-
PDF
- Split View
-
Views
-
Cite
Cite
S T O Othim, S Ramasamy, R Kahuthia-Gathu, T Dubois, S Ekesi, K K M Fiaboe, Effects of Host Age and Density on the Performance of Apanteles hemara (Hymenoptera: Braconidae), a Larval Endoparasitoid of Spoladea recurvalis (Lepidoptera: Crambidae), Journal of Economic Entomology, Volume 112, Issue 5, October 2019, Pages 2131–2141, https://doi.org/10.1093/jee/toz165
Close - Share Icon Share
Abstract
The amaranth leaf-webber, Spoladea recurvalis (Fabricius; Lepidoptera: Crambidae), is a serious pest of Amaranthus sp. in Africa and Asia. Apanteles hemara (Nixon; Hymenoptera: Braconidae) is by far the most important larval endoparasitoid of the amaranth leaf-webber. We examined the effects of host density and age on the biological characteristics of A. hemara. The regression model of the number of hosts supplied to A. hemara against the number of larvae parasitized resulted in a curve corresponding to type II functional response, with a significant increase in the number of hosts parasitized up to the density of 30 hosts before being constant up to 40 hosts. In contrast, the parasitism rate decreased linearly with increasing host densities. Development time, sex ratio, and adult longevity were not significantly affected by host density. The immature parasitoid mortality was significantly higher at higher host densities. Apanteles hemara did not parasitize 7-d-old larvae and beyond, while parasitism was significantly higher among 1- to 2-d-old compared with 3- to 4-d-old larvae. Immature parasitoid mortality was 2.6 times higher in 1- to 2-d-old larvae compared with 5- to 6-d-old larvae. The developmental period of the parasitoid from egg to adult was longest among 1- to 2-d-old larvae and least among 5- to 6-d-old larvae. Nonreproductive mortality was markedly higher among 1- to 2-d-old larvae compared with the older larvae. Adult female A. hemara were significantly larger on 3- to 4-d-old larvae compared with either 1- to 2-d-old or 5- to 6-d-old larvae. We discuss the implications of our results for the interpretation of functional response in parasitoids, mass rearing, conservation, and augmentative biological control of S. recurvalis.
The amaranth leaf-webbers Herpetogramma bipunctalis (Fabricius; Lepidoptera: Crambidae), Psara basalis (Walker; Lepidoptera: Crambidae), and Spoladea recurvalis (Fabricius; Lepidoptera: Crambidae) and leaf-worms Spodoptera exigua (Hübner; Lepidoptera: Noctuidae), S. frugiperda (J.E. Smith), and S. littoralis (Boisduval) are listed among the major arthropod pests of amaranth worldwide (Sithanantham et al. 1997; Clarke-Harris et al. 2004; Sharma and Ramamurthy 2009; James et al. 2010; Aderolu et al. 2013; Kagali et al. 2013; Mureithi et al. 2017; Othim et al. 2017,2018a,b). Of all these pests, the amaranth leaf-webber S. recurvalis causes damage of economic importance and has a potential to cause up to 100% yield losses during severe outbreaks (Clarke-Harris et al. 2004; James et al. 2010; Aderolu et al. 2013; Othim et al. 2018a,b). The amaranth leaf-webbers cause damage by wrapping and rolling leaves of amaranth to form shelters from which they feed by chewing between leaf veins resulting in the skeletonization of the foliage and dark or brown frass deposits (James et al. 2010, Grovida 2015, Othim et al. 2017). With the increasing demand for amaranth and the adverse challenge arising from pest attacks, farmers have been prompted to use various classes of synthetic insecticides (including pyrethroids and carbamates) that are often applied indiscriminately and nonjudiciously (Clarke-Harris et al. 2004, Losenge 2005, Aderolu et al. 2013, Kagali et al. 2013). Synthetic insecticides often pose health risks to consumers in addition to being ineffective in most cases. These chemicals may also lead to the resurgence and secondary outbreak of pests, development of insecticide resistance among pests, and destruction of potential predators and parasitoids (Chahal et al. 1997, Ekesi 1999, Losenge 2005, Arivudainambi et al. 2010, Srinivasan 2012). The unavailability of many biopesticide options has further complicated the management of S. recurvalis (Opisa et al. 2018). It has, therefore, become imperative to investigate and develop alternative but effective, safe, and sustainable integrated pest management approaches to manage the amaranth leaf-webber.
Some of the IPM strategies being recommended for the management of S. recurvalis include the use of pest-resistant varieties (Othim et al. 2018a,b,c), biopesticides (Opisa et al. 2018), blends of synthetic floral attractants and pheromones (Othim et al. 2018b), and natural enemies (Fernandez-Triana et al. 2017; Othim et al. 2017; Othim et al. 2018a,b). Among the natural enemies, studies have paid particular attention to the parasitoids. In India, a parasitism rate of 11.46% by Apanteles delhiensis (Mues and Subba-Rao; Hymenoptera: Braconidae) was reported by Bhattacherjee and Ramdas (1964) while Narayanan et al. (1957) observed field parasitism of up to 62% by Apanteles sp. on S. recurvalis. Within East Africa, open field parasitism by A. hemara on S. recurvalis was 6.2% in Kenya (Othim et al. 2018b) while it caused between 16.56% and 30.60% parasitism on both S. recurvalis and P. basalis in Tanzania (Othim et al. 2018a). Laboratory experiments have also shown high parasitism rates by A. hemara on S. recurvalis and Udea ferrugalis (Hübner; Lepidoptera: Crambidae) ranging from 42.63 to 94.67% at low and high parasitoid densities, respectively (Othim et al. 2017). Apanteles hemara has also proven to be effective in managing the pest on different host plants (Othim et al. 2019). These reports show that A. hemara has a potential to manage S. recurvalis in a conservation or augmentative biological control program. Furthermore, the occurrence of A. hemara has been reported in several countries of Asia, Europe, Africa, and in Australia (Kedar and Kumaranag 2013; Madl and van Achterberg 2014; Fernandez-Triana et al. 2017; Othim et al. 2017, 2018a).
It is essential to evaluate various biological and ecological factors that could influence the performance of a parasitoid before it is recommended for use in classical, conservation, or augmentative (inundative and inoculative) biological control (van Lenteren et al. 2003). Environmental and physiological factors, either abiotic (e.g., temperature) or biotic (e.g., density, size, and age of the host), are known to affect specific life history parameters of a parasitoid, including development, reproduction, and survival (Harbison et al. 2001, Uckan and Ergin 2003, Dannon et al. 2010). The functional response, which refers to parasitized hosts as a function of host density, can define the efficiency with which a parasitoid searches for its host and provide a better understanding of the interaction between the parasitoid and its host (Holling 1959, Islam et al. 2006, Harbi et al. 2018). Information on the functional response is also a critical element of population models portraying the impacts of parasitoids on the populations of its hosts (Islam et al. 2006, Tonnang et al. 2009). Understanding the functional response exhibited by a parasitoid is, therefore, critical for decision making in the implementation of successful biological control programs as it will show how the parasitoid responds to increasing or decreasing host populations.
Studies also report on the correlation between the age or size of a host insect with its nutritional quality, such that larger hosts are thought to contain more resources for the parasitoid (Godfray 1994, Rivero 2000, Chau and Mackauer 2001, Ode and Heinz 2002). The general hypothesis is that larger adult parasitoids are obtained from large hosts, and when female parasitoids choose to oviposit on larger hosts, more female offspring are produced (Godfray 1994, Harvey 2005). While this hypothesis may hold among idiobiont parasitoids, variations are likely to occur among koinobionts (such as A. hemara) with important implications for mass rearing of the parasitoid and timing of its release for biological control (Harvey et al. 2004).
No studies on the effects of these host physiological factors on the biological characteristics of A. hemara on any of its hosts exist. Therefore, the focus of this study was to investigate the effects of host-larval age on the performance of A. hemara in terms of its biological characteristics, including development time from egg to adult, parasitism rate, sex ratio, adult longevity, and adult size. Also, we assessed the functional response of A. hemara and the effects of host age on its biological characteristics.
