-
PDF
- Split View
-
Views
-
Cite
Cite
Phyllis G. Weintraub, Sonja J. Scheffer, Diedrich Visser, Graciela Valladares, Alberto Soares Correa, B. Merle Shepard, Aunu Rauf, Sean T. Murphy, Norma Mujica, Charles MacVean, Jürgen Kroschel, Miriam Kishinevsky, Ravindra C. Joshi, Nina S. Johansen, Rebecca H. Hallett, Hasan S. Civelek, Bing Chen, Helga Blanco Metzler, The Invasive Liriomyza huidobrensis (Diptera: Agromyzidae): Understanding Its Pest Status and Management Globally, Journal of Insect Science, Volume 17, Issue 1, January 2017, 28, https://doi.org/10.1093/jisesa/iew121
Close - Share Icon Share
Liriomyza huidobrensis (Blanchard) is native to South America but has expanded its range and invaded many regions of the world, primarily on flowers and to a lesser extent on horticultural product shipments. As a result of initial invasion into an area, damage caused is usually significant but not necessarily sustained. Currently, it is an economic pest in selected native and invaded regions of the world. Adults cause damage by puncturing abaxial and adaxial leaf surfaces for feeding and egg laying sites. Larvae mine the leaf parenchyma tissues which can lead to leaves drying and wilting. We have recorded 365 host plant species from 49 families and more than 106 parasitoid species. In a subset of the Argentinian data, we found that parasitoid community composition attacking L. huidobrensis differs significantly in cultivated and uncultivated plants. No such effect was found at the world level, probably due to differences in collection methods in the different references. We review the existing knowledge as a means of setting the context for new and unpublished data. The main objective is to provide an update of widely dispersed and until now unpublished data, evaluate dispersion of the leafminer and management strategies in different regions of the world, and highlight the need to consider the possible effects of climate change on further regional invasions or expansions.
Introduction
Liriomyza huidobrensis (Blanchard) (Diptera: Agromyzidae) is a globally invasive leafmining fly that feeds on hundreds of plant species, including many important fruit, vegetable, and flower crops. The pest status of this leafminer represents is a classic case of secondary pest outbreak: adults became resistant to insecticides as a result of spraying against another pest. Specifically, in South America in the 1970s the gelechid potato moth, Tuta (= Scrobipalpula) absoluta (Meyrick), was the focus of much insecticide attention and the non-pestiferous leafminer was drenched in the process (Chavez and Raman 1987). Heavy insecticide use imposed selection pressure on L. huidobrensis and, by the time, the leafminer was carried to Europe and beyond, adults were resistant to many conventional insecticides.
The main objectives of this forum article are to provide an update of widely dispersed and until now unpublished data, evaluate dispersion of the leafminer and management strategies in different regions of the world, and highlight the need to consider the possible effects of climate change on further regional invasions or expansions.
Taxonomy
Liriomyza is a large genus of 456 primarily leafmining species (ITIS 2016) within the entirely phytophagous Agromyzidae, a family of more than 2,600 described species. Most Liriomyza are not considered pests, but L. huidobrensis is one of the three polyphagous, globally invasive, and highly destructive species in this genus (Spencer 1973). Liriomyza huidobrensis was first described from Argentina as Agromyza huidobrensis Blanchard, mining leaves of Cineraria sp. in Buenos Aires (Blanchard 1926). The same author later transferred it to Liriomyza and added faba bean (Vicia faba L.) as an additional host (Blanchard 1938). On the basis of color and host variations, two more species were also described from Buenos Aires province: the light colored L. cucumifoliaeBlanchard (1938) from melon (Cucumis melo L.) and the noticeably darker L. decoraBlanchard (1954) from faba bean.
In the USA, Frick (1951) described L. langei Frick from peas (Pisum sativum L.) in California. Spencer (1973), after examining specimens of these species, subsequently synonymized all with L. huidobrensis as the two species appeared identical externally as well as in structure of the male genitalia. This meant that effectively the distribution of L. huidobrensis was exceedingly large, ranging from southern South America to the west coast of the US. However, recent molecular research (Scheffer 2000, Scheffer and Lewis 2001) found that the North America populations in California and Hawai’i are distinct from L. huidobrensis in South America, and the former species, Liriomyza langei, was resurrected. Subsequently, Takano et al. (2008) detected reproductive isolation between L. huidobrensis and L. langei, providing additional strong evidence for the species rank of L. langei.
Despite the evidence that L. huidobrensis and L. langei are distinct species, they cannot be distinguished using external morphological characters. This is common with several agromyzid flies, and identification of species generally requires dissection and examination of male genitalia. However, it is also not possible to distinguish these two species using dissection of the gentitalia. Currently, the only unambiguous means of identifying L. huidobrensis is with molecular data, preferably with DNA barcoding, in which a portion of the gene sequence of mitochondrial cytochrome oxidase I of an unknown specimen is compared for similarity with those previously identified and available on GenBank or Barcode of Life Database (BOLD). However, a certain amount of care must be taken when using a sequence database for identification, not all the sequences in these databases are identified correctly (S.J.S., personal observations). At this time, there are 196 DNA barcode sequences for L. huidobrensis on GenBank and 21 on BOLD. Other molecular methods have been developed for specifically distinguishing L. huidobrensis from L. langei using PCR-RFLP methodology (Scheffer et al. 2001) and multiplex PCR (Scheffer et al. 2014). Under some conditions, the former method may yield ambiguous results. For this reason, the multiplex PCR method is preferable (Scheffer et al. 2014).
External characteristics
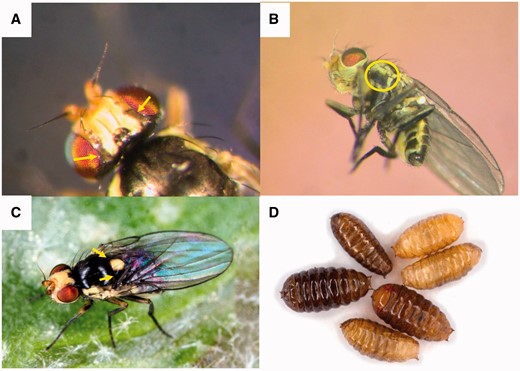
Female Liriomyza huidobrensis using her ovipositor to puncture the surface of a potato leaf.
Characteristics of Liriomyza huidobrensis. (A) Vertical bristles on a brownish yellow background contiguous with black hind margin of the eye (note arrows). (B) Mesopleuron mostly black (note circle) of male L. huidobrensis. (C) Mesonotum with dark edges (note arrows). (D) Various aged and sized pupae.
Egg and larval characteristics are too similar among Liriomyza species to be useful for identification. The posterior spiracles of the puparia (Fig. 2D) are sometimes used to distinguish L. huidobrensis pupae (6–9 pores) from those of the pests L. sativae Blanchard and L. trifolii (Burgess) (3 pores) with which it may co-occur (Spencer 1973). However, overlap in the number and arrangement of pores on the posterior spiracles is common among various Liriomyza species, and, therefore, this character cannot be considered for diagnostics except in limited circumstances where the only other Liriomyza leafminers present are L. sativae, and L. trifolii.
Biology and behavior
The life-cycle parameters of L. huidobrensis have been well studied under different temperature regimes and host plants (Prando and da Cruz 1984, Lanzoni et al. 2002, Videla et al. 2006, and references therein). Adult flies demonstrate clear diel activity (Weintraub and Horowitz 1996, Mujica et al. 2000). Typically, the first signs of the presence of L. huidobrensis, as well as other leafminers, are the punctures made predominantly in the upper leaf surface by the female ovipositor (Fig. 1). Most do not contain an egg and are used by both male and female flies for feeding on plant ‘sap’. Some punctures made by the females contain an egg. Females lay whitish, translucent eggs; they are laid singly but often in close proximity and on both leaf surfaces. Leaf stippling and egg/puncture ratios vary among host plants (Martin et al. 2005c), e.g., females laid an egg every 5 feeding punctures on Vicia faba, but every 125 punctures on Cucurbita maxima Duchesne (Videla et al. 2006). Pisum sativum, Apium graveolens (Mill.) Pers. Solanum tuberosum L., and Lactuca sativa L.were less preferred for L. huidobrensis oviposition than were Cucumis sativus and Brassica alboglabra L. Salas et al. (1988) reported that 87% of eggs laid develop to first instar. Larvae hatch from the eggs and feed in the spongy or palisade mesophyll or even alternate between them. Three larval instars develop in the leaf and the mines become progressively larger with each molt. Larval stages vary in size depending on elevation gradients (Tantowijoya and Hoffmann 2011 and references therein), from different host plants (Musundire et al. 2012), and under different constant temperature regimes (Head et al. 2002).
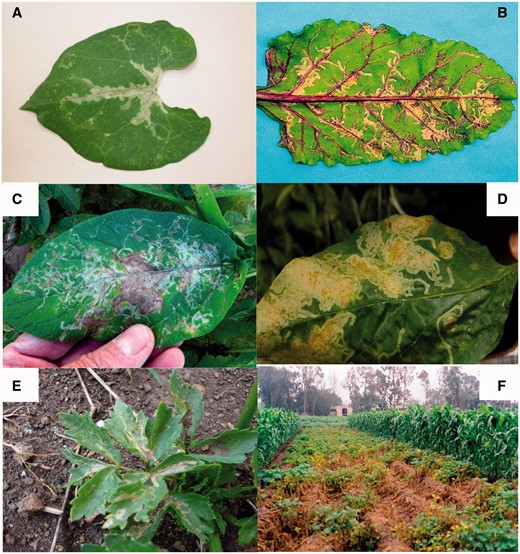
Leaf and field damage of Liriomyza huidobrensis on (A) bean, (B) beet, (C) potato, (D) sweet pepper (black line in tunnels is excrement from the larvae), (E) celery, and (F) potato field in Peru.
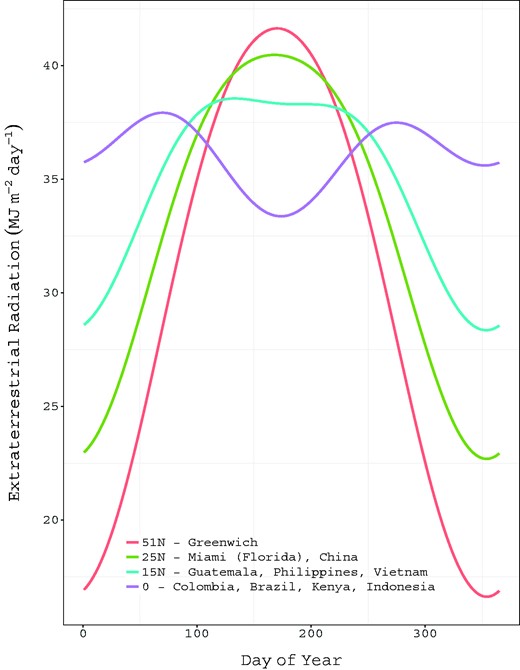
Environmental temperature governs the distribution and activity of the leafminer; in northern latitudes pupae serve as an overwintering stage and can survive up to 30 frost days with minimum temperatures of −11.5 °C (van der Linden 1993) to −20.6 °C (Chen and Kang 2004). Overwintering pupae are able to survive in cold field conditions by gradual adaptation as temperatures decline and supercooling; i.e., the accumulation of cryoprotectants, such as glycerol (Chen and Kang 2004), to protect against ice formation within their body.
Variation in intensity of solar radiation as a function of latitude and day of the year.
Host plant use
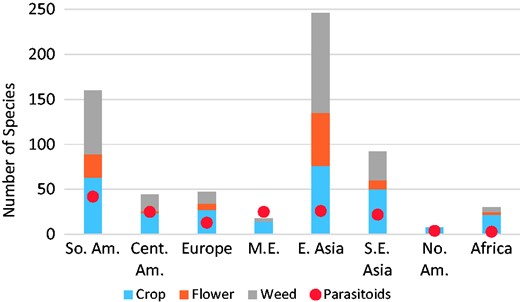
Total number of plant types (cultivated crop, cultivated flower, uncultivated/weed) and parasitoid species per world region by general order of invasion: South America (So. Am.), Central America (Cent. Am.), Europe, western Middle East (M.E.), East Asia (E. Asia), Southeast Asia (S.E. Asia), North America (No. Am.), and Africa.
Families and species of host plants for Liriomyza huidobrensis world-wide
| Plant family . | Host plant species . | Countrya . |
|---|---|---|
| Adoxaceae | Sambucus sp. | GT |
| Aizoaceae | Trianthema portulacastrum | PE |
| Alstroemeriaceae | Alstroemeria aurea | CN |
| Alismataceae | Sagittaria sagittifolia | CN |
| Amaranthaceae | Alternanthera philox eroides | CN |
| Amaranthus sp. | AR, CL, CR, GT, ID, KE, MY, TH | |
| Amaranthus caudatus | CN | |
| Amaranthus hybridus | PE | |
| Amaranthus lividus | CN | |
| Amaranthus lividus ascendens | JP | |
| Amaranthus manostanus | CN | |
| Amaranthus retroflexus | CN, CO, ID, KE | |
| Amaranthus viridis | CO, TW, VE | |
| Beta vulgaris | CR, GT, ID, JP, KE, ES, SK, VE, VN, PE | |
| Beta vulgaris cicla | AR, CN, LB, PE, VE | |
| Beta vulgaris rapacea | AR | |
| Beta vulgaris rubra | AR, ID, TW | |
| Beta vulgaris vulgaris | AR | |
| Celosia argentea | CN | |
| Celosia cristata | CN | |
| Chenopodium sp. | AR | |
| Chenopodium album | AR, CL, NC | |
| Chenopodium ambrosioides | AR, CN, CL, PE | |
| Chenopodium hircinum | PE | |
| Chenopodium murale | PE | |
| Chenopodium paniculatum | CO | |
| Chenopodium quinoa | ID, PE | |
| Deeringia amarantoides | ID | |
| Gomphrena globasa | CN | |
| Amaryllidaceae | Allium sp. | SK |
| Allium ampeloprasum | CO, ID | |
| Allium cepa | CL, CN, CO, CR, DE, GT, ID, KE, PH, ES, TW, VN | |
| Allium cepa aggregatum | ID, PE, PH | |
| Allium chinensis | CN | |
| Allium fistulosum | CN, CR, ID, IT, TW, VN | |
| Allium porrum | GT | |
| Allium sativum | CL, CN, CO, ID, ES | |
| Allium schoenprasum | VE | |
| Spinacia oleracea | AR, CA, CN, ID, IT, JP, KE, PE, TW, VN | |
| Apiaceae | Apium sp. | AR, LB, ES |
| Apium graveolens | AR, CA, CN, CR, DE, GT, ID, IL, IT, ES, SK | |
| Apium graveolens dulce | CN, PE, PH, VN | |
| Bupleurum sp. | CN | |
| Centella asiatica | CN | |
| Coriandrum sativum | CL, CN, GT, TW | |
| Daucus carota | ID, PE, PH | |
| Daucus sativa | CN, CR | |
| Hydrocotyle umbellata | PE | |
| Impatiens caeruleum | CN | |
| Levisticum officinale | GT | |
| Oenanthe benghalensis | CN | |
| Oenanthe javanica | CN | |
| Petroselinum sp. | CO, JP, ES | |
| Apocynaceae | Catharanthus roseus | CN |
| Araceae | Colocasia esculenta | CN |
| Araliaceae | Hydrocotyle sp. | AR |
| Hydrocotyle ranunculoides | AR | |
| Hydrocotyle umbellata | CN, CO, CR | |
| Asparagaceae | Asparagus officinalis | CL, ID |
| Chionodoxa luciliae | CN | |
| Asphodelaceae | Hemerocallis fulva | CN |
| Asteraceae | Arctium minus | AR |
| Arctium lappa | CL, PH | |
| Argyranthemum sp. | NO | |
| Artemisia annua | CN | |
| Artemisia argyi | CN | |
| Aster sp. | AR, CN | |
| Bellis perennis | AR, CN, VN | |
| Bidens pilosa | AR, CN, CR | |
| Bidens sp. | AR | |
| Calendula sp. | KE, ES, NO | |
| Calendula officinalis | AR, CN, PE | |
| Callistephus chinensis | AR, CN, ES | |
| Carduus crispus | CN | |
| Carduus nutans | AR | |
| Carthamus tinctorius | CN | |
| Centaurea cyanus | CN | |
| Chicorium sp. | GT | |
| Chrysanthemum sp. | AR, CN, CO, ID, NY, NO, PH, PT, SK, VN | |
| Chrysanthemum coronarium | CN, VN | |
| Chrysanthemum leucanthemum | AR | |
| Chrysanthemum morifolium | AR, CN, CO, IT | |
| Chrysanthemum segetum | ID, VN | |
| Cichorium sp. | AR | |
| Cichorium endivia | VE | |
| Cichorium intybus | AR, GT | |
| Cineraria sp. | AR | |
| Cineraria cruenta | CN | |
| Conoclinium coelestinum | CN | |
| Conyza sp. | AR | |
| Conyza bonariensis | AR | |
| Conyza canadensis | CN, ES | |
| Cosmos bipinnatus | CN | |
| Craspedia globosa | CN | |
| Crassocephalum rubens | CN, TW | |
| Crepis pulchra | AR | |
| Cynara sp. | CL, ES | |
| Cynara cardunculus scolymus | CL, CO | |
| Cynara scolymus | KE, PE, ES | |
| Dahlia sp. | ID, MY, NO | |
| Dahlia imperialis | CR, ID | |
| Dahlia pinnata | AR, CN | |
| Dahlia variabilis | AR | |
| Dendranthema mortifolium | CN | |
| Dichrocephala auriculata | CN | |
| Echinops ritro | CN | |
| Eclipta prostrata | CN | |
| Emilia sonchifolia | CN, CO, ID, PH, SK, TW | |
| Erechtites hieracifolia | CN, CR, CL, ID | |
| Erigeron briviscapus | CN | |
| Gaillardia pulchella | CN | |
| Galinsoga sp. | CR | |
| Galinsoga caracasana | CR, CL, VE | |
| Asteracea | Galinsoga ciliata | CN, CO, CR, PE |
| Galinsoga parviflora | CN, CO | |
| Galinsoga urticifolia | GT | |
| Galisonga caracasana | CO | |
| Galisonga ciliata | IT | |
| Gazania sp. | CN | |
| Gerbera sp. | GT, LB, NO, TH, VN | |
| Gerbera jamesonii | CN, ID, IT, MY, PT | |
| Gnaphalium afffine | CN | |
| Gynura crepidioides | CN | |
| Helianthus sp. | AR, CN, NO | |
| Helianthus annuus | AR, CN, PE | |
| Helichrysum sp. | NO | |
| Helichrysum bracteatum | CL | |
| Helipterum roseum | CN | |
| Hemistepta lyrata | CN | |
| Kalimeris indica | CN, KE, ES, TW | |
| Lactuca capitata | CL, CN | |
| Lactuca indica | ID, MY, TW | |
| Lactuca sativa | AR, CA, CL, CN, CO, DE, ID, GT, IL, IT, LB, PE, PT, ES, TW, VN | |
| Lactuca sativa angustata | CN | |
| Lactuca sativa asparagina | CN | |
| Lactuca sativa capitata | PH | |
| Lactuca sativa crispa | CN | |
| Lactuca sativa intybeca | CN, TW | |
| Lactuca sativa romana | CN | |
| Lactuca vulgaris | VE | |
| Osteospermum sp. | NO | |
| Pyrethrum cinerariifolium | CN | |
| Schistocarpha platyphylla | GT | |
| Senecia cruentus | IT, PH, ES | |
| Solidago sp. | CN KE, ES | |
| Sonchus sp. | PE | |
| Sonchus asper | CN, CO, LB | |
| Sonchus brachyotus | CN | |
| Sonchus oleraceus | AR, CN, CO, CR, GT, KE | |
| Synedrella nodiflora | ID, LB | |
| Tagetes sp. | AR, NO | |
| Tagetes erecta | CN, KE, PE | |
| Tagetes patula | CL, CN | |
| Tagetes tenuitolia | AR | |
| Tanacetum parthenium | CN | |
| Taraxacum mongolicum | CN | |
| Taraxacum officinal | AR | |
| Zinnia elegans | AR, CN | |
| Balsaminaceae | Impatiens balsamina | CN |
| Basellaceae | Basella alba | CN, ID, ES |
| Basella rubra | CN | |
| Brassicaceae | Barbarea sp. | CR, ID, TW |
| Brassica sp. | CO | |
| Brassica alboglabra | CA | |
| Brassica campestris | CL, CN, CR, ID | |
| Brassica campestris pekinensis | CN, PE | |
| Brassica campestris rapa | CO, PE | |
| Brassica chinensis | ID, MY | |
| Brassica juncea | CA, CL, CN, ID, PH, SK, VN | |
| Brassica napus | CN | |
| Brassica oleracea | CN, CR KE, ES, SK, ID | |
| Brassicaceae | Brassica oleracea acephala | CN |
| Brassica oleracea botrytis | CN, ID, PE | |
| Brassica oleracea capitata | CN, CO, GT, PE, PH, VN | |
| Brassica oleracea caulorapa | CN | |
| Brassica oleracea geminifera | GT | |
| Brassica oleracea italica | CN, CO, GT, ID, PE, PH, | |
| Brassica oleracea pekinensis | CN, CO | |
| Brassica rapa | AR, CN, ID, PH | |
| Brassica rapa chinensis | CN, ID, MY, TH, VN | |
| Capsella bursa-pastoris | CO, GT, IT, CN | |
| Cardamine hirouta | CN | |
| Diplotaxis muralis | PE | |
| Hirschfeldia sp. | CN, ES | |
| Lebnlaria mariema | CN | |
| Matthiola sp. | CN | |
| Matthiola incana | CN | |
| Nasturtium indicum | ID | |
| Nasturtium officinal | CN, ID, JP, PH | |
| Raphanus sativus | CN, CO, GT, ID, PE, PJ, ES | |
| Rorippa indica | CN, PJ | |
| Rorippa montan | CN | |
| Rorippa palustris | CN | |
| Campanulaceae | Campanula medium | CN |
| Platycodon grandiflorus | CN | |
| Caryophyllaceae | Dianthus sp. | NO, PH, VN |
| Dianthus barbatus | CN | |
| Dianthus caryophyllus | AR, CL, CN, ID | |
| Dianthus chinensis | CN | |
| Dianthus hybridus | CN | |
| Gypsophila elegans | AR, CN | |
| Gypsophila paniculata | CN, CO, ES | |
| Gypsophila sp. | CN, CO, NO | |
| Silene gallica | CO | |
| Stellaria alsine | CN | |
| Stellaria media | CN, PE | |
| Stellaria yunnansis | CN | |
| Vaccaria pyramidata | ID, JP | |
| Convolvulaceae | Calystegia hederacea | CN |
| Calystegia sepium | CN | |
| Ipomoea aquatica | CN | |
| Ipomoea batatas | CN, ID, TW | |
| Cucurbitaceae | Benincasa hispida | CN |
| Citrullus lanatus | CN, KE, ES | |
| Citrullus vulagris | PE | |
| Cucumis melo | AR, BR, CN, ID, ES | |
| Cucumis sativus | AR, BR, CA, CL, CN, CO, DE, ID, IT, JP, LB, PE, ES, NO, TW, TR, VN | |
| Cucurbita sp. | NO, PH, VN | |
| Cucurbita maxima | AR, CN, KE, LB, PE, TW | |
| Cucurbita maxima zapallito | AR | |
| Cucurbita moschata | AR, CN, KE, LB, TW | |
| Cucurbita pepo | CN, EU, KE, PE, PH, SK, VE | |
| Cucurbita pepo ovifera | CN | |
