-
PDF
- Split View
-
Views
-
Cite
Cite
Gautam U Mehta, Dale Ding, Mohana Rao Patibandla, Hideyuki Kano, Nathaniel Sisterson, Yan-Hua Su, Michal Krsek, Ahmed M Nabeel, Amr El-Shehaby, Khaled A Kareem, Nuria Martinez-Moreno, David Mathieu, Brendan McShane, Kevin Blas, Douglas Kondziolka, Inga Grills, John Y Lee, Roberto Martinez-Alvarez, Wael A Reda, Roman Liscak, Cheng-Chia Lee, L Dade Lunsford, Mary Lee Vance, Jason P Sheehan, Stereotactic Radiosurgery for Cushing Disease: Results of an International, Multicenter Study, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 11, 1 November 2017, Pages 4284–4291, https://doi.org/10.1210/jc.2017-01385
Close - Share Icon Share
Abstract
Cushing disease (CD) due to adrenocorticotropic hormone–secreting pituitary tumors can be a management challenge.
To better understand the outcomes of stereotactic radiosurgery (SRS) for CD and define its role in management.
International, multicenter, retrospective cohort analysis.
Ten medical centers participating in the International Gamma Knife Research Foundation.
Patients with CD with >6 months endocrine follow-up.
SRS using Gamma Knife radiosurgery.
The primary outcome was control of hypercortisolism (defined as normalization of free urinary cortisol). Radiologic response and adverse radiation effects (AREs) were recorded.
In total, 278 patients met inclusion criteria, with a mean follow-up of 5.6 years (0.5 to 20.5 years). Twenty-two patients received SRS as a primary treatment of CD. Mean margin dose was 23.7 Gy. Cumulative initial control of hypercortisolism was 80% at 10 years. Mean time to cortisol normalization was 14.5 months. Recurrences occurred in 18% with initial cortisol normalization. Overall, the rate of durable control of hypercortisolism was 64% at 10 years and 68% among patients who received SRS as a primary treatment. AREs included hypopituitarism (25%) and cranial neuropathy (3%). Visual deficits were related to treatment of tumor within the suprasellar cistern (P = 0.01), whereas both visual (P < 0.0001) and nonvisual cranial neuropathy (P = 0.02) were related to prior pituitary irradiation.
SRS for CD is well tolerated and frequently results in control of hypercortisolism. However, recurrences can occur. SRS should be considered for patients with persistent hypercortisolism after pituitary surgery and as a primary treatment in those unfit for surgery. Long-term endocrine follow-up is essential after SRS.
Cushing disease (CD) is caused by adrenocorticotropic hormone (ACTH)–secreting pituitary adenomas and can cause severe morbidity due to cortisol hypersecretion. Symptoms can include central obesity, hypertension, diabetes, and psychiatric and neurocognitive changes (1). Furthermore, CD has an increased mortality ratio of 5 compared with the general population (2). First-line therapy for CD is pituitary surgery, typically via a transsphenoidal approach, which can often be curative. However, approximately 10% to 35% of patients do not achieve endocrine remission after surgery, and an additional proportion of patients has recurrence over time (3–6). Persistent hypercortisolemia can, in some cases, be controlled by medication, but this requires lifelong therapy. Radiation targeting adenomatous tissue in the sella may also be used to achieve control of hypercortisolism. Studies have shown initial efficacy with both multisession radiation therapy as well as single-session, focused stereotactic radiosurgery (SRS) for CD, the most established being Gamma Knife radiosurgery (GKRS) (7, 8). As recurrences may occur many years after normalization of cortisol secretion, longitudinal follow-up is critical to evaluate therapeutic options for CD. As such, interpretation of these prior single-center studies has been limited both by patient number and length of follow-up. To better define the endocrine outcomes and risks of SRS for CD, we analyzed the results of GKRS using a large, international, multicenter, retrospective cohort design.
Subjects and Methods
Patients
Patients who were treated with GKRS for CD between 1990 and 2016 were identified at 10 institutions participating in the International Gamma Knife Research Foundation (protocol R-16-10). All patients were within institutional review board–approved retrospective databases. These institutions included the University of Virginia Medical Center (134 patients), the University of Pittsburgh Medical Center (37 patients), Taipei Veterans General Hospital (34 patients), Charles University in Prague (28 patients), Gamma Knife Center Cairo (18 patients), Ruber International Hospital (17 patients), Université de Sherbrooke (6 patients), University of Pennsylvania (2 patients), Beaumont Health System (1 patient), and New York University Lagone Medical Center (1 patient). Individual patient data were de-identified and pooled for analyses.
Patients were confirmed to have a diagnosis of CD and thus included in the study by endocrine testing based on serum cortisol, ACTH, and 24-hour urinary free cortisol (UFC), as appropriate. Furthermore, all patients in this study had either radiologic identification of a pituitary adenoma and/or pathologic confirmation of an ACTH-secreting pituitary adenoma after surgery. Patients with <6 months of endocrine follow-up or lacking follow-up endocrine data were excluded.
