-
PDF
- Split View
-
Views
-
Cite
Cite
Naiyarat Prasongsook, Aditi Kumar, Ashish V Chintakuntlawar, Robert L Foote, Jan Kasperbauer, Julian Molina, Yolanda Garces, Daniel Ma, Michelle A Neben Wittich, Joseph Rubin, Ronald Richardson, John Morris, Ian Hay, Vahab Fatourechi, Bryan McIver, Mabel Ryder, Geoffrey Thompson, Clive Grant, Melanie Richards, Thomas J Sebo, Michael Rivera, Vera Suman, Sarah M Jenkins, Robert C Smallridge, Keith C Bible, Survival in Response to Multimodal Therapy in Anaplastic Thyroid Cancer, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 12, 1 December 2017, Pages 4506–4514, https://doi.org/10.1210/jc.2017-01180
Close - Share Icon Share
Abstract
Historical outcomes in anaplastic thyroid cancer (ATC) have been dismal.
To determine whether an initial intensive multimodal therapy (MMT) is associated with improved ATC survival.
MMT was offered to all patients with newly diagnosed ATC treated at the Mayo Clinic from 2003 through 2015; MMT vs care with palliative intent (PI) was individualized considering clinical status and patient preferences. Outcomes were retrospectively analyzed by American Joint Committee on Cancer stage and treatments compared with patient cohort data from 1949 through 1999.
Forty-eight patients (60% male; median age, 62 years); 18 treated with PI, 30 with MMT.
Overall survival (OS) and progression-free survival determined by Kaplan-Meier method.
Median OS and 1-year survival for the later cohort were 9 months [95% confidence interval (CI), 4 to 22 months] and 42% (95% CI, 28% to 56%) vs 3 months and 10% for the earlier cohort. Median OS was 21 months compared with 3.9 months in the pooled MMT vs PI groups for the later cohort [hazard ratio (HR), 0.32; P = 0.0006]. Among only patients in the later cohort who had stage IVB disease, median OS was 22.4 vs 4 months (HR, 0.12; 95% CI, 0.03 to 0.44; P = 0.0001), with 68% vs 0% alive at 1 year (MMT vs PI). Among patients with stage IVC cancer, OS did not differ by therapy.
MMT appears to convey longer survival in ATC among patients with stage IVA/B disease.
Anaplastic thyroid cancer (ATC) is a rare, undifferentiated carcinoma that is nearly universally fatal (1). ATC constitutes 1% to 2% of all thyroid cancers but leads to a disproportionately high fraction (∼50%) of thyroid cancer-related deaths (2, 3), with a median historical survival of 3 to 6 months (4–6). Optimal treatment strategies for ATC are poorly defined, but early concurrent chemotherapy and radiation therapy (RT) appear beneficial (7–10). Surgical resection has been associated with improved survival, but this is debated (5, 6, 8, 9, 11, 12). To date, doxorubicin is the only US Food and Drug Administration-approved agent for the treatment of ATC, but it has very limited efficacy (13). New treatment strategies and agents are sorely needed.
By 2003, intensity modulated radiation therapy (IMRT) became more widely available, offering more homogeneous and complete tumor coverage with a lower dose to the larynx, esophagus, and brachial plexus; emerging data also suggested the efficacy of taxanes in treating ATC (14). Therefore, in 2003, we initiated a practice change at the Mayo Clinic involving patients with ATC who are treated under our direction. We hypothesized that more intensive initial multimodal therapy (MMT) including surgery (when feasible), combination taxane-based chemotherapy, and IMRT might lead to improved survival.
Our pilot results using this approach in 10 consecutively treated patients with stages IVA and IVB ATC demonstrated better overall survival (OS) when compared with an historical cohort encompassing the preceding 50 years (60-month vs 5- to 6-month OS) (5, 15). In the present study, we extend our pilot experience to include data from all 30 patients treated with MMT at our center during a contemporary 13-year period (2003 through 2015), compared with parallel outcome data from 18 patients who instead self-elected, and received, care with palliative intent (PI), as well as compared with outcomes data from a historical cohort (1949 through 1999).
Methods
Patients and clinical data
This single-institution retrospective cohort study was approved by the Mayo Clinic Institutional Review Board. All patients who provided informed consent for use of their medical data for research purposes and who received all or part of their primary therapy for ATC at Mayo Clinic between 1 January 2003 and 31 December 2015 were included; one patient was excluded owing to lack of permission to use medical records for research.
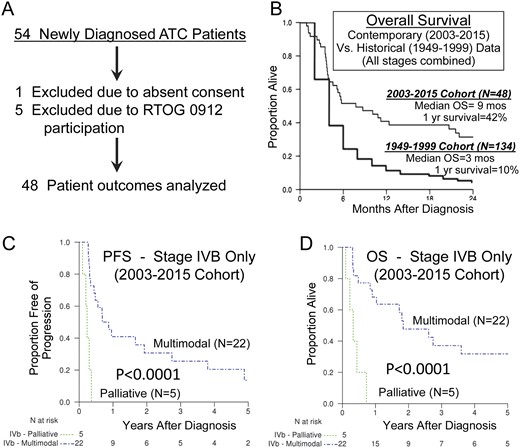
A new diagnosis of histologically confirmed ATC was required, confirmed by at least one expert thyroid pathologist at our institution [when available for rereview, ATC was also confirmed by one author (T.J.S.) in central review]. Patients with histologically uncertain diagnosis, poorly differentiated thyroid cancer, or tumors that were diagnosed only at autopsy or by death certificate were excluded. Patients (n = 5) who were treated under the Radiation Therapy Oncology Group 0912 trial (NCT01236547) were also excluded. To be classified as having received MMT, patients must have received IMRT and concurrent chemotherapy; analysis was on an intention-to-treat basis. Reasons for patient exclusion are depicted in Fig. 1A.
