-
PDF
- Split View
-
Views
-
Cite
Cite
Robert L. Koch, Daniela T. Pezzini, Andrew P. Michel, Thomas E. Hunt, Identification, Biology, Impacts, and Management of Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) of Soybean and Corn in the Midwestern United States, Journal of Integrated Pest Management, Volume 8, Issue 1, January 2017, 11, https://doi.org/10.1093/jipm/pmx004
Close - Share Icon Share
Stink bugs (Hemiptera: Heteroptera: Pentatomidae) are an emerging threat to soybean and corn production in the midwestern United States. An invasive species, the brown marmorated stink bug, Halyomorpha halys (Stål), is spreading through the region. However, little is known about the complex of stink bug species associated with corn and soybean in the midwestern United States. In this region, particularly in the more northern states, stink bugs have historically caused only infrequent impacts to these crops. To prepare growers and agricultural professionals to contend with this new threat, we provide a review of stink bugs associated with soybean and corn in the midwestern United States. Descriptions and images of common stink bug species are provided as a diagnostic aid. The biologies and impacts of stink bugs to crops are discussed, with particular attention to differences among species. Based primarily on information from southern states, scouting, thresholds, and insecticide-based management of these pests are discussed. It is hoped that this review will provide stakeholders sufficient information for management of these pests, until more region-specific research can be performed on stink bugs in soybean and corn in the midwestern United States.
The midwestern United States is the top Glycine max L. Merrill (soybean)- and Zea mays L. (corn)-producing region of the United States (National Agricultural Statistics Service [NASS] 2015). In this region, the stink bug (Hemiptera: Heteroptera: Pentatomidae) fauna is relatively diverse, comprising 45–57 species per state (McPherson 1982, Packauskas 2012, Rider 2012, Sites et al. 2012, Swanson 2012, Koch et al. 2014) and includes species known to be pests of corn and soybean in some regions (McPherson and McPherson 2000, Panizzi et al. 2000).
The significance of stink bugs in the midwestern United States is increasing. First, an invasive species of Asian origin, Halyomorpha halys (Stål) (brown marmorated stink bug), is invading the region (Tindall et al. 2012, Koch 2014). This species was first collected in North America in Pennsylvania in 1996 (Hoebeke and Carter 2003) and has since spread throughout much of the continental United States (Leskey et al. 2012a, Rice et al. 2014). Halyomorpha halys is a pest of many crops, including soybean and corn (Leskey et al. 2012a, Lee et al. 2013, Rice et al. 2014). Economically significant infestations of this pest in fruit, vegetable, and field crops had been limited primarily to the mid-Atlantic region of the United States (Leskey et al. 2012a, Rice et al. 2014) but are expanding westward into the midwestern United States (http://www.stopbmsb.org/where-is-bmsb/state-by-state/). As populations of this species increase in the midwestern United States, increasing frequencies of economically significant infestations are likely. Since 2012, H. halys has been regularly found in Ohio soybean fields and sometimes at economic levels with other stink bug species (Michel et al. 2013). Another invasive species, Piezodorus guildinii (Westwood) (redbanded stink bug), is currently established in the southeastern United States and may be expanding its range northward (Tindall and Fothergill 2011). Piezodorus guildinii is a significant pest of soybean (McPherson and McPherson 2000, Panizzi et al. 2000). In addition to the spread of new invasive species, the abundance of native stink bug species appears to be increasing in the midwestern United States (Hunt et al. 2011, 2014; Michel et al. 2013). In particular, Chinavia hilaris (Say) (green stink bug), Euschistus servus (Say) (brown stink bug), Euschistusvariolarius (Palisot de Beauvois) (onespotted stink bug), and Thyanta custator accerra McAtee (redshouldered stink bug) have been increasing in abundance and frequency for the past several years (Michel and Hunt, personal observations).
Owing to the growing attention stink bugs are receiving in the midwestern United States and the lack of a recent, comprehensive resource accessible to agricultural professionals, agency and Extension staff, and producers, we compiled this review of the identification, biology, impacts, and management of stink bugs in soybean and corn in the midwestern United States.
