-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth T. Cirulli, Mohamed A. F. Noor, Localization and Characterization of X Chromosome Inversion Breakpoints Separating Drosophila mojavensis and Drosophila arizonae, Journal of Heredity, Volume 98, Issue 2, March/April 2007, Pages 111–114, https://doi.org/10.1093/jhered/esl065
Close - Share Icon Share
Abstract
Ectopic exchange between transposable elements or other repetitive sequences along a chromosome can produce chromosomal inversions. As a result, genome sequence studies typically find sequence similarity between corresponding inversion breakpoint regions. Here, we identify and investigate the breakpoint regions of the X chromosome inversion distinguishing Drosophila mojavensis and Drosophila arizonae. We localize one inversion breakpoint to 13.7 kb and localize the other to a 1-Mb interval. Using this localization and assuming microsynteny between Drosophila melanogaster and D. arizonae, we pinpoint likely positions of the inversion breakpoints to windows of less than 3000 bp. These breakpoints define the size of the inversion to approximately 11 Mb. However, in contrast to many other studies, we fail to find significant sequence similarity between the 2 breakpoint regions. The localization of these inversion breakpoints will facilitate future genetic and molecular evolutionary studies in this species group, an emerging model system for ecological genetics.
Chromosomal inversions are major players in many important evolutionary processes. Inversions prevent genetic recombination between homologous chromosomes because products of such recombination in inverted regions either are not incorporated into egg nuclei or produce inviable gametes. The lack of crossover products stemming from heterozygotes in inverted regions can create patterns of linkage disequilibrium across many loci that facilitate adaptation or speciation. For example, Dobzhansky's classic studies in Drosophila pseudoobscura showed that certain environmental conditions (e.g., temperature) repeatedly favored specific arrangements, demonstrating selective factors affecting these allele complexes in nature (see Lewontin et al. 1981). Similarly, reducing recombination may allow hybridizing species to persist and continue to diverge rather than fuse (see reviews in Ortiz-Barrientos et al. 2002; Butlin 2005).
For many years, researchers suggested that repetitive sequences, such as transposable elements, may contribute to the formation of chromosomal inversions via ectopic exchange (for reviews, see Charlesworth et al. 1994; Coghlan et al. 2005; Noor and Chang 2006). Under this model, similar sequences at disjunct sites on a chromosome act as sites of homology and thereby are targets for ectopic exchange and reversals of sequence. Evidence for this has been obtained from plants, fungi, and various animals, wherein inversion breakpoints are very frequently associated with similar sequence motifs (Coghlan et al. 2005). An association between inversion breakpoints and repetitive sequence has been particularly well demonstrated in many Drosophila species (e.g., Lim 1988; Caceres et al. 1999; Evgen'ev et al. 2000; Richards et al. 2005).
In this study, we examine a fixed X chromosome inversion difference distinguishing the cactophilic species Drosophila mojavensis from its sister Drosophila arizonae. On this chromosome, D. arizonae bears the ancestral arrangement (shared with Drosophila navojoa and other out-group species), whereas D. mojavensis differs by one inversion (designated Xe, Ruiz et al. 1990), and neither species appears to be polymorphic for arrangements on this chromosome. These species diverged approximately 2 million years ago (Mills et al. 1986; Matzkin and Eanes 2003) and have been instrumental in our understanding of ecological and evolutionary genetics of host-plant adaptation and speciation (see review in Etges et al. 1999).
The genome sequence assembly of D. mojavensis is now available, but we know of no plans for a comparable effort in D. arizonae. Hence, we document the positions of the X chromosome inversion breakpoints separating these species by comparing the D. mojavensis genome sequence assembly and linkage map (Staten et al. 2004) to the results from our mapping crosses of D. arizonae. We also examine whether the 2 breakpoints may be associated with repetitive elements within the genome. Identifying the inversion breakpoints allows for subsequent work to examine the evolutionary forces that may have driven its spread and facilitates interpretation of population genetic research on these important sister species.
Materials and Methods
A female-parent backcross using D. arizonae strains 15081-1271.15 and 15081-1271.16 was reared, and 96 female progeny were genotyped for 7 previously described X-linked markers (Staten et al. 2004) as part of a previous study (Counterman and Noor 2006). Comparison of the resultant linkage map to the D. mojavensis sequence assembly (Comparative Analysis Freeze 1) localized 1 of the 2 X chromosomal inversion breakpoints to a 2.2-Mb region between markers DMOJX501 and DMOJX100, within scaffold 6473. The second breakpoint was only shown to be further away from DMOJX501 than DMOJX500 and DMOJA514 (>10 Mb away) but not localized further. In this study, we have extended the localizations greatly by developing 28 novel variable markers across both breakpoint regions (see Supplementary Table 1) and serially scoring 12 of these markers in backcross progeny, along with the markers previously used in this region.
