-
PDF
- Split View
-
Views
-
Cite
Cite
Jessica Leung, Kathleen Dooling, Mona Marin, Tara C Anderson, Rafael Harpaz, The Impact of Universal Varicella Vaccination on Herpes Zoster Incidence in the United States: Comparison of Birth Cohorts Preceding and Following Varicella Vaccination Program Launch, The Journal of Infectious Diseases, Volume 226, Issue Supplement_4, 1 November 2022, Pages S470–S477, https://doi.org/10.1093/infdis/jiac255
Close - Share Icon Share
Abstract
When the US varicella vaccination program was introduced in 1995, its impacts on the epidemiology of herpes zoster (HZ) were not precisely known. We used a large claims database to examine HZ incidence in the US during 1998–2019 among persons aged ≥30 years (the prevaccine cohort, born before 1990), and aged 1–29 years (includes the postvaccine cohort, born since 1990). We defined incident HZ as the first instance of an outpatient or emergency department (ED) claim with an HZ diagnostic code. Additionally, we examined the proportion of HZ visits among all ED visits as a complementary method to assess for healthcare-seeking artifacts in the findings. In persons aged ≥30 years (prevaccine cohort), we observed age-specific increases in HZ incidence during the earlier study years, with decelerations in later years, starting in 2007 with oldest age groups. Similar patterns were seen when we examined HZ visits as a proportion of all ED visits. For persons aged 1–29 years, age-specific HZ incidence increased early in the study period for the oldest age groups who were born prevaccine, but later declined in a stepwise pattern once each age group was comprised of persons born in the postvaccine period. Our results, corroborated with previously published studies, do not support prior modeling predictions that the varicella vaccination program would increase HZ incidence among adult cohorts who previously experienced varicella. Our findings also suggest that continued declines in age-specific HZ incidence as varicella-vaccinated cohorts age are likely.
The introduction of universal 1-dose childhood varicella vaccination in the United States (US) in 1995 resulted in sharp reductions (approximately 90%) in the burden of varicella within the first decade of the program [1–4], preventing varicella zoster virus (VZV) infection at both the individual level (through individual immunity) and population level (through reduced VZV force of infection). The addition of a routine second dose of varicella vaccine in 2007 further reduced that burden [5–8]. Herpes zoster (HZ) is caused by reactivation of latent VZV years to decades after initial infection; therefore, the varicella vaccination program would also be expected to impact the burden of this common, often debilitating condition. At the start of the varicella vaccination program, its impacts on HZ epidemiology were not precisely known.
Latent VZV can be wild-type VZV (wt-VZV) from natural infection, vaccine-strain VZV (vs-VZV) from immunization with live attenuated vaccine, or both. Based on available data in children and adolescents, the risk of HZ from vs-VZV appears to be approximately 80% lower than the risk from wt-VZV, with a lower incidence found in 2-dose as compared to 1-dose vaccine recipients [9, 10]. In addition to variation due to viral strain, HZ risk is determined by host immunologic factors, including general immunocompetence (ie, reduced with age and by immunocompromising conditions and therapies), age at VZV infection, and HZ vaccination [11]. More speculatively, immunological control may be boosted by exogenous exposures to VZV from contacts with varicella, and endogenously by subclinical VZV reactivation or by HZ itself. One influential paper suggested that decreased exposures to varicella following a universal varicella vaccination program would lead to decreased exogenous boosting, resulting in increases in HZ cases for the first 20 years after the start of a varicella vaccination program. The authors predicted a major epidemic of HZ, affecting >50% of those aged 10–44 years at the introduction of vaccination [12].