Identification
General
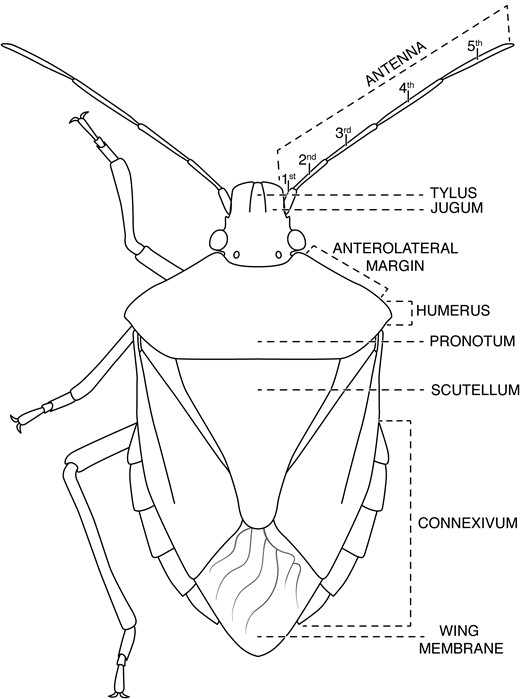
Line drawing of stink bug adult showing body parts important for discrimination of species common in soybean and corn in the midwestern United States (image credit: A.K. Tran).
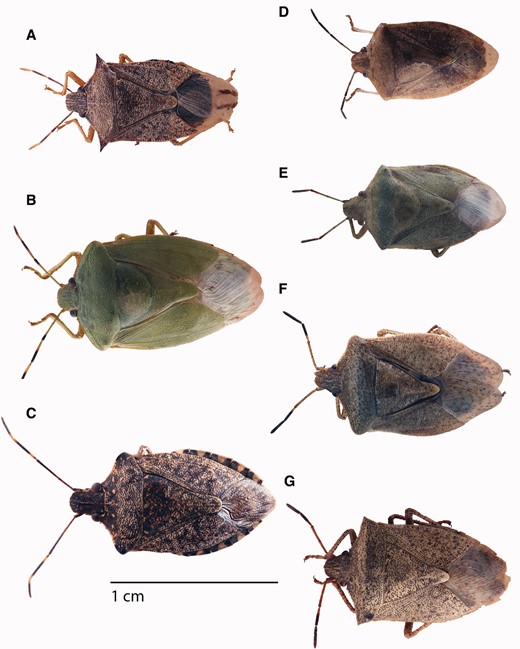
Stink bug adults commonly encountered in soybean and corn of the midwestern United States: (A) Podisus maculiventris, (B) Chinavia hilaris, (C) Halyomorpha halys, (D) Thyanta custator accerra (green), (E) Thyanta custator accerra (brown), (F) Euschistus servus euschistoides, and (G) Euschistus variolarius (photo credits: C. Kurtz and C. Philips, and modified by D. Pezzini).
Plant-feeding stink bugs potentially encountered in soybean and corn of the midwestern United States (“X” indicates reported presence of a species in a particular crop)
| Scientific name . | Common name . | Soybean . | Corn . |
|---|---|---|---|
| Banasa dimidiata (Say)a,b,c,d,e | Xg | ||
| Banasa euchlora Ståla,b,c | Xg | ||
| Chinavia hilaris (Say)a,b,c,d,e | Green stink bug | Xf,g,h,j,k,l | Xf,h,i,j |
| Chinavia pensylvanica (Gmelin)c,d,e | Xg | ||
| Chlorochroa persimilis Horvatha,b,c,d,e | Xl | Xf,h,i,j | |
| Coenus delius (Say)a,b,c,d,e | Xf,l | ||
| Cosmopepla lintneriana Kirkaldya,b,c,d,e | Xl | Xj | |
| Euschistus ictericus (L.)b,c,d,e | Xf,g,k,l | Xi,o | |
| Euschistus servus (Say)a,b,c,d,e | Brown stink bug | Xf,g,h,j,k,l | Xf,h,i,j,o |
| Euschistus tristigmus (Say)a,b,c,d,e | Dusky stink bug | Xf,g,h,k,l | Xi,o |
| Euschistus variolarius (Palisot de Beauvois)a,b,c,d,e | Onespotted stink bug | Xf,g,h,j,l | Xf,g,o |
| Halyomorpha halys (Stål)c,d,e | Brown marmorated stink bug | Xm | Xm |
| Holcostethus limbolarius (Stål)a,b,c,d,e | Xf,g,l | Xf | |
| Hymenarcys nervosa (Say)a,c,d | Xf,g,j | Xn | |
| Mecidea major Sailera,c,e | Xj | ||
| Mormidea lugens (F.)a,b,c,d,e | Xg,l | ||
| Murgantia histrionica (Hahn)a,b,c,e | Harlequin bug | Xf | |
| Neottiglossa undata (Say)b,c,d,e | Xl | ||
| Oebalus pugnax (F.)a,c,d,e | Rice stink bug | Xf,g,j,k | Xf,i,j |
| Piezodorus guildinii (Westwood)c | Redbanded stink bug | Xg,h,j,k | |
| Proxys punctulatus (Palisot de Beauvois)c | Xf,g | ||
| Thyanta calceata (Say)c,d | Xf,g | ||
| Thyanta custator accerra McAteea,b,c,d,e | Redshouldered stink bug | Xf,g,j,k,l | Xf,j |
| Thyanta pallidovirens (Stål)a | Xg |
| Scientific name . | Common name . | Soybean . | Corn . |
|---|---|---|---|
| Banasa dimidiata (Say)a,b,c,d,e | Xg | ||
| Banasa euchlora Ståla,b,c | Xg | ||
| Chinavia hilaris (Say)a,b,c,d,e | Green stink bug | Xf,g,h,j,k,l | Xf,h,i,j |
| Chinavia pensylvanica (Gmelin)c,d,e | Xg | ||
| Chlorochroa persimilis Horvatha,b,c,d,e | Xl | Xf,h,i,j | |
| Coenus delius (Say)a,b,c,d,e | Xf,l | ||
| Cosmopepla lintneriana Kirkaldya,b,c,d,e | Xl | Xj | |
| Euschistus ictericus (L.)b,c,d,e | Xf,g,k,l | Xi,o | |
| Euschistus servus (Say)a,b,c,d,e | Brown stink bug | Xf,g,h,j,k,l | Xf,h,i,j,o |
