-
PDF
- Split View
-
Views
-
Cite
Cite
Allison E. Aiello, Genevra F. Murray, Vanessa Perez, Rebecca M. Coulborn, Brian M. Davis, Monica Uddin, David K. Shay, Stephen H. Waterman, Arnold S. Monto, Mask use, hand hygiene, and seasonal influenza-like illness among young adults: A randomized intervention trial, The Journal of Infectious Diseases, Volume 201, Issue 4, 15 February 2010, Pages 491–498, https://doi.org/10.1086/650396
Close - Share Icon Share
Abstract
Background. During the influenza A(H1N1) pandemic, antiviral prescribing was limited, vaccines were not available early, and the effectiveness of nonpharmaceutical interventions (NPIs) was uncertain. Our study examined whether use of face masks and hand hygiene reduced the incidence of influenza-like illness (ILI).
Methods. A randomized intervention trial involving 1437 young adults living in university residence halls during the 2006–2007 influenza season was designed. Residence halls were randomly assigned to 1 of 3 groups—face mask use, face masks with hand hygiene, or control— for 6 weeks. Generalized models estimated rate ratios for clinically diagnosed or survey-reported ILI weekly and cumulatively.
Results. We observed significant reductions in ILI during weeks 4–6 in the mask and hand hygiene group, compared with the control group, ranging from 35% (confidence interval [CI], 9%–53%) to 51% (CI, 13%–73%), after adjusting for vaccination and other covariates. Face mask use alone showed a similar reduction in ILI compared with the control group, but adjusted estimates were not statistically significant. Neither face mask use and hand hygiene nor face mask use alone was associated with a significant reduction in the rate of ILI cumulatively.
Conclusions. These findings suggest that face masks and hand hygiene may reduce respiratory illnesses in shared living settings and mitigate the impact of the influenza A(H1N1) pandemic.
Trial Registration. ClinicalTrials.gov identifier: NCT00490633.
February 2007, the Centers for Disease Control and Prevention (CDC), in collaboration with other federal agencies and with educational institutions, businesses, health care providers, and private enterprises, developed an interim planning guide on the use of nonpharmaceutical interventions (NPIs) to mitigate an influenza pandemic [1]. These measures include voluntary home quarantine, social distancing, personal protection (use of face masks and hand hygiene), and school dismissal; similar measures have been recommended for mitigating severe acute respiratory syndrome (SARS). Use of NPIs occurred during the international SARS outbreak that began in early 2003 [2] and is ongoing in the current novel influenza A(H1N1) (hereafter “nH1N1”) pandemic.
Although several of these measures can be evaluated during seasonal influenza outbreaks, many are difficult or impossible to evaluate in advance of a pandemic. School closure has been implemented during seasonal influenza outbreaks and the current nH1N1 pandemic, but it has been difficult to assess this intervention on a large enough scale or before the peak of illness to provide inferences for future pandemics [3–5]. In contrast, use of face masks and hand hygiene interventions can be evaluated during seasonal influenza outbreaks to provide concrete evidence for the potential effectiveness of these measures during the current nH1N1 pandemic. We conducted a cluster randomized intervention study to assess the impact of face masks and hand hygiene on the incidence of influenza-like illness (ILI) symptoms among students living in university residence halls during the 2006–2007 influenza season. We examined the effects of face masks alone and face masks with provision of alcohol-based hand sanitizer, compared with a control group that received no intervention.
Methods
Study design and eligibility. The study design was a cluster randomized trial with 3 arms, conducted among university students living in residence halls. The CONSORT checklist is available in Table A1 in the Appendix, which is not available in the print edition of the Journal. On the basis of the size (>100 residents) and demographic similarity of the residential halls, 7 of 15 available residence halls were included as potential intervention or control units.
The largest of the 7 residence halls housed 1240 residents. The 6 smaller residence halls ranged from 110 to 830 residents. The 6 smaller halls were combined into 2 similar sized units, to create a comparable size to the largest residence hall; all 3 similar sized units were then randomized to the intervention or control arms. The residence hall units were randomized by blindly selecting a uniform ticket with the name of each hall out of a container (A.S.M. and A.A.) for randomization assignment to each study arm. The largest single residence hall was randomized to the mask plus alcohol-based hand sanitizer (62% ethyl alcohol in a gel base) group (hereafter, the “face mask and hand hygiene” group), a cluster composed of 4 residence halls was randomized to the face mask—only group, and the remaining 2 residence halls served as the control group (Figure A1 in the Appendix, which is not available in the print edition of the Journal).