Radiosurgical approach
Single-session Gamma Knife SRS was performed as previously described (8). In brief, frame-based stereotaxy was performed using a Leksell Model G frame (Elekta AB, Stockholm, Sweden) and thin-slice magnetic resonance imaging (MRI) for planning. GKRS was then planned by a multidisciplinary team, including a neurosurgeon, a radiation oncologist, and a medical physicist. Maximal point optic nerve, chiasm, and tract dosage were typically kept below 8 to 10 Gy. Radiosurgical parameters, including the margin and maximum dose, the isodose line, the tumor volume within the prescription isodose, and the number of isocenters, were recorded. The optic apparatus maximal dose was recorded.
Follow-up
Patients were typically followed with biochemical testing, including 24-hour UFCs, and MRI at 6-month intervals for the first 2 years and annually thereafter. Control of hypercortisolism was defined as normalization of 24-hour UFC off medications to control hypercortisolism, as determined by institutional reference ranges. Endocrine recurrence was defined as an increase in 24-hour UFC or serum cortisol above normal institutional reference ranges. Patient data were censored at the time of any additional treatments. Tumor progression was defined as changes in overall dimensions that would constitute an increase in volume >20% of pre-SRS volume (9). Tumor response was defined as a decrease in volume >20% of pre-SRS volume. Adverse radiation effects (AREs) were carefully recorded and included new or worsened non-ACTH pituitary insufficiency after treatment, as well as cranial neuropathies that were determined to be unrelated to tumor growth or subsequent therapies. Visual changes were included to assess for injury to the optic nerves, chiasm, or tracts. If subjective or objective visual changes were noted in follow-up, formal visual field testing was performed. Pituitary insufficiency was defined as new hormone deficit as defined by institutional reference ranges.
Statistical analyses
Primary outcome data (control of hypercortisolism) were evaluated using Kaplan-Meier analysis to determine progression-free survival from the time of treatment. The effect of preoperative and treatment variables on treatment outcome was analyzed using a Cox proportional hazards model by the Breslow method. Logistic regression and Fisher exact text were used to assess the relationship of continuous and binary treatment variables, respectively, with AREs. Multivariate analyses were performed if >10 events were recorded and included variables with a two-sided P value of <0.10 (10). Statistical significance was determined using a two-sided P value of <0.05. Analyses were performed using the software R (R version 3.11; The R Foundation for Statistical Computing, Vienna, Austria) and Prism (GraphPad Software, La Jolla, CA).
Results
Patient characteristics
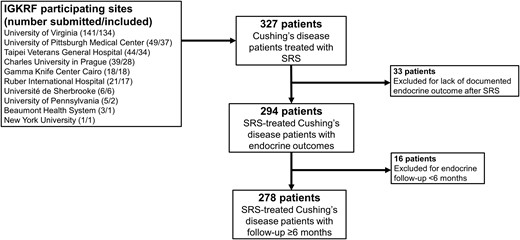
Among 10 institutions, 327 patients were identified with CD who were treated with single-session SRS. Forty-nine patients were excluded with follow-up <6 months (16 patients) or without endocrine follow up-data (33 patients), resulting in 278 patients available for analyses (Fig. 1). Mean age was 41.4 years (range, 10 to 80.6 years; Table 1). Most patients (80%) were female and had undergone prior resection (92%), whereas only 8% received SRS as a primary treatment of CD (Table 1). SRS was performed for residual tumor in 221 patients (79%) and recurrent tumor in 34 patients (12%). Sixteen patients (6%) received fractionated radiation therapy before SRS. Mean endocrine follow-up after SRS was 5.6 years (median, 4.3 years; range, 0.5 to 20.5 years).
Flowchart of 278 patients included for analyses. In total, 327 patients were submitted for analysis by International Gamma Knife Research Foundation (IGKRF) participating sites. A total of 49 patients were excluded due to limited follow-up, leaving 278 patients for final analyses.
