-
PDF
- Split View
-
Views
-
Cite
Cite
Christian Cajochen, Mirjam Münch, Szymon Kobialka, Kurt Kräuchi, Roland Steiner, Peter Oelhafen, Selim Orgül, Anna Wirz-Justice, High Sensitivity of Human Melatonin, Alertness, Thermoregulation, and Heart Rate to Short Wavelength Light, The Journal of Clinical Endocrinology & Metabolism, Volume 90, Issue 3, 1 March 2005, Pages 1311–1316, https://doi.org/10.1210/jc.2004-0957
Close - Share Icon Share
Light can elicit acute physiological and alerting responses in humans, the magnitude of which depends on the timing, intensity, and duration of light exposure. Here, we report that the alerting response of light as well as its effects on thermoregulation and heart rate are also wavelength dependent. Exposure to 2 h of monochromatic light at 460 nm in the late evening induced a significantly greater melatonin suppression than occurred with 550-nm monochromatic light, concomitant with a significantly greater alerting response and increased core body temperature and heart rate (∼2.8 × 1013 photons/cm2/sec for each light treatment). Light diminished the distal-proximal skin temperature gradient, a measure of the degree of vasoconstriction, independent of wavelength. Nonclassical ocular photoreceptors with peak sensitivity around 460 nm have been found to regulate circadian rhythm function as measured by melatonin suppression and phase shifting. Our findings—that the sensitivity of the human alerting response to light and its thermoregulatory sequelae are blue-shifted relative to the three-cone visual photopic system—indicate an additional role for these novel photoreceptors in modifying human alertness, thermophysiology, and heart rate.
THE HUMAN CIRCADIAN timing system is sensitive to ocular light exposure. The effects of light depend on the circadian phase at which light is administered: light given after the core body temperature (CBT) nadir advances the phase of circadian rhythms, whereas light given before the CBT nadir induces delays. This can be quantified by a so-called “human phase-response curve to light” (1, 2). Besides the timing of exposure, the intensity of light (i.e. irradiance) also plays a crucial role in human circadian-phase resetting (3, 4). The irradiance dose-response function to a single episode of light in the phase-delay region can be characterized by a logistic function with high sensitivity, such that half of the maximal resetting response achieved in response to bright light (9100 lux) is obtained with just 1% of this light (dim room light of ∼100 lux; see Ref.4). Recent results indicate that very low intensity monochromatic light in the short-wave range (460 nm) also affects the human circadian timing system and is capable of inducing a significantly greater phase shift than monochromatic light at 555 nm (the peak of the three-cone photopic visual system) (5). Furthermore, short wavelength light between 436 and 456 nm induced a phase advance similar to that for polychromatic light (i.e. white light) containing 185-fold more photons (6). These studies clearly demonstrate that the human circadian timing system is highly sensitive to ocular light exposure, particularly in the short wavelength range.
Besides circadian phase shifts, light also elicits acute physiological effects in humans such as a rapid suppression of melatonin at night (for review, see Ref.7), an increase in CBT (8–11) and heart rate (12), and an immediate dose-dependent alerting response, measured subjectively and objectively via the electroencephalogram (10). Brainard et al. (13, 14) have consistently shown that short wavelength light at around 460 nm is most effective in acutely suppressing human melatonin levels. Furthermore, Hankins and Lucas (15) have recently shown that acute light responses in the human electroretinogram (ERG) are highly dependent on wavelength, such that light at 483 nm elicited the strongest reduction in cone ERG b wave–implicit time.
The acute effects of light, as well as the circadian effects, seem to be mediated by the eyes. Thus, acute elevation of body temperature and suppression of melatonin are not observed when the eyes are covered (11, 16) or when light is administered to the skin in the popliteal region (17–19). There is mounting evidence that nonrod and noncone photoreceptors might form the basis of this nonimage-forming photoreceptive pathway mediating both the circadian and direct effects of light in rodents (20, 21) (for review, see Ref.22). Therefore, we hypothesized that the acute effect of light on melatonin, alertness, thermoregulation, and heart rate is blue-shifted, such that short wavelength light at 460 nm induces a greater melatonin-suppressing, alerting, hyperthermic, and tachycardic effect than light at 550 nm.