(A) Patient selection schema. (B) Kaplan-Meir estimates of OS for the entire contemporary (2003 through 2015) ATC cohort compared with 50-year (1949 through 1999) historical survival outcomes. Kaplan-Meir estimates of (C) PFS or (D) OS for patients in the later cohort with American Joint Committee on Cancer stage IVB disease treated with either PI or MMT. The MMT group demonstrated significantly better PFS and OS when compared with the PI group (P < 0.0001 for both).
The standard American Joint Committee on Cancer Staging Manual (seventh edition) (16) was used to define stages IVA, IVB, and IVC (IVA: intrathyroidal disease and N0; IVB: extrathyroidal disease, any N; IVC: metastatic disease). However, because American Joint Committee on Cancer (AJCC) stage IVB includes patients with widely differing extents of locoregional disease, we also alternatively categorized patients more precisely as follows: class 1, thyroid-confined disease with no extracapsular extension and N0; class 2, gross extrathyroid extension to neck only including lymph nodes; class 3, mediastinal involvement (but no visceral metastasis); class 4, visceral metastasis; and class 5, any of the previous characteristics with brain metastasis.
All patients were evaluated by our highly specialized group experienced in treating ATC (17). MMT vs PI and specific approaches used were self-elected by patients, personalized in response to extensive and inclusive discussions of available approaches, expected toxicities of competing therapies, dire prognosis, and historical outcomes. In addition to examining pooled all-stage data, we separately studied data from patients with stage IVB disease in isolation to minimize stage-associated outcome bias in the later cohort (2003 through 2015).
Statistics
Date of diagnosis, locoregional recurrence, distant metastasis (DM), and death or last follow-up were collected from electronic medical records of consented patients. OS was defined as survival from date of ATC diagnosis to death from any cause. Progression was defined as date of any development of locoregional relapse, DM, or death. OS and progression-free survival (PFS) were estimated using the Kaplan-Meier method from dates of ATC diagnoses, and median survival, and 1- and 2-year survival were estimated. The calculation of OS and PFS based on dates of ATC diagnoses, and not otherwise based on treatment initiation dates, was elected to allow comparisons of patient-cohort outcomes wherein no ATC-directed therapies were elected in patients in the PI group; this also avoided confusion related to variously defining therapy otherwise based on start date of therapies that differed among patients in the MMT group, and was analogous to survival calculation per the historical reference cohort (1949 through 1999). Survival was compared among groups and stages with log-rank tests. Clinical class was also assessed as an ordinal predictor with Cox proportional hazards regression, and the hazard ratio (HR) reported. The 95% confidence intervals (CIs) were calculated. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC) or R version 3.1.1 (https://cran.r-project.org) (18).
Results
Clinical and demographic characteristics
We identified 54 patients with new ATC diagnoses during the 13-year study period. One patient did not provide research authorization and, therefore, was excluded from analysis; another five patients were part of the current Radiation Therapy Oncology Group 0912 protocol and were accordingly also excluded (Fig. 1A). Final analyses, therefore, included 48 patients, 30 (63%) in the MMT group and 18 (37%) in the PI group. Demographic and pathological characteristics are listed in Table 1; 29 patients (60%) were male and, of these, seven (39%) were in the PI group and 22 (73%) were in the MMT group. The overall median age was 62 years (range, 37 to 88 years); for the PI and MMT groups, median age was 67.5 years (range, 50 to 88 years) and 60 years (range, 37 to 84 years), respectively; 43 patients (90%) were white.
Demographic and Pathologic Characteristics
| . | PI Group (n = 18) . | Intensive MMT Group (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Sex | |||
| Male | 7 (39) | 22 (73) | 29 (60) |
| Female | 11 (61) | 8 (27) | 19 (40) |
| Age at diagnosis, median (range), y | 67.5 (50.0–88.0) | 60.0 (37.0–84.0) | 62.0 (37.0–88.0) |
| Histology | |||
| ATC only | 13 (72) | 16 (53) | 29 (60) |
| Associated with DTC | 5 (28) | 14 (47) | 19 (40) |
| Type of ATC | |||
| Nondescript | 11 (61) | 22 (73) | 33 (69) |
| Sarcomatoid | 5 (28) | 7 (23) | 12 (25) |
| Others | 2 (11) | 1 (3) | 3 (6) |
| AJCC stagea | |||
| IVa | 0 (0.0) | 2 (7) | 2 (4) |
| IVb | 5 (29) | 22 (73) | 27 (57) |
| IVc | 12 (71) | 6 (20) | 18 (38) |
| Clinical disease extent, classa | |||
| 1 | 0 (0) | 2 (7) | 2 (4) |
| 2 | 5 (29) | 17 (57) | 22 (47) |
| 3 | 1 (6) | 6 (20) | 7 (15) |
| 4 | 10 (59) | 5 (17) | 15 (32) |
| 5 | 1 (6) | 0 (0) | 1 (2) |
| . | PI Group (n = 18) . | Intensive MMT Group (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Sex | |||
| Male | 7 (39) | 22 (73) | 29 (60) |
| Female | 11 (61) | 8 (27) | 19 (40) |
| Age at diagnosis, median (range), y | 67.5 (50.0–88.0) | 60.0 (37.0–84.0) | 62.0 (37.0–88.0) |
| Histology | |||
| ATC only | 13 (72) | 16 (53) | 29 (60) |
| Associated with DTC | 5 (28) | 14 (47) | 19 (40) |
| Type of ATC | |||
| Nondescript | 11 (61) | 22 (73) | 33 (69) |
| Sarcomatoid | 5 (28) | 7 (23) | 12 (25) |
| Others | 2 (11) | 1 (3) | 3 (6) |
| AJCC stagea | |||
| IVa | 0 (0.0) | 2 (7) | 2 (4) |
| IVb | 5 (29) | 22 (73) | 27 (57) |
| IVc | 12 (71) | 6 (20) | 18 (38) |
| Clinical disease extent, classa | |||
| 1 | 0 (0) | 2 (7) | 2 (4) |
| 2 | 5 (29) | 17 (57) | 22 (47) |
| 3 | 1 (6) | 6 (20) | 7 (15) |
| 4 | 10 (59) | 5 (17) | 15 (32) |
| 5 | 1 (6) | 0 (0) | 1 (2) |
Data given as no. (%) unless otherwise indicated.
