-
PDF
- Split View
-
Views
-
Cite
Cite
Roger A. Lobo, Where Are We 10 Years After the Women's Health Initiative?, The Journal of Clinical Endocrinology & Metabolism, Volume 98, Issue 5, 1 May 2013, Pages 1771–1780, https://doi.org/10.1210/jc.2012-4070
Close - Share Icon Share
The media attention surrounding the publication of the initial results of WHI in 2002 led to fear and confusion regarding the use of hormonal therapy (HT) after menopause. This led to a dramatic reduction in prescriptions for HT in the United States and around the world. Although in 2002 it was stated that the results pertained to all women receiving HT, subsequent studies from the Women's Health Initiative (WHI) and others clearly showed that younger women and those close to menopause had a very beneficial risk-to-benefit ratio. Indeed, the results showed similar protective effects for coronary disease and a reduction in mortality that had been shown in earlier observational studies, which had also focused on younger symptomatic women. In younger women, the increased number of cases of venous thrombosis and ischemic stroke was low, rendering them “rare” events using World Health Organization nomenclature. Breast cancer rates were also low and were found to be decreased with estrogen alone. In women receiving estrogen and progestogen for the first time in the WHI, breast cancer rates did not increase significantly for 7 years. Other data suggest that other regimens and the use of other progestogens may also be safer. It has been argued that in the 10 years since WHI, many women have been denied HT, including those with severe symptoms, and that this has significantly disadvantaged a generation of women. Some reports have also suggested an increased rate of osteoporotic fractures since the WHI. Therefore, the question is posed as to whether we have now come full circle in our understanding of the use of HT in younger women. Although it is appropriate to treat women with symptoms at the onset of menopause, because there is no proven therapy for primary prevention, in some women the use of HT for this role may at least be entertained.
The Women's Health Initiative (WHI) was a National Institutes of Health (NIH)-sponsored multi-outcome study comprised of 4 separate trials. These were studies on a low-fat diet, calcium-vitamin supplementation, estrogen and progestin therapy (conjugated equine estrogen 0.625 mg together with medroxyprogesterone acetate 2.5 mg), and estrogen-alone therapy (conjugated equine estrogen 0.625 mg). When we discuss WHI today, we generally refer to the results of the hormonal therapy (HT) intervention studies. The costs for this undertaking have been estimated to be $1 billion.
For at least 2 decades before WHI, data from large observational trials had suggested that HT resulted in a reduction in coronary heart disease (CHD) and mortality (1–4). Because of inherent biases in observational data, it was necessary to carry out prospective randomized trials to confirm these findings. WHI was designed to evaluate the long-term benefits and risks of postmenopausal HT with the understanding that women would be taking estrogen long term for the prevention of heart disease, not for the treatment of symptoms. However, most women in the United States initiated HT for the treatment of symptoms, not for the prevention of osteoporosis or heart disease (5).
Although considered to be a primary prevention trial, WHI did not study primary prevention in that most women were largely asymptomatic and were many years past menopause. Women up to the age of 79 years were included, and the average age of the participants was 63 years, which was on average approximately 12 years past menopause (6). Figure 1 depicts the normal age-related development and progression of atherosclerosis in the years following menopause. The percentage of women participating in WHI by years since menopause is also depicted, showing that approximately 83% of the WHI participants were more than 5 years from menopause (7). This contrasts sharply with the age of the observational cohorts who had provided the beneficial data on cardiovascular disease and mortality. These women were younger, were closer to menopause, and had received HT for symptoms of menopause.
Progression of coronary atherosclerosis by age in postmenopausal women and the ages of women participating in the hormonal trial of the WHI. [Adapted from T. B. Clarkson: The new conundrum: do estrogens have any cardiovascular benefits? Int J Fertil Womens Med. 2002;47:61–68 (7), with permission. © U.S. International Foundation for Studies in Reproduction, Inc.]
By the time WHI was under way, several secondary prevention trials (women with known coronary disease who were prescribed HT) looking at hard end-points of myocardial infarction (MI) and death (8), as well as some angiographic trials (9, 10) had shown that there was no coronary benefit with HT at standard doses. In some studies, “early harm” occurred, defined as more coronary events in the first 1–2 years, when compared to placebo. In 2002, the estrogen plus progestin (E+P) trial of the WHI was terminated after 5.6 years, having found no coronary benefit and a rate of breast cancer that crossed preset boundaries (6).
