-
PDF
- Split View
-
Views
-
Cite
Cite
Scott W Olesen, Elizabeth A Torrone, John R Papp, Robert D Kirkcaldy, Marc Lipsitch, Yonatan H Grad, Azithromycin Susceptibility Among Neisseria gonorrhoeae Isolates and Seasonal Macrolide Use, The Journal of Infectious Diseases, Volume 219, Issue 4, 15 February 2019, Pages 619–623, https://doi.org/10.1093/infdis/jiy551
Close - Share Icon Share
Abstract
Rising azithromycin nonsusceptibility among Neisseria gonorrhoeae isolates threatens current treatment recommendations, but the cause of this rise is not well understood. We performed an ecological study of seasonal patterns in macrolide use and azithromycin resistance in N. gonorrhoeae, finding that population-wide macrolide use is associated with increased azithromycin nonsusceptibility. These results, indicative of bystander selection, have implications for antibiotic prescribing guidelines.
Rising azithromycin minimum inhibitory concentrations (MICs) among Neisseria gonorrhoeae isolates may prompt reconsideration of current treatment recommendations for gonorrhea [1, 2]. However, the cause of this rise is not well understood, with recent studies reporting apparently contradictory results. One study [3] found that azithromycin MICs were higher in gonorrhea patients at a Dutch sexual health clinic who had recent azithromycin use. In contrast, a study [4] of gonorrhea patients from the United Kingdom found no link between azithromycin MICs and recent treatment for other sexually transmitted infections for which azithromycin is the typical therapy. Finally, a US ecological study [5] found that temporal and geographical variations in population-wide macrolide use did not explain differences in MICs, highlighting the role of importation of isolates with azithromycin nonsusceptibility. While importation of strains from outside the United States likely plays a role in azithromycin nonsusceptibility [5], Neisseria gonorrhoeae’s genomic population structure indicates it has acquired nonsusceptibility multiple times while circulating in the United States [6].
Our objective was to identify a population-level association between macrolide use and N. gonorrhoeae azithromycin MICs. Because seasonal variations in macrolide use are similar in magnitude to variations in use across geographical regions and across decades of time [5, 7], we examined seasonal patterns in macrolide use and azithromycin MICs and linked the 2 with a mathematical model [8]. Associations between seasonal antibiotic use and resistance have been examined for other pathogens and antibiotics [7, 9], and seasonal MICs patterns have been reported for N. gonorrhoeae and drugs besides azithromycin [10].
METHODS
We used a mathematical model [8] to link seasonal antibiotic use and MICs, substituting MIC for the model’s original outcome (ie, the proportion of isolates with resistance). In this model, antibiotic use and MICs are assumed to have a fixed, year-round average value. In contrast with a traditional regression model approach, in which the model predicts the year-round average MIC from year-round average antibiotic use, here we hypothesize that the seasonal deviations in antibiotic use from the year-round average exert a fluctuating selection pressure for higher MICs. When antibiotic use is above average, the selection pressure is greater than average, leading to increasing MICs. Conversely, when use is below average, MICs decrease. Thus, the seasonally varying MICs are expected to be proportional to the time derivative of seasonally varying antibiotic use [11]. This model makes no assertions about the specific mechanism by which antibiotic use selects for resistance.
where is the year-round mean MIC, and is the amplitude of the seasonal mean MIC. The 2 amplitudes are related by the selection coefficient , measured as the difference in mean MIC per unit difference in monthly population-wide antibiotic use. If antibiotic use varies like a sine, MICs vary like a cosine (ie, lagged by 3 months) because the model asserts that use determines the rate of change in resistance. Thus, if antibiotic use peaks in winter, MICs will peak in spring, reflecting the cumulative effect of the entire winter’s above-average antibiotic use. MICs decrease into summer as antibiotic use falls below the year-round average [11].
The original model [8] allowed for an ad hoc “restoring force” that counteracts the effects of deviations in antibiotic use on resistance. A nonzero restoring force alters the phase delay and the selection coefficient between antibiotic use and resistance. Because our results are consistent with the 3-month phase delay predicted from a zero restoring force, we used the simple form of the model discussed above.
