-
PDF
- Split View
-
Views
-
Cite
Cite
James P. Alexander, Kristen Ehresmann, Jane Seward, Gary Wax, Kathleen Harriman, Susan Fuller, Elizabeth A. Cebelinski, Qi Chen, Olen M. Kew, Mark A. Pallansch, M. Steven Oberste, Mark Schleiss, Jeffrey P. Davis, Bryna Warshasky, Susan Squires, Harry F. Hull, for the Vaccine-Derived Poliovirus Investigations Group, Transmission of Imported Vaccine-Derived Poliovirus in an Undervaccinated Community in Minnesota, The Journal of Infectious Diseases, Volume 199, Issue 3, 1 February 2009, Pages 391–397, https://doi.org/10.1086/596052
Close - Share Icon Share
Abstract
Background.Oral poliovirus vaccine (OPV) has not been used in the United States since 2000. Type 1 vaccinederived poliovirus (VDPV) was identified in September 2005, from an unvaccinated Amish infant hospitalized in Minnesota with severe combined immunodeficiency. An investigation was conducted to determine the source of the virus and its means of transmission.
Methods. The infant was tested serially for poliovirus excretion. Investigations were conducted to detect poliovirus infections or paralytic poliomyelitis in Amish communities in Minnesota, neighboring states, and Ontario, Canada. Genomic sequences of poliovirus isolates were determined for phylogenetic analysis.
Results. No source for the VDPV could be identified. In the index community, 8 (35%) of 23 children tested, including the infant, had evidence of type 1 poliovirus or VDPV infection. Phylogenetic analysis suggested that the VDPV circulated in the community for ∼2 months before the infant's infection was detected and that the initiating OPV dose had been given before her birth. No paralytic disease was found in the community, and no poliovirus infections were found in other Amish communities investigated.
Conclusions. This is the first demonstrated transmission of VDPV in an undervaccinated community in a developed country. Continued vigilance is needed in all countries to identify poliovirus infections in communities at high risk of poliovirus transmission.
Because of successful vaccination programs, endemic poliomyelitis was eliminated from the United States by the mid-1970s. In 1979, after importation of wild poliovirus, the last US polio outbreak occurred in unvaccinated Amish communities, involving 13 cases of paralytic disease in 3 states and subclinical infections in 3 other states as well as 2 cases of paralytic disease in Ontario, Canada [1, 2]. From 1980 to 1997, a mean of 9 reported paralytic poliomyelitis cases occurred annually in the United States [3, 4]. Nearly all were vaccineassociated paralytic polio (VAPP), underscoring the ongoing risk associated with oral poliovirus vaccine (OPV), estimated to be 1 case of VAPP per 2.9 million doses distributed [4]. As the occurrence of wild poliovirus importations declined to only 5 in the 1980s and to 2 in the 1990s [3, 4], the risk-benefit equation changed in favor of using inactivated poliovirus vaccine (IPV) routinely. From 1997 to 2000, the transition in vaccination policy from an all-OPV to an all-IPV schedule eliminated VAPP in the United States; the last endemically acquired case of VAPP occurred in 1999 [4–6].
In addition to causing VAPP, OPV use is also associated with the rare occurrence of genetically drifted vaccine-derived polioviruses (VDPVs) that can circulate in undervaccinated populations (circulating VDPVs [cVDPVs]) or cause persistent infections in immunodeficient individuals (immunodeficiency- related VDPVs [iVDPVs]) [7-9]. In September 2005, a poliovirus infection in an unvaccinated Amish infant was identified by the Minnesota Department of Health and reported to the Centers for Disease Control and Prevention. The virus was identified as type 1 VDPV. The present report describes the epidemiologic and laboratory investigations and control efforts regarding the first VDPV with community transmission detected in the United States.
Methods
Index patient investigation. We reviewed the infant's medical, family, and social history to identify potential sources for exposure to polioviruses. We confirmed the infant's VDPV infection and monitored her for ongoing excretion of VDPVs during treatment of the underlying immunodeficiency, using approximately weekly testing (from late September 2005 through January 2006) of serum samples for neutralizing antibody to polioviruses and of stool specimens for VDPVs.
