-
PDF
- Split View
-
Views
-
Cite
Cite
Michael Boschmann, Jochen Steiniger, Uta Hille, Jens Tank, Frauke Adams, Arya M. Sharma, Susanne Klaus, Friedrich C. Luft, Jens Jordan, Water-Induced Thermogenesis, The Journal of Clinical Endocrinology & Metabolism, Volume 88, Issue 12, 1 December 2003, Pages 6015–6019, https://doi.org/10.1210/jc.2003-030780
Close - Share Icon Share
Abstract
Drinking lots of water is commonly espoused in weight loss regimens and is regarded as healthy; however, few systematic studies address this notion. In 14 healthy, normal-weight subjects (seven men and seven women), we assessed the effect of drinking 500 ml of water on energy expenditure and substrate oxidation rates by using whole-room indirect calorimetry. The effect of water drinking on adipose tissue metabolism was assessed with the microdialysis technique. Drinking 500 ml of water increased metabolic rate by 30%. The increase occurred within 10 min and reached a maximum after 30–40 min. The total thermogenic response was about 100 kJ. About 40% of the thermogenic effect originated from warming the water from 22 to 37 C. In men, lipids mainly fueled the increase in metabolic rate. In contrast, in women carbohydrates were mainly used as the energy source. The increase in energy expenditure with water was diminished with systemic β-adrenoreceptor blockade. Thus, drinking 2 liters of water per day would augment energy expenditure by approximately 400 kJ. Therefore, the thermogenic effect of water should be considered when estimating energy expenditure, particularly during weight loss programs.
“DRINK LOTS OF water and keep yourself on schedule” is an old health adage. Nevertheless, few data underscore this homily. Recent studies have demonstrated that drinking water is, indeed, associated with a substantial physiological response. For example, water drinking increases systolic blood pressure more than 30 mm Hg in patients with severe autonomic failure. The pressor response was apparent within 5 min, reached a maximum after approximately 35 min, and was sustained for more than 60 min (1, 2). Water drinking increases blood pressure moderately in older but not younger control subjects (2). The pressor response may be mediated by the sympathetic nervous system (2–4). In healthy subjects, water drinking increases muscle sympathetic nerve traffic (3) and venous plasma norepinephrine concentrations (2, 3, 5). Furthermore, the pressor response can be abolished with systemic ganglionic blockade (2). The sympathetic nervous system is important in regulating energy metabolism and fuel utilization. Sympathetic activation increases cellular glucose uptake and metabolism and stimulates lipolysis (6). We tested the hypothesis that the sympathetic stimulus provided by water drinking might increase metabolic rate. Moreover, we determined the effect of water drinking on fuel mobilization and utilization systemically and at the adipose tissue level in normal men and women. Finally, we reasoned that the metabolic water effect might be attenuated with systemic or locally applied β-adrenoreceptor blockade.
Subjects and Methods
Subjects
Fourteen healthy subjects [seven men: age, 29 ± 3 yr; body mass index (BMI), 24.20 ± 0.94 kg/m2; seven women: age, 27 ± 2 yr; BMI, 20.80 ± 0.88 kg/m2; P < 0.05 for BMI] participated in the metabolic studies. In eight healthy subjects (two men and six women; age, 25 ± 1 yr), we determined the effect of water drinking on plasma osmolarity. All subjects were drug-free and nonsmoking. The institutional review board approved all studies, and written informed consent was obtained before study entry.
Protocol
Subjects did not eat 12.5 h before and did not drink 1.5 h before testing. Three separate studies were conducted. In the first study, we assessed the effect of drinking 500 ml of water on energy expenditure and substrate oxidation rates by using indirect calorimetry. In the second study, we used the microdialysis technique to characterize the effect of drinking 500 ml of water on adipose tissue blood flow and metabolism. In a subgroup, studies were conducted twice, once after ingestion of placebo and once after ingestion of 100 mg of the β-adrenoreceptor blocker metoprolol (Stada Arzneimittel AG, Bad Vilbel, Germany). The medications were ingested in a single-blinded fashion 1 h before water drinking. In the third study, we determined venous plasma osmolarity at baseline and 30 and 60 min after water drinking.