Materials and Methods
Host Plants
One improved breeding line of amaranth (Amaranthus dubius, line RVI00053) exhibiting moderate pest resistance (i.e., lower rates of infestation and damage by S. recurvalis compared to a known susceptible accession) and possessing desirable horticultural traits (such as broad leaves and rapid growth) was selected from both open field and laboratory screening experiments conducted in 2016 and 2017 using 36 accessions and lines obtained from the World Vegetable Center’s (WorldVeg) gene banks in Shanhua, Taiwan and Arusha, Tanzania (Othim et al. 2018a,c, 2019). This breeding line of amaranth was also favorable for the development of A. hemara (Othim et al. 2019). The selected amaranth line was raised in the screen house at WorldVeg Eastern and Southern Africa in Arusha (3.38°S, 36.8°E). Sowing of the seeds and subsequent transplanting was done as in Othim et al. (2019) after which the plants were used either for insect colony maintenance or for experiments.
Amaranth Leaf-Webber Colony
A colony of S. recurvalis, originating from adults and larvae collected within the WorldVeg experimental fields in Arusha, was initiated and maintained in the entomology laboratory at WorldVeg on A. dubius line for at least five generations before its use in experiments as described in Othim et al. (2018c). The colony was replenished quarterly with freshly caught adults and larvae from the amaranth fields within WorldVeg. The adult moths were kept in ventilated cages (40 × 40 × 45 cm) where they were fed with 10% honey solution and provided with potted amaranth plants for oviposition as described by Othim et al. (2018c). Hatched larvae were transferred into ventilated plastic containers (15 × 7 × 5 cm) with a lining of paper towel on the sides to absorb excess moisture and provided with a daily supply of freshly cut leaves of amaranth until pupation and adult emergence as described in Othim et al. (2019) with similar laboratory conditions.
Apanteles hemara Colony
A colony of the koinobiont larval endoparasitoid, A. hemara, was initiated at the entomology laboratory at WorldVeg, Arusha from pupae recovered from S. recurvalis larvae collected from amaranth fields at WorldVeg, Arusha. The colony was infused with new field collections performed every quarter to avoid effects of inbreeding and sustain colony fitness. Adult parasitoids were kept in ventilated perspex cage (40 × 40 × 45 cm), fed honey on paper strips and provided with larvae to oviposit under laboratory conditions of 25 ± 2°C, 50–70% RH, and 12:12 (L:D) h photoperiod as described by Othim et al. (2019). Handling of the parasitized larvae followed the method described by Othim et al. (2019) where they were fed in ventilated plastic containers (15 × 7 × 5 cm) having a lining of paper towel until they pupated and the pupae incubated in Petri dishes (9 cm diameter) until adult eclosion. Mass rearing of these parasitoids was done continuously for several generations using S. recurvalis larvae feeding on A. dubius as hosts before they could be used in the experiments with different host densities and age groups. Freshly emerged parasitoids were transferred to a cage where they were fed honey on strips of paper for 2 d to allow the females to mate before they could be used in the experiments.
Assessing the Effect of Larval Density of S. recurvalis on the Performance of A. hemara
Four potted amaranth plants of line RVI00053 were exposed to 10 adult females of S. recurvalis in a cage similar to that described above for 24 h for oviposition to occur. The plants were then transferred to separate ventilated holding cages of similar dimensions until the eggs hatched. The newly hatched larvae were fed on the leaves of the whole amaranth plants up to the second instar when they were ready to be used for the experiments. One leaf infested with 10-, 20-, 30- or 40-s instar S. recurvalis larvae was detached from the plant and gently placed in an aerated cylindrical container (10 × 5 cm). A 2-d-old mated naïve (with no previous oviposition experience) A. hemara female was then introduced into the container for 24 h and was fed honey as described by Othim et al. (2019). Subsequently, the exposed larvae were incubated in ventilated plastic containers (15 × 7 × 5 cm), fed, and monitored daily until F1 generation parasitoid cocoon formation and adult parasitoid or host emergence following the method described by Othim et al. (2019). The emerged adult parasitoids were transferred singly into plastic vials (20 ml) covered with a netting material, fed honey, and monitored daily until death. The same procedure was repeated with each larval density and replicated five times at every density. For every replicate, a control consisting of larvae that were not exposed to the parasitoid was set up. The laboratory conditions were maintained at 25 ± 2°C, 50–70% RH, and 12:12 (L:D) h photoperiod during the experiment.
Assessing the Influence of Larval Age on Parasitism and Other Life History Parameters of A. hemara Reared on S. recurvalis
Four potted amaranth plants of line RVI00053 were exposed to 10 S. recurvalis female adults for oviposition followed by a holding period in separate cages as described in the previous section. Preliminary experiments set ahead of the present study and aiming to determine the egg duration and viability revealed that the eggs take 3.05 ± 0.02 d to hatch and have 95.0 ± 2.55% viability. Upon hatching, 25 larvae at 1–2, 3–4, 5–6, and 7–9 d old were transferred gently into a ventilated cylindrical container (10 × 5 cm). For each larval age group, a 2-d-old mated naïve A. hemara female was introduced and left for 24 h as described in the previous section. The exposed larvae of each age group were incubated, fed, and monitored daily until parasitoid pupal cocoons were formed and the adults emerged as described in Othim et al. (2019). The emerging adult parasitoids were individually transferred into plastic vials as described in the previous section and monitored daily until they died. The experiment was replicated five times at each larval age group, and for each replicate, a control was included where no parasitoids were introduced. Laboratory conditions were similar to those of the previous experiment.
The parameters recorded for the assessment of the parasitoid’s performance at different larval densities and larval age groups were adopted from Othim et al. (2017, 2019). The parameters included the number of parasitized host larvae, number of emerged adult parasitoids, parasitoid pupal mortality, developmental time, longevity, sex ratios, and sizes of at least 15 randomly chosen parasitoids of each sex, as well as host mortalities. The length of forewings and hind tibia of the parasitoid was measured using a stereomicroscope LEICA EZ4D (Leica Microsystems Inc., Buffalo Grove, IL) at ×30 magnification.
Data Analysis
The effect of larval host density and larval host age on the rate of parasitism, pupal survival rate (cocoons that gave rise to adults), nonreproductive larval and pupal mortalities, length of forewings and hind-tibia of F1 parasitoids, and sex ratio (proportion of F1 females) was compared by performing one-way analysis of variance (ANOVA). Percentage data (such as the proportion of females, pupal survival rate, and nonreproductive mortalities) were square-root transformed to obtain normality before ANOVA. However, untransformed data were presented in the Tables 1–6. The significance of nonreproductive mortality was evaluated using Wilcoxon test to compare natural mortalities in the control and mortalities in the presence of the parasitoid. The nonreproductive host-larval and pupal mortality was identified using Abbott’s formula (Abbott 1925). The generalized linear model (GLM) with log10-link and Poisson distribution error was used to compare the factors: number of days taken for larval and pupal development, duration of the whole development cycle, and adult longevity of A. hemara. The effect of a factor for a GLM is reflected in the deviance (likelihood ratio test statistic) that has an appropriate χ2 distribution; hence, the χ2 values are presented as test statistics (O’Hara and Kotze 2010). Where significant differences occurred, the Tukey HSD test was used for separation of means. The sex ratios within each treatment were analyzed using the χ2 test. Independent samples t-test was used to compare the length of forewings and hind-tibia between male and female F1 parasitoids. Nonlinear least-squares regression was used to model the relationship between larval density and the number of hosts parasitized while a simple linear regression was used to determine the relationship between larval density and the rate of parasitism. Parasitism rate was calculated according to Othim et al. (2017) as the percentage of the number of parasitoid cocoons divided by the sum of pupae of the host and parasitoid cocoons. All statistical analyses were conducted using R-Software version 3.5.1 (R Development Core Team, Vienna, Austria).