The well-orchestrated release of information to the media in the summer of 2002 was problematic on several fronts. Principal investigators of WHI did not have an opportunity to review the data, which was not completely adjudicated, and the results were rushed to publication and disseminated to the media. Here the statements were dogmatic, and there was no explanation of the differences between relative risk and absolute or attributable risk. Relative risk describes the degree of change in a risk over the baseline rate, whereas the absolute risk provides the actual number of cases that would be increased or decreased in a given population. Even if the relative risks were statistically valid, some of which changed with time (11), the absolute risks were small, making these events “rare” using World Health Organization terminology, which will be detailed below (11). It was emphatically stated that “the adverse effects of estrogen plus progestin applied to all women, irrespective of age, ethnicity or disease status” (12). The NIH director of the study was quoted as saying that NIH was going “for high impact” with the goal “to shake up the medical establishment and change the thinking about hormones” (13). By 2007, with subsequent release of data, various media reports appeared, which is typified by the following statement in the Wall Street Journal “… some aspects of what was reported were misleading or just wrong …. Women in their 50s had a 30% lower risk of dying” (14).
It has been well documented that since the initial publication of WHI in 2002, hormonal use has decreased substantially (15) (Figure 2). Our most recent data from NHANES (2009–2010) suggests that the current use of HT in women over 40 is 4.7%, and in women aged 50–59 years it was 6.7%, compared to a total rate of use of 38.3% in 1999–2000 (16). By 2004–2005, there were data to suggest that the age-adjusted osteoporosis-related fractures had increased compared to 2000–2001 (17); an observational study of 80 955 women followed for 6.5 years since 2002 found an increased rate of hip fractures among women discontinuing HT compared to those who remained on it (18).
Quarterly claim volume per 10 000 covered members, overall by prescriber type. Shaded area indicates period before release of initial WHI results. Ob/Gyn, obstetrician/gynecologists. [Reproduced from B. Ettinger et al: Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause. 2012;19:610–615 (15), with permission. © The North American Menopause Society.]
As early as 2006, data emerged from the subset of younger women in WHI that did not show an increased risk, but showing a strong trend to decreased risk that was more consistent with data from the older observational data. For estrogen-alone therapy, a composite coronary score was significantly decreased in the women aged 50–59 years (19); these women also had decreased coronary calcium scores (20). In a combined analysis of younger women in WHI on estrogen alone and E+P, total mortality was also decreased (21). These and more recent data will be reviewed in Coronary Heart Disease Findings.
In 2011, a consensus statement by the International Menopause Society (IMS) regarding HT stated the following: “The excessive conservatism engendered by the presentation to the media of the first results of the WHI in 2002 has disadvantaged nearly a decade of women who may have missed the therapeutic window to reduce their future cardiovascular, fracture, and dementia risk” (22). This potentially provocative statement will be critically assessed in light of the existing literature to date.
Coronary Heart Disease Findings
The coronary findings in WHI for the E+P trial were of borderline significance 1.24 (1.00–1.54) (23). Furthermore, the reported data (point estimates of risk) varied over several publications (11). However, there was some evidence for early harm among the older women in the E+P trial, as had been suggested earlier in the Heart and Estrogen/Progestin Replacement Study (HERS) (8), but no increase in younger women. Indeed, the point estimates in the younger group showed a trend to benefit. In women receiving E+P < 10 years from menopause, the hazard ratio was 0.89, and it was > 1 in the older age groups. In the estrogen-alone trial in hysterectomized women, there was clearly benefit in using a composite coronary score: 0.66 (0.45–0.96) (19). In WHI and in a recent case-control study, coronary calcium scores were significantly reduced in women on estrogen (20, 24). A meta-analysis of women receiving HT who were under 60 years old, including data from WHI, showed a statistically significant reduction in coronary disease (25).
In a WHI publication that combined data from the HT groups stratified by recency of menopause, women < 10 years from menopause had a hazard ratio for CHD of 0.76 (0.5–1.16), with a significant trend for worsening with time since menopause in the other groups (21). There was also a 30% reduction in all-cause mortality as discussed earlier: 0.70 (0.51–0.96). A 10-year follow-up of women in the estrogen-alone trial in WHI showed that the 50- to 59-year-old group had a significantly reduced risk of MI (0.54 [0.34–0.86]) and CHD (0.59 [0.38–0.90]). In this study, total mortality (mainly cardiovascular) was also reduced 0.73 (0.59–1.00) (26).
