentc | 3889 (34.1) | 1027 (23.2) | 11,169 (20.9) | <0.001 |
| Steroid | 804 (7.0) | 278 (6.3) | 4105 (7.7) | <0.001 |
| Nitrate | 723 (6.3) | 166 (3.7) | 2360 (4.4) | <0.001 |
| NSAID | 2824 (24.8) | 1082 (24.4) | 12,726 (23.8) | 0.072 |
| PPI | 292 (2.6) | 62 (1.4) | 1429 (2.7) | <0.001 |
| SSRI | 241 (2.1) | 56 (1.3) | 850 (1.6) | <0.001 |
| Characteristics . | Statin User . | Other Lipid-Lowering Agent User . | Nonuser . | P Value . |
|---|---|---|---|---|
| Patients, no. | 11,409 | 4430 | 53,493 | |
| Mean age (standard deviation), y | 62.2 (11.5) | 57.9 (12.0) | 63.0 (13.3) | <0.001 |
| Male | 5019 (44.0) | 2402 (54.2) | 26,731 (50.0) | <0.001 |
| Outpatient visits related to metabolism and endocrinology in the last year | <0.001 | |||
| 0 visits | 9563 (83.8) | 3962 (89.4) | 47,253 (88.3) | |
| 1−5 visits | 1146 (10.0) | 323 (7.3) | 4387 (8.2) | |
| 6−10 visits | 451 (4.0) | 96 (2.2) | 1192 (2.2) | |
| >10 visits | 249 (2.2) | 49 (1.1) | 661 (1.2) | |
| Outpatient visits to cardiologist in the last year | <0.001 | |||
| 0 visits | 7470 (65.5) | 3340 (75.4) | 38,941 (72.8) | |
| 1−5 visits | 2254 (19.8) | 684 (15.4) | 9535 (17.8) | |
| 6−10 visits | 1044 (9.2) | 232 (5.2) | 2964 (5.5) | |
| >10 visits | 641 (5.6) | 174 (3.9) | 2053 (3.8) | |
| Duration of DM, mo (IQR) | 22 (3–58) | 14 (3–47) | 12 (3–45) | <0.001 |
| aDCSI scorea | 3.5 (2.2) | 2.9 (2.0) | 3.3 (2.2) | <0.001 |
| Comorbidities | ||||
| Hypertension | 9138 (80.1) | 3331 (75.2) | 38,532 (72.0) | <0.001 |
| CAD | 5761 (50.5) | 1912 (43.2) | 23,430 (43.8) | <0.001 |
| Cerebrovascular disease | 3753 (32.9) | 1106 (25.0) | 16,477 (30.8) | <0.001 |
| Heart failure | 1664 (14.6) | 468 (10.6) | 7986 (14.9) | <0.001 |
| CKD | 2172 (19.0) | 773 (17.4) | 10,204 (19.1) | 0.029 |
| Liver disease | 4700 (41.2) | 2045 (46.2) | 21,713 (40.6) | <0.001 |
| Atrial fibrillation | 336 (2.9) | 78 (1.8) | 2006 (3.8) | <0.001 |
| Valvular heart disease | 1244 (10.9) | 330 (7.4) | 5329 (10.0) | <0.001 |
| Cancer | 1134 (9.9) | 374 (8.4) | 6111 (11.4) | <0.001 |
| Peptic ulcer disease | 6075 (53.2) | 2230 (50.3) | 28,071 (52.5) | 0.004 |
| Physically limiting conditions | 1466 (12.8) | 449 (10.1) | 6613 (12.4) | <0.001 |
| Autoimmune disease | 577 (5.1) | 155 (3.5) | 2466 (4.6) | <0.001 |
| Antidiabetic drug | ||||
| Insulin | 297 (2.6) | 76 (1.7) | 1058 (2.0) | <0.001 |
| Metformin | 4732 (41.5) | 1747 (39.4) | 14,895 (27.8) | <0.001 |
| Sulfonylurea | 4208 (36.9) | 1594 (36.0) | 13,942 (26.1) | <0.001 |
| Thiazolidinedione | 929 (8.1) | 229 (5.2) | 1733 (3.2) | <0.001 |
| Meglitinide | 483 (4.2) | 156 (3.5) | 1583 (3.0) | <0.001 |
| DPP-4 inhibitor | 165 (1.4) | 44 (1.0) | 267 (0.5) | <0.001 |
| Acarbose | 773 (6.8) | 288 (6.5) | 1962 (3.7) | <0.001 |
| CCI scoreb | 4.8 (2.4) | 4.4 (2.3) | 4.8 (2.6) | <0.001 |
| Antihypertensive drug | ||||
| α-Blocker | 404 (3.5) | 126 (2.8) | 1752 (3.3) | 0.080 |
| ACE inhibitor or ARB | 4021 (35.2) | 1165 (26.3) | 11,596 (21.7) | <0.001 |
| β-Blocker | 2193 (19.2) | 905 (20.4) | 7863 (14.7) | <0.001 |
| Calcium channel blocker | 3845 (33.7) | 1297 (29.3) | 14,039 (26.2) | <0.001 |
| Diuretics | 1806 (15.8) | 575 (13.0) | 6984 (13.1) | <0.001 |
| Other antihypertensive drug | 186 (1.6) | 134 (3.0) | 1079 (2.0) | <0.001 |
| Other concomitant medications | ||||
| Antiplatelet agentc | 3889 (34.1) | 1027 (23.2) | 11,169 (20.9) | <0.001 |
| Steroid | 804 (7.0) | 278 (6.3) | 4105 (7.7) | <0.001 |
| Nitrate | 723 (6.3) | 166 (3.7) | 2360 (4.4) | <0.001 |
| NSAID | 2824 (24.8) | 1082 (24.4) | 12,726 (23.8) | 0.072 |
| PPI | 292 (2.6) | 62 (1.4) | 1429 (2.7) | <0.001 |
| SSRI | 241 (2.1) | 56 (1.3) | 850 (1.6) | <0.001 |
All data are described as number (%) except for mean age and propensity score.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; DPP-4, dipeptidyl peptidase-4; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump inhibitors; SSRI, selective serotonin reuptake inhibitor.