Mean ± SE of parasitism rate, number of hosts parasitized, pupal survival, and sex ratio of A. hemara and host-larval and pupal mortality at different host densities
| Larval density . | Parasitism (%) . | Number of hosts parasitized . | Host-larval mortality . | Host pupal mortality . | Parasitoid pupal survival (%) . | Sex ratio (% Female) . | χ2 . | df . | P-value . |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 90.20 ± 6.45a | 6.14 ± 0.91b | 3.29 ± 0.75b | 1.5 ± 0.5b | 63.55 ± 9.65a | 47.52 ± 6.55a | 0 | 1 | 1 |
| 20 | 72.31 ± 6.91b | 10.50 ± 1.28ab | 5.67 ± 0.49b | 2.0 ± 0.26b | 37.01 ± 7.15b | 39.67 ± 4.87a | 2.27 | 1 | 0.132 |
| 30 | 68.08 ± 2.62b | 12.40 ± 1.72a | 12.0 ± 2.0a | 3.2 ± 0.37b | 43.21 ± 5.23ab | 66.96 ± 8.73a | 3.5 | 1 | 0.061 |
| 40 | 48.36 ± 3.40c | 12.20 ± 1.28a | 15.0 ± 1.22a | 6.0 ± 0.95a | 27.82 ± 7.77b | 47.22 ± 12.11a | 0.11 | 1 | 0.739 |
| F-value | 9.27 | 5.70 | 22.97 | 10.61 | 3.76 | 2.41 | |||
| df | 3,19 | 3,19 | 3,19 | 3,14 | 3,19 | 3,13 | |||
| P-value | 0.001 | 0.006 | < 0.001 | 0.001 | 0.028 | 0.113 |
| Larval density . | Parasitism (%) . | Number of hosts parasitized . | Host-larval mortality . | Host pupal mortality . | Parasitoid pupal survival (%) . | Sex ratio (% Female) . | χ2 . | df . | P-value . |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 90.20 ± 6.45a | 6.14 ± 0.91b | 3.29 ± 0.75b | 1.5 ± 0.5b | 63.55 ± 9.65a | 47.52 ± 6.55a | 0 | 1 | 1 |
| 20 | 72.31 ± 6.91b | 10.50 ± 1.28ab | 5.67 ± 0.49b | 2.0 ± 0.26b | 37.01 ± 7.15b | 39.67 ± 4.87a | 2.27 | 1 | 0.132 |
| 30 | 68.08 ± 2.62b | 12.40 ± 1.72a | 12.0 ± 2.0a | 3.2 ± 0.37b | 43.21 ± 5.23ab | 66.96 ± 8.73a | 3.5 | 1 | 0.061 |
| 40 | 48.36 ± 3.40c | 12.20 ± 1.28a | 15.0 ± 1.22a | 6.0 ± 0.95a | 27.82 ± 7.77b | 47.22 ± 12.11a | 0.11 | 1 | 0.739 |
| F-value | 9.27 | 5.70 | 22.97 | 10.61 | 3.76 | 2.41 | |||
| df | 3,19 | 3,19 | 3,19 | 3,14 | 3,19 | 3,13 | |||
| P-value | 0.001 | 0.006 | < 0.001 | 0.001 | 0.028 | 0.113 |
Mean ± SE followed by the same letter within a column are not significantly different at P < 0.05 (Tukey’s test). Chi-square values are presented for comparison between F1 females and males.
Mean ± SE of parasitism rate, number of hosts parasitized, pupal survival, and sex ratio of A. hemara and host-larval and pupal mortality at different host densities
| Larval density . | Parasitism (%) . | Number of hosts parasitized . | Host-larval mortality . | Host pupal mortality . | Parasitoid pupal survival (%) . | Sex ratio (% Female) . | χ2 . | df . | P-value . |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 90.20 ± 6.45a | 6.14 ± 0.91b | 3.29 ± 0.75b | 1.5 ± 0.5b | 63.55 ± 9.65a | 47.52 ± 6.55a | 0 | 1 | 1 |
| 20 | 72.31 ± 6.91b | 10.50 ± 1.28ab | 5.67 ± 0.49b | 2.0 ± 0.26b | 37.01 ± 7.15b | 39.67 ± 4.87a | 2.27 | 1 | 0.132 |
| 30 | 68.08 ± 2.62b | 12.40 ± 1.72a | 12.0 ± 2.0a | 3.2 ± 0.37b | 43.21 ± 5.23ab | 66.96 ± 8.73a | 3.5 | 1 | 0.061 |
| 40 | 48.36 ± 3.40c | 12.20 ± 1.28a | 15.0 ± 1.22a | 6.0 ± 0.95a | 27.82 ± 7.77b | 47.22 ± 12.11a | 0.11 | 1 | 0.739 |
| F-value | 9.27 | 5.70 | 22.97 | 10.61 | 3.76 | 2.41 | |||
| df | 3,19 | 3,19 | 3,19 | 3,14 | 3,19 | 3,13 | |||
| P-value | 0.001 | 0.006 | < 0.001 | 0.001 | 0.028 | 0.113 |
| Larval density . | Parasitism (%) . | Number of hosts parasitized . | Host-larval mortality . | Host pupal mortality . | Parasitoid pupal survival (%) . | Sex ratio (% Female) . | χ2 . | df . | P-value . |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 90.20 ± 6.45a | 6.14 ± 0.91b | 3.29 ± 0.75b | 1.5 ± 0.5b | 63.55 ± 9.65a | 47.52 ± 6.55a | 0 | 1 | 1 |
| 20 | 72.31 ± 6.91b | 10.50 ± 1.28ab | 5.67 ± 0.49b | 2.0 ± 0.26b | 37.01 ± 7.15b | 39.67 ± 4.87a | 2.27 | 1 | 0.132 |
| 30 | 68.08 ± 2.62b | 12.40 ± 1.72a | 12.0 ± 2.0a | 3.2 ± 0.37b | 43.21 ± 5.23ab | 66.96 ± 8.73a | 3.5 | 1 | 0.061 |
| 40 | 48.36 ± 3.40c | 12.20 ± 1.28a | 15.0 ± 1.22a | 6.0 ± 0.95a | 27.82 ± 7.77b | 47.22 ± 12.11a | 0.11 | 1 | 0.739 |
| F-value | 9.27 | 5.70 | 22.97 | 10.61 | 3.76 | 2.41 | |||
| df | 3,19 | 3,19 | 3,19 | 3,14 | 3,19 | 3,13 | |||
| P-value | 0.001 | 0.006 | < 0.001 | 0.001 | 0.028 | 0.113 |
Mean ± SE followed by the same letter within a column are not significantly different at P < 0.05 (Tukey’s test). Chi-square values are presented for comparison between F1 females and males.
Results
Effect of Host Density on Parasitism and Other Biological Parameters of A. hemara
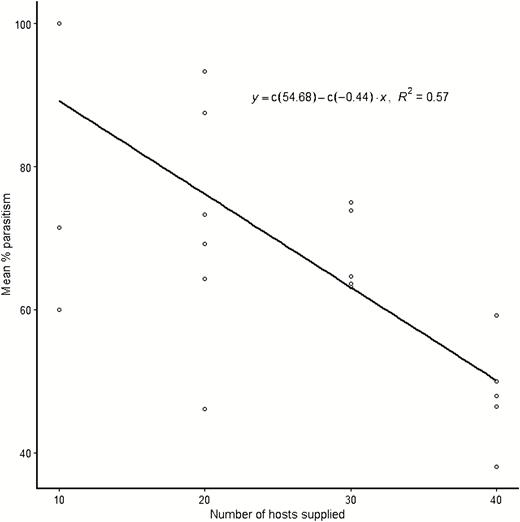
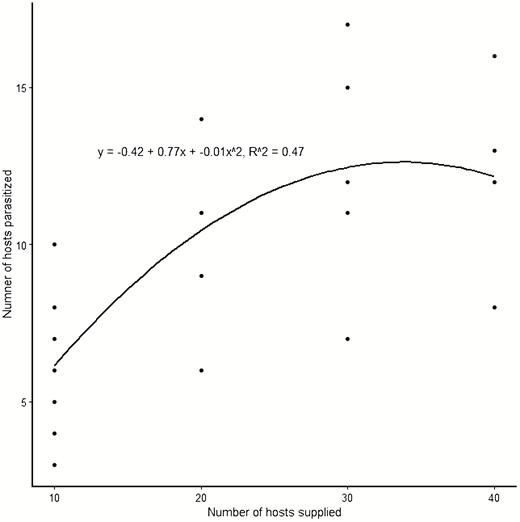
Spoladea recurvalis larval density significantly affected the percent parasitism by A. hemara such that higher parasitism was recorded at low larval densities (F = 9.27; df = 3, 19; P = 0.001). The cohort of 10 host larvae led to significantly higher parasitism rates compared with 20, 30, and 40 larval densities. No significant difference was seen in parasitism between 20 and 30 larval densities, whereas the highest larval density led to the least percent parasitism (Table 1). A significant inverse linear correlation was observed between the number of host larvae supplied and percent parasitism by A. hemara (y = −0.44x + 54.68; R2 = 0.57; F = 27.54; df = 1,21; P < 0.001) (Fig. 1), denoting a decreasing parasitism rate with increasing host density. On the contrary, a higher number of larvae was parasitized at higher host densities with cohorts of 30 and 40 larvae recording significantly higher parasitization than the cohort of 10 larvae (F = 5.70; df = 3,19; P = 0.006) (Table 1). A regression model of the number of hosts supplied to the parasitoid against the number of larvae parasitized resulted in a curve showing a gradual increase in the number of larvae parasitized up to the density of 30 hosts supplied and then a plateau with an insignificant decrease at the highest density of 40 hosts (y = −0.42 + 0.77x + −0.01x2, R2 = 0.47, F = 8.99; df = 2,20; P = 0.002) (Fig. 2).