Universal varicella vaccination may have complex individual- and population-level impacts on HZ epidemiology and risk by modifying the likelihood and age of exposure to and infection with VZV, whether vs-VZV (1 or 2 doses), wt-VZV, both, or neither (ie, VZV naive). These impacts would differ among those born before program introduction (prevaccine) and those born during a period when they were likely to be included in a routine vaccination program or catch-up campaign (postvaccine). For example, those reaching adulthood prior to launch of the varicella vaccination program were almost all latently infected with wt-VZV by the age of 15 years [13]. Declines in circulating VZV post–varicella vaccine introduction reduced opportunities for exogenous boosting and thereby, potentially, HZ-specific control. Persons born later in the postvaccine cohort, after 2002, had few opportunities for wt-VZV exposure and mostly harbor vs-VZV, with some proportion avoiding both vaccination and disease (ie, VZV-naive).
We previously reported age-specific trends in the incidence of HZ in the US [14–16]. Here, we update the analysis through 2019 and report the findings specifically in the context of the varicella vaccination program, distinguishing HZ incidence among those in prevaccine vs postvaccine cohorts.
METHODS
Data Sources
We conducted a retrospective cohort study of HZ incidence using data from the 1998–2019 IBM MarketScan® Research Databases, which include data from public and private employers and Medicare. Though data are available from 1993, we restricted our analysis to data from 1998 or later when individual-level enrollment data were available, thus allowing for age stratification; before 1998, only aggregate data with predetermined age groups were available. The MarketScan databases contain demographic data, insurance coverage, inpatient and outpatient diagnostic codes, and outpatient prescription claims. These healthcare claims databases are de-identified; therefore, upon review by the Centers for Disease Control and Prevention (CDC), this study was not considered human subjects research and thus did not require CDC Institutional Review Board approval.
Study Definitions
HZ cases were identified using outpatient and emergency department (ED) (including outpatient and inpatient) claims, with International Classification of Diseases, Ninth and Tenth Revision (ICD-9/ICD-10) diagnostic codes for HZ (053.xx/B02.xx) in the primary or secondary diagnostic position, excluding postherpetic complications (053.12–053.13/B02.2x). HZ diagnostic codes map almost 1-to-1 from ICD-9 to ICD-10. An HZ incident case was defined as the first instance an enrollee had a claim with an HZ diagnostic code.
Analyses
HZ Incidence in Persons Aged ≥30 Years (Prevaccine Cohort)
This cohort consisted of persons born before 1990, 5 years before the start of the varicella vaccination program (prevaccine cohort). Age-specific HZ incidence was calculated for 1998–2019 by dividing the annual age-specific number of incident HZ cases from outpatient settings and outpatient ED by the annual age-specific MarketScan population. HZ incidence data before 1998 were previously published [14, 16] (Supplementary Figure 1). To examine whether trends in HZ incidence among adults aged ≥30 years may have been affected by biases related to health-seeking behavior and potential case ascertainment, we also assessed the proportion of visits for HZ among all ED visits for 1998–2019 by age group. Because data on inpatient ED visits were not available before 2003, for this analysis we used outpatient ED visits for 1998–2002 and both outpatient and inpatient ED visits for 2003–2019.
HZ Incidence in Persons Aged 1–29 Years (Postvaccine Cohort)
This cohort consisted of persons born in 1990 or after (ie, ≤5 years of age at the time of varicella vaccine introduction, therefore age-eligible for routine vaccination or a catch-up campaign), defined as the postvaccine cohort. To compare HZ incidence in 1- to 29-year-olds over time, we also included analysis of persons who were born before 1990 and were aged 1–29 years during the study period in an analysis of HZ incidence by calendar time, stratified by age group. Different from our prior analyses [15, 16], we excluded persons aged <1 year from both the numerator and the denominator because they have no or very little opportunity for infection with wt-VZV or vs-VZV, and therefore no or low risk for HZ. Age-specific HZ incidence was calculated for 1998–2019 by dividing the annual age-specific number of incident HZ cases from outpatient settings and outpatient ED by the annual age-specific MarketScan population.
We also examined HZ incidence by age, stratified by birth cohort: 1981–1989 (prevaccine), 1990–2001 (early postvaccine), and 2002–2019 (late postvaccine).