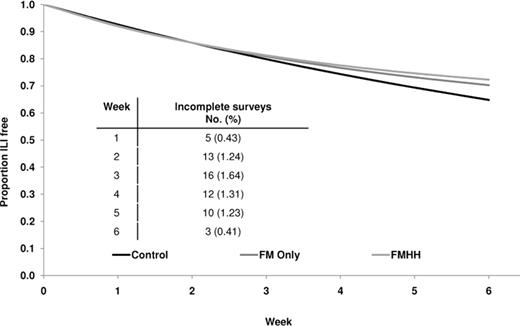
Adjusted Kaplan-Meier survival curve. The figure shows the proportion of participants that are ILI-free by intervention arm over the 6-week study period adjusted for age, sex, race/ethnicity, handwashing practices, sleep quality, stress, alcohol consumption, and influenza vaccination (n = 1042).
We estimated a sample size of 750 participants per intervention group to demonstrate a reduction in ILI incidence of 40% between each intervention and the control group [6], based on a 10% ILI attack rate in the control group, with an α level of 0.05 and statistical power of at least 80%. The total number of eligible participants was 1372 (96% retention rate among allocated participants). Additional details on the sample size are available in Section A1 of the Appendix.
Students living in these residence halls were eligible for participation if they were at least 18 years of age and willing to wear a face mask, use alcohol-based hand sanitizer, have a throat swab specimen collected when ill, and complete the baseline and weekly surveys over the 6-week study period. Potential participants reporting a skin allergy to alcohol were excluded. Written informed consent was obtained from all participants. The study was approved by the University of Michigan Institutional Review Board.
Recruitment and intervention methods. Recruitment began in November 2006 and continued until 2 weeks after the intervention period started. The intervention started during the week of 22 January 2007, after laboratory confirmation of influenza infection on the University of Michigan campus. The intervention materials and educational component were provided to participants on 26 and 27 January, and enrollment continued until 16 February. The study ended on 16 March 2007. Over the study period, a majority of residents left campus (24 February to 4 March) during a 1-week spring break. Excluding spring break, the intervention lasted 6 weeks total.
All participants received basic hand hygiene education (proper hand hygiene practices and cough etiquette) through an email video link and the study Web site. In addition, face mask and hand hygiene group participants received written materials detailing appropriate hand sanitizer and mask use; mask group participants received written materials regarding proper face mask use only. Participants in the mask intervention residence halls received standard medical procedure masks with ear loops (TECNOL procedure masks; Kimberly-Clark), which they were asked to wear as much as possible in their residence hall during the intervention period and encouraged to use outside the halls as well. Compliance with masks while sleeping was optional. Participants were instructed in correct and incorrect mask use, change of provided masks daily, and use of provided resealable plastic bags for mask storage when not in use (eg, eating) and for disposal. Mask and hand hygiene group participants also received alcohol-based hand sanitizer (portable 2 oz squeeze bottle; 8 oz pump) for use throughout the study. Additional information on supply distribution is available in Section A1 of the Appendix.
Weekly surveys. At baseline, participants were asked to self-report data on demographic information (age, sex, and race/ethnicity), hand hygiene behavior (handwashing frequency, duration, and hand sanitizer ownership), health behaviors (sleep quality, alcohol consumption, smoking habits, and influenza vaccination status), and levels of perceived stress. Additional details on behavioral measures are available in Section A1 of the Appendix. Participants were also asked to complete the baseline and weekly Web-based surveys concerning the occurrence of respiratory illness symptoms and the use of interventions during the study. The weekly surveys included questions regarding ILI symptoms, intervention compliance, and health and hygiene behaviors. In addition, trained staff stationed in residence hall common areas observed participant compliance. A detailed description of all compliance measures is available in Section A1 of the Appendix.
Report of ILI symptoms and laboratory testing. All residents in participating halls received promotional materials describing the ILI case definition (presence of cough and at least 1 constitutional symptom [fever/feverishness, chills, or body aches]) [7] and phone numbers for contacting the nursing staff to assess for ILI symptoms. During scheduled participant visits, study nurses ascertained date of illness onset, temperature, use of antipyretics, and reported symptoms (cough, feverishness, chills, body aches, headache, nasal congestion, and sore throat).
Students with ILI were offered $25.00 for providing a throat specimen. With a cotton swab, nurses collected specimens and transferred them to veal infusion broth. Samples were processed and analyzed using standard laboratory methods as described in Section A1 of the Appendix.