Baseline Characteristics of 278 Patients With CD treated With GKRS
| Variable . | Value . |
|---|---|
| Age at treatment, y | 41.4 ± 13.9 (10–80.6) |
| Females | 222 (80) |
| Prior pituitary surgery | 255 (92) |
| Time from most recent surgery to GKRS, y | 1.9 ± 3.1 (0.02–26.8) |
| Prior radiation therapy | 16 (6) |
| Radiosurgery indication | |
| Primary treatment | 23 (8) |
| Residual tumor | 221 (79) |
| Recurrent tumor | 34 (12) |
| Endocrinopathy before treatment | 80 (29) |
| Thyroid | 50 (18) |
| Testosterone/estrogen | 30 (11) |
| Growth hormone | 12 (4) |
| Diabetes insipidus | 18 (6) |
| Visual deficits before treatment | 37 (13) |
| Visual field deficit | 27 (10) |
| Diplopia | 10 (4) |
| Medical therapy before treatment | 87 (31) |
| Medical therapy held before treatment | 28 (32)a |
| No visible tumor on pretreatment MRI | 32 (12)b |
| Tumor volume,c cm3 | 1.7 ± 2.0 (0.01–12.4) |
| Pretreatment morning serum cortisol,d mcg/dL | 26.0 ± 19.7 (0.5–137) |
| Pretreatment 24-hour UFC,e mcg | 355.2 ± 819.5 (1.1–9589) |
| Endocrine follow-up, y | 5.6 ± 4.3 (0.5–20.5) |
| Variable . | Value . |
|---|---|
| Age at treatment, y | 41.4 ± 13.9 (10–80.6) |
| Females | 222 (80) |
| Prior pituitary surgery | 255 (92) |
| Time from most recent surgery to GKRS, y | 1.9 ± 3.1 (0.02–26.8) |
| Prior radiation therapy | 16 (6) |
| Radiosurgery indication | |
| Primary treatment | 23 (8) |
| Residual tumor | 221 (79) |
| Recurrent tumor | 34 (12) |
| Endocrinopathy before treatment | 80 (29) |
| Thyroid | 50 (18) |
| Testosterone/estrogen | 30 (11) |
| Growth hormone | 12 (4) |
| Diabetes insipidus | 18 (6) |
| Visual deficits before treatment | 37 (13) |
| Visual field deficit | 27 (10) |
| Diplopia | 10 (4) |
| Medical therapy before treatment | 87 (31) |
| Medical therapy held before treatment | 28 (32)a |
| No visible tumor on pretreatment MRI | 32 (12)b |
| Tumor volume,c cm3 | 1.7 ± 2.0 (0.01–12.4) |
| Pretreatment morning serum cortisol,d mcg/dL | 26.0 ± 19.7 (0.5–137) |
| Pretreatment 24-hour UFC,e mcg | 355.2 ± 819.5 (1.1–9589) |
| Endocrine follow-up, y | 5.6 ± 4.3 (0.5–20.5) |
Values are presented as number (%) or mean ± standard deviation (range).
Proportion of patients who were on medical therapy before GKRS.
Data available for 266 patients.
Data available for 234 patients with visible tumor on MRI.
Data available for 245 patients.
Data available for 228 patients—includes 27 patients with normal values (6 on medication at treatment, 21 patients treated for known residual).
Baseline Characteristics of 278 Patients With CD treated With GKRS
| Variable . | Value . |
|---|---|
| Age at treatment, y | 41.4 ± 13.9 (10–80.6) |
| Females | 222 (80) |
| Prior pituitary surgery | 255 (92) |
| Time from most recent surgery to GKRS, y | 1.9 ± 3.1 (0.02–26.8) |
| Prior radiation therapy | 16 (6) |
| Radiosurgery indication | |
| Primary treatment | 23 (8) |
| Residual tumor | 221 (79) |
| Recurrent tumor | 34 (12) |
| Endocrinopathy before treatment | 80 (29) |
| Thyroid | 50 (18) |
| Testosterone/estrogen | 30 (11) |
| Growth hormone | 12 (4) |
| Diabetes insipidus | 18 (6) |
| Visual deficits before treatment | 37 (13) |
| Visual field deficit | 27 (10) |
| Diplopia | 10 (4) |
| Medical therapy before treatment | 87 (31) |
| Medical therapy held before treatment | 28 (32)a |
| No visible tumor on pretreatment MRI | 32 (12)b |
| Tumor volume,c cm3 | 1.7 ± 2.0 (0.01–12.4) |
| Pretreatment morning serum cortisol,d mcg/dL | 26.0 ± 19.7 (0.5–137) |
| Pretreatment 24-hour UFC,e mcg | 355.2 ± 819.5 (1.1–9589) |
| Endocrine follow-up, y | 5.6 ± 4.3 (0.5–20.5) |
| Variable . | Value . |
|---|---|
| Age at treatment, y | 41.4 ± 13.9 (10–80.6) |
| Females | 222 (80) |
| Prior pituitary surgery | 255 (92) |
| Time from most recent surgery to GKRS, y | 1.9 ± 3.1 (0.02–26.8) |
| Prior radiation therapy | 16 (6) |
| Radiosurgery indication | |
| Primary treatment | 23 (8) |
| Residual tumor | 221 (79) |
| Recurrent tumor | 34 (12) |
| Endocrinopathy before treatment | 80 (29) |
| Thyroid | 50 (18) |
| Testosterone/estrogen | 30 (11) |
| Growth hormone | 12 (4) |
| Diabetes insipidus | 18 (6) |
| Visual deficits before treatment | 37 (13) |
| Visual field deficit | 27 (10) |
| Diplopia | 10 (4) |
| Medical therapy before treatment | 87 (31) |
| Medical therapy held before treatment | 28 (32)a |
| No visible tumor on pretreatment MRI | 32 (12)b |
| Tumor volume,c cm3 | 1.7 ± 2.0 (0.01–12.4) |
| Pretreatment morning serum cortisol,d mcg/dL | 26.0 ± 19.7 (0.5–137) |
| Pretreatment 24-hour UFC,e mcg | 355.2 ± 819.5 (1.1–9589) |
| Endocrine follow-up, y | 5.6 ± 4.3 (0.5–20.5) |
Values are presented as number (%) or mean ± standard deviation (range).
Proportion of patients who were on medical therapy before GKRS.
Data available for 266 patients.
Data available for 234 patients with visible tumor on MRI.
Data available for 245 patients.
Data available for 228 patients—includes 27 patients with normal values (6 on medication at treatment, 21 patients treated for known residual).