| Euschistus tristigmus (Say)a,b,c,d,e | Dusky stink bug | Xf,g,h,k,l | Xi,o |
| Euschistus variolarius (Palisot de Beauvois)a,b,c,d,e | Onespotted stink bug | Xf,g,h,j,l | Xf,g,o |
| Halyomorpha halys (Stål)c,d,e | Brown marmorated stink bug | Xm | Xm |
| Holcostethus limbolarius (Stål)a,b,c,d,e | Xf,g,l | Xf | |
| Hymenarcys nervosa (Say)a,c,d | Xf,g,j | Xn | |
| Mecidea major Sailera,c,e | Xj | ||
| Mormidea lugens (F.)a,b,c,d,e | Xg,l | ||
| Murgantia histrionica (Hahn)a,b,c,e | Harlequin bug | Xf | |
| Neottiglossa undata (Say)b,c,d,e | Xl | ||
| Oebalus pugnax (F.)a,c,d,e | Rice stink bug | Xf,g,j,k | Xf,i,j |
| Piezodorus guildinii (Westwood)c | Redbanded stink bug | Xg,h,j,k | |
| Proxys punctulatus (Palisot de Beauvois)c | Xf,g | ||
| Thyanta calceata (Say)c,d | Xf,g | ||
| Thyanta custator accerra McAteea,b,c,d,e | Redshouldered stink bug | Xf,g,j,k,l | Xf,j |
| Thyanta pallidovirens (Stål)a | Xg |
References for occurrence in midwestern United States:
References for crop associations:
Plant-feeding stink bugs potentially encountered in soybean and corn of the midwestern United States (“X” indicates reported presence of a species in a particular crop)
| Scientific name . | Common name . | Soybean . | Corn . |
|---|---|---|---|
| Banasa dimidiata (Say)a,b,c,d,e | Xg | ||
| Banasa euchlora Ståla,b,c | Xg | ||
| Chinavia hilaris (Say)a,b,c,d,e | Green stink bug | Xf,g,h,j,k,l | Xf,h,i,j |
| Chinavia pensylvanica (Gmelin)c,d,e | Xg | ||
| Chlorochroa persimilis Horvatha,b,c,d,e | Xl | Xf,h,i,j | |
| Coenus delius (Say)a,b,c,d,e | Xf,l | ||
| Cosmopepla lintneriana Kirkaldya,b,c,d,e | Xl | Xj | |
| Euschistus ictericus (L.)b,c,d,e | Xf,g,k,l | Xi,o | |
| Euschistus servus (Say)a,b,c,d,e | Brown stink bug | Xf,g,h,j,k,l | Xf,h,i,j,o |
| Euschistus tristigmus (Say)a,b,c,d,e | Dusky stink bug | Xf,g,h,k,l | Xi,o |
| Euschistus variolarius (Palisot de Beauvois)a,b,c,d,e | Onespotted stink bug | Xf,g,h,j,l | Xf,g,o |
| Halyomorpha halys (Stål)c,d,e | Brown marmorated stink bug | Xm | Xm |
| Holcostethus limbolarius (Stål)a,b,c,d,e | Xf,g,l | Xf | |
| Hymenarcys nervosa (Say)a,c,d | Xf,g,j | Xn | |
| Mecidea major Sailera,c,e | Xj | ||
| Mormidea lugens (F.)a,b,c,d,e | Xg,l | ||
| Murgantia histrionica (Hahn)a,b,c,e | Harlequin bug | Xf | |
| Neottiglossa undata (Say)b,c,d,e | Xl | ||
| Oebalus pugnax (F.)a,c,d,e | Rice stink bug | Xf,g,j,k | Xf,i,j |
| Piezodorus guildinii (Westwood)c | Redbanded stink bug | Xg,h,j,k | |
| Proxys punctulatus (Palisot de Beauvois)c | Xf,g | ||
| Thyanta calceata (Say)c,d | Xf,g | ||
| Thyanta custator accerra McAteea,b,c,d,e | Redshouldered stink bug | Xf,g,j,k,l | Xf,j |
| Thyanta pallidovirens (Stål)a | Xg |
| Scientific name . | Common name . | Soybean . | Corn . |
|---|---|---|---|
| Banasa dimidiata (Say)a,b,c,d,e | Xg | ||
| Banasa euchlora Ståla,b,c | Xg | ||