Abbreviation: DTC, differentiated thyroid cancer.
Missing data for one patient.
Demographic and Pathologic Characteristics
| . | PI Group (n = 18) . | Intensive MMT Group (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Sex | |||
| Male | 7 (39) | 22 (73) | 29 (60) |
| Female | 11 (61) | 8 (27) | 19 (40) |
| Age at diagnosis, median (range), y | 67.5 (50.0–88.0) | 60.0 (37.0–84.0) | 62.0 (37.0–88.0) |
| Histology | |||
| ATC only | 13 (72) | 16 (53) | 29 (60) |
| Associated with DTC | 5 (28) | 14 (47) | 19 (40) |
| Type of ATC | |||
| Nondescript | 11 (61) | 22 (73) | 33 (69) |
| Sarcomatoid | 5 (28) | 7 (23) | 12 (25) |
| Others | 2 (11) | 1 (3) | 3 (6) |
| AJCC stagea | |||
| IVa | 0 (0.0) | 2 (7) | 2 (4) |
| IVb | 5 (29) | 22 (73) | 27 (57) |
| IVc | 12 (71) | 6 (20) | 18 (38) |
| Clinical disease extent, classa | |||
| 1 | 0 (0) | 2 (7) | 2 (4) |
| 2 | 5 (29) | 17 (57) | 22 (47) |
| 3 | 1 (6) | 6 (20) | 7 (15) |
| 4 | 10 (59) | 5 (17) | 15 (32) |
| 5 | 1 (6) | 0 (0) | 1 (2) |
| . | PI Group (n = 18) . | Intensive MMT Group (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Sex | |||
| Male | 7 (39) | 22 (73) | 29 (60) |
| Female | 11 (61) | 8 (27) | 19 (40) |
| Age at diagnosis, median (range), y | 67.5 (50.0–88.0) | 60.0 (37.0–84.0) | 62.0 (37.0–88.0) |
| Histology | |||
| ATC only | 13 (72) | 16 (53) | 29 (60) |
| Associated with DTC | 5 (28) | 14 (47) | 19 (40) |
| Type of ATC | |||
| Nondescript | 11 (61) | 22 (73) | 33 (69) |
| Sarcomatoid | 5 (28) | 7 (23) | 12 (25) |
| Others | 2 (11) | 1 (3) | 3 (6) |
| AJCC stagea | |||
| IVa | 0 (0.0) | 2 (7) | 2 (4) |
| IVb | 5 (29) | 22 (73) | 27 (57) |
| IVc | 12 (71) | 6 (20) | 18 (38) |
| Clinical disease extent, classa | |||
| 1 | 0 (0) | 2 (7) | 2 (4) |
| 2 | 5 (29) | 17 (57) | 22 (47) |
| 3 | 1 (6) | 6 (20) | 7 (15) |
| 4 | 10 (59) | 5 (17) | 15 (32) |
| 5 | 1 (6) | 0 (0) | 1 (2) |
Data given as no. (%) unless otherwise indicated.
Abbreviation: DTC, differentiated thyroid cancer.
Missing data for one patient.
ATC was associated with underlying differentiated thyroid cancer in 40% of patients. Nondescript ATC histology was most common (69%), whereas sarcomatoid appearance (25%) was the next most common histology. AJCC stages IVA, IVB, and IVC were distributed as none, five (29%), and 12 (71%) patients, respectively, in the PI group; in one patient, stage could not be reliably determined at time of diagnosis. In the MMT group, AJCC stages IVA, IVB, and IVC were distributed as two (7%), 22 (73%), and six (20%) patients, respectively.
Treatment
Treatment details for the contemporary cohorts are listed in Table 2. In general, patients either elected to receive MMT or PI, principally defined by willingness to undergo IMRT and chemotherapy initially. However, as indicated, the PI group clearly encompassed a greater proportion of patients with stage IVC disease (71%) compared with the MMT group (20%), presumably reflecting lesser enthusiasm among patients and providers for the application of toxic intensive MMT, including surgery and IMRT in the setting of imminently threatening advanced disease.