Although the data cited above are strongly suggestive of a coronary benefit in younger women receiving estrogen, the data are based on subanalyses of large randomized trials, and therefore should be interpreted with some caution. In younger women, the hard end-points of MI or death are relatively rare. Therefore, an extremely large, adequately powered study of sufficient duration to prove this suggested beneficial effect would be extremely difficult to achieve.
The “timing” hypothesis suggests that younger symptomatic women at the onset of menopause may be protected from CHD, whereas older women treated for the first time have no benefit from HT and may have early harm. The Kronos Early Estrogen Prevention Study (KEEPS) was not designed to test the timing hypothesis but studied surrogate markers with HT in women at the onset of menopause. KEEPS was a randomized clinical trial (RCT) in recently (<3 y) postmenopausal women who received placebo, conjugated equine estrogen 0.45 mg, or transdermal estradiol 0.05 mg continuously for 4 years. All women had a uterus and received micronized progesterone 200 mg (or progesterone placebo) for 12 days each month. Only intermediate or surrogate end-points could be assessed in this short-duration study. The primary end-point was carotid intima-media thickness (IMT), and the secondary end-point was changes in coronary calcium. Multiple other end-points were assessed including symptoms, biochemical parameters, quality of life, and cognitive function. Only preliminary data have been presented at this time (27). Carotid IMT rose to the same degree in all groups, but all were lower than the rate of increase noted in a previous RCT where oral estradiol was shown to attenuate the progression of IMT (28). There was also a nonsignificant trend for coronary calcium to progress less in the 2 estrogen groups compared to placebo. Again, these very healthy women had very little coronary calcium (having coronary calcium was an exclusionary criterion at entry), and it may be that in KEEPS, there was too little atherosclerosis progression to pick up any potential changes with estrogen. Symptoms, quality of life parameters, and biochemical changes were confirmatory of the known effects of estrogen, and there were no significant adverse effects. There was no deterioration of cognitive function and some trend to improvement in verbal memory (29).
The Danish Osteoporosis Prevention Study (DOPS) was recently published (30). This was a RCT of 1006 younger women at the onset of menopause that provided more evidence in support of the timing hypothesis. Because a composite end-point was used that included coronary disease and mortality, the data will be reviewed here and in the next section. These women were treated in an open-label fashion with oral estradiol and norethindrone acetate or estradiol alone for 10 years and followed for up to 16 years. A combined end-point of mortality and hospitalizations for congestive heart failure or MI was significantly reduced in those women randomized to HT compared to the control women randomized to no treatment (30) (Figure 3). Moreover, the data suggested that the younger the women were in the trial, the more they showed a benefit. The DOPS has been criticized on the grounds that cardiovascular health was not the primary outcome of the study because it was designed to assess osteoporosis, no placebo was used, and there may not have been sufficient power. However, the hard end-points studied were well validated in the national data base, and the significant findings reported render this study noteworthy. At the same time, these younger women in DOPS had no increase in thrombosis, stroke, or any cancer. In DOPS, the younger women treated were similar to those women in the observational studies of the past, which consistently showed this potential benefit of HT. Although it could be argued that among the RCTs no one study is definitive, and some of the evidence is based on subanalyses of randomized trials as noted above, the totality of the data is consistent in showing benefit in younger women and not harm.
Risk of death or admission to hospital due to heart failure or MI (primary end-point) over 16-year follow-up, including 11 years of randomized treatment. Comparison of women randomized to hormonal treatment compared to controls randomized to no treatment. [Reproduced from I. L. Schierbeck et al: Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomized trial. BMJ. 345:e6409 (30). © BMJ Publishing Group Ltd.]