| Chinavia hilaris (Say)a,b,c,d,e | Green stink bug | Xf,g,h,j,k,l | Xf,h,i,j |
| Chinavia pensylvanica (Gmelin)c,d,e | Xg | ||
| Chlorochroa persimilis Horvatha,b,c,d,e | Xl | Xf,h,i,j | |
| Coenus delius (Say)a,b,c,d,e | Xf,l | ||
| Cosmopepla lintneriana Kirkaldya,b,c,d,e | Xl | Xj | |
| Euschistus ictericus (L.)b,c,d,e | Xf,g,k,l | Xi,o | |
| Euschistus servus (Say)a,b,c,d,e | Brown stink bug | Xf,g,h,j,k,l | Xf,h,i,j,o |
| Euschistus tristigmus (Say)a,b,c,d,e | Dusky stink bug | Xf,g,h,k,l | Xi,o |
| Euschistus variolarius (Palisot de Beauvois)a,b,c,d,e | Onespotted stink bug | Xf,g,h,j,l | Xf,g,o |
| Halyomorpha halys (Stål)c,d,e | Brown marmorated stink bug | Xm | Xm |
| Holcostethus limbolarius (Stål)a,b,c,d,e | Xf,g,l | Xf | |
| Hymenarcys nervosa (Say)a,c,d | Xf,g,j | Xn | |
| Mecidea major Sailera,c,e | Xj | ||
| Mormidea lugens (F.)a,b,c,d,e | Xg,l | ||
| Murgantia histrionica (Hahn)a,b,c,e | Harlequin bug | Xf | |
| Neottiglossa undata (Say)b,c,d,e | Xl | ||
| Oebalus pugnax (F.)a,c,d,e | Rice stink bug | Xf,g,j,k | Xf,i,j |
| Piezodorus guildinii (Westwood)c | Redbanded stink bug | Xg,h,j,k | |
| Proxys punctulatus (Palisot de Beauvois)c | Xf,g | ||
| Thyanta calceata (Say)c,d | Xf,g | ||
| Thyanta custator accerra McAteea,b,c,d,e | Redshouldered stink bug | Xf,g,j,k,l | Xf,j |
| Thyanta pallidovirens (Stål)a | Xg |
References for occurrence in midwestern United States:
References for crop associations:
Among the species potentially encountered in soybean and corn in the midwestern United States (Table 1), several are encountered with frequency. Koch and Pahs (2014, 2015) and Koch and Rich (2015) recently performed surveys of the stink bugs associated with soybean and corn in Minnesota. In Minnesota soybean, E. variolarius, Euschistusservus euschistoides, and C. hilaris comprise 68–90% of stink bug adults (Koch and Pahs 2014, Koch and Rich 2015). In Minnesota corn, E. variolarius and E. servus euschistoides comprised 95–100% of stink bug adults (Koch and Pahs 2015). A predatory species, Podisus maculiventris (spined soldier bug) is also observed in corn and soybean (Koch and Pahs 2014, 2015; Koch and Rich 2015). Further work is needed to characterize the stink bug community associated with these crops in other states in the region. Understanding of stink bug anatomy (i.e., body parts) is important for identification of these species (Fig. 1).
Euschistus servus (Brown Stink Bug)
Euschistus servus occurs throughout the midwestern United States and much of North America (McPherson 1982), and is the most common species of Euschistus in the United States (Slater and Baranowski 1978). This species is composed of two subspecies (McPherson 1982). Euschistus servus euschistoides occurs in the northern United States (Fig. 2), and E. servus servus occurs in the southern United States. These two subspecies are known to interbreed