Treatment Characteristics
| . | PI (n = 18) . | Intensive MMT (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Surgery | |||
| No | 13 (72) | 3 (10) | 16 (33) |
| Yes | 5 (28) | 27 (90) | 32 (68) |
| Type of resectiona | |||
| R0 | 1 (6) | 8 (28) | 9 (20) |
| R1 | 1 (6) | 14 (48) | 15 (32) |
| R2 | 2 (12) | 4 (14) | 6 (13) |
| Biopsy only | 13 (76) | 3 (10) | 16 (35) |
| Combined chemoradiation therapy | |||
| No | 10 (56) | 0 (0) | 10 (21) |
| Yes | 8 (44) | 30 (100) | 38 (79) |
| RT (with or without chemotherapy) | |||
| No | 1 (6) | 0 (0) | 1 (2) |
| Yes | 17 (94) | 30 (100) | 47 (98) |
| RT dose, median (range) | 30.0 (20.0–66.0) | 66.0 (46.0–70.0) | 64.2 (20.0–70.0) |
| Chemotherapy agents | |||
| Doxorubicin and docetaxel | 6 (75) | 19 (63) | 25 (66) |
| Carboplatin and paclitaxel | 1 (12.5) | 5 (17) | 6 (16) |
| Doxorubicin only | 0 (0.0) | 4 (13) | 4 (10) |
| Cisplatin only | 0 (0.0) | 2 (7) | 2 (5) |
| Paclitaxel only | 1 (12.5) | 0 (0) | 1 (3) |
| . | PI (n = 18) . | Intensive MMT (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Surgery | |||
| No | 13 (72) | 3 (10) | 16 (33) |
| Yes | 5 (28) | 27 (90) | 32 (68) |
| Type of resectiona | |||
| R0 | 1 (6) | 8 (28) | 9 (20) |
| R1 | 1 (6) | 14 (48) | 15 (32) |
| R2 | 2 (12) | 4 (14) | 6 (13) |
| Biopsy only | 13 (76) | 3 (10) | 16 (35) |
| Combined chemoradiation therapy | |||
| No | 10 (56) | 0 (0) | 10 (21) |
| Yes | 8 (44) | 30 (100) | 38 (79) |
| RT (with or without chemotherapy) | |||
| No | 1 (6) | 0 (0) | 1 (2) |
| Yes | 17 (94) | 30 (100) | 47 (98) |
| RT dose, median (range) | 30.0 (20.0–66.0) | 66.0 (46.0–70.0) | 64.2 (20.0–70.0) |
| Chemotherapy agents | |||
| Doxorubicin and docetaxel | 6 (75) | 19 (63) | 25 (66) |
| Carboplatin and paclitaxel | 1 (12.5) | 5 (17) | 6 (16) |
| Doxorubicin only | 0 (0.0) | 4 (13) | 4 (10) |
| Cisplatin only | 0 (0.0) | 2 (7) | 2 (5) |
| Paclitaxel only | 1 (12.5) | 0 (0) | 1 (3) |
Data given as no. (%) unless otherwise indicated.
Missing data for two patients.
Treatment Characteristics
| . | PI (n = 18) . | Intensive MMT (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Surgery | |||
| No | 13 (72) | 3 (10) | 16 (33) |
| Yes | 5 (28) | 27 (90) | 32 (68) |
| Type of resectiona | |||
| R0 | 1 (6) | 8 (28) | 9 (20) |
| R1 | 1 (6) | 14 (48) | 15 (32) |
| R2 | 2 (12) | 4 (14) | 6 (13) |
| Biopsy only | 13 (76) | 3 (10) | 16 (35) |
| Combined chemoradiation therapy | |||
| No | 10 (56) | 0 (0) | 10 (21) |
| Yes | 8 (44) | 30 (100) | 38 (79) |
| RT (with or without chemotherapy) | |||
| No | 1 (6) | 0 (0) | 1 (2) |
| Yes | 17 (94) | 30 (100) | 47 (98) |
| RT dose, median (range) | 30.0 (20.0–66.0) | 66.0 (46.0–70.0) | 64.2 (20.0–70.0) |
| Chemotherapy agents | |||
| Doxorubicin and docetaxel | 6 (75) | 19 (63) | 25 (66) |
| Carboplatin and paclitaxel | 1 (12.5) | 5 (17) | 6 (16) |
| Doxorubicin only | 0 (0.0) | 4 (13) | 4 (10) |
| Cisplatin only | 0 (0.0) | 2 (7) | 2 (5) |
| Paclitaxel only | 1 (12.5) | 0 (0) | 1 (3) |
| . | PI (n = 18) . | Intensive MMT (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Surgery | |||
| No | 13 (72) | 3 (10) | 16 (33) |
| Yes | 5 (28) | 27 (90) | 32 (68) |
| Type of resectiona | |||
| R0 | 1 (6) | 8 (28) | 9 (20) |
| R1 | 1 (6) | 14 (48) | 15 (32) |
| R2 | 2 (12) | 4 (14) | 6 (13) |
| Biopsy only | 13 (76) | 3 (10) | 16 (35) |
| Combined chemoradiation therapy | |||
| No | 10 (56) | 0 (0) | 10 (21) |
| Yes | 8 (44) | 30 (100) | 38 (79) |
| RT (with or without chemotherapy) | |||
| No | 1 (6) | 0 (0) | 1 (2) |
| Yes | 17 (94) | 30 (100) | 47 (98) |
| RT dose, median (range) | 30.0 (20.0–66.0) | 66.0 (46.0–70.0) | 64.2 (20.0–70.0) |
| Chemotherapy agents | |||
| Doxorubicin and docetaxel | 6 (75) | 19 (63) | 25 (66) |
| Carboplatin and paclitaxel | 1 (12.5) | 5 (17) | 6 (16) |
| Doxorubicin only | 0 (0.0) | 4 (13) | 4 (10) |
| Cisplatin only | 0 (0.0) | 2 (7) | 2 (5) |
| Paclitaxel only | 1 (12.5) | 0 (0) | 1 (3) |
Data given as no. (%) unless otherwise indicated.