What then did we learn from WHI in terms of CHD? We did learn that older women who are distant from menopause, who have established atherosclerosis (Figure 1), and who receive standard doses of oral HT are at increased risk for coronary plaque instability, mural rupture, and thrombosis. Although speculative, a hypothesis has been put forth to explain the observed findings. It has been hypothesized that this occurs because oral estrogen increases proinflammatory factors such as matrix metalloproteinase (MMP)-9 (31), which digests away the gelatinous matrix of the atheromatous plaque causing instability and rupture (32) This causes the phenomenon of early harm observed in several secondary prevention trials such as HERS (8) and the null effects in women with established coronary disease (9, 10, 33), where in the setting of significant atherosclerosis, beneficial estrogen action is impeded. Although oral estrogen increases MMPs, in younger women, without significant plaque there is no substrate on which MMPs would act. Indeed in younger women who were studied prospectively in several trials of HT, there was no evidence of this phenomenon of early harm. (34)
Mortality Changes with HT
Early observational data in several cohorts of women initiating HT for symptoms at the onset of menopause have provided consistent data showing a reduction in all-cause mortality in the range of 30% (3, 4, 35, 36). In WHI, the combined hormonal arm in women < 10 years from menopause showed a statistical reduction in mortality: 0.70 (0.51–0.96) (21). A Bayesian meta-analysis looking at both observational data and RCTs showed a statistically significant reduction in all-cause mortality in younger women (0.73 [0.52–0.96]) for the RCT data (37). In the 10-year follow-up data from the estrogen-alone trial in WHI, the 50- to 59-year-old age group had an identical point estimate of 0.73 (0.53–1.0). In DOPS, although the combined end-point of mortality, congestive heart failure and MI, was significantly reduced, mortality alone did not achieve statistical significance, most likely because of insufficient power in studying these younger women (0.57 [0.30–1.08]) (30). The data noted above appear to be extremely consistent in showing a decrease in all-cause mortality with HT in younger women. This reduction in the range of 30% is principally due to a reduction in CHD, and these data are remarkably in line with the previous data from observational studies (3, 4, 35, 36)
Stroke with HT
The effect of HT on stroke risk has been controversial. This is largely because there are many clinical variables that confound the data (primarily obesity and hypertension) and because the risk is of borderline significance. Nevertheless, several meta-analyses and observational data have shown a small increase (approximately 30%) in ischemic stroke (not hemorrhagic stroke) even in younger women receiving standard doses of oral estrogen (38, 39). In WHI, whereas there was an overall increase in the entire group with both E+P and estrogen alone, an increased risk was less evident in younger women. There was no significant increase observed with E+P in younger women, but in the estrogen-alone trial, although subgroup analysis in the 50- to 59-year-old group did not show a statistically significant increase, the women < 10 years from menopause had a statistically significant increase based on small numbers (40). Note that adjustment for this not having been the primary outcome of the trial would have rendered this finding not statistically significant. It may therefore be concluded that there may be some risk (perhaps of borderline significance) even in younger women. This is consistent with the suggestion of a small increased risk of ischemic stroke in reproductive-aged women using oral contraceptives. Recent data suggest that a small risk is observed if enough women are followed (41). The mechanism of increased risk of ischemic stroke in younger women, however, is thrombotic and not atherosclerotic as it is in older women, and could be on the basis of unknown thrombophilic sensitivities to estrogen (42). Consistent with this view is that ischemic stroke has not been observed with transdermal estrogen, unless high doses are used (43), or with lower oral doses (38, 39)
Although there may be an increased risk of ischemic stroke even in younger women receiving standard doses of oral estrogen, the absolute risk is small. The background risk of ischemic stroke in a 50- to 54-year-old woman is 3.8/10 000 woman years (39). Therefore, if we assume an approximate 30% increased risk, this would result in 1 or 2 more cases per 10 000 woman years, rendering this a “rare” occurrence. Furthermore, it may be suggested that this putative risk may be nullified by lower oral doses or the use of transdermal estrogen.
Venous Thrombosis with HT
The discussion above is also extremely relevant to the findings of venous thrombosis risk with HT. However, it has been well established that oral estrogen increases the risk of thrombosis. As observed in WHI, the risk of standard doses of oral estrogen increases the risk of venous thromboembolism about 2-fold, with most cases occurring in the first or second year of therapy, but with no changes in mortality. In WHI, some women who had a previous history of thrombosis were enrolled into the trial. The data in the estrogen-alone trial were not statistically significant. Although this was a more obese cohort, many of the women, having had a hysterectomy, had been on hormones previously. Accordingly, it could be envisioned that less vulnerable women who had been exposed to estrogen in the past were enrolled in the trial and did not have a thrombotic event. There are very consistent data that the risk is not increased with transdermal estrogen (44, 45). Other data have also suggested that various progestogens may increase the risk of thrombosis over that of estrogen. This includes norpregnane derivatives (nomegestrol acetate, promegestone) (46) as well as medroxyprogesterone acetate (47). The latter study also suggested that there was an increased risk with a continuous regimen of estrogen and progestogen (47), which was the type of regimen used in WHI.