Hospital and health care investigations and interventions. In October 2005, we conducted investigations at the 4 hospitals (hospitals 1–4) to which the infant had been admitted, to determine whether she had been infected in the health care setting or whether her infection had been transmitted to others. Hospital staff offered IPV to health care workers and patients with recent or ongoing exposure who were not current for polio vaccination [6]. At hospitals 1–3, where the infant had been admitted during July–August 2005, hospital infection control staff reviewed health care worker and patient records to identify contacts. We surveyed contact health care workers and infection control staff reviewed contact patient records regarding relevant recent illness and immunologic status. At hospital 4, where the infant remained hospitalized in October 2005, we surveyed staff who reported exposure to the infant or her environment without the use of contact precautions regarding polio vaccination status, immunologic status, and recent illnesses in themselves or family members. Hospital staff collected stool specimens from potentially exposed staff and from patients who were located in the same medical units as the infant.
To identify other potential health care sources of the infant's infection, we interviewed staff at the 4 hospitals, including pediatric and adult immunologists, to identify health care workers or Amish patients in Minnesota with chronic immunodeficiencies treated since 1 January 2003, and we contacted specialists at 3 internationally recognized Minnesota hospitals to identify foreign-born or foreign-resident children <10 years old with chronic immunodeficiencies treated since 1 January 2003 or with poliomyelitis-like conditions treated since 1 January 2005.
Community investigations and interventions. Beginning in early October 2005, we conducted investigations in the index and other Minnesota Amish communities, Amish communities in Wisconsin where the infant's grandparents lived or had close contacts, and Amish communities in Michigan, Missouri, and Ontario that had had recent contact with the index community. During each investigation, we notified local Amish bishops about the situation in the index community, sought permission to conduct an investigation, inquired about illness, and attempted to obtain stool, throat swab, or serum specimens from community members for laboratory testing. In the index community and Wisconsin investigations, we also inquired about polio vaccination status, history of poliomyelitis, and contact with other Amish communities. To minimize risk of VDPV transmission in each of the communities investigated, IPV was offered to all members of the community or to persons who lacked documentation of complete polio vaccination. We also conducted retrospective and prospective active surveillance for polio-compatible diseases, including aseptic meningitis diagnoses at central Minnesota hospitals serving Amish communities in July–December 2005; hospitalizations for aseptic meningitis at 2 Minnesota children–s hospitals, with a focus on patients from counties with Amish populations in 2003–2005; and hospitalizations or medical visits for acute flaccid paralysis, Guillain-Barré syndrome, and transverse myelitis at all Minnesota hospitals and hospitals in bordering states serving significant numbers of Minnesota residents in 2003–2005.
Virus characterization. Polioviruses were isolated by culture in rhesus monkey kidney cells. Poliovirus isolates were initially identified by patterns of neutralization with Lim Benyesh- Melnick antiserum pools [10, 11] and were confirmed by neutralization with P1-specific antiserum, diagnostic polymerase chain reaction [12, 13], and sequencing of the viral protein (VP) 1 region, as described elsewhere [14, 15]. Nearly complete (nt 27–7441) genomic sequencing was performed as described elsewhere [14, 16]. Sequence relationships among substitutions at 2- and 4-fold degenerate sites (closely approximating synonymous substitutions) in the complete open reading frame (ORF; nt 743-7369) of all VDPV isolates were summarized in a phylogenetic tree rooted to the sequence of Sabin 1 inferred by Bayesian Markov chain Monte Carlo analysis using the BEAST program (version 1.4) [17]. The ratio of nonsynonymous to synonymous substitutions (Ka/Ks ratio) within the neutralizing antigenic (NAg) sites (as defined in figure 3) were determined using the Pamilo-Bianchi-Li method [18, 19] as implemented in the MEGA3 software package [20].