Tumor and treatment characteristics
Most patients (88%) had visible tumor on MRI before SRS. Mean tumor volume was 1.7 cm3 (range, 0.01 to 12.4 cm3). Radiosurgical dose planning included suprasellar tumor extension in 17%, cavernous sinus involvement in 46%, and/or targeted the entire sella in 28% (Table 2). Altogether mean treatment volume was 2.1 cm3 (range, 0.09 to 17 cm3). Treatment characteristics of SRS are summarized in Table 2.
Treatment Characteristics of GKRS for 278 Patients With CD
| Variable . | Value . |
|---|---|
| Treatment volume,a cm3 | 2.1 ± 2.2 (0.09–17) |
| Cavernous sinus targeted | 129 (46) |
| Suprasellar component targeted | 41 (17) |
| Whole sella targeted | 78 (28) |
| Margin dose, Gy | 23.7 ± 6.2 (3–40) |
| Maximal dose, Gy | 46.3 ± 12.5 (10–80) |
| Isodose line, % | 52.1 ± 9.6 (30–90) |
| Isocenters | 7.0 ± 5.4 (1–25) |
| Maximum optic apparatus point dose,b Gy | 6.1 ± 2.7 (0–12.3) |
| Variable . | Value . |
|---|---|
| Treatment volume,a cm3 | 2.1 ± 2.2 (0.09–17) |
| Cavernous sinus targeted | 129 (46) |
| Suprasellar component targeted | 41 (17) |
| Whole sella targeted | 78 (28) |
| Margin dose, Gy | 23.7 ± 6.2 (3–40) |
| Maximal dose, Gy | 46.3 ± 12.5 (10–80) |
| Isodose line, % | 52.1 ± 9.6 (30–90) |
| Isocenters | 7.0 ± 5.4 (1–25) |
| Maximum optic apparatus point dose,b Gy | 6.1 ± 2.7 (0–12.3) |
Values are presented as number (%) or mean ± standard deviation (range).
Data available for 257 patients.
Data available for 272 patients.
Treatment Characteristics of GKRS for 278 Patients With CD
| Variable . | Value . |
|---|---|
| Treatment volume,a cm3 | 2.1 ± 2.2 (0.09–17) |
| Cavernous sinus targeted | 129 (46) |
| Suprasellar component targeted | 41 (17) |
| Whole sella targeted | 78 (28) |
| Margin dose, Gy | 23.7 ± 6.2 (3–40) |
| Maximal dose, Gy | 46.3 ± 12.5 (10–80) |
| Isodose line, % | 52.1 ± 9.6 (30–90) |
| Isocenters | 7.0 ± 5.4 (1–25) |
| Maximum optic apparatus point dose,b Gy | 6.1 ± 2.7 (0–12.3) |
| Variable . | Value . |
|---|---|
| Treatment volume,a cm3 | 2.1 ± 2.2 (0.09–17) |
| Cavernous sinus targeted | 129 (46) |
| Suprasellar component targeted | 41 (17) |
| Whole sella targeted | 78 (28) |
| Margin dose, Gy | 23.7 ± 6.2 (3–40) |
| Maximal dose, Gy | 46.3 ± 12.5 (10–80) |
| Isodose line, % | 52.1 ± 9.6 (30–90) |
| Isocenters | 7.0 ± 5.4 (1–25) |
| Maximum optic apparatus point dose,b Gy | 6.1 ± 2.7 (0–12.3) |
Values are presented as number (%) or mean ± standard deviation (range).
Data available for 257 patients.
Data available for 272 patients.
Eighty-seven patients (31%) were on medication to control hypercortisolism just before SRS. Among these patients, 85 were taking ketoconazole, 1 patient was taking mitotane, and 1 patient was taking metyrapone. In 59 patients, this medication was continued through the time of SRS, but in 28 patients, this medication was held 2 to 3 weeks before SRS. Pretreatment 24-hour UFC was available for 228 patients (Table 2). Among these, pretreatment 24-hour UFC was normal in 27 patients, including 6 patients who were requiring medication to control hypercortisolism at the time of SRS and 21 patients with known residual tumor who received SRS shortly after pituitary surgery.