The aDCSI is a 13-point scale from seven complication categories: retinopathy, nephropathy, neuropathy, cerebrovascular disease, cardiovascular disease, peripheral vascular disease, and metabolic disorder. Each complication produced a numeric score ranging from 0 to 2 (0, no abnormality; 1, some abnormality; 2, severe abnormality).
The CCI score is used to determine overall systemic health. With each increased level of CCI score, there are stepwise increases in cumulative mortality.
Including aspirin, clopidogrel, ticlopidine, and cilostazol.
Baseline Characteristics of Patients With Diabetes and PAD
| Characteristics . | Statin User . | Other Lipid-Lowering Agent User . | Nonuser . | P Value . |
|---|---|---|---|---|
| Patients, no. | 11,409 | 4430 | 53,493 | |
| Mean age (standard deviation), y | 62.2 (11.5) | 57.9 (12.0) | 63.0 (13.3) | <0.001 |
| Male | 5019 (44.0) | 2402 (54.2) | 26,731 (50.0) | <0.001 |
| Outpatient visits related to metabolism and endocrinology in the last year | <0.001 | |||
| 0 visits | 9563 (83.8) | 3962 (89.4) | 47,253 (88.3) | |
| 1−5 visits | 1146 (10.0) | 323 (7.3) | 4387 (8.2) | |
| 6−10 visits | 451 (4.0) | 96 (2.2) | 1192 (2.2) | |
| >10 visits | 249 (2.2) | 49 (1.1) | 661 (1.2) | |
| Outpatient visits to cardiologist in the last year | <0.001 | |||
| 0 visits | 7470 (65.5) | 3340 (75.4) | 38,941 (72.8) | |
| 1−5 visits | 2254 (19.8) | 684 (15.4) | 9535 (17.8) | |
| 6−10 visits | 1044 (9.2) | 232 (5.2) | 2964 (5.5) | |
| >10 visits | 641 (5.6) | 174 (3.9) | 2053 (3.8) | |
| Duration of DM, mo (IQR) | 22 (3–58) | 14 (3–47) | 12 (3–45) | <0.001 |
| aDCSI scorea | 3.5 (2.2) | 2.9 (2.0) | 3.3 (2.2) | <0.001 |
| Comorbidities | ||||
| Hypertension | 9138 (80.1) | 3331 (75.2) | 38,532 (72.0) | <0.001 |
| CAD | 5761 (50.5) | 1912 (43.2) | 23,430 (43.8) | <0.001 |
| Cerebrovascular disease | 3753 (32.9) | 1106 (25.0) | 16,477 (30.8) | <0.001 |
| Heart failure | 1664 (14.6) | 468 (10.6) | 7986 (14.9) | <0.001 |
| CKD | 2172 (19.0) | 773 (17.4) | 10,204 (19.1) | 0.029 |
| Liver disease | 4700 (41.2) | 2045 (46.2) | 21,713 (40.6) | <0.001 |
| Atrial fibrillation | 336 (2.9) | 78 (1.8) | 2006 (3.8) | <0.001 |
| Valvular heart disease | 1244 (10.9) | 330 (7.4) | 5329 (10.0) | <0.001 |
| Cancer | 1134 (9.9) | 374 (8.4) | 6111 (11.4) | <0.001 |
| Peptic ulcer disease | 6075 (53.2) | 2230 (50.3) | 28,071 (52.5) | 0.004 |
| Physically limiting conditions | 1466 (12.8) | 449 (10.1) | 6613 (12.4) | <0.001 |
| Autoimmune disease | 577 (5.1) | 155 (3.5) | 2466 (4.6) | <0.001 |
| Antidiabetic drug | ||||
| Insulin | 297 (2.6) | 76 (1.7) | 1058 (2.0) | <0.001 |
| Metformin | 4732 (41.5) | 1747 (39.4) | 14,895 (27.8) | <0.001 |
| Sulfonylurea | 4208 (36.9) | 1594 (36.0) | 13,942 (26.1) | <0.001 |
| Thiazolidinedione | 929 (8.1) | 229 (5.2) | 1733 (3.2) | <0.001 |
| Meglitinide | 483 (4.2) | 156 (3.5) | 1583 (3.0) | <0.001 |
| DPP-4 inhibitor | 165 (1.4) | 44 (1.0) | 267 (0.5) | <0.001 |
| Acarbose | 773 (6.8) | 288 (6.5) | 1962 (3.7) | <0.001 |
| CCI scoreb | 4.8 (2.4) | 4.4 (2.3) | 4.8 (2.6) | <0.001 |
| Antihypertensive drug | ||||
| α-Blocker | 404 (3.5) | 126 (2.8) | 1752 (3.3) | 0.080 |
| ACE inhibitor or ARB | 4021 (35.2) | 1165 (26.3) | 11,596 (21.7) | <0.001 |
| β-Blocker | 2193 (19.2) | 905 (20.4) | 7863 (14.7) | <0.001 |
| Calcium channel blocker | 3845 (33.7) | 1297 (29.3) | 14,039 (26.2) | <0.001 |
| Diuretics | 1806 (15.8) | 575 (13.0) | 6984 (13.1) | <0.001 |
| Other antihypertensive drug | 186 (1.6) | 134 (3.0) | 1079 (2.0) | <0.001 |
| Other concomitant medications | ||||
| Antiplatelet agentc | 3889 (34.1) | 1027 (23.2) | 11,169 (20.9) | <0.001 |
| Steroid | 804 (7.0) | 278 (6.3) | 4105 (7.7) | <0.001 |
| Nitrate | 723 (6.3) | 166 (3.7) | 2360 (4.4) | <0.001 |
| NSAID | 2824 (24.8) | 1082 (24.4) | 12,726 (23.8) | 0.072 |
| PPI | 292 (2.6) | 62 (1.4) | 1429 (2.7) | <0.001 |
| SSRI | 241 (2.1) | 56 (1.3) | 850 (1.6) | <0.001 |
| Characteristics . | Statin User . | Other Lipid-Lowering Agent User . | Nonuser . | P Value . |
|---|---|---|---|---|
| Patients, no. | 11,409 | 4430 | 53,493 | |
| Mean age (standard deviation), y | 62.2 (11.5) | 57.9 (12.0) | 63.0 (13.3) | <0.001 |
| Male | 5019 (44.0) | 2402 (54.2) | 26,731 (50.0) | <0.001 |