Sequences of amino acid residues within or bounding neutralizing antigenic (NAg) sites. Residues defining the type 1 poliovirus NAg sites by mutations conferring resistance to neutralization by monoclonal antibodies [24 -27] are indicated by boldface type. The trypsin cleavage site in NAg-1, characteristic of Sabin 1 [28], is underlined. cVDPV, circulating vaccine-derived poliovirus; DOR, Dominican Republic; FamC, family C; FamD, family D; Index, index patient; iVDPV, immunodeficiency-related vaccine-derived poliovirus; PHL, Philippines; TAI, Taiwan.
Serologic investigations. Serum samples were tested for levels of neutralizing antibodies to poliovirus types 1, 2, and 3 by means of a modified microneutralization assay [21]. Serum samples were tested in triplicate using serial 2-fold dilutions, starting at 1:8 and ending at 1:1024. The final titer for each serum (re- ported as reciprocal dilutions) was estimated by the Spearman- Kärber method [22].
Informed consent and human subject guidelines. The investigations conducted in the several jurisdictions were exempt from institutional review board review of the respective agencies, because they were determined to be authorized responses to a public health emergency.
Results
Index patient investigation. The index patient was born in February 2005. Her parents and the patient's 3 siblings were reportedly healthy. The parents had received OPV in 1979. The 3 siblings had received no childhood vaccines. During the spring and summer of 2005, the family visited the infant's grandparents in Wisconsin, and there were visitors to the index community from other Amish communities in Minnesota, Wisconsin, Michigan, and Ontario. No international travel to OPV-using countries by any member of the index community was identified.
During July 2005, the infant had multiple medical visits for respiratory illness and was hospitalized at a community hospital (hospital 1) for bronchiolitis and bacterial conjunctivitis. During August, she had medical visits for failure to thrive, fever, diarrhea, and anemia. Beginning 22 August 2005, she was hospitalized continuously at a regional medical center (hospital 2), a children's hospital (hospital 3), and a university hospital (hospital 4) for fever, irritability, bloody diarrhea, and recurrent infections. She never had paralysis. A stool specimen, obtained on 27 August 2005 on admission to hospital 3, was positive for an enterovirus that was ultimately confirmed to be a VDPV by the Minnesota Department of Health Public Health Laboratory on 29 September 2005.
Severe combined immunodeficiency was diagnosed in the infant at hospital 3, and she was transferred to hospital 4 for bone marrow transplantationon13 September 2005. At hospital 4, she was kept in protective isolation and treated with intravenous immunoglobulin. After identification of the VDPV, she underwent weekly testing for poliovirus excretion. She responded well to intravenous immunoglobulin but continued to excrete polioviruses until January 2006. She underwent 2 bone marrow transplant operations in December 2005 and January 2006; the second successfully corrected her immunodeficiency and eliminated poliovirus excretion. She was discharged in April 2006.
Hospital and health care investigations and interventions. At hospitals 1–3, 93 (33%) of 283 staff who reported possible contact with the index patient completed questionnaires. None reported being immunocompromised or having a polio-compatible illness. At hospital 4, 135 (89%) of 151 staff who reported direct contact with the infant during the last 2 weeks of September 2005 received booster IPV doses. Stool specimens were tested in 30 (75%) of 40 staff with possible unprotected contact with the infant or her environment and all 35 (100%) of the patients in the 2 wards in which she was hospitalized during 15–29 September 2005; all specimens were negative for polioviruses.
Investigations into other potential health care sources of the infant's VDPV infection had negative findings. No known immunodeficient persons receiving immunoglobulin therapy in Minnesota since 2003 were identified as Amish.Noforeign-born or foreign-resident children with chronic immunodeficiencies had been treated at Minnesota medical facilities since 2003. Only 1 foreign-resident child with a paralytic illness was identified at a children's orthopedics hospital in 2005; this patient, an 8-year old boy from South America, received doses of OPV as an infant and toddler and was being seen for follow-up and rehabilitation of a chronic paralytic “poliolike” condition.