Treatment outcomes
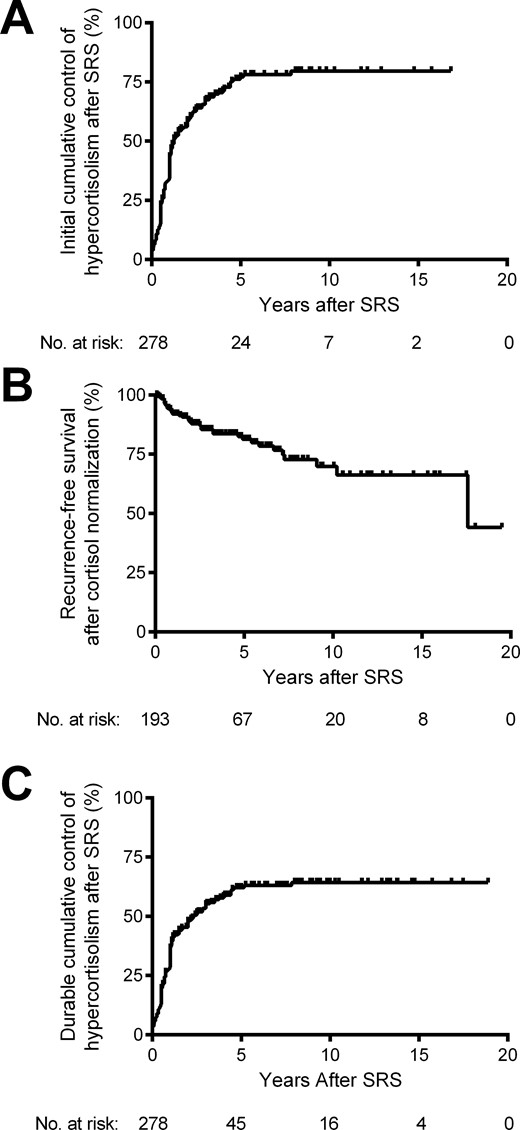
After SRS, 54 patients (19%) had uncontrolled hypercortisolemia, 31 patients (11%) were controlled on medication, and 193 patients (69%) experienced control of hypercortisolism (off medications to control hypercortisolism). By Kaplan-Meier analysis, the cumulative rate of initial control of hypercortisolism after SRS was 59%, 77%, and 80%, at 2, 5, and 10 years, respectively (Fig. 2A). Analyses after 10 years were limited by the number of patients at risk (≤7). In patients with control of hypercortisolism, mean ± standard deviation time to normalization of 24-hour UFC was 14.5 ± 14.3 months (median, 12 months). Pretreatment variables were assessed by univariate analyses to determine their effect on control of hypercortisolism. None of the tested variables were significantly related to initial response (Table 3).
Kaplan-Meier analyses of initial control of hypercortisolism after SRS for CD. (A) By analyzing initial endocrine response, the cumulative proportion of patients with control of hypercortisolism at 2, 5, 10, and 15 years after SRS was 59%, 77%, 80%, and 80%, respectively. (B) Among patients with an initial control of hypercortisolism (n = 193), the recurrence-free survival at 2, 5, 10, and 15 years after cortisol normalization was 89%, 81%, 70%, and 66%, respectively. (C) Finally, by analyzing endocrine response at last follow-up, the cumulative proportion of patients with control of hypercortisolism at 2, 5, 10, and 15 years after SRS was 48%, 62%, 64%, and 64%, respectively.
Univariate Analyses of the Relationship Between Preoperative Variables and Initial and Durable Control of Hypercortisolism After GKRS for CD
| Variable . | Initial Control of Hypercortisolism, P Value . | Durable Control of Hypercortisolism, P Value . | Recurrence After Cortisol Normalization,aP Value . |
|---|---|---|---|
| Age | 0.97 | 0.96 | 0.90 |
| Sex | 0.31 | 0.87 | 0.21 |
| Prior resection | 0.75 | 0.40 | 0.25 |
| Pretreatment urine-free cortisolb | 0.57 | 0.82 | 0.43 |
| Pretreatment serum morning cortisolc | 0.27 | 0.28 | 0.89 |
| Medication heldd | 0.10 | 0.46 | 0.20 |
| Tumor volume | 0.36 | 0.84 | 0.26 |
| Whole sella treated | 0.16 | 0.18 | 0.70 |
| Margin dose | 0.73 | 0.23 | 0.003e |
| Maximum dose | 0.86 | 0.38 | 0.009e |
| Variable . | Initial Control of Hypercortisolism, P Value . | Durable Control of Hypercortisolism, P Value . | Recurrence After Cortisol Normalization,aP Value . |
|---|---|---|---|
| Age | 0.97 | 0.96 | 0.90 |
| Sex | 0.31 | 0.87 | 0.21 |
| Prior resection | 0.75 | 0.40 | 0.25 |
| Pretreatment urine-free cortisolb | 0.57 | 0.82 | 0.43 |
| Pretreatment serum morning cortisolc | 0.27 | 0.28 | 0.89 |
| Medication heldd | 0.10 | 0.46 | 0.20 |
| Tumor volume | 0.36 | 0.84 | 0.26 |
| Whole sella treated | 0.16 | 0.18 | 0.70 |
| Margin dose | 0.73 | 0.23 | 0.003e |
| Maximum dose | 0.86 | 0.38 | 0.009e |
Among 193 patients with initial cortisol normalization.
Among 228 patients with available data.
Among 245 patients with available data.
Among 87 patients on medication at the time of SRS.
Statistically significant.