Subjects and Methods
Study participants
Ten male volunteers (age range, 21–29 yr; mean, 25.9 ± 3.8 sd) were studied. All study participants were nonsmokers, free from medical, psychiatric, and sleep disorders as assessed by history, a physical examination, and questionnaires. An ophthalmological examination was carried out before the study began and after completion of the study by one of our coauthors (S.O.) to exclude volunteers with visual impairments as well as to be certain that our light application was not harmful. The volunteers were instructed to abstain from caffeine and alcohol for 1 wk before the study; their compliance was verified with urinary toxicological analysis on the day of admission to the laboratory. They were asked to keep a regular sleep-wake schedule (bedtimes and waketimes within ±30 min of self-selected target time) during the week before their admission to the laboratory. Adherence to this regular schedule was verified with a wrist actigraph (Cambridge Neurotechnologies, Cambridge, UK) and daily sleep diaries. All volunteers gave written informed consent. The protocol, screening questionnaires, and consent form were approved by the Ethical Committee of Basel (Basel, Switzerland) and were in agreement with the Declaration of Helsinki.
Study protocol
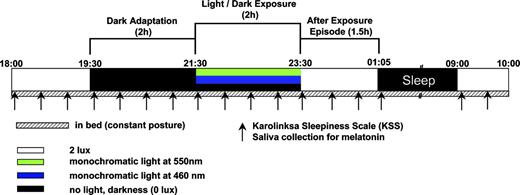
The study consisted of three arms, performed in a balanced order, separated by a 1-wk intervening period (Fig. 1). There were no changes in sleep quality or sleep-wake cycles during the intervening week. On the basis of the habitual bedtimes of the volunteers, a constant posture (CP) protocol started 10 h after usual waketime in the early evening (e.g. 1800 h) and ended the next day, 2 h after usual waketime (e.g. 1000 h). Under CP conditions, the volunteers experienced a controlled, lying-down episode of 1.5 h under 2 lux, followed by a 2-h dark adaptation episode under complete darkness (zero lux). After that, light exposure was initiated for the next 2 h. During this 2-h episode, the volunteers received either monochromatic light at 460 nm, monochromatic light at 550 nm, or no light (zero lux). After this, the volunteers remained awake for another 1.5-h episode under 2 lux (polychromatic white light), before they were allowed to sleep for 7.75 h. One study participant developed a mild cold during one of the study legs and was therefore excluded from additional analysis.
Overview of the protocol design. After 1.5 h under 2 lux, subjects were dark adapted for 2 h, followed by another 2 h in darkness or light exposure at 460 nm or 550 nm (for details about the light exposures, see Subjects and Methods). Subsequently, subjects spent 1.5 h under 2 lux before they were allowed to sleep for 8 h. The entire protocol was carried out under constant recumbent posture conditions in bed. Saliva samples were collected, and sleepiness ratings were taken, both in half-hourly intervals.
Light exposure
Monochromatic light exposure (2 h) was scheduled at a circadian phase at which polychromatic, white light exposure induces robust phase delays (1, 2, 23) and alerting effects (24). The monochromatic light was generated by a 300-W arc-ozone-free Xenon lamp (Thermo Oriel, Spectra Physics, Stratford, CT), filtered by either 460 or 550 nm (Interference filter, ±10 nm half-peak bandwidth, Spectra Physics, Stratford, CT). Monochromatic light was transmitted via two glass-fiber bundles (L.O.T. Oriel-Suisse, Romanel-sur Morges, Switzerland) through the wall, into the soundproofed and temperature-controlled chronobiology suite, onto the goggles that covered the volunteers’ eyes. The custom-built goggles (K. Haug AG, Basel, Switzerland) consisted of two spheres (27.5-mm inner radius) coated with white reflectance paint (two components polyurethane-acryl antifading paint; Lachenmeier & Co. AG, Basel, Switzerland). Each sphere was illuminated via three branches of the main fiber-optic cable to provide constant uniform illumination. Equal photon densities (2.8 × 1013 photons/cm2/sec) for the 460- and 550-nm wavelength light were administered. This irradiance level (12.1 μW/cm2 for 460 nm and 10.05 μW/cm2 for 550 nm) was chosen according to recently reported results on monochromatic light on the human circadian timing system (5). Irradiances were measured with a laser power meter (Laser Check, Coherent, Auburn, CA) before the beginning and at the end of each light exposure. During light exposure as well as during the no-light condition, volunteers were asked to keep their eyes open and to fix their gaze on the middle of the spheres. A technician checked the latter by online monitoring the polysomnographic recordings and also verifying that the subjects remained awake. The volunteers’ pupils were not dilated to avoid possible repercussions of the dilation agent per se on thermoregulation, heart rate, and alertness. However, we tested the effects of the light stimulus on pupil constriction by applying monocular light exposure (light via the goggle of the right eye) and concomitantly measuring the pupil size on the left eye via an infrared camera. The entire control protocol was conducted at the same time of day (evening), with the same light intensity on six subjects. Results from the control experiment revealed a significantly smaller pupil size after the short wavelength light at 460 nm than after light at 550 nm in comparison to the dark condition [P < 0.01; Duncan’s multiple range test performed after a one-way ANOVA for repeated measures with the factor light condition (P < 0.02; dark, 460 and 550 nm)].