Statistical analyses. Of the 1372 eligible participants, 1297 with a complete baseline survey and at least 1 weekly survey were included in analyses. Several potential covariates were examined across intervention and control groups, including age, sex, self-reported race/ethnicity, hand hygiene behaviors at baseline, sleep quality, alcohol use, smoking habits, physical activity, levels of perceived stress, reported influenza vaccination history, and mask and hand hygiene compliance over the study period. Data describing variable derivation and categorization are available in Sections A1 and A2 of the Appendix.
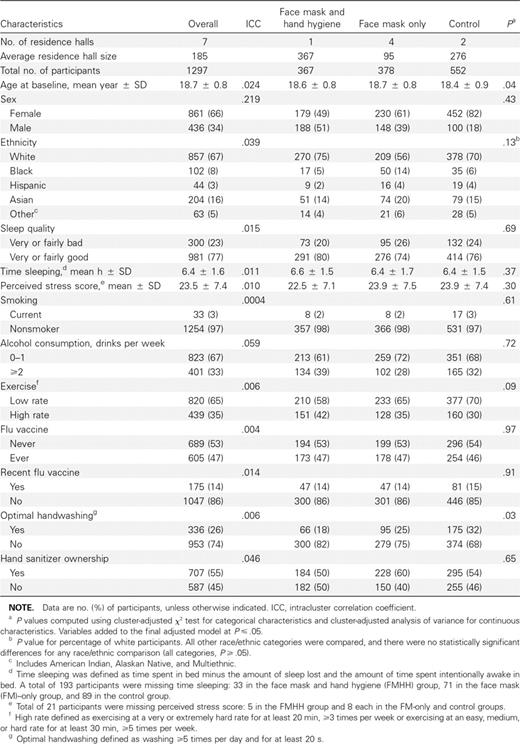
To test for potential covariate differences among intervention and control groups, baseline characteristics and hand hygiene variables were compared, using χ2 tests and analysis of variance adjusted for clustering within the 7 residence halls [8]. Intracluster correlation coefficients were calculated using the Donner method to account for grouping at the residence hall level [9]. Covariates that were significantly related to ILI rate (sex, race/ethnicity, perceived stress, sleep quality, alcohol consumption, and vaccination) at the P ⩽ .10 level or that were imbalanced across study arms at baseline (age and handwashing) at the P ⩽ .05 level were included as covariates in adjusted survival models described below.
Survival analysis. The main predictor variable was the intervention arm (ie, mask and hand hygiene or mask alone compared with control). The main outcome variable was the first reported ILI that was based on clinical ascertainment or survey report (if no available clinical report) over the 6-week study period. A small number of cases reported >1 ILI (15 cases); only the first ILI was included in our analyses.
Discrete-time survival analysis using the Proc genmod procedure in SAS (version 9.1; SAS) was used to estimate rate ratios because log-log plots demonstrated nonproportionality of the hazard lines over time [10]. A robust model-based standard error was used, assuming an exchangeable correlation structure because the number of residence hall cluster units was small (7 units) [10, 11]. Analyses were conducted using intention-to-treat [12–14]. Rate ratios and corresponding CIs were estimated for each week of the study period and cumulatively over the entire study period by fitting interaction terms between intervention group and week. Results were considered significant at P < .025 to account for comparisons across the 2 intervention and control study arms by week.
Results
The total number of participants analyzed was 1297 with 367 in the face mask and hand hygiene group (9 deemed ineligible and 26 lost to follow-up), 378 in the face mask-only group (11 deemed ineligible and 52 lost to follow-up), and 552 in the control group (19 deemed ineligible and 21 lost to follow-up) (Figure A1 in the Appendix). In total, 1297 (97%) of 1331 participants completed a baseline and at least 1 weekly survey.
Baseline characteristics of the study participants are shown in Table 1. The mean age of participants was 18.7 years (standard deviation [SD], 0.8). Sex, self-reported race/ethnicity, sleep quality, perceived stress, smoking, alcohol use, exercise, influenza vaccination, and hand sanitizer ownership were not significantly different across study arms at baseline. However, there was a significant difference between groups in the proportion of subjects who reported optimal handwashing practices, defined as handwashing for ⩾20 s at least 5 times per day; control and face mask—only groups reported a higher proportion of optimal handwashing practices than those in the face mask and hand hygiene group. Additional results on survey-reported and observed compliance are presented in Section A2 of the Appendix.