Univariate Analyses of the Relationship Between Preoperative Variables and Initial and Durable Control of Hypercortisolism After GKRS for CD
| Variable . | Initial Control of Hypercortisolism, P Value . | Durable Control of Hypercortisolism, P Value . | Recurrence After Cortisol Normalization,aP Value . |
|---|---|---|---|
| Age | 0.97 | 0.96 | 0.90 |
| Sex | 0.31 | 0.87 | 0.21 |
| Prior resection | 0.75 | 0.40 | 0.25 |
| Pretreatment urine-free cortisolb | 0.57 | 0.82 | 0.43 |
| Pretreatment serum morning cortisolc | 0.27 | 0.28 | 0.89 |
| Medication heldd | 0.10 | 0.46 | 0.20 |
| Tumor volume | 0.36 | 0.84 | 0.26 |
| Whole sella treated | 0.16 | 0.18 | 0.70 |
| Margin dose | 0.73 | 0.23 | 0.003e |
| Maximum dose | 0.86 | 0.38 | 0.009e |
| Variable . | Initial Control of Hypercortisolism, P Value . | Durable Control of Hypercortisolism, P Value . | Recurrence After Cortisol Normalization,aP Value . |
|---|---|---|---|
| Age | 0.97 | 0.96 | 0.90 |
| Sex | 0.31 | 0.87 | 0.21 |
| Prior resection | 0.75 | 0.40 | 0.25 |
| Pretreatment urine-free cortisolb | 0.57 | 0.82 | 0.43 |
| Pretreatment serum morning cortisolc | 0.27 | 0.28 | 0.89 |
| Medication heldd | 0.10 | 0.46 | 0.20 |
| Tumor volume | 0.36 | 0.84 | 0.26 |
| Whole sella treated | 0.16 | 0.18 | 0.70 |
| Margin dose | 0.73 | 0.23 | 0.003e |
| Maximum dose | 0.86 | 0.38 | 0.009e |
Among 193 patients with initial cortisol normalization.
Among 228 patients with available data.
Among 245 patients with available data.
Among 87 patients on medication at the time of SRS.
Statistically significant.
After initial cortisol normalization in 193 patients, recurrence of hypercortisolism occurred in 35 patients (18%). By Kaplan-Meier analysis, the recurrence-free survival after cortisol normalization was 89%, 81%, 70%, and 66% at 2, 5, 10, and 15 years, respectively (Fig. 2B). In this case, analyses after 15 years were limited by the number of patients at risk (≤8). Median ± standard deviation time to recurrence after cortisol normalization was 38.3 ± 44.3 months. By univariate analyses, both reduced margin (P = 0.003; hazard ratio, 0.92; 95% confidence interval, 0.87 to 0.97) and maximum (P = 0.009; hazard ratio, 0.92; 95% confidence interval, 0.87 to 0.97) treatment doses were significant predictors of recurrence, although neither was found to be statistically significant in a multivariate model (Table 3). The median margin and maximum doses for patients with recurrence were 20.5 Gy (range, 10 to 35 Gy) and 45.3 Gy (range, 22.7 to 67.3 Gy), respectively. This compared with 25 Gy (range, 12 to 40 Gy) and 50 Gy (range, 21.2 to 80 Gy) for patients without recurrences.
At last follow-up, durable control of hypercortisolism was maintained in 158 patients (57%). The cumulative rate of durable control of hypercortisolism after SRS was 48%, 62%, 64%, and 64% at 2, 5, 10, and 15 years, respectively (Fig. 2C). Again, analyses after 15 years were limited by the number of patients at risk (≤4). None of the tested pretreatment variables were significantly related to lasting control of hypercortisolism (Table 3). Among 22 patients who received SRS as a primary treatment of CD, 15 (68%) experienced durable control of hypercortisolism. Among 27 patients with normal UFC at the time of SRS, 20 (74%) had durable control of hypercortisolism. Finally, among 59 patients on medical therapy at the time of SRS, 28 (47%) experienced durable control of hypercortisolism, whereas among 28 patients whose medical therapy was held at the time of SRS, 12 (43%) experienced durable control of hypercortisolism. This difference was not statistically significant (P = 0.46).
Among patients without biochemical control, 23 subsequently underwent pituitary surgery, 18 underwent bilateral adrenalectomy, 1 underwent conventional radiotherapy, and 34 underwent repeat SRS. Complete imaging response data were available for 264 patients. Among these patients, tumors remained stable in 111 patients (42%), were reduced in size in 140 patients (53%), and were increased in size in 13 patients (5%).
AREs
SRS for CD was generally well tolerated, and AREs were limited to new endocrinopathy and cranial neuropathy (Table 4). New endocrinopathy after SRS was noted in 70 patients (25%) and was most commonly new hypothyroidism (46 patients, 17%). The development of new endocrinopathy was not related to the margin dose (P = 0.11), maximum dose (P = 0.94), or targeting of the whole sella (P = 0.65).
Complications Related to GKRS for 278 Patients With CD
| Variable . | No. (%) . |
|---|---|
| New endocrinopathy after treatment | 70 (25) |
| Thyroid | 46 (17) |
| Testosterone/estrogen | 28 (10) |
| Growth hormone | 29 (10) |
| Diabetes insipidus | 6 (2) |
| New cranial neuropathy | 7 (3) |
| CN II | 4 (1) |
| CN III | 2 (0.7) |
| CN IV | 1 (0.3) |
| CN V | 2 (0.7) |
| CN VI | 2 (0.7) |
| Variable . | No. (%) . |
|---|---|
| New endocrinopathy after treatment | 70 (25) |
| Thyroid | 46 (17) |
| Testosterone/estrogen | 28 (10) |
| Growth hormone | 29 (10) |
| Diabetes insipidus | 6 (2) |
| New cranial neuropathy | 7 (3) |
| CN II | 4 (1) |
| CN III | 2 (0.7) |
| CN IV | 1 (0.3) |
| CN V | 2 (0.7) |
| CN VI | 2 (0.7) |
Abbreviation: CN, cranial nerve.