Community investigations and interventions. The index community included 161 persons (50 adults and 111 children) in 24 families. Fifteen families agreed to be interviewed; 31 stool specimens were obtained from 5 participating families, and 10 serum specimens were obtained from 2 families. There were no cases of paralytic disease or aseptic meningitis in the community. All 3 of the infant's unvaccinated siblings had elevated titers of neutralizing antibody to poliovirus type 1 (range, 227–576) and no detectable antibodies to poliovirus types 2 and 3 (titer, <8), compatible with recent infection with a type 1 poliovirus. Stool specimens from 4 children in 2 other families were positive for type 1 VDPV; these children included a 14-year-old in family C and 3 children (aged 2, 4, and 14 years) in family D. In total, including the infant, 8 (35%) of 23 children from 3 of 5 families tested had serologic or virologic evidence of type 1 poliovirus or VDPV infection.
In Wisconsin, 17 stool and 8 serum specimens were obtained from 22 persons in 8 families from 4 communities. All stool specimens were negative and all serum samples were positive for antibodies to all 3 polioviruses. In Michigan and Missouri, all families refused to provide clinical specimens. The Amish community investigated in Ontario had 159 members in 24 families. Of these, 41 persons (26%) submitted 36 stool and 41 throat swab specimens for virus isolation. All specimens were negative for poliovirus. Surveillance in Minnesota did not disclose additional VDPV infections or polio-compatible diseases during 2003–2005.
Acceptance of IPV among the investigated communities varied widely. In the index community, a history of OPV vaccination was reported for 14 (28%) of the adults and 2 (2%) of the children, and 53 persons in 9 families accepted IPV vaccination (28% of adults and 34% of children). In other Minnesota Amish communities, rates of IPV vaccination ranged from 35% to 100%, but 1 community refused vaccination altogether. In Wisconsin, 83 (18%) of 460 individuals in 4 communities accepted IPV vaccination. In Michigan and Missouri, no families accepted vaccination. In Ontario, 19 (12%) of 159 persons had been previously vaccinated, and 17 (11%) accepted IPV vaccination.
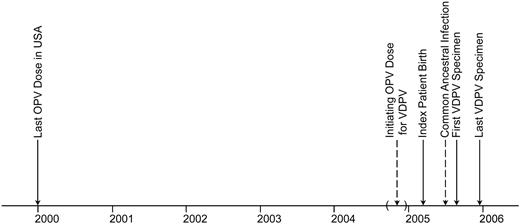
Sequence relationships among VDPV isolates. The first virus isolated from the infant was a type 1 VDPV, differing from the parental Sabin 1 OPV strain at 2.3% of VP1 nucleotides (21/ 906). VP1 sequences of 12 subsequent isolates from the infant and isolates from 4 other children in the community demonstrated that all were closely related VDPVs. Nearly complete genomic sequences of the 17 isolates revealed that all had nonrecombinant genomes. A Bayesian Markov chain Monte Carlo tree [17] was constructed from the differences in synonymous substitutions within the complete ORF sequences (6627 nt) of the 17 isolates (figure 1). Under the assumption of a strict molecular clock, nucleotide differences were scaled to time, yielding estimates at the nodes of the tree of the approximate dates of divergence of individual VDPV lineages. These estimates suggested that the initiating OPV dose for the VDPV was administered around early November 2004 (±7 weeks),∼3 months before the infant's birth (figures 1 and 2). The ancestral VDPV infection common to all community isolates was estimated to have occurred around late June 2005, ∼8 months after the initiating OPV dose and 2 months before the initial detection of VDPV in the community (figure 2). The sequence relationships among the infant's isolates (figure 1) suggest that her infection was detected soon after VDPV exposure. Although the infant's VDPV diverged into at least 3 separate lineages, the infection common to all 3 lineages was estimated to have occurred in late August, about the time of the ancestral infection linking familyD to the infant. The early divergence of the lineage leading to the family C isolate suggests that VDPV circulation in the community was more widespread than indicated by direct virus isolation, because intermediate infections almost certainly would have occurred.