| Lagenaria sp. | CN, TW | |
| Lagenaria siceraria | CN | |
| Luffa acutangula | CN | |
| Cucurbitaceae | Luffa cylindrica | AR, CN, TW |
| Melothria indica | CL, ID | |
| Momordica charantia | CL, CN, JP, KE | |
| Sechium edule | CN, ID, JP, PH, VN | |
| Euphorbiaceae | Euphorbia marginata | CN |
| Ricinus communis | CN | |
| Fabaceae | Cicer arietinum | AR, CL, ES, VN |
| Crotalaria longirostrata | GT | |
| Glycine max | AR, CN, ID, JP, ES | |
| Lablab sp. | CN | |
| Lablab purpureus | CL, CN, KE | |
| Lathyrus latifolius | AR, CN, ES | |
| Lathyrus odoratus | AR, CN, ID | |
| Lupinus mutabilis | PE | |
| Lupinus rassel | CN | |
| Lupinus sp. | CL, JP | |
| Medicago minima | CN, ES | |
| Medicago sativa | AR, CL, JP, PE, ES | |
| Melilotus suaveolens | CN | |
| Phaseolus sp. | JP, PH | |
| Phaseolus coccineus | KE | |
| Phaseolus lunatus | CL, ID, MY | |
| Phaseolus vulagris | AR, BR, CL, CN, CO, CR, ID, IT, JP, KE, LB, MY, MU, PE, ES, TW, TR, VE, VN | |
| Phaseolus vulgaris humilis | CN | |
| Phaseolus vulgaris vulgaris | PE | |
| Pisum sp. | ID, PH | |
| Pisum sativum | AR, CA, CL, GT, ID, JP, KE, LB, MY, MU, PE, ES, TW, TR, VN, CN | |
| Pisum sativum macrocarpenser | CN | |
| Pisum sativum saccharatum | GT, ID | |
| Trifolium repens | CN, CO, VN | |
| Vicia faba | AR, CL, CN, GT, ID, JP, KE, MY, PE, ES, TR, ZW | |
| Vicia sativa | CN | |
| Vigna sinensis | CN, ID, PE, VN | |
| Vigna unguiculata | CN, CO, ID, IE, PE, ES, VN | |
| Gentianaceae | Eustoma sp. | JP, NO |
| Eustoma russellianum | CN, KE | |
| Exacum sp. | NO | |
| Lisianthus sp. | GT | |
| Gesneriaceae | Streptocarpus sp. | NO |
| Hydrangeaceae | Hydrangea macrophylla | CN |
| Iridaceae | Freesia refracta | CN |
| Gladiolus hybridus | CN, MY | |
| Lamiaceae | Leonurus sybiricus | AR |
| Leonurus heterophyllus | CN | |
| Moluccella laevis | CN | |
| Ocimum basilicum | ID, MU, MA, PE | |
| Salvia splendens | CN | |
| Stachys arvensis | PE | |
| Liliaceae | Lilium sp. | CN, ID |
| Lilium davidii | CN | |
| Lilium longiflorum | CN | |
| Linaceae | Linum sp. | AR |
| Malvaceae | Abelmoschus esculentus | KE, PH |
| Alcea sp. | TH | |
| Althaea rosea | CN, PE | |
| Hibiscus trionum | CN | |
| Malva verticillata | CN, ID, JP, PH | |
| Sida sp. | PH | |
| Menispermaceae | Stephania delavayi | CN |
| Moraceae | Humulus scandens | CN |
| Onagroideae | Clarkia amoena | CN |
| Oenothera rosea | CN | |
| Oxalidaceae | Oxalis sp. | AR, CN, JP |
| Oxalis corniculata | CN | |
| Papaveraceae | Papaver sp. | TR |
| Papaver rhoeas | AR, CN, PH, ES | |
| Plantaginaceae | Plantago asiatica | CN |
| Plantago major | CN | |
| Veronica anagallis-aquatica | CN | |
| Plumbaginaceae | Limonium hybrid | CN, PH |
| Limonium latifolium | CN | |
| Limononium tataricum | CN | |
| Myosotis sylvatica | CN | |
| Poaceae | Hordeum vulgare | CN |
| Lagurus ovatus | CN | |
| Setaria viridis | CN, CO, ID, PH | |
| Triticum aestivum | CN | |
| Zea mays | AR, CN, PH | |
| Polemoniaceae | Phlox drummondii | AR, CN |
| Polygonaceae | Polygonum amphibium | CN |
| Polygonum aviculare | CN | |
| Polygonum hydropiper | CN | |
| Polygonum nepalense | CN | |
| Rumex acetosa | CN | |
| Portulacaceae | Portulaca oleracea | CO, PH |
| Primulaceae | Primula sp. | NO |
| Primula acaulis | CN | |
| Primula obconica | AR, CN | |
| Ranunculaceae | Ranunculus asiaticus | AR |
| Ranunculus sceleratus | CL | |
| Delphinium grandiflorus | CN | |
| Delphinium sp. | CN | |
| Nigella damascena | CN | |
| Ranunculus asiaticus | CN | |
| Ranunculus chinensis | CN | |
| Ranunculus sceleratus | CN | |
| Ranunculus sieboldii | CN | |
| Ranunculus viridis | CN | |
| Rosaceae | Rosa sp. | CN, TH |
| Scrophulariaceae | Calceolaria crenatiflora | CN |
| Diascia sp. | NO | |
| Nemesia sp. | NO | |
| Nemesia strumosa | CN | |
| Solanaceae | Capsicum sp. | BR, CR, ID, KE |
| Capsicum annuum | AR, CL, ID, IT, MY, NO, PE, PH, TW | |
| Capsicum baccatum | CO, PE | |
| Capsicum frutescens | CN, PE | |
| Datura sp. | NO, PH, VN | |
| Datura ferox | AR | |
| Datura stramonium | CL, CN, CO, PE, | |
| Lycium chinense | CN | |
| Nicotiana sp. | PE | |
| Solanaceae | Nicotiana glauca | PE |
| Nicotiana tabacum | CL, CN, PE, ES | |
| Petunia hybida | CN | |
| Petunia sp. | AR, CO, JP, NO | |
| Physalis angulata | CO, CR, ID, JP | |
| Solanum sp. | CL, CO | |
| Solanum americanum | ID, IT, PH | |
| Solanum melongena | AR, CL, CN, ID, IT, KE, PE, PH, VN | |
| Solanum melongena oblong | PH | |
| Solanum muricatum | CN, PH | |
| Solanum nigrum | CN | |
| Solanum oleracelus | CO, LB, PH | |
| Solanum lycopersicum | AR, CL, CN, CR, EC, GT, ID, JP, KE, KR, MY, MU, MA, NO, PE, PH PH, PT, ES, NL, TR, VE, VN | |
| Solanum tuberosum | AR, BR, CA, CL, CN, CR, EC, ID, IL, JP, KE, KR, MU, PE, PH, ZA, ES, SK, TR, VE, VN, ZW | |
| Tropaeolaceae | Nasturtium sp. | AR |
| Tropaeolum sp. | CR | |
| Tropaeolum majus | AR, CL, CN | |
| Verbenaceae | Verbena sp. | NO |
| Verbena officinalis | CN | |
| Violaceae | Viola philippica | CN |
| Viola tricolor | AR, CN, PE | |
| Viola yedensis | CN |
| Plant family . | Host plant species . | Countrya . |
|---|---|---|
| Adoxaceae | Sambucus sp. | GT |
| Aizoaceae | Trianthema portulacastrum | PE |
| Alstroemeriaceae | Alstroemeria aurea | CN |
| Alismataceae | Sagittaria sagittifolia | CN |
| Amaranthaceae | Alternanthera philox eroides | CN |
| Amaranthus sp. | AR, CL, CR, GT, ID, KE, MY, TH | |
| Amaranthus caudatus | CN | |
| Amaranthus hybridus | PE | |
| Amaranthus lividus | CN | |
| Amaranthus lividus ascendens | JP | |
| Amaranthus manostanus | CN | |
| Amaranthus retroflexus | CN, CO, ID, KE | |
| Amaranthus viridis | CO, TW, VE | |
| Beta vulgaris | CR, GT, ID, JP, KE, ES, SK, VE, VN, PE | |
| Beta vulgaris cicla | AR, CN, LB, PE, VE | |
| Beta vulgaris rapacea | AR | |
| Beta vulgaris rubra | AR, ID, TW | |
| Beta vulgaris vulgaris | AR | |
| Celosia argentea | CN | |
| Celosia cristata | CN | |
| Chenopodium sp. | AR | |
| Chenopodium album | AR, CL, NC | |
| Chenopodium ambrosioides | AR, CN, CL, PE | |
| Chenopodium hircinum | PE | |
| Chenopodium murale | PE | |
| Chenopodium paniculatum | CO | |
| Chenopodium quinoa | ID, PE | |
| Deeringia amarantoides | ID | |
| Gomphrena globasa | CN | |
| Amaryllidaceae | Allium sp. | SK |
| Allium ampeloprasum | CO, ID | |
| Allium cepa | CL, CN, CO, CR, DE, GT, ID, KE, PH, ES, TW, VN | |
| Allium cepa aggregatum | ID, PE, PH | |
| Allium chinensis | CN | |
| Allium fistulosum | CN, CR, ID, IT, TW, VN | |
| Allium porrum | GT | |
| Allium sativum | CL, CN, CO, ID, ES | |
| Allium schoenprasum | VE | |
| Spinacia oleracea | AR, CA, CN, ID, IT, JP, KE, PE, TW, VN | |
| Apiaceae | Apium sp. | AR, LB, ES |
| Apium graveolens | AR, CA, CN, CR, DE, GT, ID, IL, IT, ES, SK | |
| Apium graveolens dulce | CN, PE, PH, VN | |
| Bupleurum sp. | CN | |
| Centella asiatica | CN | |
| Coriandrum sativum | CL, CN, GT, TW | |
| Daucus carota | ID, PE, PH | |
| Daucus sativa | CN, CR | |
| Hydrocotyle umbellata | PE | |
| Impatiens caeruleum | CN | |
| Levisticum officinale | GT | |
| Oenanthe benghalensis | CN | |
| Oenanthe javanica | CN | |
| Petroselinum sp. | CO, JP, ES | |
| Apocynaceae | Catharanthus roseus | CN |
| Araceae | Colocasia esculenta | CN |
| Araliaceae | Hydrocotyle sp. | AR |
| Hydrocotyle ranunculoides | AR | |
| Hydrocotyle umbellata | CN, CO, CR | |
| Asparagaceae | Asparagus officinalis | CL, ID |
| Chionodoxa luciliae | CN | |
| Asphodelaceae | Hemerocallis fulva | CN |
| Asteraceae | Arctium minus | AR |
| Arctium lappa | CL, PH | |
| Argyranthemum sp. | NO | |
| Artemisia annua | CN | |
| Artemisia argyi | CN | |
| Aster sp. | AR, CN | |
| Bellis perennis | AR, CN, VN | |
| Bidens pilosa | AR, CN, CR | |
| Bidens sp. | AR | |
| Calendula sp. | KE, ES, NO | |
| Calendula officinalis | AR, CN, PE | |
| Callistephus chinensis | AR, CN, ES | |
| Carduus crispus | CN | |
| Carduus nutans | AR | |
| Carthamus tinctorius | CN | |
| Centaurea cyanus | CN | |
| Chicorium sp. | GT | |
| Chrysanthemum sp. | AR, CN, CO, ID, NY, NO, PH, PT, SK, VN | |
| Chrysanthemum coronarium | CN, VN | |
| Chrysanthemum leucanthemum | AR | |
| Chrysanthemum morifolium | AR, CN, CO, IT | |
| Chrysanthemum segetum | ID, VN | |
| Cichorium sp. | AR | |
| Cichorium endivia | VE | |
| Cichorium intybus | AR, GT | |
| Cineraria sp. | AR | |
| Cineraria cruenta | CN | |
| Conoclinium coelestinum | CN | |
| Conyza sp. | AR | |
| Conyza bonariensis | AR | |
| Conyza canadensis | CN, ES | |
| Cosmos bipinnatus | CN | |
| Craspedia globosa | CN | |
| Crassocephalum rubens | CN, TW | |
| Crepis pulchra | AR | |
| Cynara sp. | CL, ES | |
| Cynara cardunculus scolymus | CL, CO | |
| Cynara scolymus | KE, PE, ES | |
| Dahlia sp. | ID, MY, NO | |
| Dahlia imperialis | CR, ID | |
| Dahlia pinnata | AR, CN | |
| Dahlia variabilis | AR | |
| Dendranthema mortifolium | CN | |
| Dichrocephala auriculata | CN | |
| Echinops ritro | CN | |
| Eclipta prostrata | CN | |
| Emilia sonchifolia | CN, CO, ID, PH, SK, TW | |
| Erechtites hieracifolia | CN, CR, CL, ID | |
| Erigeron briviscapus | CN | |
| Gaillardia pulchella | CN | |
| Galinsoga sp. | CR | |
| Galinsoga caracasana | CR, CL, VE | |
| Asteracea | Galinsoga ciliata | CN, CO, CR, PE |
| Galinsoga parviflora | CN, CO | |
| Galinsoga urticifolia | GT | |
| Galisonga caracasana | CO | |
| Galisonga ciliata | IT | |
| Gazania sp. | CN | |
| Gerbera sp. | GT, LB, NO, TH, VN | |
| Gerbera jamesonii | CN, ID, IT, MY, PT | |
| Gnaphalium afffine | CN | |
| Gynura crepidioides | CN | |
| Helianthus sp. | AR, CN, NO | |
| Helianthus annuus | AR, CN, PE | |
| Helichrysum sp. | NO | |
| Helichrysum bracteatum | CL | |
| Helipterum roseum | CN | |
| Hemistepta lyrata | CN | |
| Kalimeris indica | CN, KE, ES, TW | |
| Lactuca capitata | CL, CN | |
| Lactuca indica | ID, MY, TW | |
| Lactuca sativa | AR, CA, CL, CN, CO, DE, ID, GT, IL, IT, LB, PE, PT, ES, TW, VN | |
| Lactuca sativa angustata | CN | |
| Lactuca sativa asparagina | CN | |
| Lactuca sativa capitata | PH | |
| Lactuca sativa crispa | CN | |
| Lactuca sativa intybeca | CN, TW | |
| Lactuca sativa romana | CN | |
| Lactuca vulgaris | VE | |
| Osteospermum sp. | NO | |
| Pyrethrum cinerariifolium | CN | |
| Schistocarpha platyphylla | GT | |
| Senecia cruentus | IT, PH, ES | |
| Solidago sp. | CN KE, ES | |
| Sonchus sp. | PE | |
| Sonchus asper | CN, CO, LB | |
| Sonchus brachyotus | CN | |
| Sonchus oleraceus | AR, CN, CO, CR, GT, KE | |
| Synedrella nodiflora | ID, LB | |
| Tagetes sp. | AR, NO | |
| Tagetes erecta | CN, KE, PE | |
| Tagetes patula | CL, CN | |
| Tagetes tenuitolia | AR | |
| Tanacetum parthenium | CN | |
| Taraxacum mongolicum | CN | |
| Taraxacum officinal | AR | |
| Zinnia elegans | AR, CN | |
| Balsaminaceae | Impatiens balsamina | CN |
| Basellaceae | Basella alba | CN, ID, ES |
| Basella rubra | CN | |
| Brassicaceae | Barbarea sp. | CR, ID, TW |
| Brassica sp. | CO | |
| Brassica alboglabra | CA | |
| Brassica campestris | CL, CN, CR, ID | |
| Brassica campestris pekinensis | CN, PE | |
| Brassica campestris rapa | CO, PE | |
| Brassica chinensis | ID, MY | |
| Brassica juncea | CA, CL, CN, ID, PH, SK, VN | |
| Brassica napus | CN | |
| Brassica oleracea | CN, CR KE, ES, SK, ID | |
| Brassicaceae | Brassica oleracea acephala | CN |
| Brassica oleracea botrytis | CN, ID, PE | |
| Brassica oleracea capitata | CN, CO, GT, PE, PH, VN | |
| Brassica oleracea caulorapa | CN | |
| Brassica oleracea geminifera | GT | |
| Brassica oleracea italica | CN, CO, GT, ID, PE, PH, | |
| Brassica oleracea pekinensis | CN, CO | |
| Brassica rapa | AR, CN, ID, PH | |
| Brassica rapa chinensis | CN, ID, MY, TH, VN | |
| Capsella bursa-pastoris | CO, GT, IT, CN | |
| Cardamine hirouta | CN | |
| Diplotaxis muralis | PE | |
| Hirschfeldia sp. | CN, ES | |
| Lebnlaria mariema | CN | |
| Matthiola sp. | CN | |
| Matthiola incana | CN | |
| Nasturtium indicum | ID | |
| Nasturtium officinal | CN, ID, JP, PH | |
| Raphanus sativus | CN, CO, GT, ID, PE, PJ, ES | |
| Rorippa indica | CN, PJ | |
| Rorippa montan | CN | |
| Rorippa palustris | CN | |
| Campanulaceae | Campanula medium | CN |
| Platycodon grandiflorus | CN | |
| Caryophyllaceae | Dianthus sp. | NO, PH, VN |
| Dianthus barbatus | CN | |
| Dianthus caryophyllus | AR, CL, CN, ID | |
| Dianthus chinensis | CN | |
| Dianthus hybridus | CN | |
| Gypsophila elegans | AR, CN | |
| Gypsophila paniculata | CN, CO, ES | |
| Gypsophila sp. | CN, CO, NO | |
| Silene gallica | CO | |
| Stellaria alsine | CN | |
| Stellaria media | CN, PE | |
| Stellaria yunnansis | CN | |
| Vaccaria pyramidata | ID, JP | |
| Convolvulaceae | Calystegia hederacea | CN |
| Calystegia sepium | CN | |
| Ipomoea aquatica | CN | |
| Ipomoea batatas | CN, ID, TW | |
| Cucurbitaceae | Benincasa hispida | CN |
| Citrullus lanatus | CN, KE, ES | |
| Citrullus vulagris | PE | |
| Cucumis melo | AR, BR, CN, ID, ES | |
| Cucumis sativus | AR, BR, CA, CL, CN, CO, DE, ID, IT, JP, LB, PE, ES, NO, TW, TR, VN | |
| Cucurbita sp. | NO, PH, VN | |
| Cucurbita maxima | AR, CN, KE, LB, PE, TW | |
| Cucurbita maxima zapallito | AR | |
| Cucurbita moschata | AR, CN, KE, LB, TW | |
| Cucurbita pepo | CN, EU, KE, PE, PH, SK, VE | |
| Cucurbita pepo ovifera | CN | |
| Lagenaria sp. | CN, TW | |
| Lagenaria siceraria | CN | |
| Luffa acutangula | CN | |
| Cucurbitaceae | Luffa cylindrica | AR, CN, TW |
| Melothria indica | CL, ID | |
| Momordica charantia | CL, CN, JP, KE | |
| Sechium edule | CN, ID, JP, PH, VN | |
| Euphorbiaceae | Euphorbia marginata | CN |
| Ricinus communis | CN | |
| Fabaceae | Cicer arietinum | AR, CL, ES, VN |
| Crotalaria longirostrata | GT | |
| Glycine max | AR, CN, ID, JP, ES | |
| Lablab sp. | CN | |
| Lablab purpureus | CL, CN, KE | |
| Lathyrus latifolius | AR, CN, ES | |
| Lathyrus odoratus | AR, CN, ID | |
| Lupinus mutabilis | PE | |
| Lupinus rassel | CN | |
| Lupinus sp. | CL, JP | |
| Medicago minima | CN, ES | |
| Medicago sativa | AR, CL, JP, PE, ES | |
| Melilotus suaveolens | CN | |
| Phaseolus sp. | JP, PH | |
| Phaseolus coccineus | KE | |
| Phaseolus lunatus | CL, ID, MY | |
| Phaseolus vulagris | AR, BR, CL, CN, CO, CR, ID, IT, JP, KE, LB, MY, MU, PE, ES, TW, TR, VE, VN | |
| Phaseolus vulgaris humilis | CN | |
| Phaseolus vulgaris vulgaris | PE | |
| Pisum sp. | ID, PH | |
| Pisum sativum | AR, CA, CL, GT, ID, JP, KE, LB, MY, MU, PE, ES, TW, TR, VN, CN | |
| Pisum sativum macrocarpenser | CN | |
| Pisum sativum saccharatum | GT, ID | |
| Trifolium repens | CN, CO, VN | |
| Vicia faba | AR, CL, CN, GT, ID, JP, KE, MY, PE, ES, TR, ZW | |
| Vicia sativa | CN | |
| Vigna sinensis | CN, ID, PE, VN | |
| Vigna unguiculata | CN, CO, ID, IE, PE, ES, VN | |
| Gentianaceae | Eustoma sp. | JP, NO |
| Eustoma russellianum | CN, KE | |
| Exacum sp. | NO | |
| Lisianthus sp. | GT | |
| Gesneriaceae | Streptocarpus sp. | NO |
| Hydrangeaceae | Hydrangea macrophylla | CN |
| Iridaceae | Freesia refracta | CN |
| Gladiolus hybridus | CN, MY | |
| Lamiaceae | Leonurus sybiricus | AR |
| Leonurus heterophyllus | CN | |
| Moluccella laevis | CN | |
| Ocimum basilicum | ID, MU, MA, PE | |
| Salvia splendens | CN | |
| Stachys arvensis | PE | |
| Liliaceae | Lilium sp. | CN, ID |
| Lilium davidii | CN | |
| Lilium longiflorum | CN | |
| Linaceae | Linum sp. | AR |
| Malvaceae | Abelmoschus esculentus | KE, PH |
| Alcea sp. | TH | |
| Althaea rosea | CN, PE | |
| Hibiscus trionum | CN | |
| Malva verticillata | CN, ID, JP, PH | |
| Sida sp. | PH | |
| Menispermaceae | Stephania delavayi | CN |
| Moraceae | Humulus scandens | CN |
| Onagroideae | Clarkia amoena | CN |
| Oenothera rosea | CN | |
| Oxalidaceae | Oxalis sp. | AR, CN, JP |
| Oxalis corniculata | CN | |
| Papaveraceae | Papaver sp. | TR |
| Papaver rhoeas | AR, CN, PH, ES | |
| Plantaginaceae | Plantago asiatica | CN |
| Plantago major | CN | |
| Veronica anagallis-aquatica | CN | |
| Plumbaginaceae | Limonium hybrid | CN, PH |
| Limonium latifolium | CN | |
| Limononium tataricum | CN | |
| Myosotis sylvatica | CN | |
| Poaceae | Hordeum vulgare | CN |
| Lagurus ovatus | CN | |
| Setaria viridis | CN, CO, ID, PH | |
| Triticum aestivum | CN | |
| Zea mays | AR, CN, PH | |
| Polemoniaceae | Phlox drummondii | AR, CN |
| Polygonaceae | Polygonum amphibium | CN |
| Polygonum aviculare | CN | |
| Polygonum hydropiper | CN | |
| Polygonum nepalense | CN | |
| Rumex acetosa | CN | |
| Portulacaceae | Portulaca oleracea | CO, PH |
| Primulaceae | Primula sp. | NO |
| Primula acaulis | CN | |
| Primula obconica | AR, CN | |
| Ranunculaceae | Ranunculus asiaticus | AR |
| Ranunculus sceleratus | CL | |
| Delphinium grandiflorus | CN | |
| Delphinium sp. | CN | |
| Nigella damascena | CN | |
| Ranunculus asiaticus | CN | |
| Ranunculus chinensis | CN | |
| Ranunculus sceleratus | CN | |
| Ranunculus sieboldii | CN | |
| Ranunculus viridis | CN | |
| Rosaceae | Rosa sp. | CN, TH |
| Scrophulariaceae | Calceolaria crenatiflora | CN |
| Diascia sp. | NO | |
| Nemesia sp. | NO | |
| Nemesia strumosa | CN | |
| Solanaceae | Capsicum sp. | BR, CR, ID, KE |
| Capsicum annuum | AR, CL, ID, IT, MY, NO, PE, PH, TW | |
| Capsicum baccatum | CO, PE | |
| Capsicum frutescens | CN, PE | |
| Datura sp. | NO, PH, VN | |
| Datura ferox | AR | |
| Datura stramonium | CL, CN, CO, PE, | |
| Lycium chinense | CN | |
| Nicotiana sp. | PE | |
| Solanaceae | Nicotiana glauca | PE |
| Nicotiana tabacum | CL, CN, PE, ES | |
| Petunia hybida | CN | |
| Petunia sp. | AR, CO, JP, NO | |
| Physalis angulata | CO, CR, ID, JP | |
| Solanum sp. | CL, CO | |
| Solanum americanum | ID, IT, PH | |
| Solanum melongena | AR, CL, CN, ID, IT, KE, PE, PH, VN | |
| Solanum melongena oblong | PH | |
| Solanum muricatum | CN, PH | |
| Solanum nigrum | CN | |
| Solanum oleracelus | CO, LB, PH | |
| Solanum lycopersicum | AR, CL, CN, CR, EC, GT, ID, JP, KE, KR, MY, MU, MA, NO, PE, PH PH, PT, ES, NL, TR, VE, VN | |
| Solanum tuberosum | AR, BR, CA, CL, CN, CR, EC, ID, IL, JP, KE, KR, MU, PE, PH, ZA, ES, SK, TR, VE, VN, ZW | |
| Tropaeolaceae | Nasturtium sp. | AR |
| Tropaeolum sp. | CR | |
| Tropaeolum majus | AR, CL, CN | |
| Verbenaceae | Verbena sp. | NO |
| Verbena officinalis | CN | |
| Violaceae | Viola philippica | CN |
| Viola tricolor | AR, CN, PE | |
| Viola yedensis | CN |
AR, Argentina; BR, Brazil; CA, Canada; CL, Chile; CN, China; CO, Columbia; CR, Costa Rica; EC, Ecuador; DE, Germany; GT, Guatemala; ID, Indonesia; IL, Israel; IT, Italy; JP, Japan; KE, Kenya; KR, Korea; LB, Lebanon; MY, Malaysia; MU, Mauritius; MA, Morocco; NL, The Netherlands; NO, Norway; PE, Peru; PH, Philippines; PT, Portugal; ZA, South Africa; ES, Spain; LK, Sri Lanka; TW, Taiwan; TH, Thailand; TR, Turkey; VN, Vietnam; ZW, Zimbabwe.