| Outpatient visits related to metabolism and endocrinology in the last year | <0.001 | |||
| 0 visits | 9563 (83.8) | 3962 (89.4) | 47,253 (88.3) | |
| 1−5 visits | 1146 (10.0) | 323 (7.3) | 4387 (8.2) | |
| 6−10 visits | 451 (4.0) | 96 (2.2) | 1192 (2.2) | |
| >10 visits | 249 (2.2) | 49 (1.1) | 661 (1.2) | |
| Outpatient visits to cardiologist in the last year | <0.001 | |||
| 0 visits | 7470 (65.5) | 3340 (75.4) | 38,941 (72.8) | |
| 1−5 visits | 2254 (19.8) | 684 (15.4) | 9535 (17.8) | |
| 6−10 visits | 1044 (9.2) | 232 (5.2) | 2964 (5.5) | |
| >10 visits | 641 (5.6) | 174 (3.9) | 2053 (3.8) | |
| Duration of DM, mo (IQR) | 22 (3–58) | 14 (3–47) | 12 (3–45) | <0.001 |
| aDCSI scorea | 3.5 (2.2) | 2.9 (2.0) | 3.3 (2.2) | <0.001 |
| Comorbidities | ||||
| Hypertension | 9138 (80.1) | 3331 (75.2) | 38,532 (72.0) | <0.001 |
| CAD | 5761 (50.5) | 1912 (43.2) | 23,430 (43.8) | <0.001 |
| Cerebrovascular disease | 3753 (32.9) | 1106 (25.0) | 16,477 (30.8) | <0.001 |
| Heart failure | 1664 (14.6) | 468 (10.6) | 7986 (14.9) | <0.001 |
| CKD | 2172 (19.0) | 773 (17.4) | 10,204 (19.1) | 0.029 |
| Liver disease | 4700 (41.2) | 2045 (46.2) | 21,713 (40.6) | <0.001 |
| Atrial fibrillation | 336 (2.9) | 78 (1.8) | 2006 (3.8) | <0.001 |
| Valvular heart disease | 1244 (10.9) | 330 (7.4) | 5329 (10.0) | <0.001 |
| Cancer | 1134 (9.9) | 374 (8.4) | 6111 (11.4) | <0.001 |
| Peptic ulcer disease | 6075 (53.2) | 2230 (50.3) | 28,071 (52.5) | 0.004 |
| Physically limiting conditions | 1466 (12.8) | 449 (10.1) | 6613 (12.4) | <0.001 |
| Autoimmune disease | 577 (5.1) | 155 (3.5) | 2466 (4.6) | <0.001 |
| Antidiabetic drug | ||||
| Insulin | 297 (2.6) | 76 (1.7) | 1058 (2.0) | <0.001 |
| Metformin | 4732 (41.5) | 1747 (39.4) | 14,895 (27.8) | <0.001 |
| Sulfonylurea | 4208 (36.9) | 1594 (36.0) | 13,942 (26.1) | <0.001 |
| Thiazolidinedione | 929 (8.1) | 229 (5.2) | 1733 (3.2) | <0.001 |
| Meglitinide | 483 (4.2) | 156 (3.5) | 1583 (3.0) | <0.001 |
| DPP-4 inhibitor | 165 (1.4) | 44 (1.0) | 267 (0.5) | <0.001 |
| Acarbose | 773 (6.8) | 288 (6.5) | 1962 (3.7) | <0.001 |
| CCI scoreb | 4.8 (2.4) | 4.4 (2.3) | 4.8 (2.6) | <0.001 |
| Antihypertensive drug | ||||
| α-Blocker | 404 (3.5) | 126 (2.8) | 1752 (3.3) | 0.080 |
| ACE inhibitor or ARB | 4021 (35.2) | 1165 (26.3) | 11,596 (21.7) | <0.001 |
| β-Blocker | 2193 (19.2) | 905 (20.4) | 7863 (14.7) | <0.001 |
| Calcium channel blocker | 3845 (33.7) | 1297 (29.3) | 14,039 (26.2) | <0.001 |
| Diuretics | 1806 (15.8) | 575 (13.0) | 6984 (13.1) | <0.001 |
| Other antihypertensive drug | 186 (1.6) | 134 (3.0) | 1079 (2.0) | <0.001 |
| Other concomitant medications | ||||
| Antiplatelet agentc | 3889 (34.1) | 1027 (23.2) | 11,169 (20.9) | <0.001 |
| Steroid | 804 (7.0) | 278 (6.3) | 4105 (7.7) | <0.001 |
| Nitrate | 723 (6.3) | 166 (3.7) | 2360 (4.4) | <0.001 |
| NSAID | 2824 (24.8) | 1082 (24.4) | 12,726 (23.8) | 0.072 |
| PPI | 292 (2.6) | 62 (1.4) | 1429 (2.7) | <0.001 |
| SSRI | 241 (2.1) | 56 (1.3) | 850 (1.6) | <0.001 |
All data are described as number (%) except for mean age and propensity score.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; DPP-4, dipeptidyl peptidase-4; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump inhibitors; SSRI, selective serotonin reuptake inhibitor.
The aDCSI is a 13-point scale from seven complication categories: retinopathy, nephropathy, neuropathy, cerebrovascular disease, cardiovascular disease, peripheral vascular disease, and metabolic disorder. Each complication produced a numeric score ranging from 0 to 2 (0, no abnormality; 1, some abnormality; 2, severe abnormality).
The CCI score is used to determine overall systemic health. With each increased level of CCI score, there are stepwise increases in cumulative mortality.
Including aspirin, clopidogrel, ticlopidine, and cilostazol.
After propensity score matching, 11,373 statin users were matched to 11,373 nonusers, and 4428 nonstatin lipid-lowering agent users were matched to 4428 nonusers (patient selection algorithm is shown in Fig. 1). As shown in Supplemental Tables 3 and 4, the baseline characteristics of both cohorts (including socioeconomic status, relevant comorbidities, and medication) did not differ significantly between the two groups after 1:1 matching.