Missing data for two patients.
Surgical resection was performed in 27 patients (90%) in the MMT group vs five (28%) in the PI group. Thirty patients (100%) in the MMT group received chemoradiation therapy. Alternatively, 17 patients (94%) in the PI group received nondefinitive RT for local palliation; one received definitive-intention non-IMRT radiotherapy owing to limited disease extent in the hila and mediastinum encompassed in tailored radiotherapy fields. Of the 17 patients in the PI group who received radiotherapy, nine (50%) received radiation only and eight (44%) received chemoradiation therapy. The median PI radiation dose was 30 Gy (range, 20 to 66 Gy) vs 66 Gy (range, 46 to 70 Gy) in the MMT group. Median time from surgery to radiotherapy initiation among patients in the MMT group was 27 days.
Chemotherapy was combined with RT in eight (44%) patients in the PI group and in all 30 patients in the MMT group. Although the election of chemotherapy among almost half of those in the PI group might seem discordant with a palliative approach, reluctance to undergo definitive-intention radiotherapy seemed a primary determinant in self-selection of PI vs MMT. Doxorubicin plus docetaxel (20 mg/m2 weekly, each) was the most commonly prescribed combination chemotherapy (66%), used in all patients considered vigorous. Carboplatin plus paclitaxel (area under the curve, 2; 50 mg/m2, respectively, weekly; 16%) was the next most commonly used regimen, followed by doxorubicin (20 mg/m2 weekly; 10%), cisplatin (30 mg/m2 weekly; 5%), or paclitaxel (50 mg/m2 weekly; 3%). In the PI and MMT groups, three patients (18%) and eight patients (28%), respectively, received chemotherapy after chemoradiation for 4 to 12 additional weeks. In the MMT group, median days from surgery to chemotherapy initiation was 19; 70% of patients in this group received >80% of the intended chemotherapy dosage(s) during chemoradiation.
Response and survival characteristics
Median follow-up duration was 6.8 months (range, 1 to 139.4 months) for the entire contemporary cohort, and 20.3 months (range, 2.4 to 139.4 months), and 3.8 months (range, 1 to 58.2 months) for the MMT and PI groups, respectively. Best response to therapy was assessed by either Response Evaluation Criteria in Solid Tumors version 1.1 among those with disease outside of IMRT and surgical fields (stage IVC) or by disappearance of baseline disease in response to surgery and chemoradiation among those with IVA/B ATC. Complete response was achieved by 17 patients (36%) as best response to therapy: 15 (50%) in the MMT group and two (12%) in the PI group. Partial response or stable disease was seen in four (13%) and two (12%) patients from the MMT and PI groups, respectively. Progressive disease as best response was seen in nine (30%) and seven (40%) patients in the MMT and PI groups, respectively (Table 3). Upon detection or progression of DMs or locoregional recurrence, patients were offered aggressive palliation, including stereotactic body RT of lung metastases, as well as systemic therapies per patient election regardless of initial election of PI or MMT.
Best Response to Therapy
| . | PI Groupa (n = 18) . | Intensive MMT Group (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Complete response | 2 (12) | 15 (50) | 17 (36) |
| Partial response or stable disease | 2 (12) | 4 (13) | 6 (13) |
| Progressive disease or not evaluable | 7 (41) | 9 (30) | 16 (34) |
| Not evaluable | 6 (35) | 2 (7) | 8 (17) |
| . | PI Groupa (n = 18) . | Intensive MMT Group (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Complete response | 2 (12) | 15 (50) | 17 (36) |
| Partial response or stable disease | 2 (12) | 4 (13) | 6 (13) |
| Progressive disease or not evaluable | 7 (41) | 9 (30) | 16 (34) |
| Not evaluable | 6 (35) | 2 (7) | 8 (17) |
Data given as no. (%). Best response to therapy was assessed by either Response Evaluation Criteria in Solid Tumors 1.1 among those with stage IVC disease or by disappearance of baseline disease in response to surgery and chemoradiation among those with stage IVA/B ATC.
Missing data from one patient.
Best Response to Therapy
| . | PI Groupa (n = 18) . | Intensive MMT Group (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Complete response | 2 (12) | 15 (50) | 17 (36) |
| Partial response or stable disease | 2 (12) | 4 (13) | 6 (13) |
| Progressive disease or not evaluable | 7 (41) | 9 (30) | 16 (34) |
| Not evaluable | 6 (35) | 2 (7) | 8 (17) |
| . | PI Groupa (n = 18) . | Intensive MMT Group (n = 30) . | Total (N = 48) . |
|---|---|---|---|
| Complete response | 2 (12) | 15 (50) | 17 (36) |
| Partial response or stable disease | 2 (12) | 4 (13) | 6 (13) |
| Progressive disease or not evaluable | 7 (41) | 9 (30) | 16 (34) |
| Not evaluable | 6 (35) | 2 (7) | 8 (17) |
Data given as no. (%). Best response to therapy was assessed by either Response Evaluation Criteria in Solid Tumors 1.1 among those with stage IVC disease or by disappearance of baseline disease in response to surgery and chemoradiation among those with stage IVA/B ATC.
Missing data from one patient.
Sixteen of 18 patients in the PI group died, compared with 24 of 30 patients in the MMT group. One of the two long-term survivors in the PI group had metastases to the mediastinum and hila in the absence of visceral metastases; this prohibited IMRT but enabled RT using other approaches with definitive intention, accounting for the single patient in the PI group receiving 66 Gy of radiation. For the whole cohort, median PFS was 4 months and median OS was 8.8 months (range, 4.1 to 22 months) compared with a median OS of 3 months for the previously published, 50-year, Mayo Clinic historical cohort (Fig. 1B).