RESULTS
Enrolled Population
A total of 58 363 035 persons aged ≥1 year representing approximately 256 million person-years were enrolled in MarketScan during 1998–2019, with 35 344 803 aged ≥30 years representing 166 million person-years and 25 132 743 aged 1–29 years representing 90 million person-years. Median enrollment by study period is shown in Supplementary Table 1.
HZ Incidence in Persons Aged ≥30 Years
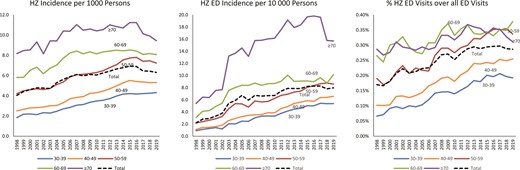
There was a total of 1 020 691 incident HZ cases among persons aged ≥30 years during 1998–2019; crude incidence increased from 4.0 to 6.3 per 1000 persons (Figure 1). HZ incidence increased with age, ranging from 2.2–4.9 per 1000 in 30- to 49-year-olds to 8.2–11.2 per 1000 in ≥70-year-olds. In the 2 oldest age groups, 60–69 and ≥70 years, rates appeared to plateau starting in 2007 and incidence in the oldest age group (≥70 years) began to decline in 2016. Rates also appeared to plateau for 40- to 49-year-olds starting in 2015 and for 50- to 59-year-olds starting in 2016. HZ incidence in ED settings, and the proportion of visits for HZ among all ED visits, showed similar patterns (Figure 1, Supplementary Figure 2).
Herpes zoster (HZ) incidence by age group among persons born before the varicella vaccination program (prevaccine cohort; persons aged ≥30 years), IBM MarketScan, United States, 1998–2019. Scales are different for the 3 graphs to better visualize the data. HZ Incidence includes incident HZ cases from outpatient and outpatient emergency department (ED) visits. To examine whether trends in HZ incidence may have been affected by case ascertainment biases due to health-seeking behavior, we examined HZ incidence in ED (HZ ED Incidence) and HZ ED visits as a proportion of all ED visits. Since our objective was to compare trends over time using these 3 methods and not to examine differences by age, the use of different scales would not affect interpretation of findings. ED data are from both inpatient and outpatient data, except for 1998–2002, which only includes outpatient data. Among HZ cases with an ED visit, 91% are from the outpatient data. The median number of total ED visits increased from 426 520 in 1998–2002 to 3 763 001 in 2015–2019; median enrollment increased from 2 273 013 to 10 066 904 during this period.
HZ Incidence in Persons Aged 1–29 Years
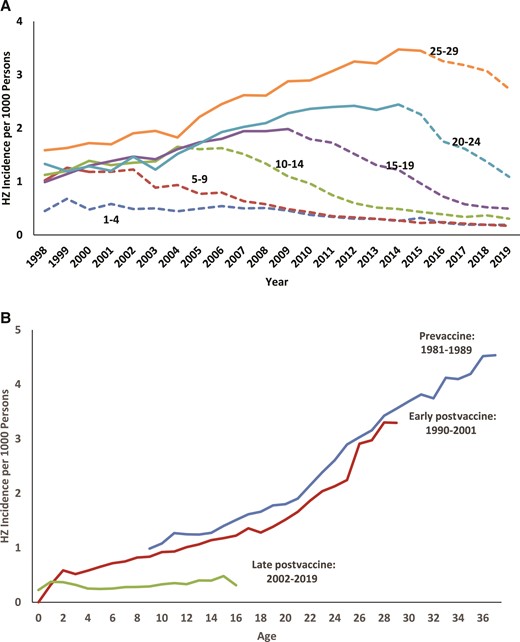
There was a total of 114 950 incident HZ cases among persons aged 1–29 years during 1998–2019. Age group–specific incidence increased in the earlier years of the study for the oldest age groups who were born prevaccine, and then declined in a stepwise fashion as each age group was dominated by the persons born postvaccine, starting in 1999 for 1- to 4-year-olds, 2002 for 5- to 9-year-olds, 2006 for 10- to 14-year-olds, 2009 for 15- to 19-year-olds, and 2014 for 20- to 29-year-olds (Figure 2A). In the 2 youngest age groups (1–4 and 5–9 years), incidence started to converge in 2009 with a rate of 0.5 per 1000, declining to 0.2 per 1000 in 2019.