Families and species of host plants for Liriomyza huidobrensis world-wide
| Plant family . | Host plant species . | Countrya . |
|---|---|---|
| Adoxaceae | Sambucus sp. | GT |
| Aizoaceae | Trianthema portulacastrum | PE |
| Alstroemeriaceae | Alstroemeria aurea | CN |
| Alismataceae | Sagittaria sagittifolia | CN |
| Amaranthaceae | Alternanthera philox eroides | CN |
| Amaranthus sp. | AR, CL, CR, GT, ID, KE, MY, TH | |
| Amaranthus caudatus | CN | |
| Amaranthus hybridus | PE | |
| Amaranthus lividus | CN | |
| Amaranthus lividus ascendens | JP | |
| Amaranthus manostanus | CN | |
| Amaranthus retroflexus | CN, CO, ID, KE | |
| Amaranthus viridis | CO, TW, VE | |
| Beta vulgaris | CR, GT, ID, JP, KE, ES, SK, VE, VN, PE | |
| Beta vulgaris cicla | AR, CN, LB, PE, VE | |
| Beta vulgaris rapacea | AR | |
| Beta vulgaris rubra | AR, ID, TW | |
| Beta vulgaris vulgaris | AR | |
| Celosia argentea | CN | |
| Celosia cristata | CN | |
| Chenopodium sp. | AR | |
| Chenopodium album | AR, CL, NC | |
| Chenopodium ambrosioides | AR, CN, CL, PE | |
| Chenopodium hircinum | PE | |
| Chenopodium murale | PE | |
| Chenopodium paniculatum | CO | |
| Chenopodium quinoa | ID, PE | |
| Deeringia amarantoides | ID | |
| Gomphrena globasa | CN | |
| Amaryllidaceae | Allium sp. | SK |
| Allium ampeloprasum | CO, ID | |
| Allium cepa | CL, CN, CO, CR, DE, GT, ID, KE, PH, ES, TW, VN | |
| Allium cepa aggregatum | ID, PE, PH | |
| Allium chinensis | CN | |
| Allium fistulosum | CN, CR, ID, IT, TW, VN | |
| Allium porrum | GT | |
| Allium sativum | CL, CN, CO, ID, ES | |
| Allium schoenprasum | VE | |
| Spinacia oleracea | AR, CA, CN, ID, IT, JP, KE, PE, TW, VN | |
| Apiaceae | Apium sp. | AR, LB, ES |
| Apium graveolens | AR, CA, CN, CR, DE, GT, ID, IL, IT, ES, SK | |
| Apium graveolens dulce | CN, PE, PH, VN | |
| Bupleurum sp. | CN | |
| Centella asiatica | CN | |
| Coriandrum sativum | CL, CN, GT, TW | |
| Daucus carota | ID, PE, PH | |
| Daucus sativa | CN, CR | |
| Hydrocotyle umbellata | PE | |
| Impatiens caeruleum | CN | |
| Levisticum officinale | GT | |
| Oenanthe benghalensis | CN | |
| Oenanthe javanica | CN | |
| Petroselinum sp. | CO, JP, ES | |
| Apocynaceae | Catharanthus roseus | CN |
| Araceae | Colocasia esculenta | CN |
| Araliaceae | Hydrocotyle sp. | AR |
| Hydrocotyle ranunculoides | AR | |
| Hydrocotyle umbellata | CN, CO, CR | |
| Asparagaceae | Asparagus officinalis | CL, ID |
| Chionodoxa luciliae | CN | |
| Asphodelaceae | Hemerocallis fulva | CN |
| Asteraceae | Arctium minus | AR |
| Arctium lappa | CL, PH | |
| Argyranthemum sp. | NO | |
| Artemisia annua | CN | |
| Artemisia argyi | CN | |
| Aster sp. | AR, CN | |
| Bellis perennis | AR, CN, VN | |
| Bidens pilosa | AR, CN, CR | |
| Bidens sp. | AR | |
| Calendula sp. | KE, ES, NO | |
| Calendula officinalis | AR, CN, PE | |
| Callistephus chinensis | AR, CN, ES | |
| Carduus crispus | CN | |
| Carduus nutans | AR | |
| Carthamus tinctorius | CN | |
| Centaurea cyanus | CN | |
| Chicorium sp. | GT | |
| Chrysanthemum sp. | AR, CN, CO, ID, NY, NO, PH, PT, SK, VN | |
| Chrysanthemum coronarium | CN, VN | |
| Chrysanthemum leucanthemum | AR | |
| Chrysanthemum morifolium | AR, CN, CO, IT | |
| Chrysanthemum segetum | ID, VN | |
| Cichorium sp. | AR | |
| Cichorium endivia | VE | |
| Cichorium intybus | AR, GT | |
| Cineraria sp. | AR | |
| Cineraria cruenta | CN | |
| Conoclinium coelestinum | CN | |
| Conyza sp. | AR | |
| Conyza bonariensis | AR | |
| Conyza canadensis | CN, ES | |
| Cosmos bipinnatus | CN | |
| Craspedia globosa | CN | |
| Crassocephalum rubens | CN, TW | |
| Crepis pulchra | AR | |
| Cynara sp. | CL, ES | |
| Cynara cardunculus scolymus | CL, CO | |
| Cynara scolymus | KE, PE, ES | |
| Dahlia sp. | ID, MY, NO | |
| Dahlia imperialis | CR, ID | |
| Dahlia pinnata | AR, CN | |
| Dahlia variabilis | AR | |
| Dendranthema mortifolium | CN | |
| Dichrocephala auriculata | CN | |
| Echinops ritro | CN | |
| Eclipta prostrata | CN | |
| Emilia sonchifolia | CN, CO, ID, PH, SK, TW | |
| Erechtites hieracifolia | CN, CR, CL, ID | |
| Erigeron briviscapus | CN | |
| Gaillardia pulchella | CN | |
| Galinsoga sp. | CR | |
| Galinsoga caracasana | CR, CL, VE | |
| Asteracea | Galinsoga ciliata | CN, CO, CR, PE |
| Galinsoga parviflora | CN, CO | |
| Galinsoga urticifolia | GT | |
| Galisonga caracasana | CO | |
| Galisonga ciliata | IT | |
| Gazania sp. | CN | |
| Gerbera sp. | GT, LB, NO, TH, VN | |
| Gerbera jamesonii | CN, ID, IT, MY, PT | |
| Gnaphalium afffine | CN | |
| Gynura crepidioides | CN | |
| Helianthus sp. | AR, CN, NO | |
| Helianthus annuus | AR, CN, PE | |
| Helichrysum sp. | NO | |
| Helichrysum bracteatum | CL | |
| Helipterum roseum | CN | |
| Hemistepta lyrata | CN | |
| Kalimeris indica | CN, KE, ES, TW | |
| Lactuca capitata | CL, CN | |
| Lactuca indica | ID, MY, TW | |
| Lactuca sativa | AR, CA, CL, CN, CO, DE, ID, GT, IL, IT, LB, PE, PT, ES, TW, VN | |
| Lactuca sativa angustata | CN | |
| Lactuca sativa asparagina | CN | |
| Lactuca sativa capitata | PH | |
| Lactuca sativa crispa | CN | |
| Lactuca sativa intybeca | CN, TW | |
| Lactuca sativa romana | CN | |
| Lactuca vulgaris | VE | |
| Osteospermum sp. | NO | |
| Pyrethrum cinerariifolium | CN | |
| Schistocarpha platyphylla | GT | |
| Senecia cruentus | IT, PH, ES | |
| Solidago sp. | CN KE, ES | |
| Sonchus sp. | PE | |
| Sonchus asper | CN, CO, LB | |
| Sonchus brachyotus | CN | |
| Sonchus oleraceus | AR, CN, CO, CR, GT, KE | |
| Synedrella nodiflora | ID, LB | |
| Tagetes sp. | AR, NO | |
| Tagetes erecta | CN, KE, PE | |
| Tagetes patula | CL, CN | |
| Tagetes tenuitolia | AR | |
| Tanacetum parthenium | CN | |
| Taraxacum mongolicum | CN | |
| Taraxacum officinal | AR | |
| Zinnia elegans | AR, CN | |
| Balsaminaceae | Impatiens balsamina | CN |
| Basellaceae | Basella alba | CN, ID, ES |
| Basella rubra | CN | |
| Brassicaceae | Barbarea sp. | CR, ID, TW |
| Brassica sp. | CO | |
| Brassica alboglabra | CA | |
| Brassica campestris | CL, CN, CR, ID | |
| Brassica campestris pekinensis | CN, PE | |
| Brassica campestris rapa | CO, PE | |
| Brassica chinensis | ID, MY | |
| Brassica juncea | CA, CL, CN, ID, PH, SK, VN | |
| Brassica napus | CN | |
| Brassica oleracea | CN, CR KE, ES, SK, ID | |
| Brassicaceae | Brassica oleracea acephala | CN |
| Brassica oleracea botrytis | CN, ID, PE | |
| Brassica oleracea capitata | CN, CO, GT, PE, PH, VN | |
| Brassica oleracea caulorapa | CN | |
| Brassica oleracea geminifera | GT | |
| Brassica oleracea italica | CN, CO, GT, ID, PE, PH, | |
| Brassica oleracea pekinensis | CN, CO | |
| Brassica rapa | AR, CN, ID, PH | |
| Brassica rapa chinensis | CN, ID, MY, TH, VN | |
| Capsella bursa-pastoris | CO, GT, IT, CN | |
| Cardamine hirouta | CN | |
| Diplotaxis muralis | PE | |
| Hirschfeldia sp. | CN, ES | |
| Lebnlaria mariema | CN | |
| Matthiola sp. | CN | |
| Matthiola incana | CN | |
| Nasturtium indicum | ID | |
| Nasturtium officinal | CN, ID, JP, PH | |
| Raphanus sativus | CN, CO, GT, ID, PE, PJ, ES | |
| Rorippa indica | CN, PJ | |
| Rorippa montan | CN | |
| Rorippa palustris | CN | |
| Campanulaceae | Campanula medium | CN |
| Platycodon grandiflorus | CN | |
| Caryophyllaceae | Dianthus sp. | NO, PH, VN |
| Dianthus barbatus | CN | |
| Dianthus caryophyllus | AR, CL, CN, ID | |
| Dianthus chinensis | CN | |
| Dianthus hybridus | CN | |
| Gypsophila elegans | AR, CN | |
| Gypsophila paniculata | CN, CO, ES | |
| Gypsophila sp. | CN, CO, NO | |
| Silene gallica | CO | |
| Stellaria alsine | CN | |
| Stellaria media | CN, PE | |
| Stellaria yunnansis | CN | |
| Vaccaria pyramidata | ID, JP | |
| Convolvulaceae | Calystegia hederacea | CN |
| Calystegia sepium | CN | |
| Ipomoea aquatica | CN | |
| Ipomoea batatas | CN, ID, TW | |
| Cucurbitaceae | Benincasa hispida | CN |
| Citrullus lanatus | CN, KE, ES | |
| Citrullus vulagris | PE | |
| Cucumis melo | AR, BR, CN, ID, ES | |
| Cucumis sativus | AR, BR, CA, CL, CN, CO, DE, ID, IT, JP, LB, PE, ES, NO, TW, TR, VN | |
| Cucurbita sp. | NO, PH, VN | |
| Cucurbita maxima | AR, CN, KE, LB, PE, TW | |
| Cucurbita maxima zapallito | AR | |
| Cucurbita moschata | AR, CN, KE, LB, TW | |
| Cucurbita pepo | CN, EU, KE, PE, PH, SK, VE | |
| Cucurbita pepo ovifera | CN | |
| Lagenaria sp. | CN, TW | |
| Lagenaria siceraria | CN | |
| Luffa acutangula | CN | |
| Cucurbitaceae | Luffa cylindrica | AR, CN, TW |
| Melothria indica | CL, ID | |
| Momordica charantia | CL, CN, JP, KE | |
| Sechium edule | CN, ID, JP, PH, VN | |
| Euphorbiaceae | Euphorbia marginata | CN |
| Ricinus communis | CN | |
| Fabaceae | Cicer arietinum | AR, CL, ES, VN |
| Crotalaria longirostrata | GT | |
| Glycine max | AR, CN, ID, JP, ES | |
| Lablab sp. | CN | |
| Lablab purpureus | CL, CN, KE | |
| Lathyrus latifolius | AR, CN, ES | |
| Lathyrus odoratus | AR, CN, ID | |
| Lupinus mutabilis | PE | |
| Lupinus rassel | CN | |
| Lupinus sp. | CL, JP | |
| Medicago minima | CN, ES | |
| Medicago sativa | AR, CL, JP, PE, ES | |
| Melilotus suaveolens | CN | |
| Phaseolus sp. | JP, PH | |
| Phaseolus coccineus | KE | |
| Phaseolus lunatus | CL, ID, MY | |
| Phaseolus vulagris | AR, BR, CL, CN, CO, CR, ID, IT, JP, KE, LB, MY, MU, PE, ES, TW, TR, VE, VN | |
| Phaseolus vulgaris humilis | CN | |
| Phaseolus vulgaris vulgaris | PE | |
| Pisum sp. | ID, PH | |
| Pisum sativum | AR, CA, CL, GT, ID, JP, KE, LB, MY, MU, PE, ES, TW, TR, VN, CN | |
| Pisum sativum macrocarpenser | CN | |
| Pisum sativum saccharatum | GT, ID | |
| Trifolium repens | CN, CO, VN | |
| Vicia faba | AR, CL, CN, GT, ID, JP, KE, MY, PE, ES, TR, ZW | |
| Vicia sativa | CN | |
| Vigna sinensis | CN, ID, PE, VN | |
| Vigna unguiculata | CN, CO, ID, IE, PE, ES, VN | |
| Gentianaceae | Eustoma sp. | JP, NO |
| Eustoma russellianum | CN, KE | |
| Exacum sp. | NO | |
| Lisianthus sp. | GT | |
| Gesneriaceae | Streptocarpus sp. | NO |
| Hydrangeaceae | Hydrangea macrophylla | CN |
| Iridaceae | Freesia refracta | CN |
| Gladiolus hybridus | CN, MY | |
| Lamiaceae | Leonurus sybiricus | AR |
| Leonurus heterophyllus | CN | |
| Moluccella laevis | CN | |
| Ocimum basilicum | ID, MU, MA, PE | |
| Salvia splendens | CN | |
| Stachys arvensis | PE | |
| Liliaceae | Lilium sp. | CN, ID |
| Lilium davidii | CN | |
| Lilium longiflorum | CN | |
| Linaceae | Linum sp. | AR |
| Malvaceae | Abelmoschus esculentus | KE, PH |
| Alcea sp. | TH | |
| Althaea rosea | CN, PE | |
| Hibiscus trionum | CN | |
| Malva verticillata | CN, ID, JP, PH | |
| Sida sp. | PH | |
| Menispermaceae | Stephania delavayi | CN |
| Moraceae | Humulus scandens | CN |
| Onagroideae | Clarkia amoena | CN |
| Oenothera rosea | CN | |
| Oxalidaceae | Oxalis sp. | AR, CN, JP |
| Oxalis corniculata | CN | |
| Papaveraceae | Papaver sp. | TR |
| Papaver rhoeas | AR, CN, PH, ES | |
| Plantaginaceae | Plantago asiatica | CN |
| Plantago major | CN | |
| Veronica anagallis-aquatica | CN | |
| Plumbaginaceae | Limonium hybrid | CN, PH |
| Limonium latifolium | CN | |
| Limononium tataricum | CN | |
| Myosotis sylvatica | CN | |
| Poaceae | Hordeum vulgare | CN |
| Lagurus ovatus | CN | |
| Setaria viridis | CN, CO, ID, PH | |
| Triticum aestivum | CN | |
| Zea mays | AR, CN, PH | |
| Polemoniaceae | Phlox drummondii | AR, CN |
| Polygonaceae | Polygonum amphibium | CN |
| Polygonum aviculare | CN | |
| Polygonum hydropiper | CN | |
| Polygonum nepalense | CN | |
| Rumex acetosa | CN | |
| Portulacaceae | Portulaca oleracea | CO, PH |
| Primulaceae | Primula sp. | NO |
| Primula acaulis | CN | |
| Primula obconica | AR, CN | |
| Ranunculaceae | Ranunculus asiaticus | AR |
| Ranunculus sceleratus | CL | |
| Delphinium grandiflorus | CN | |
| Delphinium sp. | CN | |
| Nigella damascena | CN | |
| Ranunculus asiaticus | CN | |
| Ranunculus chinensis | CN | |
| Ranunculus sceleratus | CN | |
| Ranunculus sieboldii | CN | |
| Ranunculus viridis | CN | |
| Rosaceae | Rosa sp. | CN, TH |
| Scrophulariaceae | Calceolaria crenatiflora | CN |
| Diascia sp. | NO | |
| Nemesia sp. | NO | |
| Nemesia strumosa | CN | |
| Solanaceae | Capsicum sp. | BR, CR, ID, KE |
| Capsicum annuum | AR, CL, ID, IT, MY, NO, PE, PH, TW | |
| Capsicum baccatum | CO, PE | |
| Capsicum frutescens | CN, PE | |
| Datura sp. | NO, PH, VN | |
| Datura ferox | AR | |
| Datura stramonium | CL, CN, CO, PE, | |
| Lycium chinense | CN | |
| Nicotiana sp. | PE | |
| Solanaceae | Nicotiana glauca | PE |
| Nicotiana tabacum | CL, CN, PE, ES | |
| Petunia hybida | CN | |
| Petunia sp. | AR, CO, JP, NO | |
| Physalis angulata | CO, CR, ID, JP | |
| Solanum sp. | CL, CO | |
| Solanum americanum | ID, IT, PH | |
| Solanum melongena | AR, CL, CN, ID, IT, KE, PE, PH, VN | |
| Solanum melongena oblong | PH | |
| Solanum muricatum | CN, PH | |
| Solanum nigrum | CN | |
| Solanum oleracelus | CO, LB, PH | |
| Solanum lycopersicum | AR, CL, CN, CR, EC, GT, ID, JP, KE, KR, MY, MU, MA, NO, PE, PH PH, PT, ES, NL, TR, VE, VN | |
| Solanum tuberosum | AR, BR, CA, CL, CN, CR, EC, ID, IL, JP, KE, KR, MU, PE, PH, ZA, ES, SK, TR, VE, VN, ZW | |
| Tropaeolaceae | Nasturtium sp. | AR |
| Tropaeolum sp. | CR | |
| Tropaeolum majus | AR, CL, CN | |
| Verbenaceae | Verbena sp. | NO |
| Verbena officinalis | CN | |
| Violaceae | Viola philippica | CN |
| Viola tricolor | AR, CN, PE | |
| Viola yedensis | CN |
| Plant family . | Host plant species . | Countrya . |
|---|---|---|
| Adoxaceae | Sambucus sp. | GT |
| Aizoaceae | Trianthema portulacastrum | PE |
| Alstroemeriaceae | Alstroemeria aurea | CN |
| Alismataceae | Sagittaria sagittifolia | CN |
| Amaranthaceae | Alternanthera philox eroides | CN |
| Amaranthus sp. | AR, CL, CR, GT, ID, KE, MY, TH | |
| Amaranthus caudatus | CN | |
| Amaranthus hybridus | PE | |
| Amaranthus lividus | CN | |
| Amaranthus lividus ascendens | JP | |
| Amaranthus manostanus | CN | |
| Amaranthus retroflexus | CN, CO, ID, KE | |
| Amaranthus viridis | CO, TW, VE | |
| Beta vulgaris | CR, GT, ID, JP, KE, ES, SK, VE, VN, PE | |
| Beta vulgaris cicla | AR, CN, LB, PE, VE | |
| Beta vulgaris rapacea | AR | |
| Beta vulgaris rubra | AR, ID, TW | |
| Beta vulgaris vulgaris | AR | |
| Celosia argentea | CN | |
| Celosia cristata | CN | |
| Chenopodium sp. | AR | |
| Chenopodium album | AR, CL, NC | |
| Chenopodium ambrosioides | AR, CN, CL, PE | |
| Chenopodium hircinum | PE | |
| Chenopodium murale | PE | |
| Chenopodium paniculatum | CO | |
| Chenopodium quinoa | ID, PE | |
| Deeringia amarantoides | ID | |
| Gomphrena globasa | CN | |
| Amaryllidaceae | Allium sp. | SK |
| Allium ampeloprasum | CO, ID | |
| Allium cepa | CL, CN, CO, CR, DE, GT, ID, KE, PH, ES, TW, VN | |
| Allium cepa aggregatum | ID, PE, PH | |
| Allium chinensis | CN | |
| Allium fistulosum | CN, CR, ID, IT, TW, VN | |
| Allium porrum | GT | |
| Allium sativum | CL, CN, CO, ID, ES | |
| Allium schoenprasum | VE | |
| Spinacia oleracea | AR, CA, CN, ID, IT, JP, KE, PE, TW, VN | |
| Apiaceae | Apium sp. | AR, LB, ES |
| Apium graveolens | AR, CA, CN, CR, DE, GT, ID, IL, IT, ES, SK | |
| Apium graveolens dulce | CN, PE, PH, VN | |
| Bupleurum sp. | CN | |
| Centella asiatica | CN | |
| Coriandrum sativum | CL, CN, GT, TW | |
| Daucus carota | ID, PE, PH | |
| Daucus sativa | CN, CR | |
| Hydrocotyle umbellata | PE | |
| Impatiens caeruleum | CN | |
| Levisticum officinale | GT | |
| Oenanthe benghalensis | CN | |
| Oenanthe javanica | CN | |
| Petroselinum sp. | CO, JP, ES | |
| Apocynaceae | Catharanthus roseus | CN |
| Araceae | Colocasia esculenta | CN |
| Araliaceae | Hydrocotyle sp. | AR |
| Hydrocotyle ranunculoides | AR | |
| Hydrocotyle umbellata | CN, CO, CR | |
| Asparagaceae | Asparagus officinalis | CL, ID |
| Chionodoxa luciliae | CN | |
| Asphodelaceae | Hemerocallis fulva | CN |
| Asteraceae | Arctium minus | AR |
| Arctium lappa | CL, PH | |
| Argyranthemum sp. | NO | |
| Artemisia annua | CN | |
| Artemisia argyi | CN | |
| Aster sp. | AR, CN | |
| Bellis perennis | AR, CN, VN | |
| Bidens pilosa | AR, CN, CR | |
| Bidens sp. | AR | |
| Calendula sp. | KE, ES, NO | |
| Calendula officinalis | AR, CN, PE | |
| Callistephus chinensis | AR, CN, ES | |
| Carduus crispus | CN | |
| Carduus nutans | AR | |
| Carthamus tinctorius | CN | |
| Centaurea cyanus | CN | |
| Chicorium sp. | GT | |
| Chrysanthemum sp. | AR, CN, CO, ID, NY, NO, PH, PT, SK, VN | |
| Chrysanthemum coronarium | CN, VN | |
| Chrysanthemum leucanthemum | AR | |
| Chrysanthemum morifolium | AR, CN, CO, IT | |
| Chrysanthemum segetum | ID, VN | |
| Cichorium sp. | AR | |
| Cichorium endivia | VE | |
| Cichorium intybus | AR, GT | |
| Cineraria sp. | AR | |
| Cineraria cruenta | CN | |
| Conoclinium coelestinum | CN | |
| Conyza sp. | AR | |
| Conyza bonariensis | AR | |
| Conyza canadensis | CN, ES | |
| Cosmos bipinnatus | CN | |
| Craspedia globosa | CN | |
| Crassocephalum rubens | CN, TW | |
| Crepis pulchra | AR | |
| Cynara sp. | CL, ES | |
| Cynara cardunculus scolymus | CL, CO | |
| Cynara scolymus | KE, PE, ES | |
| Dahlia sp. | ID, MY, NO | |
| Dahlia imperialis | CR, ID | |
| Dahlia pinnata | AR, CN | |
| Dahlia variabilis | AR | |
| Dendranthema mortifolium | CN | |
| Dichrocephala auriculata | CN | |
| Echinops ritro | CN | |
| Eclipta prostrata | CN | |
| Emilia sonchifolia | CN, CO, ID, PH, SK, TW | |
| Erechtites hieracifolia | CN, CR, CL, ID | |
| Erigeron briviscapus | CN | |
| Gaillardia pulchella | CN | |
| Galinsoga sp. | CR | |
| Galinsoga caracasana | CR, CL, VE | |
| Asteracea | Galinsoga ciliata | CN, CO, CR, PE |
| Galinsoga parviflora | CN, CO | |
| Galinsoga urticifolia | GT | |
| Galisonga caracasana | CO | |
| Galisonga ciliata | IT | |
| Gazania sp. | CN | |
| Gerbera sp. | GT, LB, NO, TH, VN | |
| Gerbera jamesonii | CN, ID, IT, MY, PT | |
| Gnaphalium afffine | CN | |
| Gynura crepidioides | CN | |
| Helianthus sp. | AR, CN, NO | |
| Helianthus annuus | AR, CN, PE | |
| Helichrysum sp. | NO | |
| Helichrysum bracteatum | CL | |
| Helipterum roseum | CN | |
| Hemistepta lyrata | CN | |
| Kalimeris indica | CN, KE, ES, TW | |
| Lactuca capitata | CL, CN | |
| Lactuca indica | ID, MY, TW | |
| Lactuca sativa | AR, CA, CL, CN, CO, DE, ID, GT, IL, IT, LB, PE, PT, ES, TW, VN | |
| Lactuca sativa angustata | CN | |
| Lactuca sativa asparagina | CN | |
| Lactuca sativa capitata | PH | |
| Lactuca sativa crispa | CN | |
| Lactuca sativa intybeca | CN, TW | |
| Lactuca sativa romana | CN | |
| Lactuca vulgaris | VE | |
| Osteospermum sp. | NO | |
| Pyrethrum cinerariifolium | CN | |
| Schistocarpha platyphylla | GT | |
| Senecia cruentus | IT, PH, ES | |
| Solidago sp. | CN KE, ES | |
| Sonchus sp. | PE | |
| Sonchus asper | CN, CO, LB | |
| Sonchus brachyotus | CN | |
| Sonchus oleraceus | AR, CN, CO, CR, GT, KE | |
| Synedrella nodiflora | ID, LB | |
| Tagetes sp. | AR, NO | |
| Tagetes erecta | CN, KE, PE | |
| Tagetes patula | CL, CN | |
| Tagetes tenuitolia | AR | |
| Tanacetum parthenium | CN | |
| Taraxacum mongolicum | CN | |
| Taraxacum officinal | AR | |
| Zinnia elegans | AR, CN | |
| Balsaminaceae | Impatiens balsamina | CN |
| Basellaceae | Basella alba | CN, ID, ES |
| Basella rubra | CN | |
| Brassicaceae | Barbarea sp. | CR, ID, TW |
| Brassica sp. | CO | |
| Brassica alboglabra | CA | |
| Brassica campestris | CL, CN, CR, ID | |
| Brassica campestris pekinensis | CN, PE | |
| Brassica campestris rapa | CO, PE | |
| Brassica chinensis | ID, MY | |
| Brassica juncea | CA, CL, CN, ID, PH, SK, VN | |
| Brassica napus | CN | |
| Brassica oleracea | CN, CR KE, ES, SK, ID | |
| Brassicaceae | Brassica oleracea acephala | CN |
| Brassica oleracea botrytis | CN, ID, PE | |
| Brassica oleracea capitata | CN, CO, GT, PE, PH, VN | |
| Brassica oleracea caulorapa | CN | |
| Brassica oleracea geminifera | GT | |