We measured the seasonality of macrolide antibiotic use (azithromycin, clarithromycin, and erythromycin), using the Truven Health MarketScan Research Database [12], a nationwide pharmacy prescription claims database that has previously been used to characterize antibiotic use [13], covering 2011–2015. Individuals in the database aged 10–59 years who were covered by an insurance plan for all 12 months of any year from 2011 through 2015 were included. Macrolide use, measured as outpatient pharmacy fills per 1000 insurance plan members per month, was measured for each month of 2011–2015 among 5 age groups (10–19, 20–29, 30–39, 40–49, and 50–59 years) and for each of the 4 US Census regions. We fit monthly macrolide use to a 1-year period sinusoid, using the nls function in R (version 3.4.4).
where is the MIC of the i-th isolate, is the month of collection, is the phase of the MIC seasonality, is the clinic/year of the isolate, are within-clinic/year slope terms, and are within-clinic/year intercept terms. The 10 isolates belonging to the 5 clinic/year combinations with <10 isolates were excluded because the fitting procedure could not accurately estimate within-clinic/year terms for those combinations. Because the seasonality model does not predict the year-round average MIC, our approach does not attempt to explain the differences in average MICs between clinics by using the differences in antibiotic use between clinics, nor does it attempt to explain secular trends in MICs by secular trends in antibiotic use.
The appropriateness of the seasonality model was evaluated by comparing the phases of antibiotic use and MICs , with MICs expected to peak 3 months after antibiotic use. The selection coefficient was estimated by comparing the 2 amplitudes and , with the caveats that antibiotic use was measured for a subset of the interval for which MICs were measured and that, not having access to detailed demographic information about the individuals from whom the isolates were collected, the amplitude of seasonality among gonococcus carriers could not be precisely determined.
Code used in these analyses is available at: https://github.com/gradlab/gisp-seasonal-methodology.
RESULTS
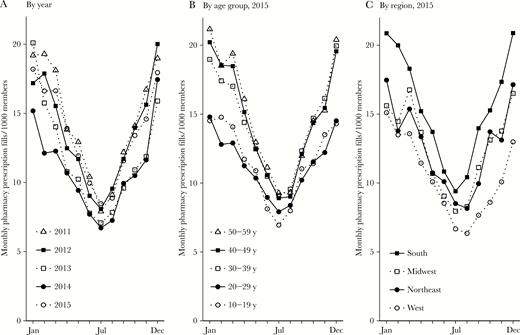
Year-round mean macrolide use was 12.8 monthly outpatient pharmacy fills per 1000 insurance plan members (95% confidence interval [CI], 12.4–13.3; Figure 1). Macrolide use was seasonal, with an amplitude of 4.8 monthly fills per 1000 members (95% CI, 4.2–5.4) and a peak in December/January (peak at 0.027 months [95% CI, –.22–.27], where 0.0 equals 1 January). Mean use and amplitude varied by age group and geographic region, but use was seasonal with a December/January peak for all data years, ages, and regions (Supplementary Table).
Seasonality in macrolide use among MarketScan members, 2011–2015. Points indicate monthly macrolide use among all included members, by year (A); among members in each age group in 2015 (B); and among members in each US Census region in 2015 (C).
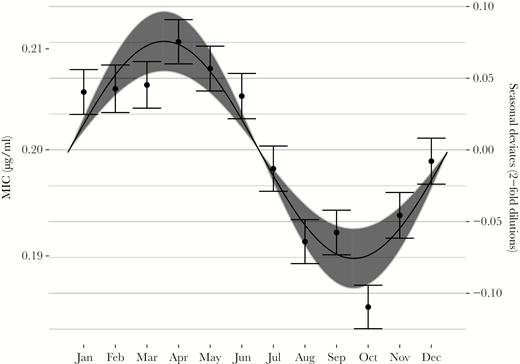
Azithromycin resistance among GISP isolates was seasonal with an amplitude of 0.076 dilutions (95% CI, .055–.097; Figure 2). As predicted by the mathematical model, seasonal resistance peaked 3 months after peak macrolide use in March/April (peak at 2.5 months [95% CI, 2.2–2.9], where 2.5 is approximately equal to 15 March).
Seasonality in Neisseria gonorrhoeae azithromycin minimum inhibitory concentrations (MICs) among GISP isolates, 2005–2015. Points indicate monthly means of the seasonal deviates; error bars show standard errors of the mean. The line indicates the point estimate for the seasonal amplitude and phase from the sinusoidal model; the gray area shows the 95% confidence interval for the amplitude. MICs (left axis) were computed using seasonal deviates from year-clinic regressions (right axis) on a baseline MIC of 0.2 μg/mL.
Using the value for and the population-wide , we back-calculated the antibiotic selection pressure coefficient b as 0.0084 MIC dilutions per additional monthly fill per 1000 people (95% CI, .0059–.0110). This value predicts that, from a baseline MIC of 0.2 μg/mL, a 10% increase in macrolide use is associated with an additive increase in mean MIC of 0.0015 μg/mL (95% CI, .0011–.0020).