Assessment of subjective sleepiness
Subjective sleepiness was assessed every 30 min on the Karolinska Sleepiness Scale (25), with a visual analog scale throughout scheduled wakefulness. Because the participants wore goggles during the light exposure and during the no-light condition, the Karolinska Sleepiness Scale and the visual analog scale were read out loud by a technician and transmitted via the interphone to the volunteers’ room.
Thermometry
CBT and eight surface skin temperatures from different body regions were recorded continuously throughout the study, using a rectal probe and skin thermocouples, with data stored in 20-sec epochs. Distal and proximal skin temperatures as well as the distal-proximal skin temperature gradient (DPG) were calculated according to the procedures described in Ref.26 .
Heart rate
Standard electrocardiogram leads were placed on the lateral thorax and on the sternum. The signal was recorded on the Vitaport-3 digital system at 256 Hz. An off-line algorithm (System Hofstetter, SHS, Allschwil, Switzerland) detected heart rate by the length of R-R intervals.
Salivary melatonin
Saliva was collected at 30-min intervals during scheduled wakefulness. A direct double-antibody RIA was used for the melatonin assay, validated by gas-chromatography-mass spectroscopy (Bühlmann Laboratories, Schönenbuch, Switzerland) (27). The minimum detectable dose of melatonin (analytical sensitivity) was determined to be 0.2 pg/ml. The functional least-detectable dose using the less than 20% coefficient of interassay variation criterion was less than 0.65 pg/ml, and individual serum and saliva melatonin profiles showed excellent parallelism (r = 0.977–0.999; slopes = 0.21–0.63) (27).
Statistics
Statistical analysis of the time course was carried out for each variable using two-way ANOVAs for repeated measures on factor light condition and time interval with Huynh-Feldt’s statistics. P values were based on corrected degrees of freedom, but the original degrees of freedom are reported. The statistical package SAS (version 6.12, SAS Institute Inc., Cary, NC) was used. For post hoc comparisons, one-sided Duncan’s multiple range tests were used. To correct for multiple comparisons, the resulting P values were alpha-corrected according to the procedures described in Ref.28 .
Results
Melatonin suppression and subjective sleepiness
Monochromatic light exposure caused a wavelength-dependent suppression of salivary melatonin (Fig. 1, top panel), as indicated by a significant two-way interaction of the factors light condition and time interval (F14,122 = 3.6; P < 0.001). Post hoc comparisons yielded a significant melatonin suppression after light at 460 nm compared with no light and to light at 550 nm 30 min after the start of light exposure, which continued for the remainder of the light-exposure episode (for post hoc comparisons, see Fig. 2). Salivary melatonin levels during monochromatic light at 550 nm were only slightly but significantly suppressed during the first hour of light exposure (Fig. 2). Subjective sleepiness ratings changed in parallel [interaction light condition × time interval (F14,112 = 1.7; P < 0.05)]. Post hoc comparisons yielded a significant decrease in subjective sleepiness during the 460-nm light exposure compared with 550-nm light exposure and no light, starting 30 min after lights on (Fig. 2, second panel). There was no significant difference in sleepiness between the 550-nm light exposure and the no-light condition.
Effects of a 2-h light exposure at 460 nm (•), 550 nm (▴), and no light (▪) in the evening under CP conditions (i.e. supine in bed) on salivary melatonin levels, subjective sleepiness as rated on the Karolinska Sleepiness Scale, CBT, and heart rate [mean values (n = 9) and ±sem]. For clarity, the sem values for the 550-nm light condition were not plotted. Significant post hoc comparisons (P < 0.05; Duncan’s multiple range test corrected for multiple comparisons) are indicated by the following symbols: *, 460-nm light vs. no light; ○, 550-nm light vs. no light; and ▿, 460-nm light vs. 550-nm light. The prelight exposure episode represents a 2-h dark adaptation episode under zero lux, whereas the light level in the 1.5-h post-light exposure was 2 lux.