Calorimetry
Oxygen uptake and carbon dioxide production were measured by using a respiratory chamber to assess changes in energy expenditure, respiratory quotient (RQ; CO2 produced/O2 consumed), and carbohydrate and lipid oxidation rates, respectively. In previous studies in 16 healthy subjects, the maximal spontaneous change in metabolic rate over a 3-h period was 0.2 ± 0.09 kJ/min (3%). Throughout the study, subjects remained seated. After a run-in period of 15 min, resting energy expenditure was determined for 30 min. Then, the subjects ingested 500 ml water (22 C). In a subgroup, we also tested the effect of 500 ml of 37 C warm water. After completion of drinking, measurements were continued for another 90 min.
Microdialysis
Microdialysis studies were conducted in the supine position as described previously (7, 8). Briefly, one (systemic β-adrenoreceptor blockade) or two (local β-adrenoreceptor blockade) microdialysis probes were inserted into sc adipose tissue at the level of the umbilicus. Before insertion of the probes, the respective area was anesthetized superficially with EMLA cream (AstraZeneca GmbH, Wedel, Germany). On the day with systemic β-adrenoreceptor blockade, the subjects ingested metoprolol (Stada Arzneimittel AG) before testing, and the probe was perfused with Ringer’s solution (Serumwerke Bernburg AG, Bernburg, Germany) supplemented with 50 mm ethanol (B. Braun Melsungen AG, Melsungen, Germany), for monitoring changes in blood flow, and 10 μm ascorbate (Jenapharm GmbH & Co. KG, Jena, Germany). On the day with local β-adrenoreceptor blockade, the subjects ingested placebo before testing, and the perfusate for one microdialysis probe was supplemented with 100 nm of the nonselective β-adrenoreceptor blocker propranolol (Obsidan; ALPHARMA-ISIS, Langenfeld, Germany). CMA/60 microdialysis probes and CMA/102 microdialysis pumps (both from CMA Microdialysis AB, Solna, Sweden) were used. The flow rate was 2 μl/min.
Analytical methods
Ethanol concentration was determined in the perfusate (inflow) and dialysate (outflow) using a standard enzymatic assay (6). Dialysate concentrations of glycerol, glucose, and lactate were determined by standardized enzymatic colorimetric methods using the CMA/600 automatic analyzer (CMA Microdialysis AB). Plasma osmolarity was determined with the freezing point depression method (Model A Osmometer, Precision Scientific, Winchester, VA).
Calculations and statistics
Energy expenditure and substrate oxidation rates were calculated according to Ferrannini (9). Changes in blood flow were determined using the ethanol dilution technique based on Fick’s principle (9–11). Accordingly, a decrease in the ratio between ethanol in the dialysate and perfusate [(EtOH)d/(EtOH)p] corresponds to an increase in blood flow and vice versa. Dialysate glycerol concentration was measured to assess changes in lipolysis and/or lipid mobilization (12). Dialysate concentrations of glucose and lactate were determined to characterize glucose supply and glycolysis, respectively. In previous studies, in situ recovery for glycerol, glucose, and lactate in the dialysate was found to be approximately 30% using near-equilibrium dialysis at 0.3 μl/min (13). All data are given as means ± sem. Statistical analyses were carried out by ANOVA with repeated measures using with β-adrenoreceptor blockade or without β-adrenoreceptor blockade and time as factors to determine the significance of differences in energy metabolism and hemodynamic and metabolic response in adipose tissue to water in normal weight men and women, respectively. For testing, a statistical program (InStat, Version 3.0; Graphpad Software Inc., San Diego, CA) was used. Significant F ratios from the ANOVA were followed by post hoc comparisons among means using Bonferroni’s multiple t test.