| Brassica oleracea italica | CN, CO, GT, ID, PE, PH, | |
| Brassica oleracea pekinensis | CN, CO | |
| Brassica rapa | AR, CN, ID, PH | |
| Brassica rapa chinensis | CN, ID, MY, TH, VN | |
| Capsella bursa-pastoris | CO, GT, IT, CN | |
| Cardamine hirouta | CN | |
| Diplotaxis muralis | PE | |
| Hirschfeldia sp. | CN, ES | |
| Lebnlaria mariema | CN | |
| Matthiola sp. | CN | |
| Matthiola incana | CN | |
| Nasturtium indicum | ID | |
| Nasturtium officinal | CN, ID, JP, PH | |
| Raphanus sativus | CN, CO, GT, ID, PE, PJ, ES | |
| Rorippa indica | CN, PJ | |
| Rorippa montan | CN | |
| Rorippa palustris | CN | |
| Campanulaceae | Campanula medium | CN |
| Platycodon grandiflorus | CN | |
| Caryophyllaceae | Dianthus sp. | NO, PH, VN |
| Dianthus barbatus | CN | |
| Dianthus caryophyllus | AR, CL, CN, ID | |
| Dianthus chinensis | CN | |
| Dianthus hybridus | CN | |
| Gypsophila elegans | AR, CN | |
| Gypsophila paniculata | CN, CO, ES | |
| Gypsophila sp. | CN, CO, NO | |
| Silene gallica | CO | |
| Stellaria alsine | CN | |
| Stellaria media | CN, PE | |
| Stellaria yunnansis | CN | |
| Vaccaria pyramidata | ID, JP | |
| Convolvulaceae | Calystegia hederacea | CN |
| Calystegia sepium | CN | |
| Ipomoea aquatica | CN | |
| Ipomoea batatas | CN, ID, TW | |
| Cucurbitaceae | Benincasa hispida | CN |
| Citrullus lanatus | CN, KE, ES | |
| Citrullus vulagris | PE | |
| Cucumis melo | AR, BR, CN, ID, ES | |
| Cucumis sativus | AR, BR, CA, CL, CN, CO, DE, ID, IT, JP, LB, PE, ES, NO, TW, TR, VN | |
| Cucurbita sp. | NO, PH, VN | |
| Cucurbita maxima | AR, CN, KE, LB, PE, TW | |
| Cucurbita maxima zapallito | AR | |
| Cucurbita moschata | AR, CN, KE, LB, TW | |
| Cucurbita pepo | CN, EU, KE, PE, PH, SK, VE | |
| Cucurbita pepo ovifera | CN | |
| Lagenaria sp. | CN, TW | |
| Lagenaria siceraria | CN | |
| Luffa acutangula | CN | |
| Cucurbitaceae | Luffa cylindrica | AR, CN, TW |
| Melothria indica | CL, ID | |
| Momordica charantia | CL, CN, JP, KE | |
| Sechium edule | CN, ID, JP, PH, VN | |
| Euphorbiaceae | Euphorbia marginata | CN |
| Ricinus communis | CN | |
| Fabaceae | Cicer arietinum | AR, CL, ES, VN |
| Crotalaria longirostrata | GT | |
| Glycine max | AR, CN, ID, JP, ES | |
| Lablab sp. | CN | |
| Lablab purpureus | CL, CN, KE | |
| Lathyrus latifolius | AR, CN, ES | |
| Lathyrus odoratus | AR, CN, ID | |
| Lupinus mutabilis | PE | |
| Lupinus rassel | CN | |
| Lupinus sp. | CL, JP | |
| Medicago minima | CN, ES | |
| Medicago sativa | AR, CL, JP, PE, ES | |
| Melilotus suaveolens | CN | |
| Phaseolus sp. | JP, PH | |
| Phaseolus coccineus | KE | |
| Phaseolus lunatus | CL, ID, MY | |
| Phaseolus vulagris | AR, BR, CL, CN, CO, CR, ID, IT, JP, KE, LB, MY, MU, PE, ES, TW, TR, VE, VN | |
| Phaseolus vulgaris humilis | CN | |
| Phaseolus vulgaris vulgaris | PE | |
| Pisum sp. | ID, PH | |
| Pisum sativum | AR, CA, CL, GT, ID, JP, KE, LB, MY, MU, PE, ES, TW, TR, VN, CN | |
| Pisum sativum macrocarpenser | CN | |
| Pisum sativum saccharatum | GT, ID | |
| Trifolium repens | CN, CO, VN | |
| Vicia faba | AR, CL, CN, GT, ID, JP, KE, MY, PE, ES, TR, ZW | |
| Vicia sativa | CN | |
| Vigna sinensis | CN, ID, PE, VN | |
| Vigna unguiculata | CN, CO, ID, IE, PE, ES, VN | |
| Gentianaceae | Eustoma sp. | JP, NO |
| Eustoma russellianum | CN, KE | |
| Exacum sp. | NO | |
| Lisianthus sp. | GT | |
| Gesneriaceae | Streptocarpus sp. | NO |
| Hydrangeaceae | Hydrangea macrophylla | CN |
| Iridaceae | Freesia refracta | CN |
| Gladiolus hybridus | CN, MY | |
| Lamiaceae | Leonurus sybiricus | AR |
| Leonurus heterophyllus | CN | |
| Moluccella laevis | CN | |
| Ocimum basilicum | ID, MU, MA, PE | |
| Salvia splendens | CN | |
| Stachys arvensis | PE | |
| Liliaceae | Lilium sp. | CN, ID |
| Lilium davidii | CN | |
| Lilium longiflorum | CN | |
| Linaceae | Linum sp. | AR |
| Malvaceae | Abelmoschus esculentus | KE, PH |
| Alcea sp. | TH | |
| Althaea rosea | CN, PE | |
| Hibiscus trionum | CN | |
| Malva verticillata | CN, ID, JP, PH | |
| Sida sp. | PH | |
| Menispermaceae | Stephania delavayi | CN |
| Moraceae | Humulus scandens | CN |
| Onagroideae | Clarkia amoena | CN |
| Oenothera rosea | CN | |
| Oxalidaceae | Oxalis sp. | AR, CN, JP |
| Oxalis corniculata | CN | |
| Papaveraceae | Papaver sp. | TR |
| Papaver rhoeas | AR, CN, PH, ES | |
| Plantaginaceae | Plantago asiatica | CN |
| Plantago major | CN | |
| Veronica anagallis-aquatica | CN | |
| Plumbaginaceae | Limonium hybrid | CN, PH |
| Limonium latifolium | CN | |
| Limononium tataricum | CN | |
| Myosotis sylvatica | CN | |
| Poaceae | Hordeum vulgare | CN |
| Lagurus ovatus | CN | |
| Setaria viridis | CN, CO, ID, PH | |
| Triticum aestivum | CN | |
| Zea mays | AR, CN, PH | |
| Polemoniaceae | Phlox drummondii | AR, CN |
| Polygonaceae | Polygonum amphibium | CN |
| Polygonum aviculare | CN | |
| Polygonum hydropiper | CN | |
| Polygonum nepalense | CN | |
| Rumex acetosa | CN | |
| Portulacaceae | Portulaca oleracea | CO, PH |
| Primulaceae | Primula sp. | NO |
| Primula acaulis | CN | |
| Primula obconica | AR, CN | |
| Ranunculaceae | Ranunculus asiaticus | AR |
| Ranunculus sceleratus | CL | |
| Delphinium grandiflorus | CN | |
| Delphinium sp. | CN | |
| Nigella damascena | CN | |
| Ranunculus asiaticus | CN | |
| Ranunculus chinensis | CN | |
| Ranunculus sceleratus | CN | |
| Ranunculus sieboldii | CN | |
| Ranunculus viridis | CN | |
| Rosaceae | Rosa sp. | CN, TH |
| Scrophulariaceae | Calceolaria crenatiflora | CN |
| Diascia sp. | NO | |
| Nemesia sp. | NO | |
| Nemesia strumosa | CN | |
| Solanaceae | Capsicum sp. | BR, CR, ID, KE |
| Capsicum annuum | AR, CL, ID, IT, MY, NO, PE, PH, TW | |
| Capsicum baccatum | CO, PE | |
| Capsicum frutescens | CN, PE | |
| Datura sp. | NO, PH, VN | |
| Datura ferox | AR | |
| Datura stramonium | CL, CN, CO, PE, | |
| Lycium chinense | CN | |
| Nicotiana sp. | PE | |
| Solanaceae | Nicotiana glauca | PE |
| Nicotiana tabacum | CL, CN, PE, ES | |
| Petunia hybida | CN | |
| Petunia sp. | AR, CO, JP, NO | |
| Physalis angulata | CO, CR, ID, JP | |
| Solanum sp. | CL, CO | |
| Solanum americanum | ID, IT, PH | |
| Solanum melongena | AR, CL, CN, ID, IT, KE, PE, PH, VN | |
| Solanum melongena oblong | PH | |
| Solanum muricatum | CN, PH | |
| Solanum nigrum | CN | |
| Solanum oleracelus | CO, LB, PH | |
| Solanum lycopersicum | AR, CL, CN, CR, EC, GT, ID, JP, KE, KR, MY, MU, MA, NO, PE, PH PH, PT, ES, NL, TR, VE, VN | |
| Solanum tuberosum | AR, BR, CA, CL, CN, CR, EC, ID, IL, JP, KE, KR, MU, PE, PH, ZA, ES, SK, TR, VE, VN, ZW | |
| Tropaeolaceae | Nasturtium sp. | AR |
| Tropaeolum sp. | CR | |
| Tropaeolum majus | AR, CL, CN | |
| Verbenaceae | Verbena sp. | NO |
| Verbena officinalis | CN | |
| Violaceae | Viola philippica | CN |
| Viola tricolor | AR, CN, PE | |
| Viola yedensis | CN |
AR, Argentina; BR, Brazil; CA, Canada; CL, Chile; CN, China; CO, Columbia; CR, Costa Rica; EC, Ecuador; DE, Germany; GT, Guatemala; ID, Indonesia; IL, Israel; IT, Italy; JP, Japan; KE, Kenya; KR, Korea; LB, Lebanon; MY, Malaysia; MU, Mauritius; MA, Morocco; NL, The Netherlands; NO, Norway; PE, Peru; PH, Philippines; PT, Portugal; ZA, South Africa; ES, Spain; LK, Sri Lanka; TW, Taiwan; TH, Thailand; TR, Turkey; VN, Vietnam; ZW, Zimbabwe.
Within the wide global host range of L. huidobrensis, local populations show strong preferences for particular plant species. In horticultural crops from central Argentina, the host plant ranking observed in the field (Valladares et al. 1996) was supported by female preferences in laboratory experiments (Videla et al. 2006), with Vicia faba and Beta vulgaris cicla L. as the preferred hosts. Moreover, Videla et al. (2012) showed that preferences of the female leafminers were strongly correlated with offspring fitness. Work on another agromyzid, L. brassicae, demonstrated that females tend to oviposit in the host that the previous generation developed on (Tavormina 1982). Conversely, no correlation between oviposition preference and larval performance was found in a study covering a different set of hosts and using a different population of the leafminer (Martin et al. 2005c). Preference for particular cultivars within a host species has also been shown by L. huidobrensis populations from southeastern Buenos Aires, where females consistently preferred certain potato cultivars over others for both feeding as well as oviposition (López et al. 2010). However, intraspecific host ranking was weaker in the laboratory, suggesting that external factors were mediating the preferences observed in the field (López et al. 2016).
The mechanisms for host preference of the leafminer were investigated in China; host plant selectivity was found to be related to many physical and nutritional factors. Selection experiments with 21 different cultivars of tomatoes (S. lycopersicum Mill.) showed that the host selectivity is negatively correlated with the quantity of leaf trichomes, while positively correlated with the content of soluble sugars in potato (S. tuberosum) leaves (Gao et al. 2006). Experiments with 11 cultivars of eggplant (S. melongena L.) also showed that host selectivity was related to the quantity of leaf trichomes, but not with protein and soluble sugars (Han et al. 2005). Host plant preference for 27 different cultivars of common bean (Phaseolus vulgaris L.) was examined (Yan et al. 2008). Chemical component analysis showed that host preference was negatively correlated with tannic acid and flavone concentrations, but was not correlated with concentrations of chlorophyll, soluble protein or soluble sugar. On an average, the most favorable/frequently attacked host plants in China are P. vulgaris, Spinacia oleracea L., L. sativa, A. graveolens, Cucumis sativus L., Gypsophila paniculata L., and S. tuberosum (Dai et al. 2001), however, there may be local preferences. In Yunan province, the leafminer’s most preferred host plants are V. faba, Beta vulgaris L., S. oleracea, A. graveolens, and L. sativa (He et al. 2001).
In agroecological zones along the Peruvian coast, the highest larval infestation intensity (percent foliar damage) was observed in crops of the families Fabaceae (45–67%), Cucurbitaceae (50%), and Solanaceae (20%) during the winter vegetation period (Mujica and Kroschel 2011). Similarly, faba bean was more attractive for L. huidobrensis than potato both under lowland and highland conditions (Mujica 2016a). However, at high altitudes, larval infestation was substantially higher in potato (99%) than in faba bean (42%). Healthy, vigorously growing potato plants are able to counteract the damaging effect of leafminers, particularly during the vegetative phase, as long as they come from high quality, pathogen-free seed potatoes and are not deficient in irrigation or fertilizer. One very unusual aspect of young potato plants is that they have an induced resistance mechanism of extruding leafminer eggs (Gonzales 1994, Mujica and Cisneros 1997, Videla and Valladares 2007). In this mechanism, cells surrounding the eggs multiply at a higher rate than normal and literally cause the egg to be pushed out of the leaf, above the cuticle layer, thus increasing risk of mortality from predation and desiccation. Researchers found that all leaves of young potato plants (leaves still expanding) extruded eggs at rates ranging from 70 to 90% and 60 to 100% of these eggs died (Mujica and Cisneros 1997, Videla and Valladares 2007).
In South Africa, Muller and Krüger (2008) demonstrated that leafminers appear to attack a field randomly, not moving from the border rows inward. Additionally, these researchers showed that yellow trap catches were 5–9 times fewer than actual field landings, as observed by foliage green bucket traps. However, a pattern of leafminer damage advancing inward from the field edge (Carmona et al. 2003), as well as vertical stratification of the damage have been observed in potato crops in Argentina and Peru, with L. huidobrensis females placing a larger number of eggs on leaves of the basal layer compared with the middle and upper layers (Facknath 2005, López et al. 2010, 2016). Seasonal variation of leafminer adult population showed a relatively slow increase during the vegetative growth and a rapid and sustained augmentation during flowering and formation of berries, followed by a decline as plants entered into plant yellowing/early maturity and senescence (Mujica 2016a). In contrast, in South Africa, it was observed that leafminer ‘attacks’ usually escalates immediately after the onset of senescence, giving an appearance of sudden and dramatic ‘invasions’ at this time (Visser 2009).
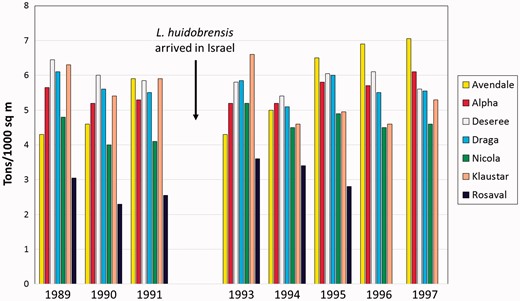
Global spread of L. huidobrensis
Native to South America, L. huidobrensis is now present on five continents and in more than 40 countries; Australia and Antarctica are the only continents yet to be colonized. The leafminer invaded first Europe, the Middle East simultaneously with East and Southeast Asia, then Africa and finally northern North America. What caused this sudden explosion six decades after it was first described is not simple to elucidate. Until the energy crisis of 1973 (World Bank 2009) Europe was self-sufficient in its supply of locally produced flowers. However, increasing costs of operating temperature-controlled greenhouses in the winter put pressure on the European flower growers. The Colombian flower industry started in the 1960s and due to fertile land and low labor costs, blossomed from the early 1970s with sharply increased exports from 1987 (Arbelaez et al. 2007). Rapid transportation was the key factor in exporting flowers; however, at that time flowers were shipped as cargo on passenger aircraft. A number of new air cargo airlines were formed in Colombia to take advantage of the opening market to Europe and the United States (http://www.airlinehistory.co.uk/Americas/Colombia/Airlines.asp).
FloraHolland flower auction in The Netherlands is the largest flower distribution market in the world. In the 1980s, it expanded into eastern Asia, thus when infested flowers arrived in Western Europe they were efficiently transported around the world. At the same time, horticultural products to the USA and Europe also saw a distinct surge due to regional development programs in Central America such as USAID’s PROEXAG, which provided technical support for what became known as ‘non-traditional agricultural exports’, or NTAE’s (Hamilton and Fischer 2003). The main pathways of movement into Europe, and beyond, were reviewed by Baker et al. (2012) but given that the leafminer is now established in several countries, the analysis also includes movement within and between countries. The three primary pathways of movement identified by the analysis were (1) plants intended for planting and any propagating material (but not seeds) of known host plants of the leafminer; (2) cut flowers and branches; and (3) plants and plant products of herbaceous species for consumption.
With regard to Europe, Pathway 1 was the least common method of movement; on the basis of data from Europhyt, from1994 to 2012, this pathway formed only 7% of total interceptions including products originating from other European Union (E.U.) states. Pathway 2 was the most common, forming 51% of total interceptions and pathway 3, the second most important, forming 40% of total interceptions. Baker et al. (2012) do stress that interpretation of these analyses need to factor in: interceptions at borders are often not differentiated at the species level (sometimes interceptions are recorded as ‘Liriomyza sp.’ or ‘Liriomyza’); not all plants are inspected; not all interceptions are reported; eggs and pupae can easily be overlooked: and detailed requirements for inspection are missing. Other pathways of movement were also considered; including natural spread, import of living Liriomyza species for research, imports of non-host commodities and packaging material, baggage and machinery; none of these pathways are considered to be significant.
Management
Chemical
Experience has shown that population of adult L. huidobrensis rapidly develop resistance to the particular conventional insecticides used in different countries. Thus, on a global basis, not all populations of the leafminer have the same resistance profile. Furthermore, larval populations have different susceptibilities to insecticides as compared with adults; in particular, larval populations are protected from contact insecticides in the leaf (MacDonald 1991, van der Staay 1992). In the early 1990s, the only effective larvicides known were abamectin and cyromazine (van der Staay 1992), so studies with the botanical insecticide extracted from the neem tree, Azadirachta indica A. Juss. (Meliaceae) were initiated in Israel and shown to be highly effective (Weintraub and Horowitz 1997). Spinosad has also provided effective control against larvae (Weintraub and Mujica 2006). Studies in Argentina have shown interesting oviposition and feeding deterrent activity using extracts of another member of the Meliaceae, i.e., Melia azedarach L., as well as translaminar effects increasing pupal mortality without negative effects on parasitism (Banchio et al. 2003). To date, only translaminar and/or systemic larvicides can be used to manage the pest at this stage (Reitz et al. 2013).
Despite large local parasitoid assemblages, control of L. huidobrensis in many countries is mainly dependent on conventional chemical insecticides, which exacerbate the populations of leafminers by killing the parasitoids that could afford natural control. For all affected areas in Costa Rica, it was believed that the spread of L. huidobrensis was due to the farmers’ abuse of chemical control and the polyphagous characteristics of L. huidobrensis. In an attempt to control the pest, farmers increased the dosage and the frequency of application of broad-spectrum insecticides; this had an indirect effect on natural enemies of L. huidobrensis, so that they could no longer manage to keep the leafminer population under control (Rodriguez et al. 1989). These researchers performed a survey of the farmers and found that they began to use higher volumes of insecticide after they observed high leafminer infestations in the crops. Different insecticides were mixed in ignorance of the toxicological group to which they belonged. Therefore, they often mixed products that had the same mode of action. Other farmers used mixtures of gasoline, oils, plant extracts, and a diversity of soaps (again without knowing the effectiveness rate of these mixtures), in an attempt to control the pest. As a result, incidence of human toxicity from treated plants was found. A similar situation was observed in Indonesia; results from farmer surveys revealed that over 60% of the farmers applied insecticides twice per week in an attempt to control leafminers in potato, although 72% of responding farmers said that control by insecticides were not effective or economical (Rauf et al. 2000).
Beginning in the 1980s, farmers in the central coast of Peru commonly used 8–13 calendar applications during the potato cropping season causing secondary infestations of the mite, Polyphagotarsonemus latus (Banks), and the bud midge, Prodiplosis longifila Gagne. Insecticide applications became the highest production costs with an average of US$600/ha (Ewell et al. 1990). However, without insecticidal control, potato yields were commonly reduced by more than 50% (Mujica and Cisneros 1997). Currently, L. huidobrensis is still the most damaging pest of potato and of numerous horticultural crops and ornamental plants in the valleys of the Peruvian coast (Mujica and Kroschel 2011, Mujica 2016b).