Herpes zoster (HZ) incidence in persons aged 1–29 years, by year (includes prevaccine and postvaccine cohorts) (A) and aged <37 years, by birth cohort (prevaccine, early postvaccine, and late postvaccine) (B), as a function of age, IBM MarketScan, United States, 1998–2019. A, Solid lines represent HZ incidence before opportunity for routine varicella vaccination and dashed lines represent HZ incidence after the opportunity for routine varicella vaccination.
When HZ incidence was examined by each birth cohort as individuals aged, the rates in the early postvaccine birth cohort were similar to the prevaccine cohort for whom all HZ was caused by wt-VZV. On the other hand, the late postvaccine birth cohort demonstrated low HZ rates without age-associated increases (Figure 2B).
DISCUSSION
The drivers of HZ epidemiology are poorly understood. Before the introduction of the varicella vaccination programs, there was relatively little study of HZ epidemiology and trends [11]. We have extended observations from earlier analyses and, by evaluating trends in HZ incidence in sequential birth cohorts that reflect the changing distribution of latent VZV strains over time and differences in corresponding HZ risks, are able to draw important conclusions. We used complementary methods to help validate these trends.
In persons aged ≥30 years (prevaccine cohort), we show that age-specific HZ incidence increased over a period of decades with recent plateau or decline, extending our earlier results [14–17] and confirming the findings of others [18–23]. These increases could not be explained by changes in healthcare-seeking behavior or in the prevalence of either immunocompromising or chronic conditions [11, 16, 17, 19–21]. The exogenous boosting hypothesis, first formulated by Hope-Simpson [24], is compelling and provides a framework for explaining increases in HZ incidence that occurred at the same time as declines in VZV circulation resulting from the varicella vaccination program. However, attribution of HZ rate increases to the varicella program has not been supported by empiric observations. First, HZ increases in the US predated the varicella program [15–17, 19, 22, 23], and analogous increases have been observed in many other countries in the absence of widespread varicella vaccination [18, 20, 25–34]. Some mathematical models attribute increases in HZ incidence to declines in exogenous boosting due to modernizing sociodemographic factors and family structure [35–38], but there is no evidence that the observed HZ increases accelerated following introduction of varicella vaccination: it is hard to attribute striking increases in HZ incidence to subtle changes in population density or family structure spread out over decades, while finding no signal as the varicella program caused a 10-fold decline in varicella incidence over a single decade. It is equally hard to reconcile these explanations with the inconsistent patterns in HZ incidence by country or region, given their substantial differences in sociodemographics and family structure [18, 39–41]. Second, observational studies in the US and elsewhere have not documented acceleration of observed HZ increases following introduction of varicella vaccination [15–18, 42]. We have previously shown that the rate of increase in HZ incidence was greater during the prevaccine period of 1993–1996 than at later times; we also showed that HZ incidence did not appear to vary by state-level varicella vaccination coverage (as might have been expected if varicella vaccination modified HZ risk) [16, 17].