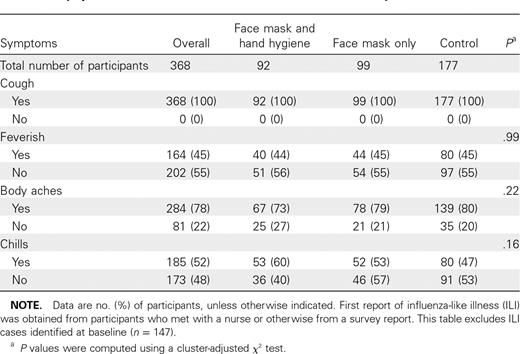
ILI symptom reports are shown in Table 2. At baseline, 147 of 1297 participants reported ILI and were therefore excluded from survival analyses. Of the 1150 who were available for analysis, 368 (32%) of 1150 participants met the definition for ILI on either their survey (274 participants) or clinical report (94 participants) and were analyzed in survival analyses. Culture and polymerase chain reaction (PCR) results of 94 clinical samples were obtained from subjects with ILI symptoms. Of these, 8 samples were positive by cell culture and 10 were positive by reverse-transcription PCR (RT-PCR) (7 for influenza A and 3 for influenza B). All specimens that tested positive by cell culture also tested positive by RT-PCR. RT-PCR—positive samples included 2 in face mask and hand hygiene, 5 in face mask alone, and 3 in the control group. The cluster-adjusted χ2P value comparing the proportion of positive samples across study groups was P = .44.
Symptom Characteristics for Influenza-like Illness Cases by Intervention Arm
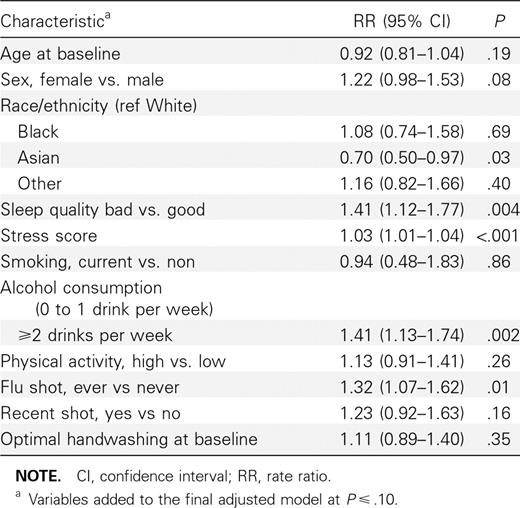
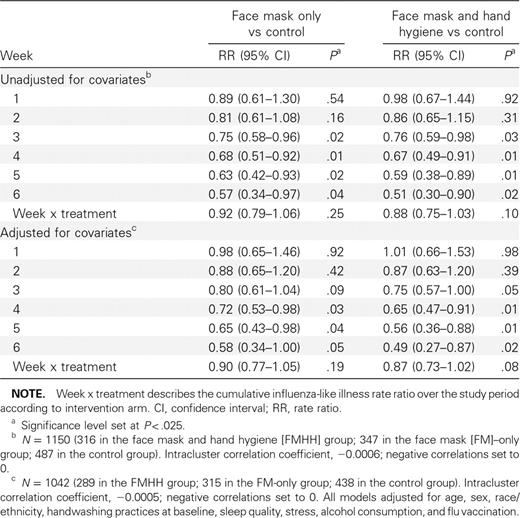
Survival analysis. Univariate analyses of characteristics with respect to the first report of ILI are shown in Table 3. Over the 6-week study period, both intervention groups showed a ∼10% reduction in cumulative ILI incidence compared with the control group in unadjusted analyses, although these results did not reach statistical significance in either group (Table 4). In addition to cumulative ILI rate over the study period, discrete-time survival analysis allowed estimation of the rate ratio over each week of the study. After the participant enrollment ended (ie, week 3 onward), significant reductions in ILI incidence were observed in the mask and hand hygiene group (weeks 4–6) and in the face mask.only group (weeks 3–5) compared with the control group. After covariate adjustment, ILI incidence was significantly lower among the mask and hand hygiene group compared with the control group from week 4 onward (Table 4; Figure 1). In the face mask—only group, adjusted results also showed a reduction in ILI incidence during week 4 onward but were not statistically significant at P < .025.