Guatemalan snow pea growers turned primarily to insecticides to manage arthropod pests, which predictably led to residue violations and regulatory restrictions on snow peas, imported from Guatemala into the United States (Hoppin 1991, Wingert 2010). Excessive use of chemicals came about primarily in response to thrips (Frankliniella spp. and Thrips tabaci Lindeman, Smith et al. 2013) and leafminers, then only known as Liriomyza spp.
Control of Liriomyza species in Kenya has mainly been made by the application of synthetic insecticides leading to the reduction of parasitism by indigenous parasitoids in vegetable fields and supporting the build-up of resistance in the pest (Guantai et al. 2015).
Because of the resistance problems and the depauperate number of effective larvicides, reliance on other measures figures greatly in leafminer management. These techniques include measures to prevent the movement of the leafminer (border interceptions, quarantine measures), local eradication, cultural methods, biological control, and other integrated pest management (IPM) strategies.
Movement restrictions
Under the European Plant Protection Organization (EPPO), phytosanitary measures are recommended to prevent further introductions of L. huidobrensis (Baker et al. 2012). Suppliers of propagation material (other than seeds) from countries outside of the E.U., where the pest occurs, are required to make monthly inspections of plant material for 3 mo prior to shipment; this covers herbaceous plants. Cut flowers should be maintained after lifting to allow all eggs to hatch followed by cold storage to kill larvae. Cut flowers and leafy vegetables should also be accompanied by a phytosanitary certificate, before shipment. However, an analysis by Baker et al. (2012) showed that not all potential host plants are covered in the regulations and thus ‘loopholes’ exist whereby the leafminer could be accidentally introduced; in particular, the regulations do not cover cut branches with foliage not intended for planting and leafy vegetables other than celery. The International Plant Protection Convention (IPPC) produced a diagnostic guide to Liriomyza species of quarantine significance under the International Standards for Phytosanitary Measures No. 27: Diagnostic protocols for regulated pests (IPPC 2006).
Liriomyza huidobrensis is the only commonly present pest in key horticultural exports to the USA (snow peas, green onions and chrysanthemums) from Guatemala and Colombia. There were 9,235 port interceptions by USDA PPQ of Liriomyza spp. into Miami from these countries between 1984 and 2006 (Borchert 2006). In the absence of information on the species of leafminers present in Guatemala, automatic detention by USDA PPQ for produce with any presence of leafminers was in effect prior to 1996. Starting in 1997, based on the findings that only L. huidobrensis was found in exported snow peas and green onions from Guatemala (MacVean and Pérez 1996, 1997), the policy was revised. Shipments bound for markets in the USA outside of Florida were allowed to move through Miami starting in 1997, although Florida maintained its quarantine restrictions for cargo destined for in-state markets. Milla and Reitz (2005) estimated that seasonal populations could establish in Florida and other parts of the USA as a result of introductions.
Cultural methods
Often small farmers do not perform any sampling to track leafminer populations. For that reason, research was performed in Costa Rica (Rodriguez and Villarreal 1989, Rodriguez et al. 1989, 1991) to evaluate types and colors of traps as well as different types of glues, with the aim of making a statistically guided decision about the most efficacious trap materials and further with the goal of standardizing the use of these traps in horticultural production. The results indicated that the largest captures of adult flies were obtained using bright yellow plastic screens, 40 × 30 cm, coated with sticky adhesives (such as Sticken®, or related products) to trap adult flies. However, these researchers also found that the use of empty bright yellow one gallon Penzoil® motor oil containers impregnated with transparent car grease was more feasible and more affordable for farmers, as these containers were discarded by local gas stations after changing engine oil.
Mass trapping Liriomyza huidobrensis in Peru. (A) Open fields with stationary traps. (B) Two farmers at dawn walking rows with oiled plastic sheet. (C) Thousands of adults caught and removed from the field. A similar device with sticky adhesive on the inside of an inverted ‘V’ frame is used in Guatemala.
Biological control
Parasitoids: In its native range, L. huidobrensis has high levels of parasitism, frequently exceeding 50% (Salvo et al. 2005). In the regions of the world where the leafminer has invaded, there are also large numbers of parasitoids attacking the larvae. Worldwide a total of at least 106 species within the families Braconidae (5 genera), Diapriidae (1 species), Eulophidae (16 genera), Pteromalidae (8 genera), Tetracampidae (2 genera), and Figitidae (8 genera) have been recorded (Table 2 and see Suppl Table 2 [online only] for complete listing and references). Names of all chalcids have been validated with the London Natural History Museum’s, Universal Chalcidoidea Database http://www.nhm.ac.uk/our-science/data/chalcidoids/.
Liriomyza huidobrensis parasitoids by superfamily, family, genus and species from countries world-wide
| Superfamily . | Family . | Parasitoid . | Countrya . |
|---|---|---|---|
| Ichneumonidea | Braconidae | Bracon intercessor | TR |
| Dacnusa sp. | CA | ||
| Dacnusa sasakawai | JP | ||
| Dacnusa sibirica | IT, PT, NL, RE | ||
| Oenanogastra sp. | CR | ||
| Opius sp. | CR, CA, ID, MY, CN, BR, CR, | ||
| CO, JP, JO, PH, IL, PE | |||
| Opius caricivorae | TW | ||
| Opius chromatomyiae | ID | ||
| Opius dimidiatus | CN, GT | ||
| Opius dissitus | CN, GT, EU, | ||
| Opius mandibularis | GT | ||
| Opius meracus | TR | ||
| Opius pallipes | NL | ||
| Opius scabriventris | AR, PE | ||
| Phaedrotoma sp. | AR | ||
| Phaedrotoma luteoclypealis | AR | ||
| Phaedrotoma mesoclypealis | AR | ||
| Phaedrotoma scabriventris | AR | ||
| Proctotrupoidea | Diapriidae | Trichopria sp. | PT |
| Chalcidoidea | Eulophidae | Asecodes sp. | ID |
| Asecodes delucchii | ID, PH | ||
| Chrysocharis sp. | AR, CR, PE, ID, PT, | ||
| Chrysocharis ainsliei | PE | ||
| Chrysocharis bedius | BR, RE | ||
| Chrysocharis brethesi | PE | ||
| Chrysocharis c.f. aluta | GT | ||
| Chrysocharis caribea | AR, PE | ||
| Chrysocharis flacilla | AR, PE, CL | ||
| Chrysocharis ignota | GT | ||
| Chrysocharis orbicularis | JO | ||
| Chrysocharis oscinidis | CA | ||
| Chrysocharis pentheus | CN, MY, JP, TW, MY, IL | ||
| Chrysocharis pubicornis | CN, JP, JO | ||
| Chrysocharis tristis | GT | ||
| Chrysocharis vonones | AR | ||
| Chrysonotomyia sp. | PE, AR | ||
| Cirrospilus ambiguus | ID | ||
| Cirrospilus vittatus | JO | ||
| Closterocerus sp. | BR, ID, PE | ||
| Closterocerus cinctipennis | PE | ||
| Closterocerus okazakii | TW | ||
| Closterocerus pulcher | GT | ||
| Diaulinopsis sp. | AR, PE | ||
| Diaulinopsis arenaria | JO | ||
| Diaulinopsis callichroma | PE | ||
| Diglyphus sp. | AR, CR, PE, CO, ZA | ||
| Diglyphus albiscapus | JP | ||
| Diglyphus begini | AR, PE, CO, CN | ||
| Diglyphus crassinervis | CN, TR, PT, JO, IL | ||
| Diglyphus intermedius | GT, CR, CN, CO | ||
| Diglyphus isaea | GT, IL, CN, IT, NL, TR, PT, JP, | ||
| JO, PH, LB, CR, | |||
| Diglyphus minoeus | PT, TR | ||
| Diglyphus pachyneurus | CN | ||
| Diglyphus pedicellus | AR | ||
| Diglyphus pedicellus | AR | ||
| Diglyphus poppoea | PT | ||
| Diglyphus pulchripes | CN | ||
| Diglyphus sp. (near intermedius) | CR | ||
| Diglyphus websteri | PE, AR, GT, | ||
| Hemiptarsenus sp. | JO | ||
| Hemiptarsenus fulvicollis | PT | ||
| Chalcidoidea | Eulophidae | Hemiptarsenus ornatus | JO |
| Hemiptarsenus unguicellus | CN | ||
| Hemiptarsenus varicornis | CN, ID, MY, SK, PH | ||
| Hemiptarsenus zilahisebessi | CN, IL, JO | ||
| Heteroschema sp. | PE | ||
| Neochrysocharis sp. | ID | ||
| Neochrysocharis beasleyi | ID, VN | ||
| Neochrysocharis diastatae | GT | ||
| Neochrysocharis formosa | CN, MY, TR, JO, IL, ID | ||
| Neochrysocharis okazakii | JP, PH | ||
| Pnigalio sp. | ID | ||
| Pediobius metallicus | CN, IL, JO | ||
| Pnigalio incompletus | JO | ||
| Pnigalio katonis | CN, PH | ||
| Pnigalio soemius | IL | ||
| Proacrias sp. | CL | ||
| Proacrias thysanoides | AR, PE | ||
| Proacrias xenodice | AR, CL | ||
| Quadrastichus sp. | GT, ID, IL | ||
| Quadrastichus liriomyzae | PH | ||
| Zagrammosoma sp. | ID, PE, PH | ||
| Zagrammosoma latilineatum | ID | ||
| Zagrammosoma multileneatum | PE | ||
| Pteromalidae | ≠ Halticoptera | PT | |
| Halticoptera sp. | AR, CO, CL, GT, PE | ||
| Halticoptera arduine | PE, AR, CL, PE | ||
| Halticoptera circulus | CA, CN, GT, JO | ||
| Halticoptera helioponi | AR | ||
| Halticoptera patellana | PE | ||
| Halticoptera peviana | AR | ||
| Notoglyptus tzeltales | GT | ||
| Pteromalidae sp. | AR, IL | ||
| Sphegigaster sp. | ID | ||
| Thinodytes cyzicus | CN | ||
| Thinodytes sp. | AR | ||
| Trichomalopsis sp. | CN | ||
| Tetracampidae | Epiclerus sp. | ES | |
| Platynocheilus cuprifrons | IL | ||
| Cynipoidea | Figitidae | Agrostocynips clavatus | AR |
| Alloxysta sp. | CL | ||
| Disorygma pacifica | GT | ||
| Ganaspidium sp. | PE | ||
| Gronotoma sp. | GT, JO | ||
| Gronotoma adachiae | CN | ||
| Gronotoma micromorpha | ID | ||
| Moneucoela sp. | GT | ||
| Tribliographa sp. | CO | ||
| Zaeucolia sp. | GT |
| Superfamily . | Family . | Parasitoid . | Countrya . |
|---|---|---|---|
| Ichneumonidea | Braconidae | Bracon intercessor | TR |
| Dacnusa sp. | CA | ||
| Dacnusa sasakawai | JP | ||
| Dacnusa sibirica | IT, PT, NL, RE | ||
| Oenanogastra sp. | CR | ||
| Opius sp. | CR, CA, ID, MY, CN, BR, CR, | ||
| CO, JP, JO, PH, IL, PE | |||
| Opius caricivorae | TW | ||
| Opius chromatomyiae | ID | ||
| Opius dimidiatus | CN, GT | ||
| Opius dissitus | CN, GT, EU, | ||
| Opius mandibularis | GT | ||
| Opius meracus | TR | ||
| Opius pallipes | NL | ||
| Opius scabriventris | AR, PE | ||
| Phaedrotoma sp. | AR | ||
| Phaedrotoma luteoclypealis | AR | ||
| Phaedrotoma mesoclypealis | AR | ||
| Phaedrotoma scabriventris | AR | ||
| Proctotrupoidea | Diapriidae | Trichopria sp. | PT |
| Chalcidoidea | Eulophidae | Asecodes sp. | ID |
| Asecodes delucchii | ID, PH | ||
| Chrysocharis sp. | AR, CR, PE, ID, PT, | ||
| Chrysocharis ainsliei | PE | ||
| Chrysocharis bedius | BR, RE | ||
| Chrysocharis brethesi | PE | ||
| Chrysocharis c.f. aluta | GT | ||
| Chrysocharis caribea | AR, PE | ||
| Chrysocharis flacilla | AR, PE, CL | ||
| Chrysocharis ignota | GT | ||
| Chrysocharis orbicularis | JO | ||
| Chrysocharis oscinidis | CA | ||
| Chrysocharis pentheus | CN, MY, JP, TW, MY, IL | ||
| Chrysocharis pubicornis | CN, JP, JO | ||
| Chrysocharis tristis | GT | ||
| Chrysocharis vonones | AR | ||
| Chrysonotomyia sp. | PE, AR | ||
| Cirrospilus ambiguus | ID | ||
| Cirrospilus vittatus | JO | ||
| Closterocerus sp. | BR, ID, PE | ||
| Closterocerus cinctipennis | PE | ||
| Closterocerus okazakii | TW | ||
| Closterocerus pulcher | GT | ||
| Diaulinopsis sp. | AR, PE | ||
| Diaulinopsis arenaria | JO | ||
| Diaulinopsis callichroma | PE | ||
| Diglyphus sp. | AR, CR, PE, CO, ZA | ||
| Diglyphus albiscapus | JP | ||
| Diglyphus begini | AR, PE, CO, CN | ||
| Diglyphus crassinervis | CN, TR, PT, JO, IL | ||
| Diglyphus intermedius | GT, CR, CN, CO | ||
| Diglyphus isaea | GT, IL, CN, IT, NL, TR, PT, JP, | ||
| JO, PH, LB, CR, | |||
| Diglyphus minoeus | PT, TR | ||
| Diglyphus pachyneurus | CN | ||
| Diglyphus pedicellus | AR | ||
| Diglyphus pedicellus | AR | ||
| Diglyphus poppoea | PT | ||
| Diglyphus pulchripes | CN | ||
| Diglyphus sp. (near intermedius) | CR | ||
| Diglyphus websteri | PE, AR, GT, | ||
| Hemiptarsenus sp. | JO | ||
| Hemiptarsenus fulvicollis | PT | ||
| Chalcidoidea | Eulophidae | Hemiptarsenus ornatus | JO |
| Hemiptarsenus unguicellus | CN | ||
| Hemiptarsenus varicornis | CN, ID, MY, SK, PH | ||
| Hemiptarsenus zilahisebessi | CN, IL, JO | ||
| Heteroschema sp. | PE | ||
| Neochrysocharis sp. | ID | ||
| Neochrysocharis beasleyi | ID, VN | ||
| Neochrysocharis diastatae | GT | ||
| Neochrysocharis formosa | CN, MY, TR, JO, IL, ID | ||
| Neochrysocharis okazakii | JP, PH | ||
| Pnigalio sp. | ID | ||
| Pediobius metallicus | CN, IL, JO | ||
| Pnigalio incompletus | JO | ||
| Pnigalio katonis | CN, PH | ||
| Pnigalio soemius | IL | ||
| Proacrias sp. | CL | ||
| Proacrias thysanoides | AR, PE | ||
| Proacrias xenodice | AR, CL | ||
| Quadrastichus sp. | GT, ID, IL | ||
| Quadrastichus liriomyzae | PH | ||
| Zagrammosoma sp. | ID, PE, PH | ||
| Zagrammosoma latilineatum | ID | ||
| Zagrammosoma multileneatum | PE | ||
| Pteromalidae | ≠ Halticoptera | PT | |
| Halticoptera sp. | AR, CO, CL, GT, PE | ||
| Halticoptera arduine | PE, AR, CL, PE | ||
| Halticoptera circulus | CA, CN, GT, JO | ||
| Halticoptera helioponi | AR | ||
| Halticoptera patellana | PE | ||
| Halticoptera peviana | AR | ||
| Notoglyptus tzeltales | GT | ||
| Pteromalidae sp. | AR, IL | ||
| Sphegigaster sp. | ID | ||
| Thinodytes cyzicus | CN | ||
| Thinodytes sp. | AR | ||
| Trichomalopsis sp. | CN | ||
| Tetracampidae | Epiclerus sp. | ES | |
| Platynocheilus cuprifrons | IL | ||
| Cynipoidea | Figitidae | Agrostocynips clavatus | AR |
| Alloxysta sp. | CL | ||
| Disorygma pacifica | GT | ||
| Ganaspidium sp. | PE | ||
| Gronotoma sp. | GT, JO | ||
| Gronotoma adachiae | CN | ||
| Gronotoma micromorpha | ID | ||
| Moneucoela sp. | GT | ||
| Tribliographa sp. | CO | ||
| Zaeucolia sp. | GT |
AR, Argentina; BR, Brazil; CA, Canada; CL, Chile; CN, China; CO, Columbia; CR, Costa Rica; GT, Guatemala; ID, Indonesia; IL, Israel; IT, Italy; JP, Japan; JO, Jordan; KE, Kenya; LB, Lebanon; MY, Malaysia; NL, The Netherlands; PE, Peru; PH, Philippines; PT, Portugal; RE, Reunion; ZA, South Africa; ES, Spain; LK, Sri Lanka; TW, Taiwan; TR, Turkey; VN, Viet Nam.
Liriomyza huidobrensis parasitoids by superfamily, family, genus and species from countries world-wide
| Superfamily . | Family . | Parasitoid . | Countrya . |
|---|---|---|---|
| Ichneumonidea | Braconidae | Bracon intercessor | TR |
| Dacnusa sp. | CA | ||
| Dacnusa sasakawai | JP | ||
| Dacnusa sibirica | IT, PT, NL, RE | ||
| Oenanogastra sp. | CR | ||
| Opius sp. | CR, CA, ID, MY, CN, BR, CR, | ||
| CO, JP, JO, PH, IL, PE | |||
| Opius caricivorae | TW | ||
| Opius chromatomyiae | ID | ||
| Opius dimidiatus | CN, GT | ||
| Opius dissitus | CN, GT, EU, | ||
| Opius mandibularis | GT | ||
| Opius meracus | TR | ||
| Opius pallipes | NL | ||
| Opius scabriventris | AR, PE | ||
| Phaedrotoma sp. | AR | ||
| Phaedrotoma luteoclypealis | AR | ||
| Phaedrotoma mesoclypealis | AR | ||
| Phaedrotoma scabriventris | AR | ||
| Proctotrupoidea | Diapriidae | Trichopria sp. | PT |
| Chalcidoidea | Eulophidae | Asecodes sp. | ID |
| Asecodes delucchii | ID, PH | ||
| Chrysocharis sp. | AR, CR, PE, ID, PT, | ||
| Chrysocharis ainsliei | PE | ||
| Chrysocharis bedius | BR, RE | ||
| Chrysocharis brethesi | PE | ||
| Chrysocharis c.f. aluta | GT | ||
| Chrysocharis caribea | AR, PE | ||
| Chrysocharis flacilla | AR, PE, CL | ||
| Chrysocharis ignota | GT | ||
| Chrysocharis orbicularis | JO | ||
| Chrysocharis oscinidis | CA | ||
| Chrysocharis pentheus | CN, MY, JP, TW, MY, IL | ||
| Chrysocharis pubicornis | CN, JP, JO | ||
| Chrysocharis tristis | GT | ||
| Chrysocharis vonones | AR | ||
| Chrysonotomyia sp. | PE, AR | ||
| Cirrospilus ambiguus | ID | ||
| Cirrospilus vittatus | JO | ||
| Closterocerus sp. | BR, ID, PE | ||
| Closterocerus cinctipennis | PE | ||
| Closterocerus okazakii | TW | ||
| Closterocerus pulcher | GT | ||
| Diaulinopsis sp. | AR, PE | ||
| Diaulinopsis arenaria | JO | ||
| Diaulinopsis callichroma | PE | ||
| Diglyphus sp. | AR, CR, PE, CO, ZA | ||
| Diglyphus albiscapus | JP | ||
| Diglyphus begini | AR, PE, CO, CN | ||
| Diglyphus crassinervis | CN, TR, PT, JO, IL | ||
| Diglyphus intermedius | GT, CR, CN, CO | ||
| Diglyphus isaea | GT, IL, CN, IT, NL, TR, PT, JP, | ||
| JO, PH, LB, CR, | |||
| Diglyphus minoeus | PT, TR | ||
| Diglyphus pachyneurus | CN | ||
| Diglyphus pedicellus | AR | ||
| Diglyphus pedicellus | AR | ||
| Diglyphus poppoea | PT | ||
| Diglyphus pulchripes | CN | ||
| Diglyphus sp. (near intermedius) | CR | ||
| Diglyphus websteri | PE, AR, GT, | ||
| Hemiptarsenus sp. | JO | ||
| Hemiptarsenus fulvicollis | PT | ||
| Chalcidoidea | Eulophidae | Hemiptarsenus ornatus | JO |
| Hemiptarsenus unguicellus | CN | ||
| Hemiptarsenus varicornis | CN, ID, MY, SK, PH | ||
| Hemiptarsenus zilahisebessi | CN, IL, JO | ||
| Heteroschema sp. | PE | ||
| Neochrysocharis sp. | ID | ||
| Neochrysocharis beasleyi | ID, VN | ||
| Neochrysocharis diastatae | GT | ||
| Neochrysocharis formosa | CN, MY, TR, JO, IL, ID | ||
| Neochrysocharis okazakii | JP, PH | ||
| Pnigalio sp. | ID | ||
| Pediobius metallicus | CN, IL, JO | ||
| Pnigalio incompletus | JO | ||
| Pnigalio katonis | CN, PH | ||
| Pnigalio soemius | IL | ||
| Proacrias sp. | CL | ||
| Proacrias thysanoides | AR, PE | ||
| Proacrias xenodice | AR, CL | ||
| Quadrastichus sp. | GT, ID, IL | ||
| Quadrastichus liriomyzae | PH | ||
| Zagrammosoma sp. | ID, PE, PH | ||
| Zagrammosoma latilineatum | ID | ||
| Zagrammosoma multileneatum | PE | ||
| Pteromalidae | ≠ Halticoptera | PT | |
| Halticoptera sp. | AR, CO, CL, GT, PE | ||
| Halticoptera arduine | PE, AR, CL, PE | ||
| Halticoptera circulus | CA, CN, GT, JO | ||
| Halticoptera helioponi | AR | ||
| Halticoptera patellana | PE | ||
| Halticoptera peviana | AR | ||
| Notoglyptus tzeltales | GT | ||
| Pteromalidae sp. | AR, IL | ||
| Sphegigaster sp. | ID | ||
| Thinodytes cyzicus | CN | ||
| Thinodytes sp. | AR | ||
| Trichomalopsis sp. | CN | ||
| Tetracampidae | Epiclerus sp. | ES | |
| Platynocheilus cuprifrons | IL | ||
| Cynipoidea | Figitidae | Agrostocynips clavatus | AR |
| Alloxysta sp. | CL | ||
| Disorygma pacifica | GT | ||
| Ganaspidium sp. | PE | ||
| Gronotoma sp. | GT, JO | ||
| Gronotoma adachiae | CN | ||
| Gronotoma micromorpha | ID | ||
| Moneucoela sp. | GT | ||
| Tribliographa sp. | CO | ||
| Zaeucolia sp. | GT |
| Superfamily . | Family . | Parasitoid . | Countrya . |
|---|---|---|---|
| Ichneumonidea | Braconidae | Bracon intercessor | TR |
| Dacnusa sp. | CA | ||
| Dacnusa sasakawai | JP | ||
| Dacnusa sibirica | IT, PT, NL, RE | ||
| Oenanogastra sp. | CR | ||
| Opius sp. | CR, CA, ID, MY, CN, BR, CR, | ||
| CO, JP, JO, PH, IL, PE | |||
| Opius caricivorae | TW | ||
| Opius chromatomyiae | ID | ||
| Opius dimidiatus | CN, GT | ||
| Opius dissitus | CN, GT, EU, | ||
| Opius mandibularis | GT | ||
| Opius meracus | TR | ||
| Opius pallipes | NL | ||
| Opius scabriventris | AR, PE | ||
| Phaedrotoma sp. | AR | ||
| Phaedrotoma luteoclypealis | AR | ||
| Phaedrotoma mesoclypealis | AR | ||
| Phaedrotoma scabriventris | AR | ||
| Proctotrupoidea | Diapriidae | Trichopria sp. | PT |
| Chalcidoidea | Eulophidae | Asecodes sp. | ID |
| Asecodes delucchii | ID, PH | ||
| Chrysocharis sp. | AR, CR, PE, ID, PT, | ||
| Chrysocharis ainsliei | PE | ||
| Chrysocharis bedius | BR, RE | ||
| Chrysocharis brethesi | PE | ||
| Chrysocharis c.f. aluta | GT | ||
| Chrysocharis caribea | AR, PE | ||
| Chrysocharis flacilla | AR, PE, CL | ||
| Chrysocharis ignota | GT | ||
| Chrysocharis orbicularis | JO | ||
| Chrysocharis oscinidis | CA | ||
| Chrysocharis pentheus | CN, MY, JP, TW, MY, IL | ||
| Chrysocharis pubicornis | CN, JP, JO | ||
| Chrysocharis tristis | GT | ||
| Chrysocharis vonones | AR | ||
| Chrysonotomyia sp. | PE, AR | ||
| Cirrospilus ambiguus | ID | ||
| Cirrospilus vittatus | JO | ||
| Closterocerus sp. | BR, ID, PE | ||
| Closterocerus cinctipennis | PE | ||
| Closterocerus okazakii | TW | ||
| Closterocerus pulcher | GT | ||
| Diaulinopsis sp. | AR, PE | ||
| Diaulinopsis arenaria | JO | ||
| Diaulinopsis callichroma | PE | ||
| Diglyphus sp. | AR, CR, PE, CO, ZA | ||
| Diglyphus albiscapus | JP | ||
| Diglyphus begini | AR, PE, CO, CN | ||
| Diglyphus crassinervis | CN, TR, PT, JO, IL | ||
| Diglyphus intermedius | GT, CR, CN, CO | ||
| Diglyphus isaea | GT, IL, CN, IT, NL, TR, PT, JP, | ||
| JO, PH, LB, CR, | |||
| Diglyphus minoeus | PT, TR | ||
| Diglyphus pachyneurus | CN | ||
| Diglyphus pedicellus | AR | ||
| Diglyphus pedicellus | AR | ||
| Diglyphus poppoea | PT | ||
| Diglyphus pulchripes | CN | ||
| Diglyphus sp. (near intermedius) | CR | ||
| Diglyphus websteri | PE, AR, GT, | ||
| Hemiptarsenus sp. | JO | ||
| Hemiptarsenus fulvicollis | PT | ||
| Chalcidoidea | Eulophidae | Hemiptarsenus ornatus | JO |
| Hemiptarsenus unguicellus | CN | ||
| Hemiptarsenus varicornis | CN, ID, MY, SK, PH | ||
| Hemiptarsenus zilahisebessi | CN, IL, JO | ||
| Heteroschema sp. | PE | ||
| Neochrysocharis sp. | ID | ||
| Neochrysocharis beasleyi | ID, VN | ||
| Neochrysocharis diastatae | GT | ||
| Neochrysocharis formosa | CN, MY, TR, JO, IL, ID | ||
| Neochrysocharis okazakii | JP, PH | ||
| Pnigalio sp. | ID | ||
| Pediobius metallicus | CN, IL, JO | ||
| Pnigalio incompletus | JO | ||
| Pnigalio katonis | CN, PH | ||
| Pnigalio soemius | IL | ||
| Proacrias sp. | CL | ||
| Proacrias thysanoides | AR, PE | ||
| Proacrias xenodice | AR, CL | ||
| Quadrastichus sp. | GT, ID, IL | ||
| Quadrastichus liriomyzae | PH | ||
| Zagrammosoma sp. | ID, PE, PH | ||
| Zagrammosoma latilineatum | ID | ||
| Zagrammosoma multileneatum | PE | ||
| Pteromalidae | ≠ Halticoptera | PT | |
| Halticoptera sp. | AR, CO, CL, GT, PE | ||
| Halticoptera arduine | PE, AR, CL, PE | ||
| Halticoptera circulus | CA, CN, GT, JO | ||
| Halticoptera helioponi | AR | ||
| Halticoptera patellana | PE | ||
| Halticoptera peviana | AR | ||
| Notoglyptus tzeltales | GT | ||
| Pteromalidae sp. | AR, IL | ||
| Sphegigaster sp. | ID | ||
| Thinodytes cyzicus | CN | ||
| Thinodytes sp. | AR | ||
| Trichomalopsis sp. | CN | ||
| Tetracampidae | Epiclerus sp. | ES | |
| Platynocheilus cuprifrons | IL | ||
| Cynipoidea | Figitidae | Agrostocynips clavatus | AR |
| Alloxysta sp. | CL | ||
| Disorygma pacifica | GT | ||
| Ganaspidium sp. | PE | ||
| Gronotoma sp. | GT, JO | ||
| Gronotoma adachiae | CN | ||
| Gronotoma micromorpha | ID | ||
| Moneucoela sp. | GT | ||
| Tribliographa sp. | CO | ||
| Zaeucolia sp. | GT |
AR, Argentina; BR, Brazil; CA, Canada; CL, Chile; CN, China; CO, Columbia; CR, Costa Rica; GT, Guatemala; ID, Indonesia; IL, Israel; IT, Italy; JP, Japan; JO, Jordan; KE, Kenya; LB, Lebanon; MY, Malaysia; NL, The Netherlands; PE, Peru; PH, Philippines; PT, Portugal; RE, Reunion; ZA, South Africa; ES, Spain; LK, Sri Lanka; TW, Taiwan; TR, Turkey; VN, Viet Nam.