Bayesian Markov chain Monte Carlo tree [17] showing estimated times of vaccine-derived poliovirus (VDPV) emergence and divergence of VDPV lineages on the basis of the rate of substitution at 2- and 4-fold degenerate sites (closely approximating synonymous codon sites) within the complete open reading frame (ORF), scaled under a model assuming a strict molecular clock with a fixation rate of 0.047 ± 0.011 synonymous substitutions/site/year, as estimated from the data set. Bootstrap values for the branch structure of the tree and estimated divergence dates of major lineages (with 95% confidence intervals in parentheses) are given at the nodes. Isolates that had identical synonymous ORF sequences (index patient isolates 7, 8, and 12) were represented only once in the tree but were included in the substitution-rate calculations.
Time line summarizing key events associated with vaccine-derived poliovirus (VDPV) circulation in the rural Minnesota community. Dates estimated from the tree shown in figure 1 are indicated by dashed arrows, and the 95% confidence interval for the date of the initiating oral poliovirus vaccine (OPV) dose is bounded by parentheses.
Genetic and antigenic evolution of the VDPVs. Key determinants of the attenuated (G480→A; VP465:Ser→Ala; VP1106: Thr→Ala) and temperature-sensitive (VP465:Ser→Ala; 3D73: His→Phe;G7441→A) phenotypes of Sabin 1 [23] had reverted in the VDPV genomes, consistent with the observed loss of the temperature-sensitive phenotype in the VDPVs and suggesting their increased neurovirulence. All VDPV isolates were antigenically distinct from Sabin 1 and had 10–11 aa replacements in or near the NAg sites (figure 3). The K2/K5 ratio within the NAg sites was high (1.17–1.96) for the Minnesota VDPVs, similar to iVDPV isolates from a Taiwanese immunodeficient patient (1.07–3.04) [16] and unlike the type 1 cVDPV isolates from Hispaniola [29] and the Philippines [30] (0.47–0.52). Only 1 VDPV isolate (from a 5-year-old child in family D) had an additional substitution in the NAg sites (figure 3), indicating that most of the antigenic evolution of the VDPV occurred before introduction into the community.
Discussion
In this report, we describe the first known occurrence of community circulation of a VDPV among an undervaccinated population in a developed country. The virus probably had circulated in the community for at least 2 months before the index patient was identified, and there was significant spread among the families tested. There was no evidence ofVDPVtransmission and no detection of polio-compatible illnesses in Minnesota or other communities investigated in the United States or Ontario, although the isolates had genetic properties typical of neurovirulent VDPV revertants [16, 29, 30].
We were unable to identify the source of the initiating OPV dose. Phylogenetic analysis excludes that the dose was given in Canada or the United States, which stopped OPV use in 1995–1996 and 2000, respectively [4, 31]. The absence in the VDPV of recombination and the relatively high concentration of amino acid substitutions in the NAg sites are more characteristic of iVDPVs than of cVDPVs [7]. Thus, we suggest that the original source of the VDPV in this outbreak was an immunodeficient person who was exposed to OPV outside the United States.
Undervaccinated populations such as this Amish community are at continued risk for poliovirus spread on introduction and present special challenges for polio outbreak control. Despite confirmed poliovirus infections in their children, <50% of the index community members accepted vaccination. Fortunately, the risk of spread among the general population of either an imported wild poliovirus or VDPV is low because of high and sustained poliovirus vaccine coverage in the United States.
OPV is the vaccine of choice for polio eradication because of its ease of administration, induction of gut immunity, and spread to unvaccinated persons, augmenting herd immunity [32]. Vaccine virus spread is also the greatest liability of OPV.On replication in vaccine recipients (usually after the first dose) or their contacts, vaccine virus can revert to neurovirulence and cause VAPP [3, 4, 32]. With the global decline of polio to low levels, many industrialized countries have changed their national vaccination schedules from OPV to IPV use [33].