Univariate Characteristics and Rate of Influenza-like Illness Symptoms
Intervention Rate Ratios, Unadjusted and Adjusted for Covariates
Discussion
Intervention studies of face masks in open, noninstitutionalized populations to protect healthy individuals from primary respiratory infections have, to our knowledge, not been previously reported. We found a significant reduction in the rate of ILI among participants randomized to the face mask and hand hygiene intervention during the latter half of this study, ranging from 35% to 51% when compared with a control group that did not use face masks.
Our results are consistent with a previous review of studies examining the effectiveness of mask use in reducing the transmission of respiratory viruses [15]. However, much of the data on natural infection derives from studies of SARS. The transmission characteristics of this pathogen may be different from those of influenza and other seasonal respiratory illnesses. Although few data are available to evaluate the efficacy of face mask use in the community setting, 2 recent randomized mask intervention studies, one in Hong Kong and the other in Australia, reported no significant reductions in secondary transmission of ILI [16, 17]. However, important methodological differences exist between our study assessing the prevention of primary infections and these earlier studies that asked participants to don masks only after identification of an influenza case residing in the household for assessment of the prevention of secondary infections. We asked participants to begin wearing the mask and using hand sanitizer at the beginning of the influenza season just after identification of the first case of influenza on campus. This fundamental study design difference may have improved our ability to identify an effect of mask and hand hygiene use, compared with studies of secondary transmission in which household members may already have been infected by the time of mask adoption.
Several factors may explain why we observed a statistically significant reduction in ILI incidence (P < .025) only during the latter half of the 6-week study period. First, we continued recruitment 2 weeks after the study started, which increased sample size by 11%. The greater participation rates later during the study may have resulted in reduced transmission of respiratory viruses within the intervention residence halls. Second, there was an almost 10% increase in the proportion of subjects in the mask and hand hygiene group who reported wearing their masks for more than average (3.5 h per day) during weeks 3–6 of the study. In contrast, this proportion only increased by 2% in the face mask—only group during the same period. Another factor that may have influenced the results included a late and mild influenza season. Laboratory-confirmed cases in Michigan and reports of ILI to University Health Services (UHS) did not substantially increase until the second week of the study. The greatest frequency of cases of ILI reported to UHS occurred during week 6, and the largest number of laboratory-confirmed cases of influenza in Michigan occurred during weeks 4 and 5 of the study. In addition, spring break travel may have influenced our results. As most students left campus during spring break (between weeks 4 and 5 of the intervention period) and were not required to continue their protective measures during this time, potential exposures during spring break may have increased illness in residence halls toward the end of the study, after break. Spring break exposures may therefore represent a confounding factor, limiting our ability to demonstrate the effectiveness of interventions. Thus, future studies are needed to identify whether the protective effects observed here can be generalized to larger influenza outbreaks, as well as the potential influence of intervention start time and interruption.
ILI incidence between the face mask and hand hygiene group and the face mask—only group were not substantially different, suggesting that the addition of a hand sanitizer component did not appreciably decrease the rate of ILI in this study population. Because the value of hand hygiene has not been established for influenza or ILI prevention during periods of confirmed viral transmission, we decided to include a trial arm in which both interventions (mask and hand hygiene) were combined. Our study, however, was not powered to detect small differences between the intervention groups, which would be expected during mild influenza seasons. Although some studies have reported a reduced risk of illness when using alcohol-based hand sanitizer in conjunction with handwashing [6, 18], the incremental effect of adding antiseptics to regular handwashing is unknown [19]. Indeed, a recent metaanalysis of community-based hand hygiene interventions reported a nonsignificant pooled reduction in respiratory illnesses based on 5 studies of alcohol-based hand sanitizer interventions in the community setting [20].
Although the mask and hand hygiene group used hand sanitizer more often, and a greater proportion of participants applied the recommended amount compared with the other study groups, this group also had a greater proportion of participants with suboptimal handwashing practices at baseline. Although we controlled for handwashing habits in our regression models, it is possible that the overall hand hygiene practices (ie, significantly higher use of hand sanitizer yet significantly lower number of handwashes per day in the face mask and hand hygiene group) were counterbalanced, such that the incremental, potentially protective effect of using alcohol-based hand sanitizer in the layered arm was matched by a greater number of handwashes per day in the mask-only arm. Nonetheless, alcohol-based hand sanitizers are more effective for inactivating a wide range of respiratory viruses, including influenza virus, compared with plain soap and water [21, 22]. It is important to note that handwashing habits were the same in both the face mask-only and control groups at baseline and over the study period, which suggests that mask use alone may provide a reduction in respiratory illnesses regardless of handwashing practices. Future work should address which particular combinations of interventions are effective in reducing ILI or other respiratory viruses, in both the health care and community settings.