Long-term risks of lower-extremities amputation, in-hospital cardiovascular death, and all-cause mortality
During the mean 5.7 years of the follow-up period, the incidence rates of any lower-extremity amputation, total lower-extremity amputation, in-hospital cardiovascular death, and all-cause mortality in the statin user cohort were lower than in the nonuser cohort (2.29 vs 3.57, 6.24 vs 8.72, and 24.38 vs 38.63 per 1000 person-years, respectively). Statin users were associated with a significantly lower risk of subsequent lower-extremity amputation events [adjusted HR (aHR), 0.75; 95% CI, 0.62 to 0.90) compared with nonusers after adjustment for various relevant factors. Regarding the risk of total lower-extremity amputation, statin users were associated with an even more risk reduction (aHR, 0.58; 95% CI, 0.36 to 0.93) compared with nonusers. When we calculated death as a competing risk, the risks of any lower-extremity amputation remained significantly decreased (but less marked) in the statin user cohort. However, the risk reduction of total lower-extremity amputation among statin users was attenuated when taking competing risk of death into consideration (Table 2).
Incidence and Risk of Lower-Extremity Amputation and Mortality Among Patients With Diabetes and PAD Before Propensity Score Matching
| . | . | Crude . | Model 1b . | Model 2c . | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events . | Person-Years . | Incidence Ratea . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Any lower-extremity amputation | |||||||||
| Nonuser | 1092 | 305,618 | 3.57 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 60 | 26,177 | 2.29 | 0.64 (0.50–0.84) | 0.001 | 0.95 (0.73–1.23) | 0.696 | 0.97 (0.75–1.26) | 0.825 |
| Statin user | 131 | 571,226 | 2.29 | 0.63 (0.53-0.76) | <0.001 | 0.75 (0.62–0.90) | 0.003 | 0.79 (0.65–0.96) | 0.016 |
| Total lower-extremity amputation | |||||||||
| Nonuser | 234 | 308204 | 0.76 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 12 | 26,314 | 0.46 | 0.60 (0.34–1.08) | 0.088 | 1.15 (0.64–2.07) | 0.647 | 1.18 (0.66–2.12) | 0.579 |
| Statin user | 19 | 57,393 | 0.33 | 0.44 (0.27–0.70) | 0.001 | 0.58 (0.36–0.93) | 0.024 | 0.62 (0.38–1.03) | 0.063 |
| In-hospital cardiovascular death | |||||||||
| Nonuser | 2689 | 308,355 | 8.72 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 114 | 26,319 | 4.33 | 0.50 (0.41–0.60) | <0.001 | 0.77 (0.64–0.93) | <0.001 | 0.82 (0.68–0.99) | 0.038 |
| Statin user | 358 | 57,383 | 6.24 | 0.75 (0.67–0.83) | <0.001 | 0.78 (0.69–0.87) | <0.001 | 0.85 (0.76–0.95) | 0.005 |
| All-cause mortality | |||||||||
| Nonuser | 11,923 | 308,616 | 38.63 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 527 | 26,326 | 20.02 | 0.52 (0.48–0.57) | <0.001 | 0.74 (0.68–0.81) | <0.001 | — | |
| Statin user | 1400 | 57,418 | 24.38 | 0.65 (0.61–0.68) | <0.001 | 0.73 (0.69–0.77) | <0.001 | — | |
| . | . | Crude . | Model 1b . | Model 2c . | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events . | Person-Years . | Incidence Ratea . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Any lower-extremity amputation | |||||||||
| Nonuser | 1092 | 305,618 | 3.57 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 60 | 26,177 | 2.29 | 0.64 (0.50–0.84) | 0.001 | 0.95 (0.73–1.23) | 0.696 | 0.97 (0.75–1.26) | 0.825 |
| Statin user | 131 | 571,226 | 2.29 | 0.63 (0.53-0.76) | <0.001 | 0.75 (0.62–0.90) | 0.003 | 0.79 (0.65–0.96) | 0.016 |
| Total lower-extremity amputation | |||||||||
| Nonuser | 234 | 308204 | 0.76 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 12 | 26,314 | 0.46 | 0.60 (0.34–1.08) | 0.088 | 1.15 (0.64–2.07) | 0.647 | 1.18 (0.66–2.12) | 0.579 |
| Statin user | 19 | 57,393 | 0.33 | 0.44 (0.27–0.70) | 0.001 | 0.58 (0.36–0.93) | 0.024 | 0.62 (0.38–1.03) | 0.063 |
| In-hospital cardiovascular death | |||||||||
| Nonuser | 2689 | 308,355 | 8.72 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 114 | 26,319 | 4.33 | 0.50 (0.41–0.60) | <0.001 | 0.77 (0.64–0.93) | <0.001 | 0.82 (0.68–0.99) | 0.038 |
| Statin user | 358 | 57,383 | 6.24 | 0.75 (0.67–0.83) | <0.001 | 0.78 (0.69–0.87) | <0.001 | 0.85 (0.76–0.95) | 0.005 |
| All-cause mortality | |||||||||
| Nonuser | 11,923 | 308,616 | 38.63 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 527 | 26,326 | 20.02 | 0.52 (0.48–0.57) | <0.001 | 0.74 (0.68–0.81) | <0.001 | — | |
| Statin user | 1400 | 57,418 | 24.38 | 0.65 (0.61–0.68) | <0.001 | 0.73 (0.69–0.77) | <0.001 | — | |