Correlation plot between host–larval density of Spoladea recurvalis and the percent parasitism rates by A. hemara
Functional response curve fit by nonlinear least-squares regression of A. hemara parasitizing its host Spoladea recurvalis at densities of 10, 20, 30, and 40 larvae.
The host-larval mortality (number of dead larvae) was significantly lower in the cohorts of 10 and 20 larvae compared with the cohorts of 30 and 40 larvae (F = 22.97; df = 3,19; P < 0.001) (Table 1). Similarly, host pupal mortality was significantly higher in the cohort of 40 larvae compared with cohorts of 10, 20, and 30 larvae (F = 10.61; df = 3,14; P = 0.001). The parasitoid’s pupal survival was significantly affected by the host–larval density with the least larval density (10 larvae) producing more viable pupae compared with 20 and 40 larval densities (F = 3.76; df = 3,19; P = 0.028) (Table 1). However, there was no significant difference in pupal viability at 20, 30, and 40 larval densities. Host–larval density did not have a significant effect on the parasitoid’s F1 sex ratio (F = 2.41; df = 3,13; P = 0.113). For all the densities tested, the sex ratio was balanced; 10 larvae (χ2 = 0; df = 1; P = 1), 20 larvae (χ2 = 2.27; df = 1; P = 0.132), 30 larvae (χ2 = 3.5; df = 1; P = 0.061), and 40 larvae (χ2 = 0.11; df = 1; P = 0.739) (Table 1).
The parasitoid’s larval (χ2 = 3.26; df = 3; P = 0.353), pupal (χ2 = 0.98; df = 3; P = 0.807), and total developmental time (χ2 = 1.52; df = 3; P = 0.679) were not significantly different across the four larval densities tested (Table 2). Similarly, the longevity of either female (χ2 = 0.19; df = 3; P = 0.98) or male (χ2 = 3.64; df = 3; P = 0.303) wasps obtained at the different larval densities did not differ significantly (Table 2).
Developmental time and adult longevity (days) (mean ± SE) of A. hemara at different densities of host S. recurvalis
| Larval density . | Larval development time . | Pupal development time . | Total development time . | Longevity . | Female longevity . | Male longevity . |
|---|---|---|---|---|---|---|
| 10 | 7.70 ± 0.20a | 3.50 ± 0.13a | 11.13 ± 0.32a | 12.24 ± 0.50a | 12.18 ± 0.86a | 12.29 ± 0.62a |
| 20 | 8.34 ± 0.14a | 3.65 ± 0.15a | 11.75 ± 0.29a | 13.50 ± 0.61a | 12.60 ± 0.85a | 14.25 ± 0.84a |
| 30 | 8.63 ± 0.13a | 3.61 ± 0.11a | 12.18 ± 0.25a | 12.24 ± 0.48a | 12.67 ± 0.69a | 11.55 ± 0.53a |
| 40 | 7.91 ± 0.22a | 4.06 ± 0.17a | 11.33 ± 0.35a | 12.30 ± 0.56a | 12.22 ± 0.81a | 12.36 ± 0.80a |
| χ2 | 3.26 | 0.98 | 1.52 | 2.06 | 0.19 | 3.64 |
| df | 3 | 3 | 3 | 3 | 3 | 3 |
| P-value | 0.353 | 0.807 | 0.679 | 0.56 | 0.98 | 0.303 |
| Larval density . | Larval development time . | Pupal development time . | Total development time . | Longevity . | Female longevity . | Male longevity . |
|---|---|---|---|---|---|---|
| 10 | 7.70 ± 0.20a | 3.50 ± 0.13a | 11.13 ± 0.32a | 12.24 ± 0.50a | 12.18 ± 0.86a | 12.29 ± 0.62a |
| 20 | 8.34 ± 0.14a | 3.65 ± 0.15a | 11.75 ± 0.29a | 13.50 ± 0.61a | 12.60 ± 0.85a | 14.25 ± 0.84a |
| 30 | 8.63 ± 0.13a | 3.61 ± 0.11a | 12.18 ± 0.25a | 12.24 ± 0.48a | 12.67 ± 0.69a | 11.55 ± 0.53a |
| 40 | 7.91 ± 0.22a | 4.06 ± 0.17a | 11.33 ± 0.35a | 12.30 ± 0.56a | 12.22 ± 0.81a | 12.36 ± 0.80a |
| χ2 | 3.26 | 0.98 | 1.52 | 2.06 | 0.19 | 3.64 |
| df | 3 | 3 | 3 | 3 | 3 | 3 |
| P-value | 0.353 | 0.807 | 0.679 | 0.56 | 0.98 | 0.303 |
Mean ± SE followed by the same letter within a column are not significantly different at P < 0.05 (Tukey’s test).
Developmental time and adult longevity (days) (mean ± SE) of A. hemara at different densities of host S. recurvalis
| Larval density . | Larval development time . | Pupal development time . | Total development time . | Longevity . | Female longevity . | Male longevity . |
|---|---|---|---|---|---|---|
| 10 | 7.70 ± 0.20a | 3.50 ± 0.13a | 11.13 ± 0.32a | 12.24 ± 0.50a | 12.18 ± 0.86a | 12.29 ± 0.62a |
| 20 | 8.34 ± 0.14a | 3.65 ± 0.15a | 11.75 ± 0.29a | 13.50 ± 0.61a | 12.60 ± 0.85a | 14.25 ± 0.84a |
| 30 | 8.63 ± 0.13a | 3.61 ± 0.11a | 12.18 ± 0.25a | 12.24 ± 0.48a | 12.67 ± 0.69a | 11.55 ± 0.53a |
| 40 | 7.91 ± 0.22a | 4.06 ± 0.17a | 11.33 ± 0.35a | 12.30 ± 0.56a | 12.22 ± 0.81a | 12.36 ± 0.80a |
| χ2 | 3.26 | 0.98 | 1.52 | 2.06 | 0.19 | 3.64 |
| df | 3 | 3 | 3 | 3 | 3 | 3 |
| P-value | 0.353 | 0.807 | 0.679 | 0.56 | 0.98 | 0.303 |
| Larval density . | Larval development time . | Pupal development time . | Total development time . | Longevity . | Female longevity . | Male longevity . |
|---|---|---|---|---|---|---|
| 10 | 7.70 ± 0.20a | 3.50 ± 0.13a | 11.13 ± 0.32a | 12.24 ± 0.50a | 12.18 ± 0.86a | 12.29 ± 0.62a |
| 20 | 8.34 ± 0.14a | 3.65 ± 0.15a | 11.75 ± 0.29a | 13.50 ± 0.61a | 12.60 ± 0.85a | 14.25 ± 0.84a |
| 30 | 8.63 ± 0.13a | 3.61 ± 0.11a | 12.18 ± 0.25a | 12.24 ± 0.48a | 12.67 ± 0.69a | 11.55 ± 0.53a |
| 40 | 7.91 ± 0.22a | 4.06 ± 0.17a | 11.33 ± 0.35a | 12.30 ± 0.56a | 12.22 ± 0.81a | 12.36 ± 0.80a |
| χ2 | 3.26 | 0.98 | 1.52 | 2.06 | 0.19 | 3.64 |
| df | 3 | 3 | 3 | 3 | 3 | 3 |
| P-value | 0.353 | 0.807 | 0.679 | 0.56 | 0.98 | 0.303 |
Mean ± SE followed by the same letter within a column are not significantly different at P < 0.05 (Tukey’s test).