Results
Energy metabolism
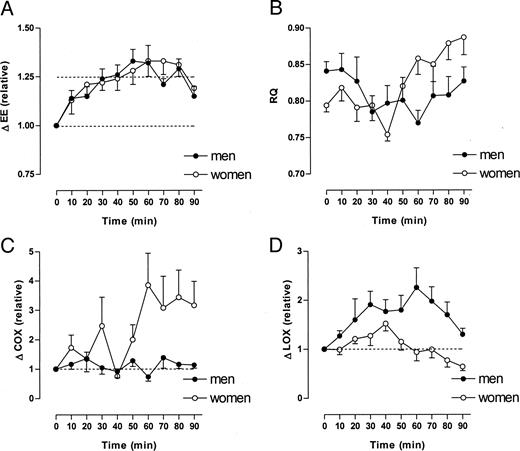
Resting energy expenditure was 5.06 ± 0.30 kJ/min in men and 4.02 ± 0.17 kJ/min in women (P < 0.001, men vs. women). Within 10 min after drinking water, energy expenditure started to increase. Sixty minutes after drinking water, energy expenditure increased 30% in men and 30% in women (Fig. 1A, P < 0.001). Resting RQ was 0.841 ± 0.013 and 0.794 ± 0.009 in men and women, respectively (Fig. 1B, P < 0.01, men vs. women). In women, RQ did not change significantly until 30 min after water drinking (Fig. 1B). After 40 min, RQ decreased significantly to a minimum of 0.75. The sharp decrease in RQ was followed by an increase up to 0.88 between 50 and 90 min (Fig. 1B). In contrast, in men, RQ decreased to 0.79 after 30 min and remained at that value for the next 30 min (Fig. 1B). RQ approached the baseline value after 90 min (Fig. 1B). Carbohydrate oxidation rate did not change significantly in men during 90 min after water drinking (Fig. 1C). In contrast, in women, carbohydrate oxidation increased about 2-fold (not significant) during the first 50 min after water drinking and about 3-fold (P < 0.05) during the following 40 min after water drinking. (Fig. 1C). During the first 40 min, the lipid oxidation rate increased in both men (+100%) and women (+50%). During the next 30 min, the lipid oxidation rate remained elevated in men, whereas it declined back to baseline values in women (Fig. 1D). After 90 min, the lipid oxidation rate was still elevated in men, whereas it decreased below baseline values in women (Fig. 1D).
Changes in energy expenditure (EE), RQ, carbohydrate oxidation rate (COX), and lipid oxidation rate (LOX) after drinking 500 ml water (22 C). Values at t = 0 min refer to baseline (before water drinking). Data are given as means ± se (n = 7 for both men and women). EE increased by about 30% in both men and women (P < 0.001) after water drinking. Resting RQ was significantly higher in men vs. women (P < 0.05). Sixty minutes after water drinking, RQ was significantly lower in men but significantly higher in women when compared with baseline (P < 0.05). COX was significantly increased in women (P < 0.001) but unchanged in men after water drinking. In contrast, LOX was significantly increased in men (P < 0.001) but unchanged in women after water drinking.
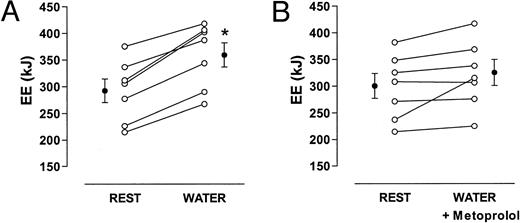
In a subset of volunteers (n = 7), the effect of systemic β-adrenoreceptor blockade on the water-induced increase in energy expenditure was tested. Again, all seven subjects showed an increase in energy expenditure from 292 ± 21 to 359 ± 23 kJ/h (Fig. 2A). In six subjects, β-adrenoreceptor blockade almost completely prevented the increase in energy expenditure after water drinking (Fig. 2B). In one woman, energy was only slightly attenuated with β-adrenoreceptor blockade.
Changes in energy expenditure (EE) after drinking 500 ml water (22 C) alone (A) or with systemic β-adrenergic blockade by metoprolol (B). Cumulative values over 1 h are given. Data are given as means ± se. *, P < 0.05 when compared with baseline (rest).
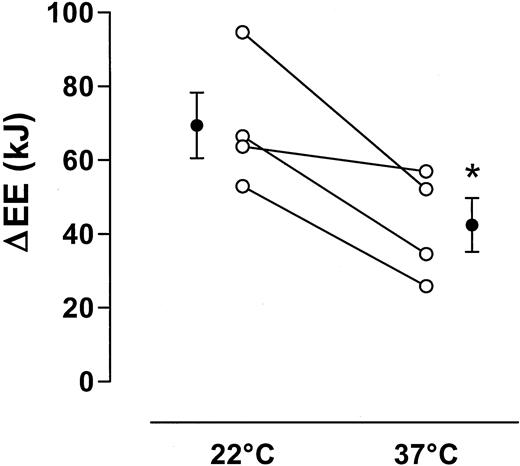
In another subset of volunteers (n = 4), the influence of water temperature on the water-induced increase in energy expenditure was tested. The water-induced change in energy expenditure was about 70 kJ at 22 C and about 40 kJ at 37 C, a difference of about 30 kJ between the two temperatures. (Fig. 3). Water drinking elicited a consistent decrease in venous osmolarity. Plasma osmolarity was 296 ± 1 mosmol/liter before water drinking and 289 ± 1 mosmol/liter after water drinking (P < 0.01).