Given the plausibility of the exogenous boosting hypothesis and the several epidemiological studies suggesting that VZV exposure can, at times, reduce HZ risk [11], our findings merit explanation. One explanation is that modelers did not account for the fact that HZ incidence was increasing in the US and elsewhere before varicella vaccination was introduced. While one would assume, as we do above, that the effects of varicella vaccination on a baseline of increasing HZ incidence would manifest as an acceleration superimposed on those increases, formal analyses of this question in modeling studies have not, to our knowledge, been conducted. Another explanation is that initial models on this topic have made simplifying assumptions that varicella transmission (and consequently benefits of exogenous exposure) would stop immediately upon program launch, whereas the varicella vaccination program took time to mature with important exogenous exposure occurring for several years after program launch. However, subsequent models have explored vaccination uptake scenarios similar to those that occurred in the US, predicting that HZ rate increases would occur and be detected in the time frame covered by our US-based empiric observations to the contrary [43, 44]. A third, perhaps most likely, explanation is supported by recent research that the effects of VZV exposure do not have a discernable population-level impact on HZ incidence because the portion of the population experiencing varicella exposures is small and the duration of boosting is short-lived [30, 45, 46].
Not only has HZ incidence not accelerated its increase since the varicella vaccine program launch, but our updated findings document age-specific plateauing or decreasing incidence over the past decade. While introduction of HZ vaccines (zoster vaccine live in 2006, recombinant zoster vaccine in 2017) may have contributed to these declines in immunocompetent adults aged ≥50 years [27, 47–50], overall, HZ vaccine coverage reached only 24% in 2018 among adults aged ≥50 years, and 34.5% among adults aged ≥60 years [51]. Furthermore, the deceleration in HZ incidence was also noted among adults aged 30–49 years who were not eligible for these vaccines. These unexplained changes in HZ incidence, like the prior increases, highlight how much we have yet to learn about the causes and epidemiology of HZ. Additionally, they provide a cautionary note for interpreting HZ vaccine effectiveness using historic controls or using population trends to calculate preventable fractions of HZ for these vaccines.
While there have been epidemiologic studies suggesting that exposures to varicella may play a role in controlling HZ [41, 45], to our knowledge, the only observational evidence that universal varicella vaccination increased HZ incidence in the general US population was our previous report of the convergence in HZ incidence in 2004 among cohorts of adults with vs without dependents aged ≤12 years (surrogates for varicella exposure) as varicella vaccine uptake increased during the years after licensure [16]. We attributed this finding to a convergence in opportunities for exogenous boosting resulting from the vaccination program. However, an updated analysis showed that once converged, the trajectories of the 2 cohorts did not then simply track each other—instead, they diverged (Supplementary Appendix). We are unable to explain this finding, but the updated data do not support our earlier interpretation. Notably, there are preliminary data from Japan [52] and Manitoba, Canada [53] that are consistent with increases in HZ incidence after introduction of varicella vaccination; these analyses should be followed over time. The Canadian authors hypothesized that the increases were more likely due to changes in demographic factors though included decreased exposure to wt-VZV as a possible factor [53]; another Canadian study found no increases in HZ in Ontario [33].
Shifting attention to persons aged 1–29 years (the postvaccine cohort), this cohort’s experience was much more variable than that of the prevaccine cohort: individuals born during the early 1990s initially had frequent opportunities for varicella exposure (like the prevaccine cohort before them), but many born during the early 1990s had not yet been infected during early childhood (by which time varicella vaccination was introduced). Opportunities for wt-VZV exposure dropped further year by year, and by the late 1990s vaccination coverage was moderate and wt-VZV circulation low. For instance, in 2001, varicella incidence was reduced by about 75%, and vaccine uptake among preschool children exceeded 70% [1, 54]. The balance in prevalence of individuals harboring VZV strains shifted accordingly, from mostly wt-VZV to both wt- and vs-VZV strains to mostly vs-VZV [9]. This interval between approximately 1990 and 2006 (ie, reflecting individuals currently aged 15–29 years) was thus highly transitional. Those born in the early postvaccine years (1990–2001) have HZ rates that increase with age similar to those born in the prevaccine era—likely a result of infection or coinfection with wt-VZV.