Thermoregulation and heart rate
Light exposure changed the time course of CBT [interaction light condition × time interval (F20,160 = 2.9; P < 0.02) (Fig. 2, third panel)]. The evening decline of CBT was significantly attenuated by light at 460 nm starting about 1 h after lights on (Fig. 2), remaining significantly higher throughout the remainder of the 1.5-h interval before sleep. No differences were found between the condition with light at 550 nm and the no-light condition. A similar pattern was found for heart rate, as indexed by beats per min [interaction light condition × time interval (F20,160 = 3.1; P < 0.01) (Fig. 2, bottom panel)]. Although the effect was short lasting, post hoc comparisons revealed a significantly higher heart rate during the 460- nm light condition starting 1.5 h after lights on compared with 550 nm and the no-light condition and lasting for the first 20 min of the after-light exposure episode.
Although repercussions of light at 460 and 550 nm were clearly visible in the time course of both proximal and distal skin temperatures, no significant interaction terms were found (Fig. 3, top two panels). However, the derived measure of the DPG that is used as an estimate of the degree of vasodilation (29) yielded a significant interaction term (F20,160 = 1.8; P < 0.03) (Fig. 3, bottom panel). Post hoc comparisons revealed a significant decrease in the DPG during both 460- and 550-nm light exposures compared with the no-light condition.
Effects of a 2-h light exposure at 460 nm (•), 550 nm (▴), and no light (▪) in the evening under CP conditions (i.e. supine in bed) on proximal and distal skin temperatures as well as the DPG [mean values (n = 9) and ±sem]. For clarity, the sem values for the 550-nm light condition were not plotted. Significant post hoc comparisons (P < 0.05; Duncan’s multiple range test corrected for multiple comparisons) are indicated by the following symbols: *, 460-nm light vs. no light; ○, 550-nm light vs. no light; and ▿, 460-nm light vs. 550-nm light. The prelight exposure episode represents a 2-h dark adaptation episode under zero lux, whereas the light level in the 1.5-h post-light exposure was 2 lux.
Discussion
These results demonstrate that the alerting response to light is wavelength dependent, such that short wavelength light (460 nm) is more effective than longer wavelength light (550 nm) in reducing sleepiness in the evening. Furthermore, our controlled study provides evidence that the effects of light on thermoregulation and heart rate are similarly wavelength dependent.
Our data are in good agreement with recent findings that the human circadian pacemaker is highly sensitive to short wavelength light (13, 30), as indexed by action spectra for human melatonin suppression and assessment of human circadian phase resetting (5, 6). On the basis of these previous studies, we expected a significantly more pronounced attenuation of the nocturnal melatonin increase after light at the shorter wavelength (460 nm), a hypothesis that was clearly verified. We have obtained very similar results as Brainard et al. (13) who reported approximately 60% suppression of melatonin after 2 h of light at 460 nm and at 12.1 μW/cm2. Therefore, melatonin levels in 460 nm did not increase during the light exposure, whereas in the 550-nm condition, they additionally increased very similarly as shown by Lockley et al. (5). To our knowledge, this is the first report showing that human alertness levels as well as thermophysiology are highly sensitive to this short wavelength light. With the exception of the proximal and distal skin temperatures, all other variables (i.e. salivary melatonin, subjective sleepiness, CBT, and heart rate) responded more strongly to 460- than 550-nm light. However, light at 550 nm was not inactive because it induced a subtle, short-lasting but significant melatonin suppression. What is interesting is that both wavelengths decreased the DPG to a similar extent. Why the effects of light on the skin temperatures were not wavelength dependent remains to be elucidated. Although our study was conducted under very controlled laboratory conditions (i.e. CP, room temperature, and food intake), skin temperatures exhibit large inter- and intraindividual variance (31). Therefore, it may be that this measure did not provide enough power to differentiate between the two wavelengths. However, there are two possibilities: 1) the DPG may indeed be a very sensitive measure for subtle illuminance changes; and/or 2) that it immediately reflects a minute-to-minute level of cognitive arousal independent of the sensory modality of the signal. The DPG increase during the dark-adaptation episode, which was unusual at this circadian phase as previously measured under 8 lux of ambient-light levels in a constant routine protocol (26, 31), can be interpreted in both ways—a diminution to zero lux and a diminution of sensory input, leading to relaxation. Interestingly, this increased DPG was also paralleled by an unusually early increase in subjective sleepiness and an unusually early evening melatonin onset. Furthermore, evidence for the responsiveness of DPG comes from the decrease in this measure seen after the 4-h dark episode in the no-light condition, when the volunteers were under 2 lux (Fig. 2, bottom panel). The DPG reflected very sensitively whether the lights were on or off. Besides the DPG, the increase in heart rate during the 460-nm light exposure may well be another indication that the autonomous nervous system acutely responds to light with an increase in sympathetic tone—a response that seems particularly susceptible to short wavelength light.