and create a hybrid population, where their two populations meet in a swath extending from roughly Kansas to Maryland (McPherson 1982). These subspecies and the hybrid are similar in size (11.0–15.0 mm long; McPherson 1982) and color (brown or light brown). However, the subspecies can be distinguished from each other based on close examination of the head, antennae, and edge of the abdomen (i.e., connexivum). The tip of the head of E. servus euschistoides appears notched because the juga are longer than the tylus, whereas the tip of the head of E. servus servus does not appear notched, because the juga and tylus are equal or nearly equal in length (Paiero et al. 2013). In addition, the last two segments of the antennae (i.e., segments four and five) are usually dark brown in E. servus euschistoides and yellowish-brown or reddish-brown in E. servus servus (Paiero et al. 2013). Finally, the edge of the abdomen is completely or nearly completely covered by the front wing in E. servus euschistoides, whereas the edge of the abdomen is more exposed in E. servus servus (Paiero et al. 2013). Hybrid adults present a combination of the characters of the two subspecies (McPherson 1982).
Euschistus variolarius (Onespotted Stink Bug)
Euschistus variolarius occurs throughout the midwestern United States and much of North America (McPherson 1982). This is the most common stink bug in northern states, but it is relatively uncommon in the southern United States, particularly below 37° latitude (Parish 1934, Slater and Baranowski 1978). Adults of E. variolarius are yellowish-brown and 11.0–15.0 mm long (McPherson 1982, Panizzi et al. 2000; Fig. 2). Males have a large black spot on the underside near the tip of the abdomen (i.e., on the pygophore), hence the common name “onespotted stink bug” (Paiero et al. 2013). Euschistus variolarius and E. servus can be found in the same habitats at the same time (Koch and Pahs 2014, 2015) and look very similar to one another. However, E. variolarius can be fairly easily distinguished from E. servus euschistoides (i.e., the more common subspecies in much of the midwestern United States) by examination of the tip of the head and the “shoulders” (i.e., anterolateral margins of the pronotum; Fig. 2). The tip of the head of E. servus euschistoides appears notched, because the juga are longer than the tylus, whereas the tip of the head of E. variolarius does not appear notched, because the juga and tylus are equal or nearly equal in length (Paiero et al. 2013). The “shoulders” of E. variolarius are generally more pointed than those of E. servus euschistoides (McPherson 1982). Presence of the black spot on the pygophore of E. variolarius males is lacking in E. servus (McPherson 1982), but should not be confused with the black spots on the underside of the abdomen of a slightly smaller species, Euschistus tristigmus (Say).