Effect of water temperature (22 C or 37 C) on changes in energy expenditure after drinking of 500 ml water. Cumulative values over 1 h are given. Data are given as means ± se. *, P < 0.05, 22 C vs. 37 C.
Adipose tissue metabolism
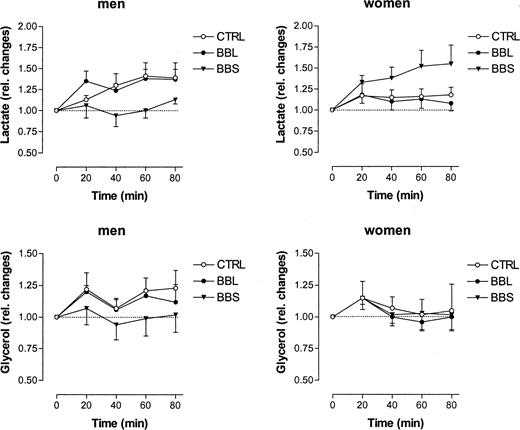
The baseline ethanol ratio was 0.39 ± 0.03 and 0.29 ± 0.05 (P < 0.05, men vs. women) in adipose tissue of men and women, respectively. Water drinking did not affect the ethanol ratio. Additionally, the ethanol ratio remained unchanged during both systemic and local β-adrenoreceptor blockade (data not shown). Baseline dialysate glucose was 0.76 ± 0.14 and 1.02 ± 0.21 mmol/liter (P < 0.05, men vs. women) in men and women, respectively. These values did not change significantly after water drinking in both groups, either in the absence or in the presence of local or systemic β-adrenoreceptor blockade (data not shown). Baseline dialysate lactate was 0.42 ± 0.12 and 0.47 ± 0.08 mmol/liter in men and women, respectively. Water drinking elicited a significant 45% increase in dialysate lactate in men (Fig. 4). Interestingly, that increase was almost completely prevented by systemic but not by local β-adrenoreceptor blockade (Fig. 4). In contrast, dialysate lactate did not change in women after water drinking. However, dialysate lactate increased 50% in women in the presence of systemic but not local β-adrenoreceptor blockade (Fig. 4). Baseline dialysate glycerol was 58 ± 15 and 96 ± 16 μmol/liter in men and women, respectively (P < 0.05, men vs. women). After water drinking, a slight but nonsignificant increase in dialysate glycerol (+20%) was noted in men, which was prevented by systemic but not by local β-adrenoreceptor blockade (Fig. 4). However, in women, no changes in dialysate glycerol were observed (Fig. 4).
Relative changes in dialysate lactate and glycerol in adipose tissue in men (n = 7) and in women (n = 7) after drinking 500 ml water (22 C). CTRL, Control, Ringer’s solution only; BBL, local β-adrenergic blockade by 100 nm propranolol added to the perfusion medium; BBS, systemic β-adrenergic blockade by ingestion of 100 mg metoprolol. Data are given as means ± se. In men, dialysate lactate increased significantly (P < 0.01) after water drinking, and this effect was prevented by systemic but not local β-adrenergic blockade. In contrast, in women, dialysate lactate did not change significantly after water drinking alone but increased significantly (P < 0.05) in the presence of systemic β-adrenergic blockade. Dialysate glycerol increased slightly but nonsignificantly in men. However, that increase was not observed during β-adrenergic blockade. In women, no changes at all were observed in dialysate glycerol with any protocol used.
Discussion
The novel finding in this study is that drinking 500 ml of water increases metabolic rate by 30% in both men and in women. The increase in metabolic rate was observed within 10 min after completion and reached a maximum 30–40 min after water drinking. The effect was sustained for more than an hour. The cardiovascular changes after water drinking that we described earlier exhibited a similar time course (1–3, 10–12). Based on our measurements, we estimate that increasing water ingestion by 1.5 liters would augment daily energy expenditure by approximately 200 kJ. Over 1 yr, energy expenditure would increase by 73,000 kJ (17,400 kcal), the energy content of 2.4 kg adipose tissue. By comparison, ingestion of 50 mg of ephedrine thrice daily increases energy expenditure by approximately 320 kJ/d (13). The substrates that fueled the increase in metabolic rate differed between men and women. In men, water drinking led to a marked increase in lipid oxidation. Carbohydrate oxidation did not change after water drinking. In contrast, in women, carbohydrates mainly fueled the increase in metabolic rate after water drinking.