Many studies have shown that exposure to white polychromatic light during the evening or nighttime increases alertness (8, 10, 24, 32–35) and CBT (8–10, 19, 34, 35). There is also evidence that light may acutely affect heart rate (12). Previously, we have found a dose-response relationship between the magnitude of the alerting response to light and its irradiance, such that half of the maximum alerting response to bright light at 9100 lux was obtained with room light of approximately 100 lux (10). However, the duration of light exposure in this study was rather long (6.5 h), and the dose relationship was only present in the latter part of the light exposure (10). In contrast, the present study revealed that light at 460 nm of very low intensity (5 photopic lux or 116.6 scotopic lux) was already effective after about 40 min of exposure, which corroborates high specificity for light in the short wavelength range, and shows that the nonimage-forming visual system does not simply count or average photons, but rather depends on exposure to particular wavelengths of energy. In fact, during the 460-nm light condition, volunteers in our experiment probably received fewer photons, because their pupil size was smaller than in the 550-nm light condition (based on our data from the control experiment; see Subjects and Methods). Therefore, the melatonin suppression and the alerting response was underestimated from what they would have been had the subjects’ pupils been artificially dilated. Despite fewer photons, 460-nm light was more efficient on the above-described variables than 550 nm, which corroborates its effectiveness also in the absence of pupil dilators.
Our results demonstrate that besides regulating human circadian rhythms, the nonclassical photoreceptors are also involved in the regulation of the acute effects of light, which has until now only been shown for ERG responses (15). Although it is possible that the central circadian pacemaker located in the suprachiasmatic nuclei (SCN) is involved in both phase shifting and acute responses to light, it is not clear that these share a common mechanism. It has been proposed that acute changes in CBT may be primary events mediating circadian phase-shift responses (36). There is, however, contrary evidence; previous administration of melatonin can completely reverse the acute CBT elevation induced by nighttime bright light without greatly altering light-induced phase shifts (23, 37, 38). Whether the delayed decline in CBT represents only an acute effect of light or whether it is the initiation of a circadian phase delay would have required a longer study. We may interpret the sustained evening maximum in CBT only after 460-nm light exposure as evidence for the latter explanation of a selective circadian phase delay shift.
The mechanisms by which light induces acute physiological responses and shifts circadian phase seem to diverge at some level. Besides the SCN, candidate retinal projections for the acute effects of light are the pretectal area (39), the intergeniculate leaflet (40), and the ventromedial preoptic area (41). It is clear that for light to rapidly suppress melatonin secretion, retinal projections to the SCN are necessary. Therefore, it has been suggested that the mechanism by which light exposure may reduce sleepiness is by its suppression of melatonin synthesis (8, 10, 24). However, there is recent evidence that these effects appear to be mediated by mechanisms that are separate from melatonin suppression (42). It is more likely to be the ventromedial preoptic area that innervates all of the major nuclei of the ascending monoaminergic and, in particular, the histaminergic system and plays a key role in wakefulness and electroencephalogram arousal (43, 44).
All of the above-mentioned brain regions receive projections from intrinsically photosensitive retinal ganglion cells for which the photopigment melanopsin has recently been identified (45). Melanopsin is present in the human retina (46), and melanopsin-containing retinal ganglion cells are directly photosensitive at a λmax of 484 nm in the rat (47). Melanopsin expression defines a subset of retinal ganglion cells that play a broad role in the regulation of nonvisual photoreception, providing projections that contribute to circadian entrainment, negative masking, the regulation of sleep-wake states, and the pupillary reflex (for citations, see Ref.45). Our results add to these functions, suggesting that changes in human alertness and aspects of autonomic control (thermoregulation and heart rate) are influenced, if not regulated, by the nonvisual system via the photopigment melanopsin. A definite answer to this would be to investigate people lacking the classical receptors, or having a melanopsin deficiency, to the see repercussions this may have on light-induced changes in alertness, thermoregulation, and heart rate. At least in blind mice, melanopsin is required for nonimage-forming photic responses. However, there is still a debate (see Ref.48) as to whether the photopigment melanopsin is the only candidate for nonvisual ocular photoreception.