Median PFS significantly differed between the MMT and PI groups in the later cohort [8.3 months (range, 4 to 20.2 months) vs 3 months (range, 2 to 3.6 months), respectively; HR, 0.32; P = 0.0027] but was not definable in the historical cohort because of limited data. Similarly, the median OS was 21 months (range, 175 to 1313 months) compared with 4 months (range, 2.7 to 5.3 months) in the MMT group vs PI group (HR, 0.32; P = 0.0006). The patients with stage IVA disease and most of those with stage IVB disease were treated with MMT. Both patients with stage IVA disease were alive and free of disease at last follow-up >5 years after diagnosis. Patients with stage IVC disease elected to receive MMT, largely based on oligometastatic disease, goals of therapy, and patient performance status. PFS and OS are shown in Supplemental Fig. 1A and 1B by AJCC stage for the PI and MMT groups.
Because comparisons of pooled ATC patient data introduce outcome bias based on stage alone differing between the PI and MMT groups, we separately analyzed only outcomes of patients with stage IVB disease. Among these patients electing MMT, median PFS was 9.4 months, with 1- and 2-year PFS of 41% (range, 20% to 61%) and 31% (range, 11% to 50%), respectively (Fig. 1C). In contrast, patients in the PI group who had stage IVB disease had a median PFS of 2.8 months; there were no patients alive at 1 year [HR, 0.11 (95% CI, 0.03 to 0.39); P < 0.0001; Fig. 1C and 1D]. Patients in the MMT group with stage IVB disease had a median OS of 22.4 months, with 1- and 2-year survival of 68% (range, 49% to 88%) and 48% (range, 26% to 69%; Table 4); patients in the PI group with stage IVB disease had a median OS of 4 months, with none alive at 1 year [HR, 0.12 (95% CI, 0.03 to 0.44); P = 0.0001; Fig. 1D]. Patients in the MMT group with stage IVC disease had a median OS of 4.6 months (range, 72 to 341 months) with 1-year OS of 0% (Table 4); MMT was not associated with significantly longer survival when compared with PI among patients with stage IVC disease (HR, 1.15; P = 0.78; Table 4), but occasional long-term survival was attained (Supplemental Fig. 1B).
OS by AJCC Staging
| . | Median OS (months) . | 1-Year OS, % (95% CI) . | 2-Year OS, % (95% CI) . | HR (95% CI) . | P Value . |
|---|---|---|---|---|---|
| Stage IVA | NA | NA | |||
| PI (n = 0) | NA | NA | NA | ||
| MMT (n = 2) | NR | 100a | 100a | ||
| Stage IVB | 0.12 (0.03–0.44) | 0.0001 | |||
| PI (n = 5) | 4 | 0a | 0a | ||
| MMT (n = 22) | 22.4 | 68 (37–74) | 48 (20–58) | ||
| Stage IVC | 1.15 (0.41–3.27) | 0.78 | |||
| PI (n = 12) | 3.7 | 21 (0–46) | 21 (0–46) | ||
| MMT (n = 6) | 4.6 | 0a | 0a |
| . | Median OS (months) . | 1-Year OS, % (95% CI) . | 2-Year OS, % (95% CI) . | HR (95% CI) . | P Value . |
|---|---|---|---|---|---|
| Stage IVA | NA | NA | |||
| PI (n = 0) | NA | NA | NA | ||
| MMT (n = 2) | NR | 100a | 100a | ||
| Stage IVB | 0.12 (0.03–0.44) | 0.0001 | |||
| PI (n = 5) | 4 | 0a | 0a | ||
| MMT (n = 22) | 22.4 | 68 (37–74) | 48 (20–58) | ||
| Stage IVC | 1.15 (0.41–3.27) | 0.78 | |||
| PI (n = 12) | 3.7 | 21 (0–46) | 21 (0–46) | ||
| MMT (n = 6) | 4.6 | 0a | 0a |
Abbreviations: NA, not applicable; NR, not reached.
CI unable to be estimated owing to low variability.
OS by AJCC Staging
| . | Median OS (months) . | 1-Year OS, % (95% CI) . | 2-Year OS, % (95% CI) . | HR (95% CI) . | P Value . |
|---|---|---|---|---|---|
| Stage IVA | NA | NA | |||
| PI (n = 0) | NA | NA | NA | ||
| MMT (n = 2) | NR | 100a | 100a | ||
| Stage IVB | 0.12 (0.03–0.44) | 0.0001 | |||
| PI (n = 5) | 4 | 0a | 0a | ||
| MMT (n = 22) | 22.4 | 68 (37–74) | 48 (20–58) | ||
| Stage IVC | 1.15 (0.41–3.27) | 0.78 | |||
| PI (n = 12) | 3.7 | 21 (0–46) | 21 (0–46) | ||
| MMT (n = 6) | 4.6 | 0a | 0a |
| . | Median OS (months) . | 1-Year OS, % (95% CI) . | 2-Year OS, % (95% CI) . | HR (95% CI) . | P Value . |
|---|---|---|---|---|---|
| Stage IVA | NA | NA | |||
| PI (n = 0) | NA | NA | NA | ||
| MMT (n = 2) | NR | 100a | 100a | ||
| Stage IVB | 0.12 (0.03–0.44) | 0.0001 | |||
| PI (n = 5) | 4 | 0a | 0a | ||
| MMT (n = 22) | 22.4 | 68 (37–74) | 48 (20–58) | ||
| Stage IVC | 1.15 (0.41–3.27) | 0.78 | |||
| PI (n = 12) | 3.7 | 21 (0–46) | 21 (0–46) | ||
| MMT (n = 6) | 4.6 | 0a | 0a |
Abbreviations: NA, not applicable; NR, not reached.