Our data strongly suggest that the increase in metabolic rate with water is related to sympathetic activation and increased stimulation of β-adrenergic receptors. Indeed, the maximal increase in metabolic rate after water drinking corresponds to the maximal sympathetic activation in previous studies (2, 3). Systemic β-adrenoreceptor blockade substantially attenuated the water-induced increase in metabolic rate. Based on this observation, one might speculate that water drinking during β-adrenoreceptor blockade may reduce body temperature. Unfortunately, we did not determine body temperature. We propose that limb vasoconstriction after water drinking (3) may be sufficient to maintain thermal homeostasis even in the absence of an increase in metabolic rate.
We used the microdialysis technique to monitor metabolic changes, both systemically and at the tissue level. Water drinking did not change adipose tissue blood flow as determined by the ethanol dilution technique (7, 8, 14, 15). Therefore, changes in metabolite concentrations after water drinking cannot be explained by local blood flow changes. We were particularly interested to learn whether or not the oxidized lipids were derived from sc stores. In men, interstitial glycerol increased substantially after water drinking. The response was abolished by systemic but not local β-adrenoreceptor blockade. Thus, in men, water drinking increases lipid mobilization through stimulation of β-adrenoreceptors. However, the lipids are not derived from sc abdominal adipose tissue. In women, dialysate glycerol did not change after water drinking regardless of the presence or absence of β-adrenoreceptor blockade.
Dialysate glucose concentrations did not change after water drinking. This observation suggests that the balance between glucose supply and glucose utilization in adipose tissue did not change with water. In men, dialysate lactate increased even though systemic carbohydrate oxidation was not increased. The increase in lactate is consistent with increased glycolysis. The effect was suppressed by systemic but not local β-adrenoreceptor blockade. Thus, the lactate is not generated in sc adipose tissue. We speculate that the increase in lactate production may result from an increase in glucose release from the liver that is suppressed with β-adrenoreceptor blockade. In women, dialysate lactate was not increased. Presumably, glucose was more completely oxidized in women, as evidenced by the increase in carbohydrate oxidation. Paradoxically, dialysate lactate increased after water drinking during systemic but not local β-adrenoreceptor blockade. Perhaps a decrease in lipid mobilization and oxidation was followed by an increase in glycolysis. The gender-specific effect of water might be related to differences in body composition or hormonal factors (16).
The mechanism that elicits sympathetic activation with water drinking remains unclear (17). The pressor response does not seem to be influenced by water temperature (2). Part of the increase in energy expenditure may have been due to the energy required to heat the water from room temperature to body temperature (500 ml × 15 C = 7500 cal = 30 kJ). The calculated energy expenditure attributed to heating the water closely matched the difference between the thermogenic effect of 22 C water and 37 C water in our metabolic chamber studies. Thus, approximately 60–70% of the water-induced thermogenesis cannot be attributed to the heating of the ingested water. Gastric distension increases sympathetic activity in humans (18). However, at the time of the maximal response, less than 125 ml water remain in the stomach (19). We observed a mild but nevertheless consistent reduction in plasma osmolarity after water drinking, which mirrored the time course of the metabolic response. In humans, infusion of hypo-osmolar solutions through a gastric tube causes a greater increase of sweat production, a sympathetic response, than infusion of isosmolar solutions (20). Perhaps the sympathetic activation with water drinking involves osmoreceptive or sodium-sensitive afferent nerve fibers (21, 22). One important implication of our study is that the effect of water on energy expenditure and fuel utilization should be recognized as a powerful confounding factor in metabolic studies. Indeed, water drinking-induced thermogenesis is an important and unrecognized component of daily energy expenditure. If confirmed in other studies, this cost-free intervention may be a useful adjunctive treatment in overweight and obese individuals to attain an increase in energy expenditure.
Acknowledgements
This work was supported in part by the Deutsche Forschungsgemeinschaft. J.J. is a recipient of a Helmholtz fellowship of the Max-Delbrueck-Center of Molecular Medicine.