Influence of Larval Host Age on Some Biological Parameters of A. hemara
Apanteles hemara was able to successfully parasitize larvae of all the age groups below 7 d while older larvae beyond 7 d escaped parasitism. The rate of parasitism was significantly higher in 1- to 2-d-old larvae compared with 3- to 4-d-old larvae but did not differ between 3- and 4-d-old larvae and 5- and 6-d-old larvae (F = 4.0; df = 2,15; P = 0.04) (Table 3). The survival of A. hemara pupae was significantly higher among 5- to 6-d-old larvae compared with both 1- to 2-d-old and 3- to 4-d-old larvae (F = 6.4; df = 2,15; P = 0.01). The proportion of female parasitoids obtained from all the three larval age groups did not differ significantly (F = 0.644; df = 2,15; P = 0.539). However, within each larval age group, male biased sex ratio was obtained among 5- to 6-d-old larvae (χ2 = 6.7; df = 1; P = 0.01) while both 1- to 2-d-old (χ2 = 1.68; df = 1; P = 0.194) and 3- to 4-d-old larvae (χ2 = 0.00; df = 1; P = 1.00) had balanced sex ratios (Table 3).
Mean ± SE of parasitism, pupal survival and sex ratio (%) of A. hemara at different host–larval age groups
| Host-larval agea . | Parasitism (%) . | Parasitoid pupal survival . | Sex ratio (% female) . | χ2 . | df . | P-value . |
|---|---|---|---|---|---|---|
| 1–2 d | 79.17 ± 7.22a | 49.29 ± 9.85b | 60.00 ± 16.96a | 1.68 | 1 | 0.194 |
| 3–4 d | 52.64 ± 5.29b | 43.75 ± 8.29b | 43.75 ± 14.06a | 0.00 | 1 | 1.00 |
| 5–6 d | 61.82 ± 6.64ab | 80.51 ± 5.17a | 37.08 ± 3.95a | 6.70 | 1 | 0.010 |
| F-value | 4.0 | 6.4 | 0.644 | |||
| df | 2,15 | 2,15 | 2,15 | |||
| P-value | 0.04 | 0.01 | 0.539 |
| Host-larval agea . | Parasitism (%) . | Parasitoid pupal survival . | Sex ratio (% female) . | χ2 . | df . | P-value . |
|---|---|---|---|---|---|---|
| 1–2 d | 79.17 ± 7.22a | 49.29 ± 9.85b | 60.00 ± 16.96a | 1.68 | 1 | 0.194 |
| 3–4 d | 52.64 ± 5.29b | 43.75 ± 8.29b | 43.75 ± 14.06a | 0.00 | 1 | 1.00 |
| 5–6 d | 61.82 ± 6.64ab | 80.51 ± 5.17a | 37.08 ± 3.95a | 6.70 | 1 | 0.010 |
| F-value | 4.0 | 6.4 | 0.644 | |||
| df | 2,15 | 2,15 | 2,15 | |||
| P-value | 0.04 | 0.01 | 0.539 |
aNo parasitism was obtained on 7-d-old larvae and beyond. Mean ± SE followed by the same letter within a column are not significantly different at P < 0.05 (Tukey’s test).
Mean ± SE of parasitism, pupal survival and sex ratio (%) of A. hemara at different host–larval age groups
| Host-larval agea . | Parasitism (%) . | Parasitoid pupal survival . | Sex ratio (% female) . | χ2 . | df . | P-value . |
|---|---|---|---|---|---|---|
| 1–2 d | 79.17 ± 7.22a | 49.29 ± 9.85b | 60.00 ± 16.96a | 1.68 | 1 | 0.194 |
| 3–4 d | 52.64 ± 5.29b | 43.75 ± 8.29b | 43.75 ± 14.06a | 0.00 | 1 | 1.00 |
| 5–6 d | 61.82 ± 6.64ab | 80.51 ± 5.17a | 37.08 ± 3.95a | 6.70 | 1 | 0.010 |
| F-value | 4.0 | 6.4 | 0.644 | |||
| df | 2,15 | 2,15 | 2,15 | |||
| P-value | 0.04 | 0.01 | 0.539 |
| Host-larval agea . | Parasitism (%) . | Parasitoid pupal survival . | Sex ratio (% female) . | χ2 . | df . | P-value . |
|---|---|---|---|---|---|---|
| 1–2 d | 79.17 ± 7.22a | 49.29 ± 9.85b | 60.00 ± 16.96a | 1.68 | 1 | 0.194 |
| 3–4 d | 52.64 ± 5.29b | 43.75 ± 8.29b | 43.75 ± 14.06a | 0.00 | 1 | 1.00 |
| 5–6 d | 61.82 ± 6.64ab | 80.51 ± 5.17a | 37.08 ± 3.95a | 6.70 | 1 | 0.010 |
| F-value | 4.0 | 6.4 | 0.644 | |||
| df | 2,15 | 2,15 | 2,15 | |||
| P-value | 0.04 | 0.01 | 0.539 |
aNo parasitism was obtained on 7-d-old larvae and beyond. Mean ± SE followed by the same letter within a column are not significantly different at P < 0.05 (Tukey’s test).
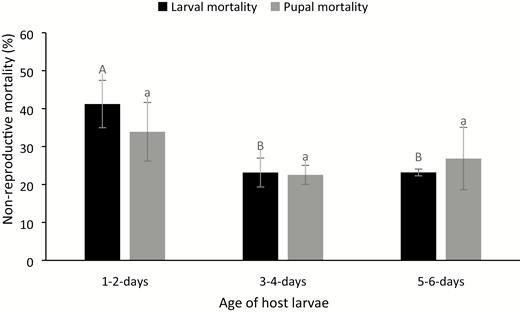
Significant nonreproductive larval mortality caused by A. hemara was observed among 1- to 2-d-old larvae (W = 0; P = 0.027), 3- to 4-d-old larvae (W = 2; P = 0.004), and 5- to 6-d-old larvae (W = 3.5; P = 0.025). The nonreproductive larval mortality was significantly higher among 1- to 2-d-old larvae compared with 3- to 4-d-old larvae and 5- to 6-d-old larvae (F = 5.37; df = 2,16; P = 0.017) (Fig. 3). No significant difference was observed in nonreproductive larval mortality between 3- and 4-d-old larvae and 5- and 6-d-old larvae. Similarly, nonreproductive pupal mortality did not differ significantly across the three larval age groups (F = 0.67; df = 2, 13; P = 0.528).
Nonreproductive larval and pupal mortality (mean ± SE) by A. hemara at different age groups of Spoladea recurvalis (mean larval (pupal) mortalities). The same upper (lower) case alphabet is not significantly different at P < 0.05 (Tukey’s test).
The age of the host–larva significantly influenced the parasitoid’s larval development time. The larval development time was prolonged in the younger age groups compared with the older ones. Development in 1- to 2-d-old host larvae resulted in longer development time compared with 3- to 4-d-old larvae while 5- to 6-d-old larvae had the shortest larval development time (χ2 = 23.53; df = 2; P < 0.001) (Table 4). On the contrary, the pupal (cocoon) development time was not significantly influenced by the age of their host larvae (χ2 = 0.96; df = 2; P = 0.619). Subsequently, the total developmental time of the parasitoid was 1.57 and 2.48 d shorter when 3- to 4-d-old and 5- to 6-d-old larvae, respectively, were parasitized, compared with development time in 1- to 2-d-old larvae (F = 8.20; df = 2; P = 0.017). The total development time was also significantly shorter in 5- to 6-d-old larvae compared with 3- to 4-d-old larvae. No significant difference in the longevity of either F1 male (χ2 = 1.45; df = 2; P = 0.483) or female wasps (χ2 = 0.003; df = 2; P = 0.998) was observed when larvae of all the tested age groups were parasitized by their mothers.