A more recently recognized problem related to OPV use is the emergence of VDPVs. Where polio control programs succeed in eliminating wild polioviruses but fail to maintain high population immunity, undervaccinated populations become susceptible to transmission of VDPVs (cVDPVs). At least 8 polio outbreaks caused by cVDPVs have occurred in countries in which vaccine coverage was low [7, 8]; outbreaks have been associated with VDPVs of all 3 serotypes [7], and it appears that cVDPVs present a similar paralysis risk as wild polioviruses [7, 29, 34, 35]. These outbreaks have been controlled with mass OPV immunization campaigns. The threat posed by cVDPVs can only disappear whenOPVuse is terminated rapidly after global eradication [33].
A potential longer-term risk to global polio eradication is the chronic infection of immunodeficient persons with VDPVs (iVDPVs) [8, 9]. Long-term persistent infections have been documented among persons with severe combined immunodeficiency, common variable immunodeficiency, and agammaglobulinemia. Many case subjects have died of their underlying immunodeficiency, and some have spontaneously cleared their infection [36]. One healthy individual with common variable immunodeficiency has excreted iVDPVs for ∼20 years [37]. The global prevalence of persistent iVDPV infection is unknown, because most such infections are detected only after the infected individual develops paralytic disease. The potential for iVDPV spread is unclear; most long-term excreters are likely to live in developed countries where they survive longer, but most of their close contacts are protected through immunization and repeated boosting by exposure to the virus [38].
While OPV use continues globally, IPV-using countries should remain vigilant to the potential for importation, chronic excretion, and circulation of wild polioviruses and VDPV in undervaccinated populations and should maintain laboratory capacity for poliovirus identification [8]. Antiviral compounds capable of clearing chronic infections are urgently needed [39]. Global cessation of OPV use and management of the risks related to polioviruses in the posteradication era, including those posed by VDPVs, will be essential to protect the gains achieved by global eradication of wild polioviruses.
Vaccine-Derived Poliovirus Investigations Group
Minnesota-Minnesota Department of Health: L. Bahta, J. Bartkus, J. Harper, R. Lynfield, C. Miller, J. Rainbow; Todd County Health Department: C. Schneider; Clearwater County Health Department: B. Engen; Fillmore County Health Department: S. Serfling; Otter Tail County Health Department: D. Thorson; Wadena County Health Department: K. Nelson; Winona County Health Department: L. Theurer; Children's Hospitals and Clinics of Minnesota: P. Ackerman. Wisconsin-Wisconsin Department of Health and Family Services: J. Berg, J. Gabor, D. Hopfensperger, M. Sotir; Monroe County Health Department: T. Clark, S. Nelson, P. Rainwater, L. Smith, B. Wolf; Eau Claire City-County Health Department: T. Weegman. Michigan-Michigan Department of Community Health: J. Blostein. Missouri-Missouri Department of Health and Senior Services: B.-P. Zhu, H. Marx. Georgia-Centers for Disease Control and Prevention: K. Kenyan, A. Parker, N. Dybdahl- Sissoko. Canada-Public Health Agency of Canada: S. Bishway, P. Varughese; Middlesex-London Health Unit, Ontario: C. Egan, A. Locker, G. Pollett, M. A. Simpson.
Acknowledgments
We thank J. Goldsmith, D. Scott, and J. Epstein, Food and Drug Administration, for assistance in identifying intravenous immunoglobulin products with high titers of antibody to poliovirus type 1; C. John, University of Minnesota Medical Center, and R. Andersen, Children's Hospitals and Clinics of Minnesota, for care of the patient; occupational health and infection control staff at the 4 hospitals, for their help with the hospital investigations; the staff of the Todd County Health Department, for their assistance with the investigation in central Minnesota; the staff of the Vaccine Preventable Disease and Infectious Disease Control Teams, Middlesex-London Health Unit, for their assistance with the investigation in Ontario; N. Crouch and J. Besser, Minnesota Department of Health (MDH), for management of the laboratory investigation in Minnesota; A. J. Williams and J. Iber, Centers for Disease Control and Prevention (CDC), for assistance with the laboratory investigation at the CDC; J. Stieger, MDH, and C. Allen, K. Sheedy, and C. Thompson, CDC, for assistance with communications to other public health agencies, the media, and the public; G. Wallace, A. Calugar, and E. Wilder, CDC, for technical assistance with vaccine-supply issues; N. Khetsuriani and J. Orta, CDC, for technical and programmatic assistance; and H. Jafari, D. Johnson, L. Venczel, and H. Sandhu, CDC, for liaison with the World Health Organization and its regional offices.