For the 11 new microsatellite markers used, polymerase chain reaction (PCR) was performed in 10 μl reactions containing 1 μl of flyprep DNA (Gloor and Engels 1992), using the following touchdown cycling protocol: 95 °C for 1 min; 3 cycles of 95 °C for 30 s, 56 °C for 30 s, 72 °C for 30 s; 3 cycles of 95 °C for 30 s, 53 °C for 30 s, 72 °C for 30 s; 30 cycles of 95 °C for 30 s, 50 °C for 30 s, 72 °C for 30 s (Palumbi 1996). Products were visualized on a polyacrylamide gel using a LiCor DNA analyzer.
We generated DNA sequences in one region of interest from the 2 parental lines of D. arizonae used in the cross (GenBank accession numbers DQ885915 and DQ885916). From these sequences, we designed one restriction fragment length polymorphism (RFLP) marker that distinguishes the 2 lines (DMOJX1.30a). Following PCR as above, the products were digested with 4 U MseI for 1 h at 37 °C. The digests were visualized on a 2% agarose gel.
We identified the linear order of all the markers and estimated the crossover frequencies in Kosambi (1944) centiMorgans. The order of markers in D. arizonae was compared with the D. mojavensis order as detailed in the genome sequence assembly (Gilbert 2005) and linkage map (Staten et al. 2004). Sequence analyses were performed with BLAST (Altschul et al. 1990), RepeatMasker (Smit et al. 2004), and DroSpeGe (Gilbert 2005), all with default settings. We also directly consulted the published Drosophila melanogaster genome sequence assembly (Adams et al. 2000) for some comparisons.
Results and Discussion
Our primary goals were 1) to precisely identify one of the X chromosome inversion breakpoints, separating D. mojavensis and D. arizonae and 2) to coarsely localize the other breakpoint. With this information, we also sought to describe the features of the breakpoint region and to determine if any sequences were shared between the 2 breakpoint regions that may have facilitated the generation of this inversion in D. mojavensis.
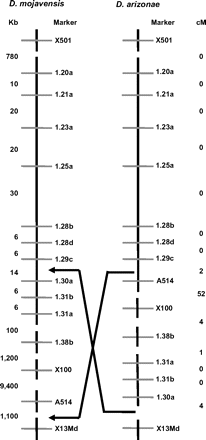
Mapping 10 of the new microsatellites (Supplementary Table 1) in D. arizonae, we initially localized one end of the X chromosome inversion to an interval of approximately 20 kb between markers 1.29c and 1.31b (Figure 1). This breakpoint region spanned 2 D. mojavensis sequence contigs, 01_9263 and 01_9264, with a small (∼200 bp) region in the middle of unresolved sequence. Subsequently, we scored the backcross progeny for an RFLP marker (DMOJX1.30a) within this interval, and we confirmed that locus was still within the inverted region, narrowing the breakpoint further to a 13.7-kb region between markers 1.29c and 1.30a (Figure 1, Supplementary Table 1), all within contig 01_9263 and with no ambiguous bases.
X chromosome inversion breakpoint regions identified via mapping. The left panel indicates physical distances between markers in the D. mojavensis genome sequence assembly. The right panel indicates recombinational distances between these markers in D. arizonae, and the arrows designate the localized inversion breakpoint regions.
We localized the other X chromosome breakpoint by amplifying microsatellite marker DMOJX13Md, which is 1.1 Mb from DMOJA514 (both also in D. mojavensis genome sequence scaffold 6473). We found that these 2 markers were freely recombining in D. arizonae, yet DMOJX13Md was tightly linked to DMOJX1.30a (Figure 1) in D. arizonae; the latter 2 markers are separated by more than 12 Mb in D. mojavensis. As such, we conclude that DMOJX13Md is outside of the inverted region (Figure 1) and that the other breakpoint is within this 1.1-Mb interval. These results define the size of the X chromosome inversion separating these species at between 10.7 and 11.8 Mb.
We used BLAST (Altschul et al. 1990) to search for sequences in the 13.7-kb region of D. mojavensis that resemble repetitive elements (via DroSpeGe, Gilbert 2005). No significant similarity was found to regions outside this interval except a few small approximately 20- to 30-bp hits. However, within this interval, we found a large sequence that is repeated and inverted. The 2 sequences are 1066 bp in length, 97% similar (1038/1066, no gaps), and separated by an 865-bp stretch. This repeated sequence does not bear substantial DNA sequence similarity to other regions of the D. mojavensis genome, but a translated BLAST search shows that this region has significant similarity to the D. melanogaster–predicted serine proteinase gene CG2056.