Developmental time and adult longevity (days; mean ± SE) of A. hemara at different age groups of its host S. recurvalis
| Host-larval age . | Larval development time . | Pupal development time . | Total development time . | Longevity . | Female longevity . | Male longevity . |
|---|---|---|---|---|---|---|
| 1–2 d | 8.70 ± 0.12a | 4.45 ± 0.36a | 12.77 ± 0.41a | 11.75 ± 0.71a | 12.18 ± 0.94a | 11.22 ± 1.12a |
| 3–4 d | 7.71 ± 0.13b | 3.85 ± 0.29a | 11.20 ± 0.30b | 12.42 ± 0.76a | 12.11 ± 0.99a | 12.70 ± 1.18a |
| 5–6 d | 6.19 ± 0.10c | 4.08 ± 0.16a | 10.29 ± 0.18c | 12.61 ± 0.43a | 12.10 ± 0.69a | 12.86 ± 0.55a |
| χ2 | 23.53 | 0.96 | 8.2 | 0.77 | 0.003 | 1.45 |
| df | 2 | 2 | 2 | 2 | 2 | 2 |
| P | <0.001 | 0.619 | 0.017 | 0.682 | 0.998 | 0.483 |
| Host-larval age . | Larval development time . | Pupal development time . | Total development time . | Longevity . | Female longevity . | Male longevity . |
|---|---|---|---|---|---|---|
| 1–2 d | 8.70 ± 0.12a | 4.45 ± 0.36a | 12.77 ± 0.41a | 11.75 ± 0.71a | 12.18 ± 0.94a | 11.22 ± 1.12a |
| 3–4 d | 7.71 ± 0.13b | 3.85 ± 0.29a | 11.20 ± 0.30b | 12.42 ± 0.76a | 12.11 ± 0.99a | 12.70 ± 1.18a |
| 5–6 d | 6.19 ± 0.10c | 4.08 ± 0.16a | 10.29 ± 0.18c | 12.61 ± 0.43a | 12.10 ± 0.69a | 12.86 ± 0.55a |
| χ2 | 23.53 | 0.96 | 8.2 | 0.77 | 0.003 | 1.45 |
| df | 2 | 2 | 2 | 2 | 2 | 2 |
| P | <0.001 | 0.619 | 0.017 | 0.682 | 0.998 | 0.483 |
Mean ± SE followed by the same letter within a column are not significantly different at P < 0.05 (Tukey’s test).
Developmental time and adult longevity (days; mean ± SE) of A. hemara at different age groups of its host S. recurvalis
| Host-larval age . | Larval development time . | Pupal development time . | Total development time . | Longevity . | Female longevity . | Male longevity . |
|---|---|---|---|---|---|---|
| 1–2 d | 8.70 ± 0.12a | 4.45 ± 0.36a | 12.77 ± 0.41a | 11.75 ± 0.71a | 12.18 ± 0.94a | 11.22 ± 1.12a |
| 3–4 d | 7.71 ± 0.13b | 3.85 ± 0.29a | 11.20 ± 0.30b | 12.42 ± 0.76a | 12.11 ± 0.99a | 12.70 ± 1.18a |
| 5–6 d | 6.19 ± 0.10c | 4.08 ± 0.16a | 10.29 ± 0.18c | 12.61 ± 0.43a | 12.10 ± 0.69a | 12.86 ± 0.55a |
| χ2 | 23.53 | 0.96 | 8.2 | 0.77 | 0.003 | 1.45 |
| df | 2 | 2 | 2 | 2 | 2 | 2 |
| P | <0.001 | 0.619 | 0.017 | 0.682 | 0.998 | 0.483 |
| Host-larval age . | Larval development time . | Pupal development time . | Total development time . | Longevity . | Female longevity . | Male longevity . |
|---|---|---|---|---|---|---|
| 1–2 d | 8.70 ± 0.12a | 4.45 ± 0.36a | 12.77 ± 0.41a | 11.75 ± 0.71a | 12.18 ± 0.94a | 11.22 ± 1.12a |
| 3–4 d | 7.71 ± 0.13b | 3.85 ± 0.29a | 11.20 ± 0.30b | 12.42 ± 0.76a | 12.11 ± 0.99a | 12.70 ± 1.18a |
| 5–6 d | 6.19 ± 0.10c | 4.08 ± 0.16a | 10.29 ± 0.18c | 12.61 ± 0.43a | 12.10 ± 0.69a | 12.86 ± 0.55a |
| χ2 | 23.53 | 0.96 | 8.2 | 0.77 | 0.003 | 1.45 |
| df | 2 | 2 | 2 | 2 | 2 | 2 |
| P | <0.001 | 0.619 | 0.017 | 0.682 | 0.998 | 0.483 |
Mean ± SE followed by the same letter within a column are not significantly different at P < 0.05 (Tukey’s test).
Host-larval age significantly influenced the morphometric features of the F1 parasitoids of A. hemara. The forewing length of female wasps obtained when 3- to 4-d-old larvae were parasitized was significantly longer compared with those obtained when 1- to 2-d-old and 5- to 6-d-old larvae were parasitized (F = 4.88; df = 2,63; P = 0.011) (Table 5). No significant difference was observed in the forewing length of male wasps across the tested age groups (F = 2.44; df = 2,82; P = 0.094). The forewings length of female wasps obtained when 3- to 4-d-old larvae were parasitized was significantly longer than that of their male counterparts (t = 3.75; df = 41; P = 0.001) while there was no significant difference in the forewings length between male and female wasps when 1- to 2-d-old (t = 1.12; df = 76; P = 0.268) and 5- to 6-d-old larvae (t = 1.84; df = 30; P = 0.075) were parasitized.
Forewing length (mm) of female and male (mean ± SE) F1 progeny of A. hemara obtained from different age groups of host larvae
| Host-larval age . | Female forewing . | Male forewing . | t . | df . | P-value . |
|---|---|---|---|---|---|
| 1–2 d | 2.81 ± 0.02bA | 2.78 ± 0.02aA | 1.12 | 76 | 0.268 |
| 3–4 d | 2.92 ± 0.03aA | 2.77 ± 0.03aB | 3.75 | 41 | 0.001 |
| 5–6 d | 2.80 ± 0.05bA | 2.69 ± 0.04aA | 1.84 | 30 | 0.075 |
| F-value | 4.88 | 2.44 | |||
| df | 2,63 | 2,82 | |||
| P-value | 0.011 | 0.094 |
| Host-larval age . | Female forewing . | Male forewing . | t . | df . | P-value . |
|---|---|---|---|---|---|
| 1–2 d | 2.81 ± 0.02bA | 2.78 ± 0.02aA | 1.12 | 76 | 0.268 |
| 3–4 d | 2.92 ± 0.03aA | 2.77 ± 0.03aB | 3.75 | 41 | 0.001 |
| 5–6 d | 2.80 ± 0.05bA | 2.69 ± 0.04aA | 1.84 | 30 | 0.075 |
| F-value | 4.88 | 2.44 | |||
| df | 2,63 | 2,82 | |||
| P-value | 0.011 | 0.094 |
Means followed by the same lower (upper) case letter within a column (row) are not significantly different at P < 0.05 (Tukey’s test (within column), t-test (within row)).
Forewing length (mm) of female and male (mean ± SE) F1 progeny of A. hemara obtained from different age groups of host larvae
| Host-larval age . | Female forewing . | Male forewing . | t . | df . | P-value . |
|---|---|---|---|---|---|
| 1–2 d | 2.81 ± 0.02bA | 2.78 ± 0.02aA | 1.12 | 76 | 0.268 |
| 3–4 d | 2.92 ± 0.03aA | 2.77 ± 0.03aB | 3.75 | 41 | 0.001 |
| 5–6 d | 2.80 ± 0.05bA | 2.69 ± 0.04aA | 1.84 | 30 | 0.075 |
| F-value | 4.88 | 2.44 | |||
| df | 2,63 | 2,82 | |||
| P-value | 0.011 | 0.094 |
| Host-larval age . | Female forewing . | Male forewing . | t . | df . | P-value . |
|---|---|---|---|---|---|
| 1–2 d | 2.81 ± 0.02bA | 2.78 ± 0.02aA | 1.12 | 76 | 0.268 |
| 3–4 d | 2.92 ± 0.03aA | 2.77 ± 0.03aB | 3.75 | 41 | 0.001 |
| 5–6 d | 2.80 ± 0.05bA | 2.69 ± 0.04aA | 1.84 | 30 | 0.075 |
| F-value | 4.88 | 2.44 | |||
| df | 2,63 | 2,82 | |||
| P-value | 0.011 | 0.094 |
Means followed by the same lower (upper) case letter within a column (row) are not significantly different at P < 0.05 (Tukey’s test (within column), t-test (within row)).