Incidence and Risk of Lower-Extremity Amputation and Mortality Among Patients With Diabetes and PAD Before Propensity Score Matching
| . | . | Crude . | Model 1b . | Model 2c . | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events . | Person-Years . | Incidence Ratea . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Any lower-extremity amputation | |||||||||
| Nonuser | 1092 | 305,618 | 3.57 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 60 | 26,177 | 2.29 | 0.64 (0.50–0.84) | 0.001 | 0.95 (0.73–1.23) | 0.696 | 0.97 (0.75–1.26) | 0.825 |
| Statin user | 131 | 571,226 | 2.29 | 0.63 (0.53-0.76) | <0.001 | 0.75 (0.62–0.90) | 0.003 | 0.79 (0.65–0.96) | 0.016 |
| Total lower-extremity amputation | |||||||||
| Nonuser | 234 | 308204 | 0.76 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 12 | 26,314 | 0.46 | 0.60 (0.34–1.08) | 0.088 | 1.15 (0.64–2.07) | 0.647 | 1.18 (0.66–2.12) | 0.579 |
| Statin user | 19 | 57,393 | 0.33 | 0.44 (0.27–0.70) | 0.001 | 0.58 (0.36–0.93) | 0.024 | 0.62 (0.38–1.03) | 0.063 |
| In-hospital cardiovascular death | |||||||||
| Nonuser | 2689 | 308,355 | 8.72 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 114 | 26,319 | 4.33 | 0.50 (0.41–0.60) | <0.001 | 0.77 (0.64–0.93) | <0.001 | 0.82 (0.68–0.99) | 0.038 |
| Statin user | 358 | 57,383 | 6.24 | 0.75 (0.67–0.83) | <0.001 | 0.78 (0.69–0.87) | <0.001 | 0.85 (0.76–0.95) | 0.005 |
| All-cause mortality | |||||||||
| Nonuser | 11,923 | 308,616 | 38.63 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 527 | 26,326 | 20.02 | 0.52 (0.48–0.57) | <0.001 | 0.74 (0.68–0.81) | <0.001 | — | |
| Statin user | 1400 | 57,418 | 24.38 | 0.65 (0.61–0.68) | <0.001 | 0.73 (0.69–0.77) | <0.001 | — | |
| . | . | Crude . | Model 1b . | Model 2c . | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events . | Person-Years . | Incidence Ratea . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Any lower-extremity amputation | |||||||||
| Nonuser | 1092 | 305,618 | 3.57 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 60 | 26,177 | 2.29 | 0.64 (0.50–0.84) | 0.001 | 0.95 (0.73–1.23) | 0.696 | 0.97 (0.75–1.26) | 0.825 |
| Statin user | 131 | 571,226 | 2.29 | 0.63 (0.53-0.76) | <0.001 | 0.75 (0.62–0.90) | 0.003 | 0.79 (0.65–0.96) | 0.016 |
| Total lower-extremity amputation | |||||||||
| Nonuser | 234 | 308204 | 0.76 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 12 | 26,314 | 0.46 | 0.60 (0.34–1.08) | 0.088 | 1.15 (0.64–2.07) | 0.647 | 1.18 (0.66–2.12) | 0.579 |
| Statin user | 19 | 57,393 | 0.33 | 0.44 (0.27–0.70) | 0.001 | 0.58 (0.36–0.93) | 0.024 | 0.62 (0.38–1.03) | 0.063 |
| In-hospital cardiovascular death | |||||||||
| Nonuser | 2689 | 308,355 | 8.72 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 114 | 26,319 | 4.33 | 0.50 (0.41–0.60) | <0.001 | 0.77 (0.64–0.93) | <0.001 | 0.82 (0.68–0.99) | 0.038 |
| Statin user | 358 | 57,383 | 6.24 | 0.75 (0.67–0.83) | <0.001 | 0.78 (0.69–0.87) | <0.001 | 0.85 (0.76–0.95) | 0.005 |
| All-cause mortality | |||||||||
| Nonuser | 11,923 | 308,616 | 38.63 | Reference | Reference | Reference | |||
| Nonstatin lipid-lowering agent user | 527 | 26,326 | 20.02 | 0.52 (0.48–0.57) | <0.001 | 0.74 (0.68–0.81) | <0.001 | — | |
| Statin user | 1400 | 57,418 | 24.38 | 0.65 (0.61–0.68) | <0.001 | 0.73 (0.69–0.77) | <0.001 | — | |
Meanwhile, nonstatin lipid-lowering agent users were not associated with a significant risk reduction of lower-extremity amputation events (aHR, 0.95; 95% CI, 0.73 to 1.23). Both statin users and users of nonstatin lipid-lowering agents were associated with lower risks of in-hospital cardiovascular death and all-cause mortality (Table 2).
In the propensity score–matched analysis (Table 3), statin users had a lower risk of any lower-extremity amputation (HR, 0.75; 95% CI, 0.60 to 0.94), total lower-extremity amputation (HR, 0.48; 95% CI, 0.28 to 0.83), in-hospital cardiovascular death (HR, 0.75; 95%, CI 0.66 to 0.87), and all-cause mortality (HR, 0.72; 95% CI, 0.67 to 0.77) compared with matched nonusers. When death was considered as a competing risk, the risks of any lower-extremity amputation (HR, 0.77; 95% CI, 0.61 to 0.97) and total lower-extremity amputation (HR, 0.50; 95% CI, 0.29 to 0.87) remained significantly decreased in the statin user cohort. However, the nonstatin lipid-lowering agent users had a merely neutral effect on the risk of any lower-extremity amputation (HR, 0.92; 95% CI, 0.64 to 1.31), total lower-extremity amputation (HR, 1.15; 95% CI, 0.50 to 2.65), and in-hospital cardiovascular death (HR, 0.89; 95% CI, 0.69 to 1.15).