References
Potential conflicts of interest: none reported.
Presented in part: National Vaccine Advisory Committee, Washington, DC, 8 February 2006; National Immunization Conference, Atlanta, 7 March 2006.
Financial support: none reported.
Author notes
Present affiliations: California Department of Public Health, Immunization Branch, Epidemiology and Surveillance Section, Richmond (K.H.); HF Hull Associates, St. Paul, Minnesota (H.F.H.); HealthEast Care System, St. Joseph's Hospital, St. Paul, Minnesota (G.W.).
Study group members are listed after the text.




![Sequences of amino acid residues within or bounding neutralizing antigenic (NAg) sites. Residues defining the type 1 poliovirus NAg sites by mutations conferring resistance to neutralization by monoclonal antibodies [24 -27] are indicated by boldface type. The trypsin cleavage site in NAg-1, characteristic of Sabin 1 [28], is underlined. cVDPV, circulating vaccine-derived poliovirus; DOR, Dominican Republic; FamC, family C; FamD, family D; Index, index patient; iVDPV, immunodeficiency-related vaccine-derived poliovirus; PHL, Philippines; TAI, Taiwan.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/199/3/10.1086_596052/2/m_199-3-391-fig003.jpeg?Expires=1716312839&Signature=uO4ZZx8yzqWzCyvwGjck3KyyK9RRHqnfZYX7vdCuje8tPZPHtUjSU3Z96D8uvHIGenJ-RNkZwk6dEJktoKhAISWJkmcoz3AbneXrBrFSrQhSvC8KMbRpTWC4WkryhN3zaujBuU3oSsHxvunQjudlniGCnJwqDzlQiruiTD8avhKVleZTkPKiDI3hLGu4lJ52P9HwN4gXeCs3F4lk5ez5mvn2FvijG5rn8osFpQgIWWIZhs2xYJe5aLcVue63k05IZtO5vtaDPhqani2h1MCdAVpHG1I4qQ3u6Rf89jnbLpqQQF~LYpF5PzM-0DDFunil4ihaDNBuAO79Hmp0CvTwTw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Bayesian Markov chain Monte Carlo tree [17] showing estimated times of vaccine-derived poliovirus (VDPV) emergence and divergence of VDPV lineages on the basis of the rate of substitution at 2- and 4-fold degenerate sites (closely approximating synonymous codon sites) within the complete open reading frame (ORF), scaled under a model assuming a strict molecular clock with a fixation rate of 0.047 ± 0.011 synonymous substitutions/site/year, as estimated from the data set. Bootstrap values for the branch structure of the tree and estimated divergence dates of major lineages (with 95% confidence intervals in parentheses) are given at the nodes. Isolates that had identical synonymous ORF sequences (index patient isolates 7, 8, and 12) were represented only once in the tree but were included in the substitution-rate calculations.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/199/3/10.1086_596052/2/m_199-3-391-fig001.jpeg?Expires=1716312839&Signature=csWdrJylwo3zA33X3xrzO0mGi7RcU6rHXBR8SAwhmapg-MQD7xjAo2jN52IS-7fKnpvazy37d~adSutkSGqmDMdt9v-nrxVs5DwLZ4~W5KLh7OEx32~lelqDKIGtwhB1DvNCPMp5jarSDvvDfJQdqWtpV3QFijEAHOmIpJmHumtOjoSVqkho7qvZJVoezc6eflYC7lO~0U-htavRJbXyhGzwA5mnoi8LXw-7kWFfXH7JDNP4b0pl1MstV-sLDi6ZMkPu40D~cq0-zPBEVMJsfMciZgtb8Du2WLP-ggVPRk0eXQUugquvvb8Zp6KAPAH~4HUx88KCSQ8Imnk9cODG1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)