Many adult parasitoids exhibit host-feeding behavior to enhance protein levels during ova formation and maturation (Jervis and Kidd 1986). Due to the large variety of parasitoids attacking L. huidobrensis worldwide, virtually all behavioral and reproductive strategies of parasitic Hymenoptera are found; for example, Diglyphus isaea Walker and Hemiptarsenus varicornis (Girault) are ectoparasitic (feeding externally on the leafminer larvae from within the leaf mines), synovigenic (adult females continue to produce and mature eggs throughout their entire lives), and idiobiont (adult females paralyze and arrest the development of the leafminer larvae) wasps and prefer almost exclusively third instars for oviposition. On the contrary, Dacnusa sibirica Telenga is an endoparasite (eggs are laid in the leafminer larva), proovigenic (adult females have a full complement of mature eggs upon eclosion), and koinobiont (the parasitized leafminer larva is not paralyzed and continues to develop) wasp and will attack all stages of the leafminer larvae.
Host plant influences are not limited to the leafminer, but extend also to its parasitoids, as indicated by plant driven variation in parasitism rates (Videla et al. 2006, 2012) and parasitoid performance (Salvo and Valladares 2002). In Argentina, 23 parasitoid species attack L. huidobrensis, with higher diversity and impact of parasitoids in agricultural than in natural habitats (Salvo et al. 2005). Total parasitism rates of the main parasitoids vary among crops from 30% (Vicia faba) to 70% (Cucurbita maxima), with Chrysocharis flacilla (Walker), Phaedrotoma scabriventris (Nixon), and Halticoptera helioponi De Santis (Salvo et al. 2005, Videla et al. 2006). Given the presence of this leafminer in native plants and weeds in Argentina and considering the generalist habits of its parasitoids, Valladares and Salvo (1999) proposed the use of wild plants hosting non-pest leafminers, as reservoirs of parasitoids which could provide open rearing systems for the biological control of L. huidobrensis.
Along the Peruvian coast many parasitoid species were identified from L. huidobrensis, Table 3 (revised from Mujica and Kroschel 2011). Halticoptera arduine (Walker) was the dominant species both in lowland and highland agroecological zones. Mean parasitism and fly–parasitoid ratios were not affected by altitude, but varied with planting date. Parasitoid diversity decreased with altitude, and both altitude and host crop affected parasitism and preference of parasitoids (Mujica and Kroschel 2011). Results suggest that weather conditions, natural enemies, and plant quality attributes are the main determinants of the population dynamics of L. huidobrensis.
Contribution of the main parasitoids species to the total Liriomyza huidobrensis parasitism along the subtropical regions of the Peruvian coast
| Scientific name of host plants . | Foliar damagea . | No. of parasitoid species . | Parasitism (%) by species . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chrysocharis brethesi . | Chrysocharis caribea . | Chrysocharis flacilla . | Diglyphus begini . | Diglyphus websteri . | Ganaspidium sp. . | Halticoptera arduine . | Others . | Total parasitism . | |||
| Allium cepa aggregatum | Low | 3 | 0.0 | 9.1 | 0.0 | 0.0 | 9.1 | 0.0 | 45.5 | 0.0 | 63.6 |
| Apium graveolens dulce | Middle–high | 7 | 0.0 | 12.1 | 0.0 | 15.9 | 3.2 | 11.8 | 18.2 | 2.6 | 63.7 |
| Beta vulgaris cicla | Low | 5 | 11.1 | 22.2 | 5.6 | 0.0 | 22.2 | 0.0 | 22.2 | 0.0 | 83.3 |
| Beta vulgaris vulgaris | Low–middle | 8 | 0.4 | 1.5 | 9.8 | 1.1 | 6.9 | 0.7 | 22.9 | 0.0 | 43.3 |
| Brassica campestris rapa | Low | 2 | 0.0 | 0.0 | 34.0 | 0.0 | 0.0 | 0.0 | 30.0 | 2.0 | 66.0 |
| Brassica oleracea capitata | High | 3 | 0.0 | 5.1 | 0.2 | 0.0 | 0.3 | 0.9 | 16.5 | 0.2 | 23.2 |
| Brassica campestris pekinensis | 8 | 0.0 | 0.0 | 0.0 | 0.0 | 20.0 | 0.0 | 60.0 | 0.0 | 80.0 | |
| Capsicum annuum | Low | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | 0.0 | 50.0 |
| Capsicum baccatum | Low | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cucumis sativus | Low | 4 | 8.3 | 16.7 | 0.0 | 0.0 | 8.3 | 0.0 | 16.7 | 0.0 | 50.0 |
| Cucurbita maxima | Middle–high | 7 | 0.0 | 0.3 | 6.1 | 0.5 | 0.5 | 0.3 | 10.8 | 0.5 | 18.9 |
| Cucurbita pepo | Low | 3 | 0.0 | 3.0 | 0.0 | 0.0 | 7.3 | 0.0 | 22.0 | 0.6 | 32.9 |
| Lactuca sativa | Middle–high | 6 | 0.4 | 1.8 | 4.3 | 0.0 | 0.0 | 1.4 | 69.6 | 0.6 | 78.2 |
| Lycopersicon esculentum | Low–high | 6 | 0.1 | 1.5 | 2.1 | 0.0 | 0.8 | 0.1 | 33.2 | 0.2 | 38.0 |
| Medicago sativa | Middle–high | 11 | 0.1 | 6.4 | 15.4 | 0.6 | 2.1 | 1.1 | 5.7 | 7.6 | 39.0 |
| Ocimum basilicum | Low | 3 | 0.0 | 3.6 | 0.0 | 0.0 | 0.0 | 0.0 | 39.3 | 0.0 | 42.9 |
| Phaseolus vulgaris | Low–high | 17 | 1.0 | 4.2 | 2.0 | 0.0 | 8.0 | 0.7 | 15.1 | 1.9 | 32.9 |
| Phaseolus vulgaris vulgaris | High | 4 | 0.0 | 0.0 | 17.0 | 0.0 | 4.9 | 0.0 | 26.6 | 0.3 | 48.9 |
| Pisum sativum | Low–high | 16 | 0.4 | 6.1 | 3.2 | 0.7 | 4.7 | 1.7 | 19.8 | 2.4 | 38.9 |
| Raphanus sativus | Low–middle | 6 | 0.0 | 4.8 | 5.7 | 0.0 | 1.0 | 10.5 | 21.0 | 1.0 | 43.8 |
| Solanum tuberosum | Low–high | 16 | 0.0 | 0.2 | 5.1 | 0.0 | 2.2 | 0.8 | 16.2 | 1.8 | 26.3 |
| Spinacea oleracea | Low | 3 | 0.0 | 0.0 | 5.9 | 0.0 | 5.9 | 0.0 | 23.5 | 0.0 | 35.3 |
| Tagetes erecta | Middle | 5 | 20.0 | 0.0 | 4.0 | 0.0 | 0.0 | 8.0 | 20.0 | 4.0 | 56.0 |
| Vicia faba | Middle–high | 20 | 0.1 | 0.4 | 3.7 | 2.5 | 2.6 | 2.1 | 9.7 | 3.5 | 24.6 |
| Scientific name of host plants . | Foliar damagea . | No. of parasitoid species . | Parasitism (%) by species . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chrysocharis brethesi . | Chrysocharis caribea . | Chrysocharis flacilla . | Diglyphus begini . | Diglyphus websteri . | Ganaspidium sp. . | Halticoptera arduine . | Others . | Total parasitism . | |||
| Allium cepa aggregatum | Low | 3 | 0.0 | 9.1 | 0.0 | 0.0 | 9.1 | 0.0 | 45.5 | 0.0 | 63.6 |
| Apium graveolens dulce | Middle–high | 7 | 0.0 | 12.1 | 0.0 | 15.9 | 3.2 | 11.8 | 18.2 | 2.6 | 63.7 |
| Beta vulgaris cicla | Low | 5 | 11.1 | 22.2 | 5.6 | 0.0 | 22.2 | 0.0 | 22.2 | 0.0 | 83.3 |
| Beta vulgaris vulgaris | Low–middle | 8 | 0.4 | 1.5 | 9.8 | 1.1 | 6.9 | 0.7 | 22.9 | 0.0 | 43.3 |
| Brassica campestris rapa | Low | 2 | 0.0 | 0.0 | 34.0 | 0.0 | 0.0 | 0.0 | 30.0 | 2.0 | 66.0 |
| Brassica oleracea capitata | High | 3 | 0.0 | 5.1 | 0.2 | 0.0 | 0.3 | 0.9 | 16.5 | 0.2 | 23.2 |
| Brassica campestris pekinensis | 8 | 0.0 | 0.0 | 0.0 | 0.0 | 20.0 | 0.0 | 60.0 | 0.0 | 80.0 | |
| Capsicum annuum | Low | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | 0.0 | 50.0 |
| Capsicum baccatum | Low | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cucumis sativus | Low | 4 | 8.3 | 16.7 | 0.0 | 0.0 | 8.3 | 0.0 | 16.7 | 0.0 | 50.0 |
| Cucurbita maxima | Middle–high | 7 | 0.0 | 0.3 | 6.1 | 0.5 | 0.5 | 0.3 | 10.8 | 0.5 | 18.9 |
| Cucurbita pepo | Low | 3 | 0.0 | 3.0 | 0.0 | 0.0 | 7.3 | 0.0 | 22.0 | 0.6 | 32.9 |
| Lactuca sativa | Middle–high | 6 | 0.4 | 1.8 | 4.3 | 0.0 | 0.0 | 1.4 | 69.6 | 0.6 | 78.2 |
| Lycopersicon esculentum | Low–high | 6 | 0.1 | 1.5 | 2.1 | 0.0 | 0.8 | 0.1 | 33.2 | 0.2 | 38.0 |
| Medicago sativa | Middle–high | 11 | 0.1 | 6.4 | 15.4 | 0.6 | 2.1 | 1.1 | 5.7 | 7.6 | 39.0 |
| Ocimum basilicum | Low | 3 | 0.0 | 3.6 | 0.0 | 0.0 | 0.0 | 0.0 | 39.3 | 0.0 | 42.9 |
| Phaseolus vulgaris | Low–high | 17 | 1.0 | 4.2 | 2.0 | 0.0 | 8.0 | 0.7 | 15.1 | 1.9 | 32.9 |
| Phaseolus vulgaris vulgaris | High | 4 | 0.0 | 0.0 | 17.0 | 0.0 | 4.9 | 0.0 | 26.6 | 0.3 | 48.9 |
| Pisum sativum | Low–high | 16 | 0.4 | 6.1 | 3.2 | 0.7 | 4.7 | 1.7 | 19.8 | 2.4 | 38.9 |
| Raphanus sativus | Low–middle | 6 | 0.0 | 4.8 | 5.7 | 0.0 | 1.0 | 10.5 | 21.0 | 1.0 | 43.8 |
| Solanum tuberosum | Low–high | 16 | 0.0 | 0.2 | 5.1 | 0.0 | 2.2 | 0.8 | 16.2 | 1.8 | 26.3 |
| Spinacea oleracea | Low | 3 | 0.0 | 0.0 | 5.9 | 0.0 | 5.9 | 0.0 | 23.5 | 0.0 | 35.3 |
| Tagetes erecta | Middle | 5 | 20.0 | 0.0 | 4.0 | 0.0 | 0.0 | 8.0 | 20.0 | 4.0 | 56.0 |
| Vicia faba | Middle–high | 20 | 0.1 | 0.4 | 3.7 | 2.5 | 2.6 | 2.1 | 9.7 | 3.5 | 24.6 |
Low = 1–25%; middle = 26–50%; high > 50%.
Contribution of the main parasitoids species to the total Liriomyza huidobrensis parasitism along the subtropical regions of the Peruvian coast
| Scientific name of host plants . | Foliar damagea . | No. of parasitoid species . | Parasitism (%) by species . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chrysocharis brethesi . | Chrysocharis caribea . | Chrysocharis flacilla . | Diglyphus begini . | Diglyphus websteri . | Ganaspidium sp. . | Halticoptera arduine . | Others . | Total parasitism . | |||
| Allium cepa aggregatum | Low | 3 | 0.0 | 9.1 | 0.0 | 0.0 | 9.1 | 0.0 | 45.5 | 0.0 | 63.6 |
| Apium graveolens dulce | Middle–high | 7 | 0.0 | 12.1 | 0.0 | 15.9 | 3.2 | 11.8 | 18.2 | 2.6 | 63.7 |
| Beta vulgaris cicla | Low | 5 | 11.1 | 22.2 | 5.6 | 0.0 | 22.2 | 0.0 | 22.2 | 0.0 | 83.3 |
| Beta vulgaris vulgaris | Low–middle | 8 | 0.4 | 1.5 | 9.8 | 1.1 | 6.9 | 0.7 | 22.9 | 0.0 | 43.3 |
| Brassica campestris rapa | Low | 2 | 0.0 | 0.0 | 34.0 | 0.0 | 0.0 | 0.0 | 30.0 | 2.0 | 66.0 |
| Brassica oleracea capitata | High | 3 | 0.0 | 5.1 | 0.2 | 0.0 | 0.3 | 0.9 | 16.5 | 0.2 | 23.2 |
| Brassica campestris pekinensis | 8 | 0.0 | 0.0 | 0.0 | 0.0 | 20.0 | 0.0 | 60.0 | 0.0 | 80.0 | |
| Capsicum annuum | Low | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | 0.0 | 50.0 |
| Capsicum baccatum | Low | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cucumis sativus | Low | 4 | 8.3 | 16.7 | 0.0 | 0.0 | 8.3 | 0.0 | 16.7 | 0.0 | 50.0 |
| Cucurbita maxima | Middle–high | 7 | 0.0 | 0.3 | 6.1 | 0.5 | 0.5 | 0.3 | 10.8 | 0.5 | 18.9 |
| Cucurbita pepo | Low | 3 | 0.0 | 3.0 | 0.0 | 0.0 | 7.3 | 0.0 | 22.0 | 0.6 | 32.9 |
| Lactuca sativa | Middle–high | 6 | 0.4 | 1.8 | 4.3 | 0.0 | 0.0 | 1.4 | 69.6 | 0.6 | 78.2 |
| Lycopersicon esculentum | Low–high | 6 | 0.1 | 1.5 | 2.1 | 0.0 | 0.8 | 0.1 | 33.2 | 0.2 | 38.0 |
| Medicago sativa | Middle–high | 11 | 0.1 | 6.4 | 15.4 | 0.6 | 2.1 | 1.1 | 5.7 | 7.6 | 39.0 |
| Ocimum basilicum | Low | 3 | 0.0 | 3.6 | 0.0 | 0.0 | 0.0 | 0.0 | 39.3 | 0.0 | 42.9 |
| Phaseolus vulgaris | Low–high | 17 | 1.0 | 4.2 | 2.0 | 0.0 | 8.0 | 0.7 | 15.1 | 1.9 | 32.9 |
| Phaseolus vulgaris vulgaris | High | 4 | 0.0 | 0.0 | 17.0 | 0.0 | 4.9 | 0.0 | 26.6 | 0.3 | 48.9 |
| Pisum sativum | Low–high | 16 | 0.4 | 6.1 | 3.2 | 0.7 | 4.7 | 1.7 | 19.8 | 2.4 | 38.9 |
| Raphanus sativus | Low–middle | 6 | 0.0 | 4.8 | 5.7 | 0.0 | 1.0 | 10.5 | 21.0 | 1.0 | 43.8 |
| Solanum tuberosum | Low–high | 16 | 0.0 | 0.2 | 5.1 | 0.0 | 2.2 | 0.8 | 16.2 | 1.8 | 26.3 |
| Spinacea oleracea | Low | 3 | 0.0 | 0.0 | 5.9 | 0.0 | 5.9 | 0.0 | 23.5 | 0.0 | 35.3 |
| Tagetes erecta | Middle | 5 | 20.0 | 0.0 | 4.0 | 0.0 | 0.0 | 8.0 | 20.0 | 4.0 | 56.0 |
| Vicia faba | Middle–high | 20 | 0.1 | 0.4 | 3.7 | 2.5 | 2.6 | 2.1 | 9.7 | 3.5 | 24.6 |
| Scientific name of host plants . | Foliar damagea . | No. of parasitoid species . | Parasitism (%) by species . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chrysocharis brethesi . | Chrysocharis caribea . | Chrysocharis flacilla . | Diglyphus begini . | Diglyphus websteri . | Ganaspidium sp. . | Halticoptera arduine . | Others . | Total parasitism . | |||
| Allium cepa aggregatum | Low | 3 | 0.0 | 9.1 | 0.0 | 0.0 | 9.1 | 0.0 | 45.5 | 0.0 | 63.6 |
| Apium graveolens dulce | Middle–high | 7 | 0.0 | 12.1 | 0.0 | 15.9 | 3.2 | 11.8 | 18.2 | 2.6 | 63.7 |
| Beta vulgaris cicla | Low | 5 | 11.1 | 22.2 | 5.6 | 0.0 | 22.2 | 0.0 | 22.2 | 0.0 | 83.3 |
| Beta vulgaris vulgaris | Low–middle | 8 | 0.4 | 1.5 | 9.8 | 1.1 | 6.9 | 0.7 | 22.9 | 0.0 | 43.3 |
| Brassica campestris rapa | Low | 2 | 0.0 | 0.0 | 34.0 | 0.0 | 0.0 | 0.0 | 30.0 | 2.0 | 66.0 |
| Brassica oleracea capitata | High | 3 | 0.0 | 5.1 | 0.2 | 0.0 | 0.3 | 0.9 | 16.5 | 0.2 | 23.2 |
| Brassica campestris pekinensis | 8 | 0.0 | 0.0 | 0.0 | 0.0 | 20.0 | 0.0 | 60.0 | 0.0 | 80.0 | |
| Capsicum annuum | Low | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 50.0 | 0.0 | 50.0 |
| Capsicum baccatum | Low | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cucumis sativus | Low | 4 | 8.3 | 16.7 | 0.0 | 0.0 | 8.3 | 0.0 | 16.7 | 0.0 | 50.0 |
| Cucurbita maxima | Middle–high | 7 | 0.0 | 0.3 | 6.1 | 0.5 | 0.5 | 0.3 | 10.8 | 0.5 | 18.9 |
| Cucurbita pepo | Low | 3 | 0.0 | 3.0 | 0.0 | 0.0 | 7.3 | 0.0 | 22.0 | 0.6 | 32.9 |
| Lactuca sativa | Middle–high | 6 | 0.4 | 1.8 | 4.3 | 0.0 | 0.0 | 1.4 | 69.6 | 0.6 | 78.2 |
| Lycopersicon esculentum | Low–high | 6 | 0.1 | 1.5 | 2.1 | 0.0 | 0.8 | 0.1 | 33.2 | 0.2 | 38.0 |
| Medicago sativa | Middle–high | 11 | 0.1 | 6.4 | 15.4 | 0.6 | 2.1 | 1.1 | 5.7 | 7.6 | 39.0 |
| Ocimum basilicum | Low | 3 | 0.0 | 3.6 | 0.0 | 0.0 | 0.0 | 0.0 | 39.3 | 0.0 | 42.9 |
| Phaseolus vulgaris | Low–high | 17 | 1.0 | 4.2 | 2.0 | 0.0 | 8.0 | 0.7 | 15.1 | 1.9 | 32.9 |
| Phaseolus vulgaris vulgaris | High | 4 | 0.0 | 0.0 | 17.0 | 0.0 | 4.9 | 0.0 | 26.6 | 0.3 | 48.9 |
| Pisum sativum | Low–high | 16 | 0.4 | 6.1 | 3.2 | 0.7 | 4.7 | 1.7 | 19.8 | 2.4 | 38.9 |
| Raphanus sativus | Low–middle | 6 | 0.0 | 4.8 | 5.7 | 0.0 | 1.0 | 10.5 | 21.0 | 1.0 | 43.8 |
| Solanum tuberosum | Low–high | 16 | 0.0 | 0.2 | 5.1 | 0.0 | 2.2 | 0.8 | 16.2 | 1.8 | 26.3 |
| Spinacea oleracea | Low | 3 | 0.0 | 0.0 | 5.9 | 0.0 | 5.9 | 0.0 | 23.5 | 0.0 | 35.3 |
| Tagetes erecta | Middle | 5 | 20.0 | 0.0 | 4.0 | 0.0 | 0.0 | 8.0 | 20.0 | 4.0 | 56.0 |
| Vicia faba | Middle–high | 20 | 0.1 | 0.4 | 3.7 | 2.5 | 2.6 | 2.1 | 9.7 | 3.5 | 24.6 |
Low = 1–25%; middle = 26–50%; high > 50%.