Incidence and Risk of Lower-Extremity Amputation and Mortality Among Statin Users, Users of Nonstatin Lipid-Lowering Agents, and Nonusers Among Patients with Diabetes and PAD After Propensity Score Matching
| . | . | . | Propensity Score Matched . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Users of Lipid-Lowering Agents . | Nonusers . | Crude . | Competing Risk . | |||||||
| No. of Events . | Person-Years . | Incidence Ratea . | No. of Events . | Person-Years . | Incidence Ratea . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Statin user vs nonuser | ||||||||||
| Any lower-extremity amputation | 131 | 57,005 | 2.30 | 170 | 55,256 | 3.08 | 0.75 (0.60–0.94) | 0.013 | 0.77 (0.61–0.97) | 0.024 |
| Total lower-extremity amputation | 19 | 57,275 | 0.33 | 38 | 55,626 | 0.68 | 0.48 (0.28–0.83) | 0.010 | 0.50 (0.29–0.87) | 0.014 |
| In-hospital cardiovascular death | 358 | 57,266 | 6.25 | 459 | 55,680 | 8.24 | 0.75 (0.66–0.87) | <0.001 | 0.78 (0.68–0.89) | <0.001 |
| All-cause mortality | 1396 | 57,300 | 24.36 | 1866 | 55,710 | 33.49 | 0.72 (0.67–0.77) | <0.001 | — | |
| User of nonstatin lipid-lowering agents vs nonuser | ||||||||||
| Any lower-extremity amputation | 60 | 26,172 | 2.29 | 63 | 25,181 | 2.50 | 0.92 (0.64–1.31) | 0.633 | 0.94 (0.66–1.34) | 0.751 |
| Total lower-extremity amputation | 12 | 26,310 | 0.45 | 10 | 25,329 | 0.39 | 1.15 (0.50–2.65) | 0.750 | 1.19 (0.51–2.75) | 0.690 |
| In-hospital cardiovascular death | 114 | 26,314 | 4.33 | 122 | 25,339 | 4.81 | 0.89 (0.69–1.15) | 0.377 | 0.92 (0.71–1.19) | 0.524 |
| All-cause mortality | 527 | 26,321 | 20.02 | 674 | 25,342 | 26.60 | 0.75 (0.67–0.84) | <0.001 | — | |
| . | . | . | Propensity Score Matched . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Users of Lipid-Lowering Agents . | Nonusers . | Crude . | Competing Risk . | |||||||
| No. of Events . | Person-Years . | Incidence Ratea . | No. of Events . | Person-Years . | Incidence Ratea . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Statin user vs nonuser | ||||||||||
| Any lower-extremity amputation | 131 | 57,005 | 2.30 | 170 | 55,256 | 3.08 | 0.75 (0.60–0.94) | 0.013 | 0.77 (0.61–0.97) | 0.024 |
| Total lower-extremity amputation | 19 | 57,275 | 0.33 | 38 | 55,626 | 0.68 | 0.48 (0.28–0.83) | 0.010 | 0.50 (0.29–0.87) | 0.014 |
| In-hospital cardiovascular death | 358 | 57,266 | 6.25 | 459 | 55,680 | 8.24 | 0.75 (0.66–0.87) | <0.001 | 0.78 (0.68–0.89) | <0.001 |
| All-cause mortality | 1396 | 57,300 | 24.36 | 1866 | 55,710 | 33.49 | 0.72 (0.67–0.77) | <0.001 | — | |
| User of nonstatin lipid-lowering agents vs nonuser | ||||||||||
| Any lower-extremity amputation | 60 | 26,172 | 2.29 | 63 | 25,181 | 2.50 | 0.92 (0.64–1.31) | 0.633 | 0.94 (0.66–1.34) | 0.751 |
| Total lower-extremity amputation | 12 | 26,310 | 0.45 | 10 | 25,329 | 0.39 | 1.15 (0.50–2.65) | 0.750 | 1.19 (0.51–2.75) | 0.690 |
| In-hospital cardiovascular death | 114 | 26,314 | 4.33 | 122 | 25,339 | 4.81 | 0.89 (0.69–1.15) | 0.377 | 0.92 (0.71–1.19) | 0.524 |
| All-cause mortality | 527 | 26,321 | 20.02 | 674 | 25,342 | 26.60 | 0.75 (0.67–0.84) | <0.001 | — | |
Death was calculated as a competing risk.
Per 103 person-years.
Incidence and Risk of Lower-Extremity Amputation and Mortality Among Statin Users, Users of Nonstatin Lipid-Lowering Agents, and Nonusers Among Patients with Diabetes and PAD After Propensity Score Matching
| . | . | . | Propensity Score Matched . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Users of Lipid-Lowering Agents . | Nonusers . | Crude . | Competing Risk . | |||||||
| No. of Events . | Person-Years . | Incidence Ratea . | No. of Events . | Person-Years . | Incidence Ratea . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Statin user vs nonuser | ||||||||||
| Any lower-extremity amputation | 131 | 57,005 | 2.30 | 170 | 55,256 | 3.08 | 0.75 (0.60–0.94) | 0.013 | 0.77 (0.61–0.97) | 0.024 |
| Total lower-extremity amputation | 19 | 57,275 | 0.33 | 38 | 55,626 | 0.68 | 0.48 (0.28–0.83) | 0.010 | 0.50 (0.29–0.87) | 0.014 |
| In-hospital cardiovascular death | 358 | 57,266 | 6.25 | 459 | 55,680 | 8.24 | 0.75 (0.66–0.87) | <0.001 | 0.78 (0.68–0.89) | <0.001 |
| All-cause mortality | 1396 | 57,300 | 24.36 | 1866 | 55,710 | 33.49 | 0.72 (0.67–0.77) | <0.001 | — | |
| User of nonstatin lipid-lowering agents vs nonuser | ||||||||||
| Any lower-extremity amputation | 60 | 26,172 | 2.29 | 63 | 25,181 | 2.50 | 0.92 (0.64–1.31) | 0.633 | 0.94 (0.66–1.34) | 0.751 |
| Total lower-extremity amputation | 12 | 26,310 | 0.45 | 10 | 25,329 | 0.39 | 1.15 (0.50–2.65) | 0.750 | 1.19 (0.51–2.75) | 0.690 |
| In-hospital cardiovascular death | 114 | 26,314 | 4.33 | 122 | 25,339 | 4.81 | 0.89 (0.69–1.15) | 0.377 | 0.92 (0.71–1.19) | 0.524 |
| All-cause mortality | 527 | 26,321 | 20.02 | 674 | 25,342 | 26.60 | 0.75 (0.67–0.84) | <0.001 | — | |
| . | . | . | Propensity Score Matched . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Users of Lipid-Lowering Agents . | Nonusers . | Crude . | Competing Risk . | |||||||
| No. of Events . | Person-Years . | Incidence Ratea . | No. of Events . | Person-Years . | Incidence Ratea . | HR (95% CI) . | P Value . | HR (95% CI) . | P Value . | |
| Statin user vs nonuser | ||||||||||
| Any lower-extremity amputation | 131 | 57,005 | 2.30 | 170 | 55,256 | 3.08 | 0.75 (0.60–0.94) | 0.013 | 0.77 (0.61–0.97) | 0.024 |
| Total lower-extremity amputation | 19 | 57,275 | 0.33 | 38 | 55,626 | 0.68 | 0.48 (0.28–0.83) | 0.010 | 0.50 (0.29–0.87) | 0.014 |
| In-hospital cardiovascular death | 358 | 57,266 | 6.25 | 459 | 55,680 | 8.24 | 0.75 (0.66–0.87) | <0.001 | 0.78 (0.68–0.89) | <0.001 |
| All-cause mortality | 1396 | 57,300 | 24.36 | 1866 | 55,710 | 33.49 | 0.72 (0.67–0.77) | <0.001 | — | |
| User of nonstatin lipid-lowering agents vs nonuser | ||||||||||
| Any lower-extremity amputation | 60 | 26,172 | 2.29 | 63 | 25,181 | 2.50 | 0.92 (0.64–1.31) | 0.633 | 0.94 (0.66–1.34) | 0.751 |
| Total lower-extremity amputation | 12 | 26,310 | 0.45 | 10 | 25,329 | 0.39 | 1.15 (0.50–2.65) | 0.750 | 1.19 (0.51–2.75) | 0.690 |
| In-hospital cardiovascular death | 114 | 26,314 | 4.33 | 122 | 25,339 | 4.81 | 0.89 (0.69–1.15) | 0.377 | 0.92 (0.71–1.19) | 0.524 |
| All-cause mortality | 527 | 26,321 | 20.02 | 674 | 25,342 | 26.60 | 0.75 (0.67–0.84) | <0.001 | — | |
Death was calculated as a competing risk.