CI unable to be estimated owing to low variability.
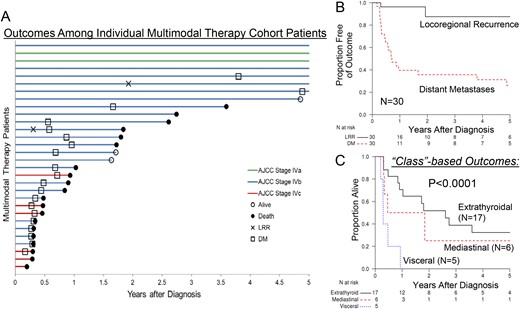
Timelines of locoregional relapse, distant relapse, and death are shown in Fig. 2A for all 30 patients in the MMT group. Locoregional relapse was seen in five of 11 patients in the PI group vs two of 27 in the MMT group. Median locoregional recurrence-free survival (LRRFS) was not reached in the MMT group (Fig. 2B, black line), with 1-year LRRFS of 96.3% (range, 89.2% to 100.0%) and 2-year LRRFS of 87.5% (range, 69.9% to 100.0%), indicating excellent locoregional disease control in response to MMT upon intention-to-treat analysis. Development of DMs, however, was common, occurring in 10 of 11 patients in the PI group and 21 of 27 patients in the MMT group, as noted on follow-up; aggressive stereotactic body RT and systemic approaches were offered in response. In the MMT group, DM-free survival at 2 years was 35.7% (range, 17.8% to 53.6%; Fig. 2B, red line). Disease class was also associated with increased risk of death with each increasing disease-extent class (1–5) when assessed for the whole cohort (HR, 2.04; P < 0.0001) or the MMT group alone (HR, 2.84; P < 0.0001; Fig. 2C).
(A) “Swimmers” plot showing the progression and survival in patients treated with MMT in the contemporary ATC cohort. The x-axis is truncated at 5 years. (B) Kaplan-Meir estimates of LRRFS or DM-free survival for the MMT group in the later ATC cohort (n = 30). (C) Kaplan-Meir estimates of OS of patients in the MMT group parsed by disease extent (or class, as defined in the article). Differences shown are significant at P < 0.001. LRR, locoregional relapse.
RT was completed as planned in 29 patients (97%) in the MMT group. Twenty of those 29 patients (70%) received ≥80% of planned chemotherapy doses during RT. Feeding tube placement was required during MMT for 18 patients (60%), but long-term requirement (>1 year) was infrequent (n = 2; one patient has feeding and tracheostomy tubes placed before treatment because of advanced local disease invading both organs, and the other patient was a >5-year survivor after a second course of chemoradiotherapy). Tracheotomy was required in three patients (10%) in the MMT group. Seventeen patients (60%) in the MMT group were hospitalized with complications, most frequently a convergence of mucositis, dehydration, and failure to thrive. Other causes for hospitalization included septic shock, respiratory failure, fever, and venous thromboembolism. One patient died during chemoradiation; the cause of death was unknown.
Discussion
Prognosis in ATC has been historically grim. Consequently, many providers have approached ATC with therapeutic nihilism; very few treatments have accordingly been investigated systematically. We aimed to improve historical ATC outcomes by applying intensive initial MMT in patients with ATC who preferred aggressive therapy. As aspired, MMT combining surgery, IMRT, and chemotherapy was associated with superior outcomes compared with our historical cohort, and also compared with a parallel PI group in the later cohort (Fig. 1).
For the entire later cohort, pooling all patients, median OS was ∼9 months, which was superior to most previously reported, single-institution or population-based studies (4, 6, 8, 19), including our own, which demonstrated 3-month historical OS from 1949 through 1999. Most recently, however, Rao et al. (20) reported a median OS of 11.9 months and 39% survival at 1 year for their entire MD Anderson ATC cohort, which are similar outcomes to our own overall contemporary cohort data and supportive of the contention that more intensive and expeditious care at referral centers is now associated with improved overall ATC outcomes. Moreover, in an analysis of more uniform patients with stage IVB ATC in our contemporary cohort, self-election of more intensive MMT was associated with significantly longer OS (22.4 months vs 4 months) than OS in patients with stage IVB disease in the PI group (HR, 0.12; 95% CI, 0.03 to 0.44; P = 0.0001). Among patients in the later cohort who had stage IVB disease and were treated with MMT, 68% were alive at 1 year vs 0% for patients in the PI group (Fig. 1D).
However, along with improved OS, we report considerable MMT-related toxicities; ∼60% of patients receiving MMT required hospitalization, 60% required at least temporary feeding tube placement (one permanent and therapy associated), and 10% required at least a temporary tracheostomy. Hence, there is an apparent trade-off between attained improved OS and imposed collateral toxicities. The question thus arises as to the conditions under which toxicities might be expected to be outweighed by survival benefit.