We hypothesized that this inverted repeat of CG2056 may be closely associated with an inversion breakpoint and sought to test this by comparison to the D. melanogaster sequence assembly (Adams et al. 2000). Drosophila melanogaster and D. mojavensis diverged about 63 million years ago (Tamura et al. 2004) and surely differ by a great many rearrangements. Nonetheless, microsynteny between these species groups still exists in some regions (Ranz et al. 2001), so we hypothesized that gene order may be retained between these species for very small windows. Correspondingly, for genes very close to the inversion breakpoints, we predict that D. melanogaster may have the same order as that of D. arizonae, whereas D. mojavensis will differ because it bears the derived arrangement.
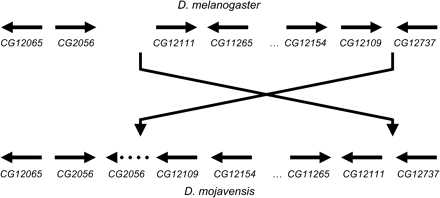
The D. melanogaster homologues of the D. mojavensis genes in or near the smaller breakpoint window are shown in Figure 2 on the lower left side (identified via DroSpeGe, Gilbert 2005). As we predicted, we observed a break in gene-order conservation between the 2 species following the inverted repeat of CG2056. Further, we found that the D. melanogaster gene order in these 2 small windows can be restored by a single reciprocal switch (see Figure 2). This order affords us compelling hypotheses regarding the exact locations of both inversion breakpoints. The region between the reversed CG2056 and CG12109 in D. mojavensis spans less than 1300 bp and bears no significant BLAST similarity to any sequenced Drosophila species or other region within D. mojavensis. The breakpoint region between CG12111 and CG12737 in D. mojavensis spans 2700 bp. It has some small unique stretches of sequence similarity to other Drosophila species, but no repetitive elements. BLAST of this region to the D. mojavensis genome sequence identified one <50-bp stretch that appears to be repeated in several places across the genome (not at the other breakpoint region, however), but no other repetitive elements. If these regions are the inversion breakpoints separating D. mojavensis and D. arizonae, the size of the inversion is approximately 10.8 Mb.
X chromosome inversion breakpoint regions identified via hypothesized microsynteny between D. melanogaster and D. mojavensis. The direction of transcription of each predicted gene is noted. Distances between transcripts are not to scale.
The absence of sequences repeated between the 2 breakpoint regions was surprising, as such repetitive sequences have been shown to be strongly associated with inversions both in Drosophila (e.g., Lim 1988; Caceres et al. 1999; Evgen'ev et al. 2000; Richards et al. 2005) and in general (for reviews, see Charlesworth et al. 1994; Coghlan et al. 2005), though there are exceptions (e.g., Wesley and Eanes 1994). In contrast to our findings here, the species D. melanogaster and D. pseudoobscura have been separated by 55 million years (Tamura et al. 2004), yet more than 60% of the 921 inversion breakpoints identified as separating these species still had BLAST similarity to other inversion breakpoints (Richards et al. 2005). As D. mojavensis and D. arizonae are separated by only approximately 2 million years (Mills et al. 1986; Matzkin and Eanes 2003), there has been much less time for the loss of such repeated sequences. A repeated sequence may have been lost in the exchange generating the inversion and could perhaps be related to the inverted repeat of CG2056, which we identified in one breakpoint region. However, at present, we only have a correlation between the inverted repeat of CG2056 and the inversion breakpoint, not direct evidence that the inversion caused the repeat.
We have identified and described the 2 breakpoint locations of the X chromosome inversion separating D. mojavensis and D. arizonae. This information will facilitate the interpretation of population genetic, molecular evolutionary, phylogenetic, phylogeographic, and genetic mapping studies in this species group (e.g., Pantazidis et al. 1993; Durando et al. 2000; Oliveira et al. 2003; Counterman and Noor 2006). Further, unlike many other well-studied Drosophila species, the ecology of these species has been studied intensively. As more genomic tools and information accumulate for “emerging model” species with well-studied ecologies such as these, we can hopefully integrate these different types of information to obtain a more complete and holistic understanding of the speciation process.
Supplementary Material
Supplementary Table 1 can be found at http://www.jhered.oxfordjournals.org/.
We thank A. Chang and one referee for comments on the manuscript, B. Counterman for providing flies for this study, and E. Gragg for technical assistance. This research was funded by National Science Foundation grants 0509780 and 0549893 to M.A.F.N. This paper is based on a presentation given at the 2006 Annual Meeting of the American Genetic Association, “Genetics of Speciation,” University of British Columbia, Vancouver, Canada, July 21–24, 2006.
References
Author notes
Corresponding Editor: Loren Rieseberg