Per 103 person-years.
Subgroup analysis of the risks of lower-extremities amputation
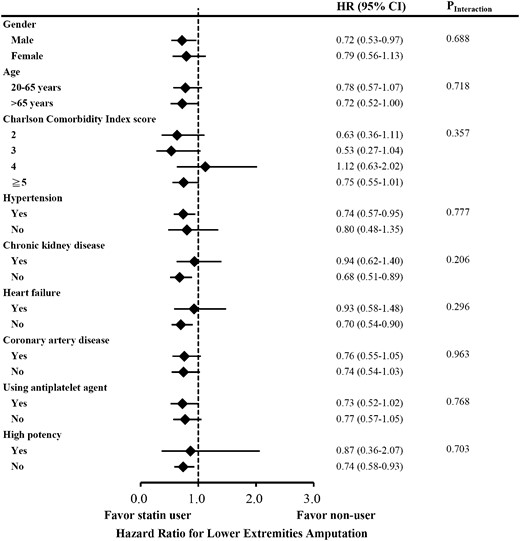
Focusing on lower-extremity amputation as outcome, tests of interactions were not significant for sex (P = 0.688), age > 65 years (P = 0.718), CCI score (P = 0.357), hypertension (P = 0.777), CKD (P = 0.206), heart failure (P = 0.296), CAD (P = 0.963), use of antiplatelet drugs (P = 0.768), and use of high-potency statin (P = 0.703). The effect of statin on the risk of any lower-extremity amputation was consistent in subgroup analysis (Fig. 2; Supplemental Table 5).
Subgroup analysis of risk of any lower-extremity amputation among statin user and matched nonuser.
Discussion
By using a nationwide DM cohort database representing most of the DM population in Taiwan from 2000 to 2011, we investigated whether the use of statins reduces lower-extremity amputation rate among DM patients with an established diagnosis of PAD compared with two propensity score–matched cohorts without statin use. In our study, statin therapy was associated with an ∼25% lower rate of lower-extremity amputations, in addition to a 22% risk reduction of cardiovascular mortality when taking into consideration the competing risk of death. These findings suggest that statin therapy not only reduces the risk of adverse cardiovascular events, but also has favorably effects on limb prognosis in DM patients with PAD.
Treatment of PAD patients should encompass three major aspects: concomitant cardiovascular risk factors modification, reservation of exercise capacity, and avoiding future limb events, including acute or chronic critical limb ischemia, revascularization, and amputation. According to previous PAD management guidelines (7–9) from the American College of Cardiology, American Heart Association, and European Society of Cardiology, the use of lipid-lowering agents is recommended to achieve target low-density lipoprotein cholesterol < 100 mg/dL, or < 70 mg/dL in high-risk individuals. The 2013 American College of Cardiology/American Heart Association guidelines further recommend that patients with clinical PAD undergo moderate to high-intensity statin therapy to reduce the risk of cardiovascular events regardless of the low-density lipoprotein cholesterol levels (10). This recommendation was mainly based on clinical evidence (21, 22) that has shown that statins reduce major cardiovascular events in PAD patients. Additionally, lipid-lowering drugs have been shown to also have a beneficial effect on improving walking distances, claudication symptoms, and pain-free walking time (11–13). In contrast, the protective effect of lipid-lowering drugs on limb outcomes is not well established, and the results of recent reports are inconclusive.
Limited clinical data support the advantage of statins on limb-related outcomes. The REACH registry (23), which enrolls a total of 5861 patients with symptomatic PAD, disclosed a significantly lower rate of limb outcomes, including revascularization and ischemic amputation, during a 4-year follow up period in statin users compared with nonusers (22.0 vs 26.2%; HR, 0.82; 95% CI, 0.72 to 0.92; P = 0.0013). In comparison, the Heart Protection Study group recruited 6748 PAD patients who were randomly allocated to simvastatin treatment or placebo groups (21). They demonstrated a significant (16%) reduction in the rate of first peripheral vascular event. However, the result was mainly driven by a 20% relative risk reduction in noncoronary revascularization (including carotid artery), and there was no effect on amputation rate. In our study, statin users had a 25% lower risk of any lower-extremity amputation and a 52% lower risk of total lower-extremity amputation compared with matched nonusers. Our present study is a real-world nationwide observational study, which is validated for insurance claim purpose, providing solid evidence for the association between statin therapy and risk reduction of lower-extremity amputation.