Similar to the discussion for stroke, the thrombosis risk with HT has a small absolute risk. The venous thromboembolism risk in younger women, assuming a 2-fold relative risk, is in the range of 30/100 000 woman years. This rare occurrence is less than the rate in normal pregnancy, approximately 60/100 000 woman years (48).
Breast Cancer Risk with HT
The major fear women have regarding HT is the potential of developing breast cancer (49). In 2002 the major reason the E+P trial was stopped was that a preset boundary for breast cancer had been crossed. Although point estimates for the risk with E+P varied in several publications (in the range of 1.24–1.28) (11) and was generally of borderline significance, the data were interpreted as being highly significant. In a follow-up publication of WHI investigators in 2006, adjustment for risk factors showed a nonsignificant increase of 1.20 (0.94–1.53) (50).
Moreover, it was clear from this and an earlier publication (51) that in women who had never received hormones in the past, this risk was not significant over the 5.6 years of the trial: 1.09 (0.86–1.40) (50). The increased risk was primarily attributable to prior users who had a greater cumulative exposure to hormones. Also, the risk was not significantly increased in younger women (51). The risk with E+P, however, does increase with time, but sensitivity analyses in adherent participants showed no increase for at least 7 years (1.23 [0.90–1.67]) (50).
More recent epidemiological data from WHI chose to combine analyses from observational and trial data, which does not appear to be valid. A “gap analysis” was carried out based on timing of initiation of hormones after menopause, which also was based on imprecise data. These analyses suggested that earlier initiation of E+P increases the risk of breast cancer in adherent women (52). However, as reviewed by a coauthor, these analyses are at odds with the actual published data of the trial and do not appear to be valid (53). It is clear that although there is increased risk with time, the risk with the E+P regimen used in WHI is greater in older rather than younger women, and that the risk does not increase for 7 years in women who have never received hormones in the past.
Although it is clear that the risk with E+P is greater than that of using estrogen alone (discussed below) the regimen may be of importance. Observational data from France have suggested that the risk is not increased with micronized progesterone or dydrogesterone (54), and in DOPS using estradiol and norethindrone acetate there was no increase in breast cancer after up to 11 years of therapy and a 16-year follow-up period, although the number of women in this trial was not large (30).
Recent iterative analyses and modeling have suggested that the effect of hormones is to promote the growth of occult breast tumors. It has been estimated that 93.3% of the breast cancers in the E+P trial were occult tumors (54), and the effect of E+P was to decrease the normal doubling time (leading to an earlier clinical detection) from 200 to 150 days (55).
In the estrogen-alone trial, breast cancer rates were seen to decrease, which was significant among adherent women (56). This was confirmed in the 10-year follow-up data of the estrogen-alone trial, where breast cancer rates were significantly decreased; among women with breast cancer, both breast cancer mortality and total mortality were significantly decreased in estrogen users (57). The reduced rate of breast cancer is thought to be due to a proapoptotic effect in women “deprived” for some time from estrogen (55). This hypothesis suggests that the initial use of estrogen (most likely dependent on dose) increases the rate of growth of occult tumors, and if not begun for a period of time, the effect of estrogen “deprivation” results in a proapoptotic effect through at least 2 mechanisms (intrinsic or mitochondrial and extrinsic) (58). Dose and duration of therapy are likely to be important, but there are few data on this issue. In an observational study of hysterectomized women using conjugated equine estrogen 0.625 mg, breast cancer risk did not increase for at least 15 years, and this was predominantly seen in lean women (59). Increased body mass index is a significant endogenous risk factor for breast cancer, which is similar to, or exceeds the risk of HT (60, 61).
Much has been made of the declining rates of breast cancer in the United States and that this may be linked to the cessation of HT use since 2002. However, this is far too simplistic a view. The decline began before 2002 when the use of HT was higher, the decreased rate occurred in all age groups including in older women not expected to be taking HT, and there clearly has been a change in breast cancer surveillance over time. The decline in HT use has occurred all over the world, yet not all countries have reported a downward trend of breast cancer rates (62). Thus, whereas some of the decline in breast cancer rates observed in some countries, such as the United States, may be related in part to a decline in hormonal use, the change in HT use does not explain the whole phenomenon.