The length of the hind-tibia of both female (F = 0.1; df = 2,61; P = 0.909) and male (F = 2.38; df = 2,76; P = 0.099) wasps obtained when larvae from the three age groups were parasitized did not differ significantly. However, within each of the three age groups, the female hind-tibia lengths were significantly longer than those of their male counterparts (Table 6).
Hind-tibia length (mm) of male and female (mean ± SE) F1 progeny of A. hemara obtained from different age groups of host larvae
| Host–larval age . | Female hind-tibia . | Male hind-tibia . | T . | df . | P-value . |
|---|---|---|---|---|---|
| 1–2 d | 0.84 ± 0.01aA | 0.76 ± 0.01aB | 5.59 | 70 | <0.001 |
| 3–4 d | 0.84 ± 0.01aA | 0.77 ± 0.02aB | 3.1 | 40 | 0.004 |
| 5–6 d | 0.83 ± 0.02aA | 0.73 ± 0.02aB | 4.04 | 29 | <0.001 |
| F-value | 0.1 | 2.38 | |||
| df | 2,61 | 2,76 | |||
| P-value | 0.909 | 0.099 |
| Host–larval age . | Female hind-tibia . | Male hind-tibia . | T . | df . | P-value . |
|---|---|---|---|---|---|
| 1–2 d | 0.84 ± 0.01aA | 0.76 ± 0.01aB | 5.59 | 70 | <0.001 |
| 3–4 d | 0.84 ± 0.01aA | 0.77 ± 0.02aB | 3.1 | 40 | 0.004 |
| 5–6 d | 0.83 ± 0.02aA | 0.73 ± 0.02aB | 4.04 | 29 | <0.001 |
| F-value | 0.1 | 2.38 | |||
| df | 2,61 | 2,76 | |||
| P-value | 0.909 | 0.099 |
Means followed by the same lower-case letter within a column and same uppercase letter within a row are not significantly different at P < 0.05 (Tukey’s test (within column), t-test (within row))
Hind-tibia length (mm) of male and female (mean ± SE) F1 progeny of A. hemara obtained from different age groups of host larvae
| Host–larval age . | Female hind-tibia . | Male hind-tibia . | T . | df . | P-value . |
|---|---|---|---|---|---|
| 1–2 d | 0.84 ± 0.01aA | 0.76 ± 0.01aB | 5.59 | 70 | <0.001 |
| 3–4 d | 0.84 ± 0.01aA | 0.77 ± 0.02aB | 3.1 | 40 | 0.004 |
| 5–6 d | 0.83 ± 0.02aA | 0.73 ± 0.02aB | 4.04 | 29 | <0.001 |
| F-value | 0.1 | 2.38 | |||
| df | 2,61 | 2,76 | |||
| P-value | 0.909 | 0.099 |
| Host–larval age . | Female hind-tibia . | Male hind-tibia . | T . | df . | P-value . |
|---|---|---|---|---|---|
| 1–2 d | 0.84 ± 0.01aA | 0.76 ± 0.01aB | 5.59 | 70 | <0.001 |
| 3–4 d | 0.84 ± 0.01aA | 0.77 ± 0.02aB | 3.1 | 40 | 0.004 |
| 5–6 d | 0.83 ± 0.02aA | 0.73 ± 0.02aB | 4.04 | 29 | <0.001 |
| F-value | 0.1 | 2.38 | |||
| df | 2,61 | 2,76 | |||
| P-value | 0.909 | 0.099 |
Means followed by the same lower-case letter within a column and same uppercase letter within a row are not significantly different at P < 0.05 (Tukey’s test (within column), t-test (within row))
Discussion
The response elicited by a parasitoid at varying host densities is an important attribute when considering an agent for biological control (Berryman 1999), because the host density has been reported in several studies to affect the performance of a parasitoid (Harbison et al. 2001, Islam et al. 2006, Zanuncio et al. 2013, de Pedro et al. 2017, Harbi et al. 2018). Parasitoid exposure to low host-larval density resulted in significantly higher rates of parasitism compared with high larval density treatments. A similar trend was reported by Harbi et al. (2018) in which higher parasitism rates by Diachasmimorpha longicaudata (Ashmead; Hymenoptera: Braconidae) were recorded at lower densities of Ceratitis capitata (Wiedeman; Diptera: Tephritidae) and Zanuncio et al. (2013) where parasitism by Campoletis flavicincta (Ashmead; Hymenoptera: Ichneumonidae) decreased with increasing density of Spodoptera frugiperda (Smith; Lepidoptera: Noctuidae). These results are contrary to the findings of Dannon et al. (2010) in which the percent parasitism of Maruca vitrata (Fabricius; Lepidoptera: Pyralidae) by Apanteles taragamae (Viereck; Hymenoptera: Braconidae) increased with larval density. The trend observed by Dannon et al. (2010) is atypical considering the trend in most solitary endoparasitoids (Montoya et al. 2000, Harbison et al. 2001, Kitthawee et al. 2004, Islam et al. 2006, Zanuncio et al. 2013, de Pedro et al. 2017, Harbi et al. 2018). While Dannon et al. (2010) point to a possible effect of the experimental set-up (arena) for their atypical observation, our results follow the prevailing trend found in most solitary endoparasitoids. This decrease in parasitism rate with increasing host density might not be the result of decreased parasitoid activity but rather a reflection of the fact that the calculation of parasitism rate itself includes an element of host density. This finding could further contribute to the conclusions of Fernández-arhex and Corley (2003), who while reviewing 32 functional response studies in parasitoids, found no clear relationship between the parasitoids’ functional response curves and their actual success in the field. We, therefore, recommend further robust studies on the best use of functional responses in parasitoids compared with their use in predators.
Nevertheless, when we consider functional response as the relationship between the number of hosts attacked by a parasitoid as a function of prey density (Holling 1959, Fernández-arhex and Corley 2003, Zanuncio et al. 2013), our data correspond to type II functional response. This means that the number of hosts parasitized by A. hemara increases with its host density but gradually decelerates to a constant, resulting in an asymptotic curve (Fernández-arhex and Corley 2003). We suggest that this is a more realistic and practical approach than the use of rate, since it can reveal the satiation level (i.e., the daily maximum oviposition potential) as a result of egg depletion in the parasitoids’ ovaries at higher host densities (Berryman 1999, Hassel 2000, Wajnberg et al. 2008, Zanuncio et al. 2013, Harbi et al. 2018). Thus, determining the highest number of eggs oviposited by female A. hemara per unit time would be useful for informing successful biological control programs, specifically parasitoid-to-host ratios to be applied.
Various studies have used either absolute number of hosts attacked (Harbison et al. 2001, Islam et al. 2006) or proportion/percent parasitism (Dannon et al. 2010, Pedro et al. 2017) to assess the functional response of parasitoids. Also, in consonance with the present study, Luna et al. (2007), Zanuncio et al. (2013), and Harbi et al. (2018) assessed functional response using both percentage of hosts parasitized and absolute number of hosts parasitized; where at higher host densities, the number of parasitized hosts increased, but the parasitism percentage declined. The conflicting results from the two different approaches call for careful verification of the methods of assessment of functional response. An evaluation of the two approaches in the present study demonstrated that the lower parasitism rates reported at higher host density did not translate into a smaller absolute number of hosts parasitized. Instead, the number of parasitized hosts increased with host density until satiation was reached, denoted by a plateau in the curve. The approach based on the number of hosts parasitized can be used to generate models to simulate the impacts of A. hemara on the populations of S. recurvalis in open field or screen house conditions and guide on how many parasitoids can be released (calibration of release) in a biological control program for effective management of the pest (Tonnang et al. 2009).
More viable parasitoid cocoons were available when the parasitoid encountered lower host larvae than when the parasitoid encountered higher densities of host larvae. This outcome might suggest that A. hemara, at low host density, can choose to lay only fit/mature eggs, while it could lay unfit/immature eggs at higher host densities. Zanuncio et al. (2013) failed to observe a significant difference in the percentage of C. flavicincta pupae that did not emerge into adults at different densities of S. frugiperda. Similarly, Harbison et al. 2001) did not see any significant difference in the pupal viability of Allorhogas pyralophagus (Marsh; Hymenoptera: Braconidae) at varying densities of Eoreuma loftini (Dyar; Lepidoptera: Pyralidae). We hypothesize that other factors (such as sex of eggs laid by the parasitoid and environmental conditions) can play a role in determining successful adult emergence; and hence, we recommend further studies to explore such factors.