One approach to assessing benefit-to-toxicity tradeoffs involves analyzing outcomes by AJCC stage. Although toxicities remained uniform, survival was best in patients with lower-stage ATC. In particular, both patients with stage IVA disease treated with MMT were long-term survivors (>5 years and still alive as of last follow-up) making patients with stage IVA disease seem especially well suited to consider intensive initial MMT. Patients in the later cohort with stage IVB disease treated with MMT also apparently had improved OS compared with their counterparts in the PI group (Fig. 1D), with ∼30% alive 5 years from diagnosis compared with none surviving even 1 year in the patients in the PI group with stage IVB disease (Fig. 1D). Some patients with IVB disease, however, fared poorly and incurred considerable toxicities; ∼20% appeared to gain little or no OS benefit from even the most aggressive care (Fig. 1D). Moreover, according to our data, patients with stage IVC ATC appeared to gain insufficient survival benefit to offset the considerable MMT-associated toxicities. However, even palliative treatment as administered by our group seems to have been of at least some benefit: Patients who received only supportive care in a large population-based study had median OS of only 15 days (4), dying quickly of local tumor invasion and asphyxiation (21).
MMT has been applied to ATC previously with conflicting results. There are three reported separate cohorts from Sweden using various chemotherapies administered with differing RT fractionation schedules (hyperfractionation) (10, 22). Consistent with our present report, however, a prospective study from France showed significantly better outcomes in association with a protocol combining surgical resection (when feasible) followed by chemoradiation; median OS was 10 months, and 1- and 3-year survival was 46% and 27%, respectively (7). Similar results were reported in newer cohorts, including a recent study reporting a 22-month median OS for patients treated with trimodal therapy (20).
RT dosage also likely matters in ATC, specifically with regard to locoregional disease control. Several other studies have also demonstrated that >45 to 50 Gy is associated with improved outcomes in ATC (6, 9, 23). A recent National Cancer Database analysis also showed that patients who were treated with >60 Gy of RT received most benefit (21). The use of accelerated and hyperfractionated RT, however, has demonstrated conflicting results (20, 24). Nevertheless, hyperfractionation, accelerated RT, and newer techniques like IMRT, all allow more homogeneous and conformal RT with potential for better results at least in terms of minimizing toxicities, as mentioned.
Recent studies, including ours, indicate that most patients with ATC now die of systemic, and not locoregional, disease (7, 10, 25). Several studies suggested that surgery likely also adds benefit (7, 8, 10, 25); however, most studies are retrospective, have significant biases, and lack comparator groups. At our institution, we have adapted a multidisciplinary approach whereby surgery is offered to patients with ATC if R0 or R1 resection is expected in those with stages IVA and IVB disease. However, laryngectomy is not entertained in our surgical approach to ATC, and patients with IVC disease are less often deemed to benefit from surgery over RT alone.
Effective and tolerable salvage approaches for recurrent ATC are sorely needed. Animal models indicate that ATC is highly immunogenic, with inflammatory cell-rich stroma (26, 27), making ATC an attractive target for immunotherapy. Incorporating immunotherapy early in the disease, possibly with MMT, could offer an avenue to improve the DM-free survival; such a trial is being planned at our institution. Other salvage strategies such as BRAF inhibitors (28, 29) or other kinase inhibitors such as lenvatinib represent additional potential salvage alternatives (30). Recently presented data examining the combination of dabrafenib (a BRAF inhibitor) and trametinib (an MEK inhibitor) in patients with BRAFV600E-mutated ATC impressively also reported an overall response rate of 69% and 1-year OS of 80% (31). These results are surprising because they closely mimic analogous outcome data in patients with BRAFV600E-mutated differentiated thyroid cancer ; confirmation of the results is awaited. Several agents have also been found to combine synergistically with taxanes in ATC, including the PPARγ agonist efatutazone (32) or pazopanib (apparently acting on the basis of cell cycle effects attributable to inhibition of aurora kinases) (33), both of which are the subject of active clinical investigations in ATC.
Regardless of presented data and attained levels of statistical significance, improved survival demonstrated in this study should be interpreted with caution. Biases due to the inherent nature of retrospective analyses and those arising from a study restricted to a single center can inadvertently occur. Similarly, comparisons with palliative cohort data without randomization impose other potential means for introduction of bias. Bearing in mind these limitations, to our knowledge, these are the best available data in the absence of multicenter, randomized studies in the context of this rare and aggressive cancer.
To summarize, MMT combining surgery, IMRT, and chemotherapy was, in our experience, feasible and associated with longer survival in nonmetastatic ATC, but it was highly toxic. DMs are frequent despite aggressive initial therapy, and patients with AJCC stage IVC disease unfortunately appear to derive limited benefit from even the most aggressive initial therapeutic approaches. Hence, MMT seems appropriate for strong consideration in patients with stages IVA and IVB disease who are seeking improved survival and are accepting of attendant therapy-related toxicities, but not among patients with IVC disease.
Abbreviations:
- AJCC
American Joint Committee on Cancer
- ATC
anaplastic thyroid cancer
- CI
confidence interval
- DM
distant metastasis
- HR
hazard ratio
- IMRT
intensity modulated radiation therapy
- LRRFS
locoregional recurrence-free survival
- MMT
multimodal therapy
- OS
overall survival
- PFS
progression-free survival
- PI
care with palliative intent
- RT
radiation therapy.
Acknowledgments
The authors thank the patients and families who participated in this research and who entrusted their care to our group, and Candace Kostelec for exemplary administrative assistance. This manuscript is dedicated to our departed colleague, Thomas J. Sebo. We remain deeply indebted to Tom for his contributions to the field of endocrine pathology.
Current Affiliation: N. Prasongsook’s current affiliation is Phramongkutklao Hospital, Bangkok, Thailand. A. Kumar’s current affiliation is Medical College of Wisconsin, Milwaukee, Wisconsin 53226. B. McIver’s current affiliation is Moffitt Cancer Center Magnolia Campus, 12902 USF Magnolia Drive Tampa, Florida 33612.
Disclosure Summary: The authors have nothing to disclose.
References