Discussion
Using a mathematical model that links seasonal patterns of antibiotic use with resistance, we found that the population-wide seasonal variation in azithromycin resistance was associated with seasonal variation in N. gonorrhoeae azithromycin MICs.
The antibiotic selection pressure coefficient we observed refines the results from the ecological study by Kirkcaldy et al [5], which estimated that a 10% increase in population-wide macrolide use was associated with a 95% CI of –.05 to .02 μg/mL for the difference in N. gonorrhoeae azithromycin MICs. Our result, that a 10% difference in population-wide macrolide use is associated with a 0.0015-μg/mL difference in MIC, significantly differs from zero and is also well within Kirkcaldy et al’s 95% CI. Our results suggest that the association between population-level macrolide use and azithromycin MICs was 10–100-fold smaller than could be detected with Kirkcaldy et al’s design. The collinearity of secular trends in use and MICs may have reduced that study’s power to detect an association [7, 9]. Predicting a difference in clinics’ MICs from their differences in local macrolide use may also have decreased the study’s power, as MICs may vary between clinics for reasons aside from antibiotic use.
We speculate that the seasonal pattern in MICs arises because N. gonorrhoeae hosts are more likely to use macrolides to treat respiratory complaints in winter than in summer, subjecting N. gonorrhoeae to a seasonal bystander effect in which the bacteria experience additional antibiotic pressure in the winter for reasons unrelated to gonorrhea. To link the individual-level and population-level effects, we used a back-of-the-envelope calculation. Our observed 12.8 macrolide claims per 1000 people per month means that <2% of people claimed a macrolide in a given month. A 10% increase in macrolide use, therefore, roughly corresponds to an extra 0.2% of people claiming a macrolide. If, as per the estimate from the Dutch individual-level study [3], azithromycin MICs double among N. gonorrhoeae carriers in the last month, then the mean N. gonorrhoeae MIC will increase by 0.2%. If a baseline of 0.2 μg/mL is assumed, a 0.2% increase corresponds to 0.0004 μg/mL, which is smaller than but comparable to our result of 0.0015 μg/mL. While acknowledging that ecological designs do not enable individual-level inference, our results suggest that the small, observed seasonal variation in azithromycin MICs is compatible with large, individual-level variations in MICs.
The principal limitation of our study is its ecological design, which prevents ruling out other seasonal effects. For example, the observed MIC seasonality could be due to hosts carrying N. gonorrhoeae with higher MICs contributing disproportionately to the sampled population during spring. However, we do not expect that the seasonality of the gonorrhea incidence, which may peak in summer [15], could explain our results: seasonal, summer use of azithromycin for gonorrhea would cause an MIC seasonality pattern opposite from what we observed. We also note that, because seasonal amplitudes of use varied by as much as 20% across age and geographic regions (Supplementary Table), and because we measured macrolide use among a convenience sample of individuals covered by private insurance, the selection coefficient b we computed should be regarded as an estimate only.
In conclusion, we found that seasonal changes in population-wide macrolide use are associated with a small but nonzero seasonal sinusoidal pattern in mean N. gonorrhoeae MIC. The relationship between macrolide use and azithromycin MICs that we observed is consistent both with Kirkcaldy et al’s 95% CI and with Wind et al’s finding that recent exposure to azithromycin is associated with large increases in N. gonorrhoeae MIC within individuals. Our results are inconsistent with a third report finding no such individual-level effect [4]. Although we cannot establish the relative effect of population-wide azithromycin use and direct treatment on MICs [3], the rise in population-wide macrolide use likely influenced the rise of gonococcal azithromycin MICs. The anticipated bystander effect of antibiotics used for conditions other than gonorrhea should be considered as treatment guidelines for gonococcal infections are updated.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. S. W. O., M. L., and Y. H. G. conceived of and designed the work. E. A. T., J. R. P., and R. D. K. acquired the data. S. W. O. analyzed the data. S. W. O. and Y. H. G. drafted the work. All authors contributed to interpretation of data, revised the work, approved the final version, and agreed to be accountable for accuracy and integrity of the work. Y. H. G. had full access to all the data in the study and had final responsibility to decide to submit for publication.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the National Institute of General Medical Sciences, or the National Institutes of Health. The funding sources had no role in the study.
Financial support. This work was supported by the National Institute of General Medical Sciences (cooperative agreement U54GM088558 to S. W. O. and M. L.) and the National Institute of Allergy and Infectious Diseases (grant R01 AI132606 to Y. H. G.).
Potential conflicts of interest. Y. H. G. has received consulting fees from GlaxoSmithKline. All other authors report no potential conflicts.