Our results show stepwise decreases in HZ incidence among persons aged 1–29 years over the study period, which extend our previously published analyses [15] and support the associated interpretations. Among age groups likely latently infected with wt-VZV, the risk of HZ increased by calendar year for unknown reasons—similar to the prevaccine cohort. But because HZ risk among vs-VZV infected individuals is much lower than that for age-matched wt-VZV infected individuals, HZ risk then subsequently decreased as the age groups became dominated by the vaccinated cohort. Our findings suggest that in the future, age-specific HZ rates will likely continue to decline in successive age groups over time. The results to date provide no evidence that the lower risk of vs-VZV–associated HZ currently observed in children and adolescents increases with age; it is important to continue monitoring HZ rates among vaccinated cohorts through adulthood.
By 2006, varicella incidence was a fraction of its prevaccination program baseline [1, 5], leaving few opportunities for wt-VZV exposure. Varicella vaccination rates approached 90% among preschool children [54]. It is reasonable to expect circumstances observed for persons born between 2006 and 2019 (ie, persons currently aged 2–15 years) to represent the establishment of a new steady-state, with approximately 90% of individuals likely infected with latent vs-VZV, and small categories of wt-VZV, mixed wt-/vs-VZV, and uninfected (ie, VZV naive) individuals. The HZ rates in this population of largely vs-VZV-infected individuals should roughly reflect the age-specific biological risk of vs-VZV reactivation. Indeed, during this period, the incidence in children aged 1–9 years seems to be stabilizing at 0.2 per 10 000 population, the lowest levels ever published for children.
Above we discuss trends in HZ incidence in the prevaccine cohort, noting that in past reports, we (and others) described increasing HZ incidence occurring over decades without clear explanation [11, 14–17, 19, 20, 22, 23, 55]. We have long recognized that epidemiological analyses that rely on clinical data can be confounded by factors affecting healthcare seeking and by barriers to the receipt of healthcare [16, 17, 56, 57]. In our current analysis, we looked at HZ visits as a proportion of all ED visits to assess care seeking over time. To conduct this assessment, we examined HZ incidence in the ED clinical setting, which itself provides some validation of our findings as compared to the general outpatient setting. We believe this approach provides reassurance that observed changing HZ patterns among adults are real.
We have highlighted the limitations of administrative data–based methods in the past. These include reliance on diagnostic coding, which can be prone to errors and which we were unable to validate. Also, although MarketScan databases capture a large population (indeed, a considerable portion of the total US population), it is a convenience sample of persons primarily covered under employer-sponsored insurance, and not representative of the US population. Birth cohorts can be an imprecise surrogate for HZ-related risk status because of variability of VZV exposure within the cohort. We could not distinguish wt-VZV vs vs-VZV strains in our study population and we did not have data on varicella vaccination status. While we used methods to try to address HZ case ascertainment, we believe that it is such an important confounder that we emphasize it again: Not all patients with HZ seek medical care, and that behavior likely depends, in part, on HZ severity, which is, in turn, affected by age and possibly VZV strain. Completeness of HZ ascertainment is particularly unknown for children who may present with only very mild symptoms and not seek care or are misdiagnosed. Nevertheless, the striking declines in pediatric HZ incidence that we observed are biologically plausible and had been predicted in the past [12, 44, 58–60]. Though we attempted to validate trends in HZ, the limitations with administrative data remain and may have still affected the trends in this analysis. Last, we did not have inpatient ED visit data for 1998–2002, though most ED visits (91%) are captured in the outpatient data.
In conclusion, aside from the direct benefits that the US varicella vaccination program has provided in reducing varicella morbidity and mortality as reported elsewhere in this supplement [5, 6], the program has also reduced HZ incidence among children and adolescents born in the vaccine era. The reduction of HZ, likely resulting from the varicella vaccination program, should ultimately extend to the entire population over time. Furthermore, our US data do not support modeled predictions that the varicella vaccination program would increase HZ incidence among persons who previously experienced varicella; these empiric data should be used in future models. These results were validated using methods to control for healthcare seeking and other artifacts. Our results provide reassuring evidence to countries considering adoption of universal varicella vaccination programs.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We would like to thank Jane Seward, MBBS, MPH, for her thoughtful review of this manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. No financial support was received for this work.
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.