Spencer (1973) reported that L. huidobrensis was extremely abundant on weedy species near crop fields and these were the source of crop infestation in Venezuela. However, in Guatemala and Peru, populations of L. huidobrensis, while common on many species of horticultural crops, are extremely rare outside of crop fields. Similar to the situation in Argentina, a number of authors (MacVean and Pérez 1996, 1997, Pérez et al. 1997, Mujica 2007) have found that host-plant use is highly biased in favor of crop species compared to wild hosts surrounding crop fields. Therefore, significant reservoirs of parasitoids exclusive to L. huidobrensis cannot be expected to exist outside the crop system; however, generalist parasitoids may be found in these weedy ecosystems. The prevalence of L. huidobrensis parasitoids, therefore, more likely depends on the dynamics within the crop, or possibly on immigration of generalist parasitoids originating from other species of leafminers. Thus, it appears that rational crop management should include conservation or augmentation of natural enemies. It is noteworthy that several samples of mined wild hosts from areas surrounding crop fields yielded no pupae or adults, which suggests effective natural mortality factors (MacVean and Pérez 1996, Pérez et al. 1997). Considering the overuse of pesticides within the crop fields, this scenario fits well with what is known of many leafminer outbreaks in other crops, such as tomatoes, where overuse of pesticides has eliminated the natural enemy complex and allowed a secondary herbivore (the leafminer) to acquire primary pest status (Oatman and Kennedy 1976).
In Costa Rica four parasitoid species, Diglyphus sp., Chrysocharis sp., Opius sp., and Oenonogastra sp. are found in low altitude zones (1,400–1,700 m.a.s.l.) while in high zones (1,700–2,400 m.a.s.l.) only Diglyphus sp. and Opius sp. are found (Carballo et al. 1990). Hidalgo (1990) and Hidalgo and Carballo (1991) suggest that the differences in parasitoid diversity occur because at high altitudes there is less quantity and diversity of weeds where these natural enemies can feed and find refuge. Additionally, potato crops grow in high altitude zones where a greater number of applications of broad-spectrum insecticides are made. In both high and low zones, the early season parasitism is low, but it increases by the end of the crop cycle, reaching up to 85% of parasitism in low lying areas.
When L. huidobrensis arrived in Israel, one primary parasitoid, i.e., D. isaea was already present. Diglyphus isaea is known to attack a number of hosts and it was clear that in Israel it expanded its host range to include L. huidobrensis very quickly. This situation, of D. isaea being the predominant parasitoid, lasted for a number of years, during which time the severity of the leafminer declined. In 2004, some 12 years after the leafminer was first discovered, leaf samples collected from 12 potato fields in the western Negev region had an assemblage of 10 additional parasitoid species. Of the 11 total parasitoids attacking L. huidobrensis, only three (D. isaea, D. crassinervis Erdos, and Pnigalio soemius [Walker]) were in common with a survey of parasitoids attacking L. trifolii (Friedberg and Gijswijt 1983).
In Indonesia, the most common and abundant parasitoid on potatoes is H. varicornis (Rauf et al. 2000), in Bali an Opius sp. is very abundant not only on potatoes, but tomato, celery and uncultivated plants (Suryawan and Reyes 2006). Damage by L. huidobrensis and the incidence of parasitism were highly variable among crops in Indonesia. For example, in potato in Cimacan, West Java, there was complete crop failure in March of 1996, with almost no parasitoids emerging from collected leaves. On the contrary, in that same area in shallot onions, nearly 100% of the leaves collected had leafminers that were parasitized mostly by H. varicornis. Other species of parasitoids have been increasing in abundance, such as Opius chromatomyiae Belokobylskij & Wharton in highland areas. It is now clear that when L. huidobrensis was accidentally introduced to Indonesia, at least some parasitoids also were introduced (Suryawan and Reyes 2006).
We tested whether the parasitoid community composition was affected by different variables at three levels: the first was the world data – 500 known host plant-parasitoid assemblages associated with L. huidobrensis (Supp Table 2 [online only]). At this level, the following variables effect was tested: region in the world, country, host-plant species and whether the plant was cultivated or not. The second level was all the data from Argentina – as it is the native region of L. huidobrensis. The third level was a subset of the Argentinian data – only parasitoids recorded in the references of Salvo and Valladares (1995, 1997, 2002), this was done to minimize the effect of different methods that were used in publications from around the world. Only the effect of cultivated versus non-cultivated plants was tested on both the second and third levels as there was not enough data to test the host plant effect.
For all analysis, we used analysis of variance of distance matrices (Adonis test, or PERMANOVA; Anderson 2001) with 1,000 permutations of the data. ‘References’ (from which these data were obtained) was used as a stratifying variable. We conducted all the above analyses in R (R Core Team 2013) using the package ‘Vegan’ (Oksannen et al. 2013). Using the subset of the Argentinian data (Salvo and Valladares 1995, 1997, 2002), whether a plant was cultivated or not significantly effected the parasitoid community composition (F = 1.694, r2 = 0.101, P = 0.045) (Table 4). This result indicates that the plants affect the parasitoid asamblage and could, perhaps, affect the biological control of the pest. At the world and Argentinian country level, the variables examined did not have a statistically significant effect on the parasitoid community (Table 4). This could be explained by the fact that there were too few references (for many countries there are only one or two researchers publishing), in countries where the leafminer has recently invaded, researchers have focused on economically important plants, the fact that different methods were used to collect the data in the different studies, or parasitoids of L. huidobrensis, are not affected by the plant on which their host is found.
Results of statistical analysis for preferred parasitoid-host plant associations for all parasitoid-host plant associations world-wide, only Argentina, and only Salvo and Valladares publications
| Data . | Variable . | F . | r2 . | P . |
|---|---|---|---|---|
| All of the world | Region | 6.152 | 0.174 | 0.174 |
| All of the world | Country | 2.002 | 0.294 | 0.139 |
| All of the world | Cultivated/wild | 3.664 | 0.009 | 0.924 |
| All of the world | Host plant | 0.512 | 0.184 | 0.177 |
| Argentina | Cultivated/wild | 0.734 | 0.013 | 0.934 |
| Salvo & Valladares | Cultivated/wild | 1.694 | 0.101 | 0.045 |
| Data . | Variable . | F . | r2 . | P . |
|---|---|---|---|---|
| All of the world | Region | 6.152 | 0.174 | 0.174 |
| All of the world | Country | 2.002 | 0.294 | 0.139 |
| All of the world | Cultivated/wild | 3.664 | 0.009 | 0.924 |
| All of the world | Host plant | 0.512 | 0.184 | 0.177 |
| Argentina | Cultivated/wild | 0.734 | 0.013 | 0.934 |
| Salvo & Valladares | Cultivated/wild | 1.694 | 0.101 | 0.045 |
Results of statistical analysis for preferred parasitoid-host plant associations for all parasitoid-host plant associations world-wide, only Argentina, and only Salvo and Valladares publications
| Data . | Variable . | F . | r2 . | P . |
|---|---|---|---|---|
| All of the world | Region | 6.152 | 0.174 | 0.174 |
| All of the world | Country | 2.002 | 0.294 | 0.139 |
| All of the world | Cultivated/wild | 3.664 | 0.009 | 0.924 |
| All of the world | Host plant | 0.512 | 0.184 | 0.177 |
| Argentina | Cultivated/wild | 0.734 | 0.013 | 0.934 |
| Salvo & Valladares | Cultivated/wild | 1.694 | 0.101 | 0.045 |
| Data . | Variable . | F . | r2 . | P . |
|---|---|---|---|---|
| All of the world | Region | 6.152 | 0.174 | 0.174 |
| All of the world | Country | 2.002 | 0.294 | 0.139 |
| All of the world | Cultivated/wild | 3.664 | 0.009 | 0.924 |
| All of the world | Host plant | 0.512 | 0.184 | 0.177 |
| Argentina | Cultivated/wild | 0.734 | 0.013 | 0.934 |
| Salvo & Valladares | Cultivated/wild | 1.694 | 0.101 | 0.045 |
Records of introduction of Liriomyza huidobrensis on the commodity/ies from which it was first reported and the current status of the leafminer in countries in the European Union
| Country . | First reported in: . | First reported on: . | Current status . | Reference . |
|---|---|---|---|---|
| Belgium | 1989/90 | Several vegetables and ornamental flowers under glass | Restricted distribution and mainly under protected cultivation | Baker et al. (2012) |
| France | Probably 1989-90 | Information not available | Widespread | Baker et al. (2012) |
| Germany | Early 1990s | Information not available | Present, few occurrences | Leuprecht (1992) |
| Greece | 1990s | Information not available | Widespread | Baker et al. (2012) |
| Italy | 1991 | Information not available | Restricted distribution | Suss (1991) |
| Ireland | 1997 | Imported flowers | Eradicated | Baker et al. (2012) |
| Netherlands | 1989 | Information not available | Present under protected culture | de Goffau (1991) |
| Portugal | 1991 | Information not available | Present in significant part of country | Baker et al. (2012) |
| Spain | Early 1992 | Information not available | Widespread | Cabello and Belda Suráez (1992) |
| United Kingdom | 1989 | Imported chrysanthemums under glass | Eradicated | Cheek et al. (1993) |
| Country . | First reported in: . | First reported on: . | Current status . | Reference . |
|---|---|---|---|---|
| Belgium | 1989/90 | Several vegetables and ornamental flowers under glass | Restricted distribution and mainly under protected cultivation | Baker et al. (2012) |
| France | Probably 1989-90 | Information not available | Widespread | Baker et al. (2012) |
| Germany | Early 1990s | Information not available | Present, few occurrences | Leuprecht (1992) |
| Greece | 1990s | Information not available | Widespread | Baker et al. (2012) |
| Italy | 1991 | Information not available | Restricted distribution | Suss (1991) |
| Ireland | 1997 | Imported flowers | Eradicated | Baker et al. (2012) |
| Netherlands | 1989 | Information not available | Present under protected culture | de Goffau (1991) |
| Portugal | 1991 | Information not available | Present in significant part of country | Baker et al. (2012) |
| Spain | Early 1992 | Information not available | Widespread | Cabello and Belda Suráez (1992) |
| United Kingdom | 1989 | Imported chrysanthemums under glass | Eradicated | Cheek et al. (1993) |
Records of introduction of Liriomyza huidobrensis on the commodity/ies from which it was first reported and the current status of the leafminer in countries in the European Union
| Country . | First reported in: . | First reported on: . | Current status . | Reference . |
|---|---|---|---|---|
| Belgium | 1989/90 | Several vegetables and ornamental flowers under glass | Restricted distribution and mainly under protected cultivation | Baker et al. (2012) |
| France | Probably 1989-90 | Information not available | Widespread | Baker et al. (2012) |
| Germany | Early 1990s | Information not available | Present, few occurrences | Leuprecht (1992) |
| Greece | 1990s | Information not available | Widespread | Baker et al. (2012) |
| Italy | 1991 | Information not available | Restricted distribution | Suss (1991) |
| Ireland | 1997 | Imported flowers | Eradicated | Baker et al. (2012) |
| Netherlands | 1989 | Information not available | Present under protected culture | de Goffau (1991) |
| Portugal | 1991 | Information not available | Present in significant part of country | Baker et al. (2012) |
| Spain | Early 1992 | Information not available | Widespread | Cabello and Belda Suráez (1992) |
| United Kingdom | 1989 | Imported chrysanthemums under glass | Eradicated | Cheek et al. (1993) |
| Country . | First reported in: . | First reported on: . | Current status . | Reference . |
|---|---|---|---|---|
| Belgium | 1989/90 | Several vegetables and ornamental flowers under glass | Restricted distribution and mainly under protected cultivation | Baker et al. (2012) |
| France | Probably 1989-90 | Information not available | Widespread | Baker et al. (2012) |
| Germany | Early 1990s | Information not available | Present, few occurrences | Leuprecht (1992) |
| Greece | 1990s | Information not available | Widespread | Baker et al. (2012) |
| Italy | 1991 | Information not available | Restricted distribution | Suss (1991) |
| Ireland | 1997 | Imported flowers | Eradicated | Baker et al. (2012) |
| Netherlands | 1989 | Information not available | Present under protected culture | de Goffau (1991) |
| Portugal | 1991 | Information not available | Present in significant part of country | Baker et al. (2012) |
| Spain | Early 1992 | Information not available | Widespread | Cabello and Belda Suráez (1992) |
| United Kingdom | 1989 | Imported chrysanthemums under glass | Eradicated | Cheek et al. (1993) |
A crab spider (Tomisidae) catching and feeding (insert) on a Liriomyza huidobrensis adult.
Three tiger/hunter fly species (Muscidae) are known to prey on L. huidobrensis. Tiger flies attack on-wing by catching the prey with their legs and then using a mouth hook at the end of the proboscis to feed on body fluids. From Portugal Coenosia attenuata Stein was recorded; unfortunately, it was found to be an effective predator on a number of other predators and parasitoids as well (Martins et al. 2012). This species has been successfully used to control L. huidobrensis in ornamental greenhouse crops in Colombia (Antioquia) (Prieto 2014). It is also used in Chile and Ecuador to reduce infestations of L. huidobrensis in horticultural and ornamental protected crops (Martinez-Sanchez et al. 2002). In Indonesia, C. humilis Fabricius is common in vegetable fields and can be effective against L. huidobrensis (Hidrayaini et al. 2005). In leafminer-infested potato fields, about 60% of the prey are L. huidobrensis adults; other prey include whiteflies, leafhoppers, and other small flying insects (Harwanto et al. 2004). Coenosia exigua Stein has been reported from Thailand and Vietnam (Ooi and Preongwitayakun 2009) on beans and ornamentals. In Thailand, breeding troughs (composed of a mixture of compost, fine soil, coconut and peanut shell parts, and green sticky rice or rice flakes constantly moisten) for C. exigua have been set in fields and seeded with fungus gnats to attract breeding populations.
Along the central coastal valleys of Peru, long-legged flies, primarily species of the genus Condylostylus (Dolichopodidae), form the most important family of foliage-inhabiting predators in the potato canopy. Condylostylus similis (Aldrich) and other species of the same and related genera have been reported to occur in large numbers near humid places with abundant vegetation (Cisneros and Mujica 1997). Condylostylus spp. are abundant in potato, beans, and other crops affected by leafminers, when insecticides are not used. The long-legged flies hunt leafminer and whitefly (Bemisia spp.) adults voraciously on the leaf surface and in flight. Similar predation behavior is exhibited by the black fly, Drapetis sp. (Empididae). In commercial potato fields, 74.3% of the foliage-inhabiting predators were represented by Condylostylus sp. followed by Drapetis sp. (20.2%) (Mujica 2016b). Thus, these two predators play an important role as potential biocontrol agents in these potato agroecological zones.
Integrated pest management
The level of IPM varies drastically in different regions of the world. Because Peru is host to the International Potato Center (CIP) and potatoes are indigenous and one of the most preferred crops, there has been intensive and comprehensive research on pest management tactics within the strategy of IPM. We present as a model the integrated management approach investigated by CIP for leafminer on the central coast of Peru which includes: cultural practices, evaluation and development of tolerant potato cultivars, trapping devices, the selective use of insecticides, and the role and use of natural enemies (Mujica and Cisneros 1997). These techniques along with monitoring methods of the fly population are the basis for structuring the integrated management of this pest (Mujica and Cisneros 1997, Cisneros and Mujica 1999a). In the last few years, IPM of L. huidobrensis has been reinforced with new ecological approaches based on (1) an overall understanding of its distribution and population dynamics in different potato agroecologies supported by phenology modeling, (2) yield loss assessments and the use of control thresholds to minimize insecticide applications, (3) habitat management with special consideration of conservation biological control, and (4) use of selective insecticides to enhance natural biological control (Kroschel et al. 2012, Mujica 2016b).
Plants that grow from low-quality seed (e.g., virus infested seed) or are deficiently irrigated and/or fertilized, show damage much earlier and leaves dry more rapidly, thus yields are affected. Balanced N-fertilization is important as high N-content in leaves promotes leafminer fly development. Continuous food availability by re-planting hosts crops will favor the abundance of the leafminer fly. Therefore, rotation with non-host crops is recommended (Mujica and Cisneros 1997).
Tolerance of foliar damage varies with potato variety. In Peru, late maturing cultivars generally compensate for higher injury levels better than early maturing cultivars (Mujica and Kroschel 2013). Action threshold at which control measures should start to prevent L. huidobrensis population from reaching an economic injury level have been established for selected potato cultivars. The use of the action threshold is suggested as a decision support tool to reduce pest management cost. IPM implemented in the Cañete valley was more effective in managing pests (L. huidobrensis and P. longifila) than the farmers’ pest management practices. IPM reduced the environmental impact quotient (EIQ) of the plant protection measures by 69.2% and achieved 35% higher potato yields resulting in higher net profits for potato farmers (US$1410/ha). This clearly indicates the benefits of using IPM in potato cultivation for both farmers and the environment (Mujica 2016b).
In Argentina, an IPM approach has been advocated (although not yet implemented) for L. huidobrensis on potato and tomato crops in Buenos Aires province (Vincini and Carmona 2006), with studies considering population dynamics of the pest and its main parasitoids, calculation of economic damage thresholds, efficiency of yellow sticky traps, and the use of cultivar resistance, based on plant traits such as foliage coloration (López et al. 2010) or egg extrusion capability (Videla and Valladares 2007).
Potato field in South Africa showing leafminer damage to the foliage (right), where the tractor-mounted spray boom (applying insecticides) did not reach.
Potato yields in seven varieties of potatoes in Israel before and after arrival of Liriomyza huidobrensis. The potato yields for the year that the leafminer arrived were intentionally removed to emphasize lack of yield changes.
Insecticide effects on parasitoids and predators
There is abundant evidence to the fact that conventional insecticide applications adversely affect leafminer parasitoids (Weintraub and Horowitz 1996, Civelek and Yoldas 2003, Prijono et al. 2004). In Israel, Weintraub and Horowitz (1996) compared the effects of management practices on the number of parasitoids caught by vacuum sampling. In fields where no insecticides were applied, a large number of parasitoids, primarily Diglyphus isaea, were caught throughout the day (parasitoid:leafminer, 30:1.7). In a commercial field with insecticide applications, the parasitoid:leafminer populations were reversed, 1.8:49. This gave the first clear evidence of the negative effects of insecticides to L. huidobrensis parasitoid populations. However, follow-up studies (Weintraub 1999) focusing only on translaminar insecticides (abamectin and cyromyzine) to reduce the number of conventional insecticide applications found that there were significant reductions in both larval leafminers and D. isaea by both insecticides.
Similarly, in Indonesia, insecticide applications reduced parasitism by indigenous parasitoids and affected populations of the predatory muscid fly, C. humilis, which in turn reduced control of leafminers (Hidrayani et al. 2005). In replicated field trials, repeated applications of profenofos and carbosulfan, while ineffective in controlling L. huidobrensis numbers on potatoes, decreased rates of parasitism by H. varicornis and O. chromatomyiae, and reduced numbers of C. humilis. These detrimental pesticide effects may have contributed to the increased damage and decreased yield in the treated fields.
It is remarkable that the overuse of insecticides over many years in Guatemala has not eliminated the natural enemy complex of L. huidobrensis. However, it is certainly likely that natural control has been hampered by chemical use.
Biodiversity and the potential for management
An increasing complexity of a landscape or cropping system can enhance the functional biodiversity in agroecosystems and can, therefore, positively contribute to the natural regulation of pests (Bianchi et al. 2006, Chaplin-Kramer et al. 2011). In the Cañete region, the Ihuanco (21% cultivated area) and Cañete (82% cultivated area) valleys were characterized as complex and simple structured landscapes, respectively. Although there were no differences in the vegetation diversity between both valleys, a higher abundance of trees and shrubs (mainly in field margins) occurred in the complex landscape. A rich arthropod fauna classified species from 119 families in 19 orders related to potato agroecosystems (without the application of insecticides). In the different functional groups, the highest abundance corresponded to neutral/unclassified species (35.1%), followed by predators (32.5%), herbivores (16.5%), and parasitoids (15.9%). Entomophagous species (predators and parasitoids) were more abundant than phytophagous species in both landscapes. The complex landscape had a higher taxonomic and functional diversity than the simple structured landscape. In contrast, entomophagous abundance was superior in the simple landscape. The potato agroecosystem shelters a diverse and abundant entomophagous guild that can be augmented with adequate management strategies on the landscape level to increase agroecosystem resilience to potato pest outbreaks.
Conservation biological control strategies, like reducing the use of broad-spectrum insecticides or increasing plant diversity in and around potato fields, were assessed for maintaining or increasing the potential of natural control. The potential of using the plant-leafminer association in maize (Zea mays L.-Liriomyza graminivora Hering) and of the weed, Galinsoga parviflora-Liriomyza sabaziae Spencer, as conservation strips in potato was evaluated in complex and simple landscapes. These alternative host plant-leafminer systems should provide an ‘open parasitoid rearing system’ (van der Linden 1993) by planting non-harmful leafminer host plants together with potato to increase natural control of L. huidobrensis. In the mixed cropping system potato-maize reduced larval infestation and leaf damage, as well as increased parasitism were observed compared with the potato-G. parviflora cropping system. A strong association of the parasitoid community to the host plant (as described previously in the Biological control section) suggests that host plant–leafminer associations determine the taxonomic composition of each parasitoid community. By contrast, the predator community, during the potato season, was more similar between landscape structures than between cropping systems. Hence, the results emphasize that biological diversity and ecological functions are not only affected by the cropping systems but also by the overall landscape characteristics. Multi-trophic interactions must be taken into account in planning conservation biological control for L. huidobrensis. The study shows that natural biological control of L. huidobrensis in the central Peruvian coast can be improved with adequate management strategies on the cropping system and landscape level which consequently increase agroecosystem resilience to potato pest outbreaks.
Distribution history and current pest status
South America
Liriomyza huidobrensis is native to the cool highlands of South America where it feeds on a variety of crops and wild host plants. It is an important pest in many countries and, in recent decades, has spread widely and invasively in many agricultural regions of the world.
Argentina: Liriomyza huidobrensis was originally identified from Buenos Aires Province (Blanchard, 1926), and since then it has been recorded in other central and northern areas of the country, including the provinces of Córdoba, Entre Ríos, Jujuy, La Pampa, Santa Fe, Tucumán (Valladares 1984, López et al. 2010). Damage varies between crops and localities, where it has been recorded on 40 different crops (Valladares et al. 1999, 2011). The leafminer is abundant not only on cultivated hosts but also on a wide range of native plants (Valladares 1984) and in natural environments such as Chaco forest (Valladares and Salvo 1999). Liriomyza huidobrensis is currently considered a key pest on potato crops in Buenos Aires province (Vincini and Carmona 2006) where it was first detected during the 1980s, with leafminer outbreaks since then being likely the result of insecticide overuse (Vincini and Carmona 2006). In other regions of the country, it is most damaging on horticultural crops like faba beans, chard, spinach, and tomato, with little impact on potato (Valladares et al. 1996, Videla et al. 2006) and, therefore, with lower economic impact.
Brazil: The historical data on L. huidobrensis distribution in crop areas are scarce due the difficulty of identifying Liriomyza species, which occur throughout the entire country. Liriomyza species were initially considered secondary pests in Brazil; however, in recent years, they have become severe agriculture pests. In the last three decades, with the expansion of Brazilian agriculture to the cerrado and caatinga biomes, L. huidobrensis outbreaks have increased in Asteraceae, Curcubitaceae, Fabaceae, and Solanaceae crops (Furiatti et al. 2008, Dequech et al. 2010, Costa-Lima et al. 2016). Liriomyza huidobrenis is also abundant on a wide range of native and ornamental plants (de Souza 1986). Currently, the leafminer is a primary pest in melon (C. melo) in northeastern Brazil, causing severe damage to this crop (Guimarães et al. 2009, Moura et al. 2014). The leafminer control is carried out mainly with insecticides (Lara et al. 2002, Bueno et al. 2007, Guimarães et al. 2009). However, high rates of natural biological control have been ascribed to braconid and eulophid parasitoids (Pereira et al. 2002, Guimarães et al. 2009, 2010, Dequech et al. 2010).