Complications Related to GKRS for 278 Patients With CD
| Variable . | No. (%) . |
|---|---|
| New endocrinopathy after treatment | 70 (25) |
| Thyroid | 46 (17) |
| Testosterone/estrogen | 28 (10) |
| Growth hormone | 29 (10) |
| Diabetes insipidus | 6 (2) |
| New cranial neuropathy | 7 (3) |
| CN II | 4 (1) |
| CN III | 2 (0.7) |
| CN IV | 1 (0.3) |
| CN V | 2 (0.7) |
| CN VI | 2 (0.7) |
| Variable . | No. (%) . |
|---|---|
| New endocrinopathy after treatment | 70 (25) |
| Thyroid | 46 (17) |
| Testosterone/estrogen | 28 (10) |
| Growth hormone | 29 (10) |
| Diabetes insipidus | 6 (2) |
| New cranial neuropathy | 7 (3) |
| CN II | 4 (1) |
| CN III | 2 (0.7) |
| CN IV | 1 (0.3) |
| CN V | 2 (0.7) |
| CN VI | 2 (0.7) |
Abbreviation: CN, cranial nerve.
Cranial neuropathy that was unrelated to tumor progression or subsequent treatments was experienced by seven patients (3%), with four patients (1%) experiencing visual deficits and four patients (1%) experiencing other cranial neuropathy (cranial nerves III to VI). One patient experienced both visual and nonvisual cranial neuropathy. Vision loss was not significantly related to the maximal optic apparatus point dose (P = 0.36). However, visual dysfunction was significantly related to both prior pituitary irradiation (P < 0.0001) and treatment of tumor within the suprasellar cistern (P = 0.01). Conversely, nonvisual cranial neuropathy was not significantly related to treatment of tumor within the cavernous sinus (P = 0.63), but it was predicted by prior pituitary irradiation (P = 0.02).
Discussion
CD
ACTH-secreting pituitary adenomas that cause CD can cause significant morbidity as well as up to a fivefold increase in standardized mortality (1, 2). CD is infrequent in the general population (3% to 6% of pituitary adenomas). Therefore, there is a paucity of large-scale studies on the outcomes of various treatments (11–13). Pituitary surgery is the treatment of choice for these lesions. However, it can be complicated by the frequent lack of identifiable tumor on imaging as well as local invasion (14). As such, rates of biochemical control after surgery can range from 50% to >80%, depending on tumor-specific factors (3, 4, 6, 15). Despite these limitations, surgery offers the greatest likelihood of immediate remission for patients with CD. Medical therapy can be used to control hypercortisolism, but it is not curative and results in biochemical control in <50% of patients (16–18). Conventional fractionated radiation therapy has been noted to result in control of hypercortisolism in approximately 80% of patients in small, single-center cohorts (n = 30 to 40 patients) (7, 19).
SRS for CD
SRS, including GKRS, is a single-session, focused radiation option for patients with CD. Compared with treatment of nonfunctioning adenomas, typically greater margin doses are advocated for functioning adenomas (20). The largest study to date (n = 96) demonstrated durable control of hypercortisolism in up to 70% of patients (8). Fifteen percent of patients demonstrated an initial cortisol normalization but then experienced recurrence of their hypercortisolism. Tumor control was noted in 98% of patients. New endocrinopathy was noted in 36% and new cranial neuropathy was noted in 5%.
Current study
Because multi-institutional long-term data on SRS for CD do not exist, we retrospectively analyzed the results of SRS for CD at multiple experienced centers using GKRS. Analysis of 278 patients who met criteria for inclusion demonstrated tumor control among 95% of patients. This rate is similar to rates previously reported in both functioning and nonfunctioning (typically at a lower margin dose) pituitary adenomas with SRS (8, 21–24).
Initial control of hypercortisolism occurred in 80% at 10 years. However, among these patients, recurrence occurred in 18%. The mean time to initial cortisol normalization was 14.5 months (median, 12 months). Although limited data exist for conventional radiotherapy for CD, the median time to cortisol normalization ranges from 18 to 42 months (7, 19, 20). This suggests that SRS may result in shorter response times than conventional radiotherapy (20). In a prior study by our group using a large, homogeneously treated cohort of patients with pituitary adenoma, we found that the rate of endocrine remission after SRS was increased by a factor of 1.30 for each 1-mL reduction in SRS treatment volume (25). Thus, a macroadenoma compared with microadenoma in patients with CD likely portends a slower rate of remission after SRS. Overall, durable control of hypercortisolism was experienced by 64% of patients at 10 years. Although holding medications to control hypercortisolism just before SRS has been previously suggested to improve the likelihood of endocrine response with CD, this study did not demonstrate such an effect (26). Little is known regarding the use of radiation therapy or SRS for CD as a primary therapy. In the current study, control of hypercortisolism was observed in 68% of patients who received SRS as a primary treatment.