It will be interesting to test whether short wavelength light is more efficient in the workplace environment, where high alertness levels are required, and in the treatment of seasonal affective disorder; although in all putative applications, the blue-light damage potential needs to be evaluated (49). An important physiological question is whether the decline in alertness and thermoregulation with age is a consequence of age-related changes in lens transmittance at the short-wave range (50).
We thank Dr. Corinna Schnitzler for medical screenings; Claudia Renz, Giovanni Balestrieri, and Marie-France Dattler for their help in data acquisition; and the volunteers for participating.
This research was supported by the Velux Foundation (Glarus, Switzerland) and in part by The Swiss National Foundation Grants START 3130-054991.98 and 3100-055385.98 (to C.C.).
First Published Online December 7, 2004
Abbreviations:
- CBT,
Core body temperature;
- CP,
constant posture;
- DPG,
distal-proximal skin temperature gradient;
- ERG,
electroretinogram;
- SCN,
suprachiasmatic nuclei.



![Effects of a 2-h light exposure at 460 nm (•), 550 nm (▴), and no light (▪) in the evening under CP conditions (i.e. supine in bed) on salivary melatonin levels, subjective sleepiness as rated on the Karolinska Sleepiness Scale, CBT, and heart rate [mean values (n = 9) and ±sem]. For clarity, the sem values for the 550-nm light condition were not plotted. Significant post hoc comparisons (P < 0.05; Duncan’s multiple range test corrected for multiple comparisons) are indicated by the following symbols: *, 460-nm light vs. no light; ○, 550-nm light vs. no light; and ▿, 460-nm light vs. 550-nm light. The prelight exposure episode represents a 2-h dark adaptation episode under zero lux, whereas the light level in the 1.5-h post-light exposure was 2 lux.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/90/3/10.1210_jc.2004-0957/1/m_zeg0030512900002.jpeg?Expires=1716323296&Signature=0k29PTgYP1baGEBMo-9UH8BS3U6ztnbkBoxYAy8BVot05ZAmQYNzVQgCp8KxCCrFYG0Tx2dtUIkX~0An43HYub5F2G5gSMnwVtAxUh9hndhFHI~0M2AjQP6~N7FlcDx8tHFG52CfQr-DGN2okRYJh8HPLAQh9bTstoZbFyK-TrJLeyDh5BlufIQRBh6OPzPjqT8CVOx4W5Rc0E68goJFTGQJexja3sAJrATX1ULtEtUUh0RZawgxgkbg8afdkRxe5wZqQB6VchnpUtpUikIyuPJigTBLzz3DMhwVqxoD8hbEkhQpJ-pn3PEK8ujJNuP17ohPWbmltuKoL8SKm4ikZA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Effects of a 2-h light exposure at 460 nm (•), 550 nm (▴), and no light (▪) in the evening under CP conditions (i.e. supine in bed) on proximal and distal skin temperatures as well as the DPG [mean values (n = 9) and ±sem]. For clarity, the sem values for the 550-nm light condition were not plotted. Significant post hoc comparisons (P < 0.05; Duncan’s multiple range test corrected for multiple comparisons) are indicated by the following symbols: *, 460-nm light vs. no light; ○, 550-nm light vs. no light; and ▿, 460-nm light vs. 550-nm light. The prelight exposure episode represents a 2-h dark adaptation episode under zero lux, whereas the light level in the 1.5-h post-light exposure was 2 lux.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/90/3/10.1210_jc.2004-0957/1/m_zeg0030512900003.jpeg?Expires=1716323296&Signature=eLRJD0soWcuGrpzCoDFALQqbyOW77XfOpVNt1jb6x9Op-R3vxj4ZRY56HFsgeF9tawm-mItfeK7julr3fGi29AVdtCLml3UdBc-JAV91SbAmFEJxHoRLkFM8qZzG8BAJTrikFx3rRWumqLZ-fE-6Axslv2bY53QgFtayD4lnHghGAPXjKhVhGMEZkP80FL--walRWGp0zDAuxpx6JeWZDkALYWLW4-VIADQnOWtprtIPMAchkJeTzi~~UEks9dcEtY2Ce2KFrdVEJ3NcFofH~nvSghvySo~84cTyFbEmCEZK3icGXBQUIzellyZfQimxtJaeqR5Lu1Kovf4NaOzOSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