Putting the potential risks of breast cancer into perspective is extremely important in discussing HT with women. Although estrogen alone may decrease the risk, it probably does not increase the risk unless large doses are used for a prolonged time in susceptible women with unknown occult tumors. With E+P, young women initiating standard dose therapy for the first time at the onset of menopause do not have an increased risk of breast cancer for at least 5 years, and probably for up to 7 years, although the risk does increase thereafter at least for the regimen studied in WHI. Other regimens and doses may be safer, but definitive data are lacking. What is the magnitude of this risk in real terms? Although there is not a statistically significant increase over 5 years with E+P in younger women who have never been on hormones, for illustrative purposes the absolute risk will be calculated assuming an overall increased risk over 5 years as originally reported by WHI (relative risk of 1.24). A 50-year-old woman may expect that her background or endogenous risk of breast cancer will be 2.8% by age 60 years. The putative increased risk of breast cancer with using HT for 5 years would increase her risk to 3.37%—an absolute increased risk of 0.67%. This is less than the risk conferred by obesity, by being a flight attendant, or by many other common exposures (61).
Cognitive and Dementia Risks/Benefits with HT
Several observational studies and meta-analysis had suggested that estrogen prescribed to younger women at the onset of menopause decreases the risk of Alzheimer's disease or delays its onset (63, 64). In WHI, when older women (> 65 y) were studied, there was a significant detrimental effect in cognitive function in women in the E+P trial (65) and only a trend to this effect with estrogen alone (66). It has been hypothesized that the timing of initiation of HT is critical here, as it is for CHD. However, no RCT to date has been able to prove a cognitive benefit. In the recently completed KEEPS, preliminary data have suggested that there is no detrimental effect of HT, and indeed a trend to benefit in certain women (29) although this short-term trial was not designed to assess the effects of HT on cognition and dementia. While we await more definitive prospective trial data, a possible benefit based on the timing of initiation of HT is consistent with the observational studies cited above (63, 64) as well as other observational studies that focused on the timing of initiation (67–69).
Mood Effects with HT
The reduction of menopausal symptoms has been associated with improvements in mood, depression scores, and insomnia in multiple clinical trials. Quality of life assessments have also shown benefit (70). However, whether this clinical benefit is exclusively due to a reduction in symptoms of menopause remains unclear, although it does explain some of this benefit. Whether there is a benefit in asymptomatic postmenopausal women has not been proven, although smaller prospective trials have suggested some benefit (71). In KEEPS, improvements in depression, anxiety, and sexual function were observed (27).
Cost Effectiveness of HT
Assessments of quality-adjusted years of life in women on HT have consistently shown that HT is cost-effective, predominantly in younger women (72, 73). In The Endocrine Society Task Force report on HT, the number of younger women benefiting from the reduction in symptomatology greatly overcomes numerically any of the attributable risks or benefits discussed above (74) (Figure 4).
Attributable or excess risk or benefit per 1000 women receiving menopausal HT for 5 years who are 50–59 years old or < 10 years from menopause who have relief from hot flushes and symptoms of vaginal atrophy. [Adapted from Santen RJ (74).]
Bone Health as an Indication for HT
It is undeniable that HT reduces fractures in women, even in those who do not have established osteoporosis or are not at a particularly high risk for fracture, as was shown in WHI (75). Earlier it was discussed that since the cessation of HT in many women after the initial publication of data from WHI, many more osteoporosis-related fractures have occurred (17, 18). Accordingly, it has been argued that HT should be an indication for the prevention and treatment of osteoporotic fractures in young women, even if they are asymptomatic (74). Recent society guidelines are consistent with this view (22, 76). The North American Menopause Society guidelines of 2012 suggest that HT may be considered in younger women at high risk for fracture (76). It remains fairly nebulous, however, how “high” this risk should be in that many women have risk factors for fracture. Clearly, other antiresorptive therapies such as bisphosphonates are not appropriate in young women, particularly those without documented osteoporosis.
HT as a Preventative Therapy in Younger Women?