New endocrinopathy after SRS was experienced by 25% of patients. This finding is consistent with data from other large series of SRS for functioning adenomas (27). The current rate of hypopituitarism appears to be lower than the rate experienced after conventional radiation for CD (57% to 76%) (7, 19). Previous studies have suggested that hypopituitarism after pituitary SRS may be related to higher margin dose and treatment of either suprasellar extension or targeting of the whole sella (8, 27). Despite these previous studies, these factors were not significant predictors of hypopituitarism in this cohort.
New cranial neuropathy in the current study occurred in 3% of patients. This result is consistent with prior large cohort studies of cranial neuropathy after SRS for all pituitary adenomas (28). Patients selected for this treatment should be carefully counseled as to this particular risk. Intuitively, targeting of tumor in the suprasellar cistern was related to visual deficits after SRS. Conversely, targeting of the cavernous sinus was not related to the incidence of nonvisual cranial neuropathy. A history of pituitary irradiation predicted the development of both visual and nonvisual cranial neuropathies. This risk should be understood for patients who are undergoing SRS after conventional radiotherapy.
Study limitations
The major limitation of this study was its retrospective design. Future prospective study will be necessary to more definitively understand the outcomes of SRS for CD. However, as CD accounts for only 3% to 6% of pituitary adenomas, it will take many years to accumulate such data with long-term follow-up (11–13). Some patients in the current study were previously reported in single-center reports from participating sites. Despite this overlap, follow-up data on all patients in the current study were updated whenever possible. SRS in this study was performed over a >2-decade period. Over this time, there have been incremental refinements in the Gamma Knife platform, neuroimaging protocols (i.e., MRI and computed tomography), and radiosurgical approach that may affect outcome. Due to data accrual over 2 decades and among 10 different centers, criteria for endocrine remission between patients were inhomogeneous and normalization of 24-hour UFC was used as a unifying primary outcome. However, UFC measurements alone may fail to capture a subset of biochemical recurrences (29).
Due to the multi-institutional, retrospective approach, a precise record of pretreatment medications to control hypercortisolism, including dosage, and start and end dates was not available. It is also likely that the risk of hypopituitarism following SRS is underestimated as systematic assessment of the non-ACTH axes after SRS was not undertaken at all centers, and such testing was occasionally performed at the discretion of the treating clinicians. Similarly, subtle changes to vision after SRS could possibly be underestimated as formal visual field testing was not routinely performed. Finally, hypofractionated SRS may also have a role in the treatment of CD, particularly for patients with lesions involving the suprasellar cistern, who may be at risk for optic apparatus injury. Further study will be required to assess the efficacy and safety of this approach.
Management of CD
The current study provides a greater rationale for the role of SRS in the management of CD. Currently, SRS and radiation therapy are considered second-line therapeutic options for CD that should be performed in conjunction with medical therapy but before bilateral adrenalectomy (30). Unlike medical therapy, SRS provides a possibility of cure and freedom from lifelong medical therapy. Furthermore, compared with bilateral adrenalectomy, SRS appears to cause less morbidity, particularly with respect to the risk of developing Nelson syndrome (31, 32). Overall, the findings of the current study support the role of SRS in management. Finally, as some patients who are elderly or otherwise medically unfit for surgery may not tolerate pituitary surgery, SRS as a primary therapy appears to be a viable option with a rate of control of hypercortisolism comparable to pituitary surgery (33).
Conclusion
These data demonstrate that SRS for CD can provide durable control of hypercortisolism in most treated patients while being generally safe and well tolerated. However, due to the possibility of recurrence after initial cortisol normalization and hypopituitarism, long-term endocrine follow-up after SRS is necessary. Finally, SRS may be a reasonable primary therapeutic option in carefully selected patients who cannot undergo pituitary surgery.
Abbreviations
- ACTH
adrenocorticotropic hormone
- ARE
adverse radiation effect
- CD
Cushing disease
- GKRS
Gamma Knife radiosurgery
- MRI
magnetic resonance imaging
- SRS
stereotactic radiosurgery
- UFC
urinary free cortisol.
Acknowledgments
We appreciate the assistance of Ms. Linda Baxendell with coordination of data for the International Gamma Knife Research Consortium.
Author Contributions: G.U.M. designed the study, collected the data, analyzed the data, drafted the manuscript, and critically revised the manuscript. D.D. collected the data, analyzed the data analysis, and critically revised the manuscript. M.R.P., H.K., N.S., Y.-H.S., M.K., A.M.N., A.E.-S., K.A.K., N.M.-M., D.M., B.M., K.S., D.K., I.G., J.Y.L., R.M.-A., W.A.R., R.L., C.-C.L., and L.D.L. collected the data and critically revised the manuscript. M.L.V. supervised the study and critically revised the manuscript. J.P.S. supervised the study, designed the study, collected the data, and critically revised the manuscript.
Disclosure Summary: I.G. reports stock ownership and serving on the Board of Directors for the Greater Michigan Gamma Knife and, through her institution, reports receiving funding for non–study-related research from Elekta. L.D.L. reports stock ownership in Elekta AB.
References
Semple PL, Vance ML, Findling J, Laws ER.