Peru: Liriomyza huidobrensis is the most damaging leafminer fly species to agriculture and is widely distributed throughout the coast and in warmer valleys of the Andes highlands. Extensive surveys along the Peruvian coast from Tumbes in the north to Tacna in the south revealed nine leafminer fly species with L. huidobrensis (88%) as clearly the most abundant and adapted species to the subtropical desert climate (mean temperature: 19ºC; precipitation: 14 mm per year) of the central and southern coastal region (Mujica and Kroschel 2011). Liriomyza huidobrensis infestation was more serious during the cold months from July to November. It is known that the highest population density and infestation levels normally occur in the central coastal region of Peru during the winter cropping season (from July to October) and the lowest population in the hottest months from December to April (Cisneros and Mujica 1999a). The adaptation of L. huidobrensis to lower temperatures was also demonstrated during the El Niño phenomenon in Peru in the years 1997–1998. An average increase of the min. temperature of 5ºC during the winter season dropped the L. huidobrensis population drastically by more than 81% (Cisneros and Mujica 1999b).
Historically, L. huidobrensis has existed in low populations in the central coast of Peru with no economic effects on the potato crop until the 1950s (Wille 1952), and increases in population densities have been mainly related to increasing and frequent use of insecticides. Coinciding with the availability of modern insecticides, farmers sprayed potato fields with organochlorine and organophosphate insecticides (e.g., DDT, parathion, BHC) to control the pinworm, T. absoluta (Campos 1976, Cisneros and Mujica 1999b), which was a minor pest that did not justify the use of chemical control. However, during this decade L. huidobrensis populations began to increase and reached harmful levels of infestation (Herrera 1963). Accordingly, insecticides were primarily used to control L. huidobrensis infestations rather than other potato pests (Campos 1978, Cisneros and Mujica 1999b). In this context, the surge of L. huidobrensis as a pest has been attributed to the destruction of its natural enemies by the use of insecticides (Cisneros 1986, Yabar 1988, Ochoa Chavarria and Carballo 1993).
Central America
Liriomyza huidobrensis was first reported from Costa Rica in Central America (Spencer 1983). It is not clear whether Central America is part of the native range of L. huidobrensis or whether Central American populations are the result of an early introduction or expansion from other regions. Because many small insects in this region were and remain understudied (MacVean et al. 2001), whether L. huidobrensis was present at low levels in Central America and surged with the expansion of crop host availability in the 1980s or whether it was introduced with the non-traditional exports boom (e.g., on propagative material) is unclear. Preliminary results from a molecular phylogeographic analysis of global L. huidobrensis populations suggest that Central American populations are, indeed, the result of a past introduction(s) (Scheffer unpub. data).
Costa Rica: The leafminer was considered a secondary pest for the majority of horticultural crops in national production until the end of the 1980s. In 1989, it reached the category of primary pest, significantly affecting horticultural crops that were destined for national consumption and for export. The first reports came from small farmers. Those farms were situated at an altitude of 1,400 m above sea level (m.a.s.l.) in the Tejar, Tablón, and San Isidro districts of the province of Cartago. Six months after those reports, other towns in the same province (San Rafael de Oreamuno and Paraíso centro) reported very high populations of leafminers in the lettuce and celery crops, causing huge economic losses to the farmers. By the end of the year, the pest had spread to most of the main towns of horticultural production in Cartago. Every town in Cartago that was situated between 1,400 and 1,800 m.a.s.l. was affected; additionally, other provinces such as Alajuela, Heredia, and Guanacaste were affected.
Guatemala: Liriomyza huidobrensis is primarily found in the highlands of Guatemala at elevations above 1,500 m (MacVean and Pérez 1996, 1997, Pérez et al. 1997), where it feeds on a variety of crops (see Table 1). The use of wild host plants by L. huidobrensis in the Guatemalan horticultural regions (central volcanic highlands) is remarkably low; only 10% of the L. huidobrensis population was found on non-crops (MacVean and Perez 1996, Pérez et al. 1997). This might seem to provide evidence that L. huidobrensis is an introduced crop specialist; however, an alternative explanation is that native populations of L. huidobrensis in Guatemala preferentially moved into agroecosystems following the movement of propagation material into the central highlands.
North America
Canada: Liriomyza huidobrensis was first discovered in Canada on various plant species in an ornamental flower greenhouse near Kettleby, Ontario, in 1998, and was thought to have arrived on plant material imported from Costa Rica (Murphy et al. 2014). In the following year, large numbers occurred in both flower and vegetable greenhouses and in field vegetable crops in the Holland Marsh region (McDonald et al. 2000, Scheffer et al. 2001). Populations reached outbreak levels for a number of years and crops experiencing high levels of damage included lettuce (L. sativa), spinach (S. oleracea), celery (A. graveolens), Asian crucifers (Brassica spp.), greenhouse ornamentals, greenhouse cucumbers (C. sativus), and onions (Allium cepa L.) (Martin et al. 2005b). The pea leafminer remained geographically isolated within the Holland Marsh region, but was unable to overwinter outside man-made structures (Martin et al. 2005a). Thus, L. huidobrensis appeared to survive the winter opportunistically within greenhouses from which they could reinfest neighboring fields each summer.
Because L. huidobrensis is unable to survive outside the greenhouse in winter, pest management recommendations were focused on effective control of L. huidobrensis in greenhouses, preventing the spread of L. huidobrensis from greenhouses in the spring, judicious use of pesticides in field vegetable crops, and conservation of natural enemies (Martin et al. 2005a, Bahlai et al. 2006). Three insecticides (abamectin, acetamiprid, and cyromazine) were given emergency and/or full registration for control of, or reduction of damage by, L. huidobrensis on leafy vegetables and leafy Brassica greens from 2001 to 2005. Liriomyza huidobrensis populations tended to reach very high levels in late August or early September (Martin et al. 2005a), leading celery growers to temporarily adopt the practice of harvesting celery before early September in order to minimize damage and yield losses.
These approaches were felt to have the potential to lessen or avoid infestations altogether (Bahlai et al. 2006) and appear to have been very successful, as L. huidobrensis is no longer a pest in Ontario. Reports of damage by L. huidobrensis have declined in Ontario since the mid-2000s (Murphy et al. 2014). Celery growers in the Holland Marsh region are no longer concerned about this pest and abandoned the practice of early harvesting a number of years ago (D. Van Dyk and M. R. McDonald, personal communications). Similarly, leafminer damage in general is quite low in greenhouse vegetable and ornamental crops, and there have been no recent reports of either presence or damage of L. huidobrensis (C. McCreary, personal communication).
United States – California:Liriomyza langei is an important, native, polyphagous vegetable pest in California. Because L. huidobrensis cannot be morphologically distinguished from L. langei, there has been the question of whether the invasive L. huidobrensis has been introduced to California, but remained undetected due to its similarity with L. langei. Scheffer et al. (2014) used a multiplex PCR method and DNA sequencing to screen 664 flies matching the description of L. huidobrensis/langei from six vegetable growing counties: Monterey, San Benito, San Luis Obispo, San Mateo, Santa Barbara, and Santa Cruz. Of the specimens that matched the morphological description, only molecular markers/sequences of L. langei were present in the samples, thus providing no evidence of invasive L. huidobrensis populations within California (Scheffer et al. 2014).
Western Europe
Liriomyza huidobrensis has been intercepted by plant quarantine authorities since the early 1980s. Cheek et al. (1993) reported that the first outbreaks in the UK occurred in 1989 and were associated with imported chrysanthemums and other ornamental plants. The outbreaks soon spread to a number of vegetable crops, where lettuce was severely affected. Between 1989 and 1992, 170 outbreaks occurred in England, one in Wales but none in Scotland or Northern Ireland. Outbreaks have been largely confined to protected crops which allows for effective pest management to be implemented (Baker et al. 2012). From 1991, a total eradication policy was adopted and applied (Bartlett 1993); this required eradication of the pest at nurseries propagating young plants and adequate control at production nurseries to reduce the risk of spread. Fortunately, all further outbreaks have been exterminated and the leafminer no longer currently does not occur in the UK (McLean 2015).
Breeding populations established in a number of European countries; records of the first outbreaks in sampled European countries, together with the mode of entry and current status, are summarized in Table 5. Pest interception data from the European network of plant health information shows that L. huidobrensis is regularly intercepted from horticulture imports into the region and sometimes similar trade within the region (Baker et al. 2012, Europhyte 2016).
The question of the source of first invasions by the leafminer into countries within the EU was analysed by Oudam et al. (1993). Electrophoretic analyses revealed high genetic distances among populations in the Netherlands, France, and Switzerland, suggesting that there have been multiple and separate introductions into Europe (Oudam et al. 1993) in contrast to an earlier suggestion by Trouvé et al. (1991) that first introductions into other European countries occurred via the Netherlands. Oudam et al. (1993) also found low genetic distances between European populations and a Peruvian population, suggesting that South America was the source of the leafminer invasions into Europe.
In general, most countries observed that after the first outbreaks of L. huidobrensis, the leafminer was increasingly attacked by local parasitoid wasps (and in some cases predatory flies) and these began to exert a high degree of mortality (Baker et al. 2012). Biological control soon became the backbone of IPM in glasshouses and several types of biological control agents were developed for use in European glasshouses. In particular, the parasitoids Dacnusa sibirica and Diglyphus isaea are important in keeping L. huidobrensis under control and are reared commercially; D. sibirica, which performs best at cool temperatures, is used early in the season sometimes with D. isaea whereas the latter species, which performs better at higher temperatures, is used alone later in the season when temperatures rise (van der Linden 2004). Currently the countries listed in Table 5 consider the leafminer to be moderate to minor problem but the importance of maintaining biological control is emphasized (Baker et al. 2012).
Scandanavia
Norway: Liriomyza huidobrensis is considered a quarantine pest (Andersen and Hofsvang 2010) and was recorded for the first time in 1995. The species was imported on Gypsophila sp. flowers for planting from Israel, and possibly the Netherlands, and established in three greenhouses in southwestern Norway. The next import of L. huidobrensis was to a greenhouse in southeastern Norway in the spring of 2002 on Chrysanthemum pot plants from the Netherlands which were distributed to greenhouses and garden centers across the country. These leafminers established and reproduced outside the primary infected greenhouses during the summer months and were found as far as 1.0 km away. Field investigations the following year concluded, however, that the species had not been able to overwinter outdoors, and there were no re-introductions from the field into the greenhouses during summer. The total economic losses for Norwegian greenhouses due to the outbreak of L. huidobrensis in 2002 was estimated to 40–50 million NOK (approximately 4.7–5.9 million USD).
Since 2002, L. huidobrensis has been imported 16 times on Exacum, Chrysanthemum and Verbena (Denmark and possibly Kenya, 2003), Osteospermum (USA, 2004), Chrysanthemum (Brazil, 2007), Exacum (Denmark, 2008), Diascia (unknown export country, 2009), Senecio, Impatiens, Tagetes and Bacoba (Denmark and unknown export country, 2011), bedding plants (unknown export country, 2014), and tomato (the Netherlands, 2015). Each time, L. huidobrensis has been successfully, actively eradicated (N. Johansen, personal communication).
The probability of establishment and spread of L. huidobrensis in Norwegian greenhouses is regarded as high, with concomitant spread and reproduction to nearby out-door crops during summer. It is unlikely that the species can survive outdoor in Norway today due to low winter temperatures, but this risk may increase with global warming. Currently, L. huidobrensis does not occur in Norway.
The Middle East
Israel: Liriomyza huidobrensis was first found in an outbreak in February 1992 in the Jordan Valley. Chrysanthemum growers were routinely treating for another agromyzid leafminer (L. trifolii), but when it could not be managed, further investigation revealed the leafminer to be L. huidobrensis (Gokkes et al. 1993). The leafminer had most likely entered Israel from Europe a year or two before the outbreak in the Jordan Valley. The leafminer quickly spread throughout the country feeding on other plants and causing economic damage in celery and supposedly potatoes.
Currently, L. huidobrensis is no longer a pest of flowers, vegetables, or any crop in Israel. Within about 10 years of L. huidobrensis arriving in the country, parasitoids of other leafminers started attacking the larvae to the point where there is now natural biological control throughout the country.
Lebanon: The leafminer started attacking Gerbera and vegetables, such as cucumber and beans, in the early 1990s, but its exact date of arrival is unknown (Noujeim et al. 2013).
Turkey: Liriomyza huidobrensis was first reported on beans and cucumber in İzmir and Adana provinces in 1995 (Yabas et al. 1995). Seven years later, in the year 2002, it was recorded in the neighboring province of Aydın, again on cucumbers in greenhouses (Yıldırım 2002). It has since been recorded in many provinces (Dursun 2008), although it is now found in low populations (Dursun 2015).
East Asia
China: Yunnan province, in southwestern China, is famous for the cultivation and production of flowers. Although L. huidobrensis had been intercepted in imported flowers by the local customs officials for years, it was first observed in Yunnan province in 1993 (Jiang 1997) where it was responsible for extensive damage to flowers and vegetables. The leafminer rapidly spread from south to north, its range encompassing the whole of China, except the Tibetan plateau, within a couple of years (Chen and Kang 2004). The leafminer also occurs on Hainan Island, and on Taiwan Island (Shiao and Wu 2000).
In southern China, e.g., Yunnan and Guangdong provinces, the species can occur in the field all year round (Chen and Kang 2004). In central China, the leafminer overwinters in the pupal stage, and continues developing in the spring. However, in northern China, beyond the overwintering limits of distribution, the leafminer cannot survive the cold winter in the field. Instead, the insects move to the protected areas such as greenhouses to overwinter. Greenhouse facilities are widespread in northern China, and are used for vegetable and fruit cultivation throughout the winter. These protected facilities provide warm shelter for the leafminers, allowing the species to spread to the most northern areas of China. This divergence of overwintering behaviors along latitudinal gradients explains the wide distribution of the leafminer in China (Chen and Kang 2004, Kang et al. 2009).
Following the outbreak of pest populations in the 1990s, a multi-pronged approach to management of L. huidobrensis was carried out by farmers and the government in China. Integrated pest management was used to control the pest, including parasitoid release, pesticide spraying, greenhouse fumigation, yellow sticky traps, cold exposure, and intercropping (Kang et al. 2009, Chen et al. 2011). The abundance of L. huidobrensis in China has dramatically decreased in the past decade, and no leafminer populations have been reported in recent years. Currently, L. huidobrensis is no longer considered a major pest in Chinese agricultural production.
Japan: Liriomyza huidobrensis was first observed in 2001 in Hokkaido (Shindo and Kinota 2005) and, by 2004, it was found in 19 locations in the Aomori Prefecture. The leafminer has been found on 30 different host plants across 14 plant families in Japan (Shindo and Kinota 2005). During that survey, eight parasitoid species were observed using L. huidobrensis as a host. Since it usually takes many years for parasitoids to switch or parasitize new host leafminer species, the fact that eight species were found parasitizing L. huidobrensis within three years of being identified in the country suggests that the leafminer had actually been in Japan some time before being discovered.
Korea: In Korea, L. huidobrensis was first recorded during a 2011–2012 survey of potato pests. Its identity was confirmed by morphological and DNA analyses (Maharjan et al. 2014).
Southeast Asia
Liriomyza huidobrensis appears to have arrived in Southeast Asia at about the same time as in East Asia. One report suggested L. huidobrensis to be present in Malaysia, presumably arriving on cut flowers from Holland (bin Hussain and Sivapragasam 2008) and Vietnam (Tran 2009). In Bangladesh, a molecular survey of leafminers from infested fields during 2008–2011 found that L. huidobrensis was not present and that the problematic leafminer was L. sativae (Amin et al. 2014). Currently, Liriomyza huidobrensis has not been reported in Australia (LaSalle, personal communication).
Indonesia: Liriomyza huidobrensis was first reported in 1994 in potato in Cisarua, West Java (Rauf 1995, Shepard et al. 1996). For the next 8–10 years, it was found in other parts of Java, in West Sumatra and South Sulawesi. This newly invasive species was particularly serious on potato, other vegetables and ornamental crops, especially those at higher elevations (800–1,700 m). Major highland vegetable growing areas where L. huidobrensis was found include Lembang, Pangalengan, Garut, and Cipanas in West Java, Banjarnegara and Magelang in Central Java, Batu in East Java, Alahan Panjang in West Sumatra, Barastagi in N. Sumatra, and Malino in South Sulawesi (Rauf et al. 2000). Over the next few years, this leafminer became the most serious leafminer species in Indonesia. Extensive surveys revealed that it attacked over 28 cultivated and weed plants that were surveyed from 19 sites in Indonesia (Rauf et al. 2000). Yield losses from this pest reached as high as 70% in some crops such as potato and snap bean. Currently, there are no outbreaks of leafminers in Indonesia such as those that occurred in the 1990s, except where insecticides are applied intensively, such as occasionally on potato.
Philippines: The first outbreak of L. huidobrensis in the Philippines was reported on potatoes in 1999, with more than 1,000 ha infested at the time (Molitas-Colting et al. 2002). Molecular analysis of 258 individuals from 26 crops found little genetic variation, as is typical of introduced species (Scheffer et al. 2006).
Vietnam: The first record of the leafminer was in 1995 in Da Lat; from greenhouses of flowers which were imported from the Netherlands (Andersen et al. 2008). These authors reported it from six provences in the south and one in central Vietnam, and reported that it is slowly spreading to other provences and predict its spread to northern Vietnam.
Africa
In 1990, L. huidobrensis was reported in Reunion, probably having been imported from France, and two years later on potatoes from Mauritius (Macdonald et al. 2002). A survey of vegetables on Reunion revealed that L. huidobrensis was primarily infesting Fabacaceae (P. vulgaris, P. sativum, Lablab purpureus (L.) Sweet and V. faba) (Vayssieres et al. 2001). Liriomyza huidobrensis has been reported in Morocco (Hanafi 2005) and Kenya (Chabi-Olaye et al. 2008). In Kenya, L. huidobrensis is a devastating pest reaching incidence up to 80% across the different crops (fresh vegetables and flowers), altitudes, and seasons (Gitonga et al. 2010). The economic situation is further exacerbated by the fact that the leafminer is included in the European Union list of quarantine pests (Foba et al. 2015), limiting the export of products to Europe.
South Africa: The events that led to the first outbreak in 1999 of L. huidobrensis in South Africa are unknown. The most plausible route of invasion of L. huidobrensis into South Africa is from Zimbabwe, as it was known to be present there before 1999 and caused considerable damage on faba beans (Musundire 2002, cited in Musundire et al. 2011). Within South Africa, the first reports of severe infestations were received during the latter parts of 1999 associated with potatoes (Visser 2012). Initially these reports only emanated from the far southwestern parts of the country, but within a few months, several other parts of the country also reported the occurrence of the leafminer. After its arrival, L. huidobrensis became one of the four major pests of potato, the other three being the potato tuber moth, nematodes, and virus-transmitting aphids (Visser 2015). A 2016 survey of potato pests in South Africa showed that L. huidobrensis was the most important pest, reported as a high priority pest in 13 of the 16 production regions. Today, it is found across all provinces and is a potential pest wherever it occurs.
Climate change and future invasions/Establishment
Computer software for modeling climate change effects on insects, Insect Life Cycle Modeling (ILCYM), is freely available. Todate, only the Peruvian researchers at CIP have examined predicted effects on L. huidobrensis which we present here; a world-wide analysis of the effects of climate change on the leafminer is beyond the perview of this publication.
Simulations and mapping of the current and future distribution of L. huidobrensis using ILCYM the software (Sporleder et al. 2009, 2013) clearly indicates that due to predicted climate change, this species is expected to expand its range to higher altitudes as we all as to the southern Andean region (Mujica 2016b) and higher altitudes globally. Further, the pest will develop more generations per year thus increasing its abundance and damage potential. For the current climate the pest phenology model estimated 9–12 generations per year for lowland conditions of the Peruvian coast and 6–9 generations for the Andean highlands, which estimations are consistent with reported field data. The expected changes in temperature will increase more rapidly under lowland valley conditions (3–5 more generations per year) in comparison with colder climates of the Andean highlands with a predicted increase of fewer than three generations per year, thus moderately increasing the pest damage potential. These early predictions should help to support farmers in the adaptation to climate change by developing and promoting adequate pest management strategies to reduce crop yield and quality losses.
Conclusions
Although L. huidobrensis is a global invasive pest, with time, it has shown very different responses around the globe: pest, present but not pest, or not present. The overview of its global history assembled in this publication, shows that the leafminer has been successfully eradicated from the United Kingdom and Norway; and has never established in Australia, New Zealand, or the Antarctica. It is well established in the western Middle East, Indonesia, and China but is no longer an economic pest in these regions. In Europe, it is easily controlled with natural enemies as the basis of IPM programs.
However, it is a severe economic pest in its native region of South America, Central America, and also in Africa. The various fates of the leafminer populations across the invaded areas do not seem to be easily attributed to any of the multiple factors here considered. For example, the leafminer is found on a wide variety of hosts and supports diverse parasitoid assemblages in South America, where it holds pest status, but also in China where it does not cause economically important losses.
Curiously North America has not been invaded, except for a limited area in Ontario, Canada, and that ‘establishment’ was short-term. Similarly, Australia and New Zealand have not been invaded, although another leafminer (L. sativae) was detected in the Torres Straits (Blacket et al. 2015). Thus, pathways for dispersal of Liriomyza species affecting horticulture are a risk for these regions. Should L. huidobrensis gain access to Australia and New Zealand, based on the situation world-wide, the leafminer would probably become established in parts of both countries where winters are not snow-bound and summer high temperatures are below 30–35 °C.
Liriomyza huidobrensis damage to crops varies from country to country. In Indonesia, potato yields have been devastated; in Israel, potato yields were never affected; in Argentina and Peru, both situations are found: potatoes are heavily affected in Buenos Aires province and central Peruvian coast, but not in Córdoba province or warmer Peruvian highland valleys where the leafminer reaches high densities on horticultural crops instead; in South Africa it is currently the most widespread and destructive pest of potato. In other countries, beans and legumes are more severely affected than other crops.
Of the large number of natural enemies reported, parasitoids are predominant. However, their efficacy is highly variable; reported control figures range between 1% and 90%. Parasitoid populations vary in abundance and efficacy with altitude and host plant(s). On the last point, non-cultivated (weedy) plants may or may not act as reservoirs for L. huidobrensis and/or associated parasitoids. For example, in Guatemala weeds are not considered as reservoirs for the leafminer or for its parasitoids; whereas, in Argentina, Costa Rica, Peru, and Venezula weeds can indeed act as reservoirs for the leafminer and/or its parasitoids. In some cases, many weeds or native plants provide alternative hosts for the parasitoids that attack L. huidobrensis. Another major factor limiting the impact of parasitoids and natural enemies in general where they do occur is the on-going use of broad spectrum insecticides by smallholder farmers, largely because they lack information about suitable control measures.
While predators are fewer in number, the most prominent ones are the dipterans, which can induce significant control of leafminer populations. The occurrence of other predators are not well documented, as predatory events are of short duration, and mostly observed by chance.
Liriomyza huidobrensis is strongly affected by temperature and seems to benefit from cooler conditions, as shown in laboratory and observational studies, including altitudinal and/or latitudinal gradients. For example, the leafminer was first observed to invade higher, cooler latitudes in Costa Rica, Guatemala and Indonesia. In warm subtropical climates, its activity is restricted to cooler months. In northern latitudes, it can physiologically adapt to sub-zero climatic conditions, but cannot survive for long periods in non-protected areas.
Finally, in the current scenario of ongoing climate change, simulation studies suggest that L. huidobrensis would expand its range to higher altitudes globally, with more generations being able to develop within a year, thus increasing the leafminer damage potential. Further studies are needed in this regard, in order to provide useful and timely information for the promotion of adequate management strategies for this leafminer pest.
Acknowledgements
Most, if not all, of the authors of this manuscript have consulted Dr. John LaSalle for identification of parasitoids. We wish to thank him for his years of dedication to parasitoid biology and identification. Fig. 4 was generated specifically for us by Dr. Or Sperling of the Agricultural Research Organization. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
References Cited
Author notes
Subject Editor: Jessica Dohmen-Vereijssen