In revisiting the 2011 IMS guidelines statement, the sentiment strongly suggests a role for HT in primary prevention, rather than merely short-term use for menopausal symptoms (22). Apart from lifestyle modification, there is no known primary prevention strategy that has been validated for women. Although HT has been shown prospectively to decrease the risk of new-onset diabetes (77, 78), the use of statins increases this risk (79, 80) and does not alter mortality (81). Although it continues to be argued by some that statin therapy may have a beneficial effect in women, there is no real evidence of its role in primary prevention in young healthy women < 60 years old (82). However, it is more clear that HT in younger women reduces all-cause mortality, as discussed above (observational studies, Refs. 3, 4, 35, and 36; randomized trials, Refs. 21 and 37; 2 additional trials barely missed statistical significance, Refs. 26 and 30). Aspirin therapy also does not have a role in primary prevention in younger women (83). There has been a suggestion that calcium supplements may increase MI and death rates, although this was shown predominantly in older women (84).
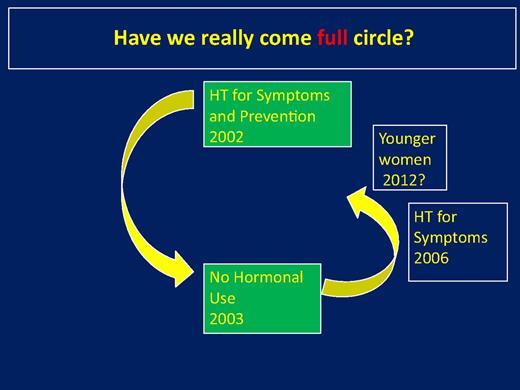
Thus, 10 years after WHI, have we come full circle? A diagram of this conundrum is depicted in Figure 5. Before WHI, many clinicians believed that estrogen protected against coronary disease and osteoporosis and had benefits in terms of treating various symptoms of menopause, and therefore should be used, in the absence of known contraindications, for prevention. What was not clear until after WHI is that age and years since menopause were significant variables. By 1 to 2 years after WHI, HT was rarely used, even for women with symptoms. Indeed, in concert with the sentiment of the IMS statement reviewed above (22), it is certain that many severely symptomatic women were made to bear their symptoms unnecessarily without treatment. However, around 2006, it was generally agreed that HT could be used for younger women to treat symptoms. The current data, particularly with estrogen alone, are highly supportive for a prevention role in reducing fractures, CHD, and mortality in younger women who initiate therapy close to menopause, as was shown originally in observational studies, with a very favorable benefit-to-risk ratio. The data with E+P are similarly suggestive but are less precise. Nevertheless, in DOPS there were statistically significant benefits with E+P, but using a different regimen and progestogen. It is likely, therefore, that different doses and regimens and the type of progestogen are probably quite important in this regard, but specific data are lacking.
Suggested use of HT from before the time of the publication of WHI to the present, posing the question as to whether we have come full circle.
Clearly, more research is needed, but it is not likely that we will have useful information any time soon. Not only will it be prohibitively expensive to conduct a WHI-type trial exclusively in younger women looking at hard end-points such as MI and death, but it will take many years to accrue these events.
In the meantime, we need to individualize therapy in those women with symptoms, with the view that in young healthy women, we probably have come full circle, and a role for HT in prevention may at least be entertained.
Acknowledgments
Disclosure Summary: The author has no real or perceived conflicts of interest with anything involving this work.
Abbreviations
- CHD
coronary heart disease
- E+P
estrogen, plus progestin
- HT
hormonal therapy
- IMT
intima-media thickness
- MI
myocardial infarction
- MMP
matrix metalloproteinase
- RCT
randomized clinical trial
- WHI
Women's Health Initiative.