Several demographic characteristics and health factors were associated with risk of ILI in our study population, including ever having an influenza vaccination, being white versus Asian race, higher levels of stress, and increased alcohol intake. Possibly, reports of “ever having an influenza vaccination” may be associated with increased ILI because young individuals who seek vaccination may be more health-conscious and likely to report ILI symptoms, compared with those who have never had a vaccination. This bias has been reported in other studies of vaccination and ILI symptom reporting [23, 24]. Reported seasonal vaccination status, on the other hand, was not protective of ILI rates. However, only 14% of the total study population reported vaccination acquisition during the corresponding influenza season. Additional discussion of demographic variables is available in the supplemental materials (Section A3 of the Appendix).
This study has several limitations. First, influenza incidence was low, so it is likely that most ILI cases were not associated with influenza infection, even though the study was conducted during the influenza season. Second, the study was underpowered to detect low reductions in the rate of ILI and across study arms. The number of clusters in this study was small, thus suggesting some potential for inflation of variance estimates [25]. However, there are several factors that support the validity of our methods and results. First, there were no significant differences in rates of ILI across the 7 residence halls at baseline, which suggests that naturally occurring differences in ILI rates across halls are unlikely to explain our findings. In addition, we observed consistent reductions in both the face mask—only and face mask and hand hygiene groups over the study period. Given that the mask-only group was composed of 4 residence hall clusters and the changes in the rates of ILI were also comparable to the mask and hand hygiene group, it is unlikely that natural variation could account for the consistency in results across study arms over time. Second, the magnitude of the design effect (ie, intracluster correlation coefficient) for both the adjusted and unadjusted models was well below 1 (see footnotes in Table 4), which suggests a lack of significant clustering of ILI by residence hall. Therefore, control for clustering along with conservative P-value cutoffs used in this study may have potentially masked statistically significant results [25]. Next, the bulk of the data was collected through Web-based weekly surveys in which participants reported their activities, symptoms, and other events during the prior week. By relying largely upon self-reported data, this study may be susceptible to reporting bias; some individuals could have reported what they thought was expected of them. The similarity in reported behavioral habits and hand hygiene practices across intervention and control groups argues against differential reporting biases. Because of the inability to blind participants to study interventions, compliance with these interventions must be considered carefully. We assessed compliance as described in Sections A1 and A2 of the Appendix, but it was not possible to gather observational data on all participants at all times and venues. Finally, given the limited age range and specialized living setting of study participants, we are not able to generalize our results to other, nonuniversity aged, community-dwelling populations. However, our findings should be applicable to individuals living in similar crowded and close-quarter living settings.
We demonstrated a protective effect of the intervention even with relatively moderate use of face masks throughout the day. We believe that during an influenza pandemic, compliance with interventions will be higher than what we found in this study, particularly if rates of serious complications are high or well publicized. If our findings also apply to laboratory-confirmed influenza infections, the effect on influenza transmission could be substantial, particularly early in a pandemic when vaccine supply will almost certainly be limited, as with the current nH1N1 pandemic [26]. Our results indicate that interventions to reduce the transmission of ILI during a winter season may have substantial effects among individuals who share crowded living conditions.
Acknowledgments
We gratefully acknowledge the thousands of participants who made this study possible and the efforts on behalf of staff volunteers, recruitment coordinators, and laboratory assistants. We are especially thankful for the assistance of the nursing staff and the generous help provided by University Health Services and University Housing. Alcohol-based hand sanitizer was generously provided by Warner Lambert, a subsidiary of Pfizer.
References
Potential conflicts of interest: none reported.
Presented in part: Workshop on personal protective equipment for health care workers in the workplace against novel H1N1 influenza, Institute of Medicine-Board on Health Sciences Policy, Washington, DC, 12–13 August 2009. The 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and 46th Annual Meeting of the Infectious Disease Society of America, Washington, DC, 25–28 October 2008.
Financial support: Centers for Disease Control and Prevention (grant U01 C1000441) (PIs: A.S.M. and A.E.A.).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC). The CDC had a role in the design and conduct of the study, interpretation of data, and preparation, review, and approval of the manuscript; they did not have a role in the collection, management, or analysis of the data. Warner Lambert provided hand sanitizer without any involvement in the study design, analysis, results, or writing of the manuscript.