The immature developmental time, sex ratio, and longevity of both male and female adults of the parasitoid did not vary across the host densities. A parasitoid’s developmental time, sex ratio, and adult longevity are often reflective of its host’s nutritional quality (Harvey 2000, Othim et al. 2017). Since the host larvae supplied to the parasitoids were of the same age and fed on the same host plant, we expected similarities in development time, sex ratio, and adult longevity. Zanuncio et al. (2013) also reported no significant difference in the sex ratio of C. flavicincta at different densities of S. frugiperda caterpillars.
Parasitism rate was significantly higher in 1- to 2-d-old larvae compared with 3- to 4-d-old larvae. A host’s morphological and physical characteristics, including size, texture, movement and defense responses, can affect a parasitoid’s attempts and capabilities to oviposit (Godfray 1994, Lauro et al. 2005, Othim et al. 2017). As the host larvae get older, they display stronger physical defense against the ovipositing female parasitoids compared with the younger and often smaller ones that can be easily handled and consequently parasitized (Brodeur et al. 1996, Shi et al. 2002). Older larvae are also usually larger compared with the parasitoid, hence, increase their handling time and present a risk to the parasitoid due to their developed mechanisms of defense. Younger S. recurvalis larvae also tend to aggregate while feeding, which could reduce the host searching time and result in higher parasitism rates. Shi et al. (2002) reported similar findings where they observed significantly higher parasitism by Cotesia vestalis (Haliday; (formerly Cotesia plutellae Kurdjumov) Hymenoptera: Braconidae) on the second and third larval instars of Plutella xylostella (Linnaeus; Lepidoptera: Plutellidae) compared with fourth instar larvae.
The developmental period of the parasitoid was most prolonged in 1- to 2-d-old larvae and least in 5- to 6-d-old larvae while cocoon survival rate was significantly lower in the younger larvae. Quality nutrition is a vital determinant of the developmental period and successful development of a parasitoid and is influenced by its host’s quality (Godfray 1994, Harvey 2000, Othim et al. 2017). In most cases, suboptimum nutrition of a host results in extended or longer developmental time in the koinobiontic parasitoids (Godfray 1994, Harvey and Strand 2003, Othim et al. 2017). Most studies have also presented nutritional richness in terms of host size and age (Godfray 1994, Harvey 2000). The model proposed by Mackauer and Sequeira (1993) supports the prolonged development time and high immature mortality in younger larvae observed in our study. The model postulates that the parasitoids attacking hosts of low-quality exhibit a lag phase in their development to allow the hosts to acquire sufficient nutrients for their development. For example, Apanteles myeloenta (Wilkinson; Hymenoptera: Braconidae) had prolonged developmental time in the first instar larvae of Ectomyelois ceratoniae (Zeller; Lepidoptera: Pyralidae) compared with the second and third instars (Farahani and Goldansaz 2013). Similarly, Meteorus pulchicornis (Wesmael; Hymenoptera: Braconidae) took longer to develop in first instar larvae of Mamestra brassicae (Linnaeus; Lepidoptera: Noctuidae) compared with the second and subsequent instars (Malcicka and Harvey 2014). The immature survival of A. hemara was also higher on the older larvae because these larvae had accumulated enough nutrients to meet the minimum threshold requirements for the developing parasitoids.
Apart from the developmental time many studies have also presented host size (quality) as a determinant of a parasitoid’s fitness (size) (Godfray 1994, Visser 1994, Harvey 2000, Ode and Heinz 2002). The age of host larvae significantly influenced the fitness of A. hemara in terms of size of F1 offspring. However, this effect of host age on female size of A. hemara was not linear. Several cases are reported in which parasitoid size is a nonlinear or increasing function of host age (Harvey et al. 1994, 1999, 2004; Harvey 2000, 2005; Harvey and Strand 2002,). Specifically, Harvey et al. (2004) demonstrated that the size of Microplitis demolitor (Wilkinson; Hymenoptera: Braconidae) did not have a linear correlation with larval age of Pseudoplusia includens (Walker; Lepidoptera: Noctuidae). While our results on parasitoid fitness can be interpreted superficially to suggest that the intermediate age group produced more fit parasitoids, the nonlinear relationship between host age and parasitoid size makes it difficult to conclude about the fitness benefits of body size (Harvey et al. 2004). Furthermore, that interpretation would not take into consideration the costs related to developmental time.
Our results show a significant nonreproductive host-larval mortality caused by A. hemara. Host feeding and host stinging behavior of the parasitoid often cause this mortality and are important contributors to pest suppression (Byeon et al. 2009, Akutse et al. 2015, Foba et al. 2015, Othim et al. 2017). Othim et al. (2017) established the possibility of superparasitism by A. hemara on S. recurvalis. Hence, the multiple visits to the same host by A. hemara can explain the high nonreproductive mortality among younger host larvae, implying repeated host stinging and resulting in numerous physical injuries (Keinan et al. 2012). The young hosts are also less mobile and incapable of self-defense, hence are more vulnerable and accessible to the parasitoid (Shi et al. 2002). Significant nonreproductive mortality has been reported to be a common phenomenon in ectoparasitoids while only a few endoparasitoids have this ability (Bernardo et al. 2006; Tran and Takagi 2005; Mafi and Ohbayashi 2010; Akutse et al. 2015; Muchemi et al. 2018a,b,c). For instance, Akutse et al. 2015 and Muchemi et al. 2018b recorded significant nonreproductive mortality in the ectoparasitoid Diglyphus isaea (Walker; Hymenoptera: Eulophidae) while Foba et al. 2015 and Muchemi et al. 2018c recorded insignificant nonreproductive mortality in the endoparasitoids Phaedrotoma scabriventris (Nixon; Hymenoptera: Braconidae), Opius dissitus (Muesebeck; Hymenoptera: Braconidae), and Halticoptera arduine (Walker; Hymenoptera: Pteromalidae) on Liriomyza sp. (Diptera: Agromyzidae). However, in congruence with our findings on A. hemara, significant nonreproductive mortality has been reported in the endoparasitoids Copidosoma koehleri (Blanchard; Hymenoptera: Encyrtidae) parasitizing potato tuber moth, Phthorimaea operculella (Zeller; Lepidoptera: Gelechiidae) (Keinan et al. 2012), D. longicaudata parasitizing C. capitata (Harbi et al. 2018), Aganaspis daci (Weld; Hymenoptera: Figitidae) parasitizing C. capitata (de Pedro et al. 2017), and Chrysocharis flacilla (Walker; Hymenoptera: Eulophidae) parasitizing Liriomyza sp. (Muchemi et al. 2018a,b).
In conclusion, A. hemara exhibited a type II functional response in which the number of hosts parasitized increased with host density then gradually decelerated to a constant regardless of any further increase in host density. We also found a different result between the two approaches used to assess the effect of host density on parasitism rate, which calls for a careful comparison of pieces of literature on the functional response of parasitoids. The age of the host larvae influenced parasitism rates, immature mortality, nonreproductive mortality, and size (fitness) of the female adult progeny. The development time of the parasitoid was prolonged in younger host larvae compared with the older larvae. For mass production of A. hemara, the shorter development time and low immature mortality achieved in the older hosts make them more favorable than the younger hosts in rearing.
Acknowledgments
We are grateful to Dr Dinssa F. Fekadu who provided the improved breeding line of amaranth used in this study, Mr Robert Mwashimaha for his technical assistance, Ms. Osogo Dolorosa for professional editing of the manuscript, and WorldVeg ESA for offering their institution and facilities for use in this study. The funding for this research was provided by the German Federal Ministry for Economic Cooperation and Development (BMZ) through icipe and the WorldVeg (Project number: 13.1432.7-001.00). We acknowledge icipe core funding provided by UK Aid from the UK Government, the Swedish International Development Cooperation Agency (SIDA), the Swiss Agency for Development and Cooperation (SDC), Government of the Federal Democratic Republic of Ethiopia and the Kenyan Government geared toward promoting the research agenda of icipe. The first author obtained a scholarship in the BMZ funded project through the Dissertation Research Internship Programme (DRIP) of icipe.