Because DM is associated with greater PAD risk, Sohn et al. (24) conducted a retrospective, observational study with a cohort of 83,593 DM cholesterol drug-naive patients. They compared the incidence of lower-extremity amputation between patients receiving statins, nonstatin cholesterol agents, and those without any cholesterol-lowering agents prescribed. After a mean follow-up period of 4.6 years, statin users had a 35% to 43% risk reduction for lower-extremity amputation than those not receiving any cholesterol-lowering agents (HR, 0.65; 95% CI, 0.42 to 0.99). In contrast, this protective effect was not seen in individuals taking nonstatin cholesterol-lowering drugs (HR, 0.95; 95% CI, 0.35 to 2.60). In our study, similar to the study design from Sohn et al., we also divided patients into statin users, other lipid-lowering agent users, and those never prescribed any lipid-lowering medications. However, our study differed in several ways. First, Sohn et al. enrolled all patients with DM, whereas we specifically focused on DM patients with an established diagnosis of PAD. Second, we recruited a larger cohort and used a longer follow-up period using data from a nationwide health insurance system. We demonstrated a protective effect of statins, but not other lipid-lowering agents, on amputation rate in the population of DM patients with known PAD. This result accords with what Sohn et al. disclosed.
Recently, Yang et al. (25) reported an association between statin use and risk of lower limb amputation in 38,973 diabetes patients from a random sample in the Taiwan NHIRD. However, their matching methods or adjustment factors to reduce confounding factors were not clearly reported. The proportion of the PAD diagnosis among patients with DM was also not clearly defined. Moreover, statin therapy is prone to have a less significant benefit among patients with underlying CAD, CKD, and PAD in their subgroup analysis. This raises the concern that death, as a competing risk, should be considered in the outcome analysis when comparing the amputation incidence among statin users and nonusers in a high-risk population of DM patients. In our study, we demonstrated a substantial reduction in the risk of amputation among patients using statins as compared with nonusers after adjusting for death as a competing risk.
The underlying mechanism for this protective effect from statins is supported by previous experimental studies. Earlier investigations of atherosclerotic plaques have identified two major risk markers for plaque vulnerability that might cause subsequent clinical symptoms in atherosclerosis diseases. The first is inflammatory cells infiltrate with the release of proangiogenic cytokines and matrix metalloproteinase proteases (26). The second is the extent of intraplaque neovascularization (27). Statins, but not other classes of lipid-lowering drugs, are known for their pleiotropic beneficial effects on atherosclerotic plaque stabilization and regression (28). The action of statin is derived from its lipid-lowering and anti-inflammatory properties in addition to its ability to improve endothelial function (29). A recently published meta-analysis of eight randomized controlled trials examining the effects of statins on vascular endothelial growth factor also suggested a statistically significant reduction of plasma vascular endothelial growth factor concentration after statin therapy (30), although the clinical impact on vascular disease is still uncertain.
One of the main strengths of our study is that it involved one of the largest cohorts of patients with type 2 DM in the world, that is, 1.2 million patients representing most of the DM population in Taiwan for the period from 2000 to 2011, thus minimizing referral bias. The use of Taiwan’s NHIRD makes it unlikely that any record of diagnosis or prescription was missed in this study. Furthermore, owing to the retrospective observational nature of this study, patients were matched and compared using propensity scores to reduce confounding effects. A time-varying analysis and a competing risk analysis were also performed. However, some limitations of our study should be addressed. First, unmeasured confounding is the primary limitation inherent in the use of administrative data. Although we used propensity score–matched analysis to balance major baseline comorbidities associated with adverse event occurrence between cohorts, bias attributable to unmeasured confounding could not be completely ruled out. Second, we lacked data on blood glucose or glycated hemoglobin levels. We matched cohorts according to aDCSI scores and the use of glucose-lowering medication, including insulin and oral antidiabetic drugs, which may partly reflect glycemic control and lessen the impact of antidiabetic drugs on risk of adverse limb outcome. Third, some lifestyle information such as smoking status, alcohol consumption, obesity, dietary habits, and exercise condition were not available through the administrative dataset. Another limitation is that there is currently no step-by-step guideline for indications of amputation. The decision to amputate may be affected by several factors other than disease severity, including surgeon’s experience, local facility, or patient preference, which could not be detected or evaluated using the information available in the database.
In conclusion, our study shows that statins, but not other lipid-lowering agents, has a protective effect on lower-extremity amputation in the population of DM patients with known PAD. The salutary property of statin might be partially derived from its pleiotropic effects, such as anti-inflammatory and antiatherogenic activities, beyond its influence on lowering cholesterol. Because a randomized placebo-controlled clinical trial would be unethical given the known beneficial effects of statins on cardiovascular outcomes, using data from a large, real-world, longitudinal cohort with properly conducted statistical analyses could provide valuable epidemiologic evidence to clarify the potential protective effects of statins on limb outcomes.
Abbreviations:
- aDCSI
adapted Diabetes Complications Severity Index
- aHR
adjusted hazard ratio
- CAD
coronary artery disease
- CCI
Charlson Comorbidity Index
- CI
confidence interval
- CKD
chronic kidney disease
- DM
diabetes mellitus
- HR
hazard ratio
- NHIRD
National Health Insurance Research Database
- PAD
peripheral arterial disease.
Acknowledgments
This work was supported, in part, by “Novel Bioengineering and Technological Approaches to Solve Two Major Health Problems in Taiwan” sponsored by Taiwan Ministry of Science and Technology Academic Excellence Program Grant MOST 105-2633-B-009-003, as well as by Ministry of Health and Welfare Grant MOHW 104-TDU-B-211-113-003. Funding agencies had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author contributions: C.-Y.H. and Y.-T.C. conceived and designed the research. Y.-T.C. and C.-C.C. performed statistical analysis. P.-H.H. and S.-J.L. handled funding and supervision. Y.-T.C. and Y.-W.S. acquired the data. C.-Y.H. and Y.-W.S. drafted the manuscript. P.-H.H. and Y.-T.C. made critical revision of the manuscript for key intellectual content. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. P.-H.H. vows that this study has been reported honestly, accurately, and transparently, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Disclosure Summary: The authors have nothing to disclose.
References
Author notes
These authors contributed equally to this study.
Address all correspondence and requests for reprints to: Po-Hsun Huang, MD, Division of Cardiology, Taipei Veterans General Hospital, No. 201, Section 2, Shipai Road, Beitou District, Taipei City 11217, Taiwan (Republic of China). E-mail: huangbs@vghtpe.gov.tw.