References



![Progression of coronary atherosclerosis by age in postmenopausal women and the ages of women participating in the hormonal trial of the WHI. [Adapted from T. B. Clarkson: The new conundrum: do estrogens have any cardiovascular benefits? Int J Fertil Womens Med. 2002;47:61–68 (7), with permission. © U.S. International Foundation for Studies in Reproduction, Inc.]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/98/5/10.1210_jc.2012-4070/3/m_zeg0051397750001.jpeg?Expires=1716472038&Signature=gDeNjsI3zbRpaP~4wMntviYQPwTdY83iFs-uqUKx~YCSqgIPwHOqIBXHUtnITcJ81IaeVSjV1O2DY2PKgeZrU2IxqC53EDgsqqnrWtW8xF1dm4Ury4z8PGI7o0bJHyvKMCuctO0D98P5GJ6lKNuMROjI64qlBOongoEqCzJyQ-5u-vofjPoNquKVLkT67yhNOTPL~Omnd5bVsUU9jJlA-4kxL2PUs3qABeT50roCBDOizETRcU2GFq9l0h9mzdGixWt0sTZ4e8lHkWYLJkpGmA43Zdplf6TwnOJbZWBsCefHm7CCA98Cx~r0JY8IEtda1jVlRuBpkBdwydMoX72rGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Quarterly claim volume per 10 000 covered members, overall by prescriber type. Shaded area indicates period before release of initial WHI results. Ob/Gyn, obstetrician/gynecologists. [Reproduced from B. Ettinger et al: Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause. 2012;19:610–615 (15), with permission. © The North American Menopause Society.]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/98/5/10.1210_jc.2012-4070/3/m_zeg0051397750002.jpeg?Expires=1716472038&Signature=hF2zyfDFB6cC~BcSHVGP5GLVJcGAJErLZs66uXfITCHfmuKSoOIava-axeGOinl3kAca1-PNas9eL57RCl0pwww08xMDsSDkc2rDoQdrx-HPx4BPEr~IfEFQM3CRj-Hck2jzjwS8LfjwPFahaeuXo3NkzjSKxdYb4w4drhjF~Wl6t0cy4kE4iT0oVmPSQUg-RcXkqDTq5r0pErFFGYjp5SVXgdlJLoYclB1AbP29CirQD6sgz3Xy8ZexHdlkQNuZcUQA68wgddDnZ7WrK2agc6vWSlfyomZtR9tFrGYAArcR2N1UYQhfP-m0ZgH-DEpswX5okdMeRGNCjQRNvLufKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Risk of death or admission to hospital due to heart failure or MI (primary end-point) over 16-year follow-up, including 11 years of randomized treatment. Comparison of women randomized to hormonal treatment compared to controls randomized to no treatment. [Reproduced from I. L. Schierbeck et al: Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomized trial. BMJ. 345:e6409 (30). © BMJ Publishing Group Ltd.]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/98/5/10.1210_jc.2012-4070/3/m_zeg0051397750003.jpeg?Expires=1716472038&Signature=qQheFjMC0bzr2T1mY8Pgq4knyFv0HQtQsKPG1jN6rGInS7k7lZBqPpRbEoEX5yqOhH2sim1knWObLmbb3g03ebhWolp2evP21DRb4F~MjPccKMNRZ0H0Vyu~9ay~Z5TFthJDPmxZjMoYHe5VrLbx54U8kycLQQ-D1wzcaPIOlcYQ91UriGan1xajl~QXAgpKGSyc01FCjyB~alvE8ajbQJhLWSfl5b17FpZ5JLkswBCWPlxR4zpXd19MjiHRSf9xyMVrDnOmXHpOKA8~cXINnee~xQuAgKtrAxx-Jw5RoGWFEHKLz4SYJqXzGRafhRKZeKMIrUjQLUvAkMn-GMXErA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Attributable or excess risk or benefit per 1000 women receiving menopausal HT for 5 years who are 50–59 years old or < 10 years from menopause who have relief from hot flushes and symptoms of vaginal atrophy. [Adapted from Santen RJ (74).]](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/98/5/10.1210_jc.2012-4070/3/m_zeg0051397750004.jpeg?Expires=1716472038&Signature=PCdAdZhTKI7UScgdF3yPeH7R9gUP3rKY-LPqQFRFfvUBFQoyrAYP9vDyUrQvenvi0IBRodjqL0FMOgM5kQlENiI0BUYoSewSDpCSGKnB3kr2VY83rnmgewU6Ba-Q7NedgrtWKarplOWFOLTtOBmAhmDCkKxZQ7uGsbwLaos9JVeHQd7byzarZN6L187f3gaZoGodDoapZdXEa64jsp5HNRufDgyARWsULhFcp9osPbksxR7UUdtfb9DV~kXeKRg-8ok2U7Uh5AEbieLDjfkzmteyEO9rbnD4s3rjzpa3DH93ylhzn~ayLM6A~sY4xigAh8ZJ~GKbRGtvVN64zOVLnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)