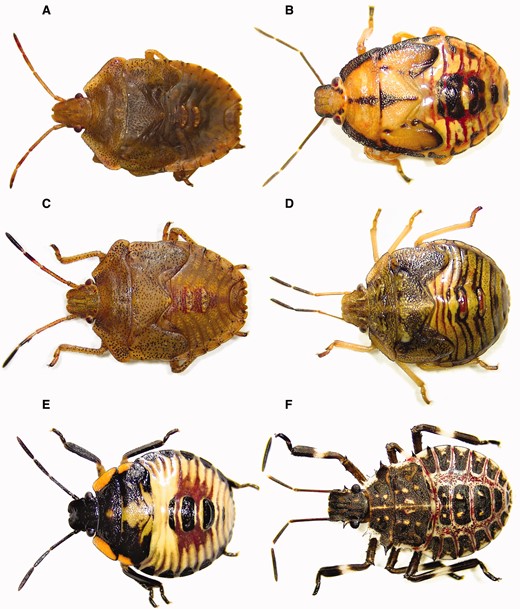
Stink bug nymphs (late-instars) encountered in soybean and corn of the midwestern United States: (A) Euschistus servus, (B) Podisus maculiventris, (C) Euschistus variolarius, (D) Thyanta custator, (E) Chinavia hilaris, and (F) Halyomorpha halys (photo credit: D. Pezzini).
Chinavia hilaris (Green Stink Bug)
Chinavia hilaris occurs throughout the midwestern United States and much of North America (McPherson 1982). This species is often referred to Acrosternum hilare (Say) (Kamminga et al. 2012). Adults of C. hilaris are green and 13.0–19.0 mm long (McPherson 1982, Panizzi et al. 2000; Fig. 2). However, an orange color form is infrequently encountered (Kamminga et al. 2012). In crops in the midwestern United States, C. hilaris is generally larger than the other green-colored stink bugs, such as Chlorochroa persimilis (11–15 mm) or T. custator accerra (see below; McPherson 1982, Rider 2012). Nymphs of C. hilaris are oval-shaped and range in size from 1.6 to 12.7 mm (DeCoursey and Esselbaugh 1962). Coloration of the nymphs transitions from mostly black with orange markings to mostly green with black and orange markings as nymphs develop (DeCoursey and Esselbaugh 1962; Fig. 3). However, as with the adults, the nymphs of C. hilaris present two different color forms (i.e., a light form and a dark form; Kamminga et al. 2012).
Thyanta custator accerra (Redshouldered Stink Bug)
Thyanta custator accerra occurs throughout the midwestern United States and large areas of North America (McPherson 1982, Rider and Chapin 1992). Adults of T. custator accerra are 9.0–13.0 mm long (McPherson 1982, Paiero et al. 2013; Fig. 2). Two color forms of this species exist; a green form in spring and summer, and a brown form in fall (McPherson 1982, Paiero et al. 2013). Some individuals of the green color form have a red- or pink-colored band across the pronotum, hence the common name “redshouldered stink bug” (Paiero et al. 2013). This species can be distinguished from P. guildinii, the redbanded stink bug, by the presence of a prominent spine extending from the base of the abdomen between the hind legs and pointing toward the head on P.guildinii, and the lack of such spine on T. custator accerra (Kamminga et al. 2009). A description of the nymphal instars of this species is provided by DeCoursey and Esselbaugh (1962). In general, nymphs range from 0.9 to 8.2 mm long (DeCoursey and Esselbaugh 1962). Coloration of the thorax and abdomen of nymphs transitions from brown with white and yellow markings to brown with white, amber, and yellow markings as nymphs progress from the first to fifth instars (DeCoursey and Esselbaugh 1962; Fig. 3). The third to fifth instars generally have a “T”-shaped mark on the pronotum (DeCoursey and Esselbaugh 1962).
Halyomorpha halys (Brown Marmorated Stink Bug)
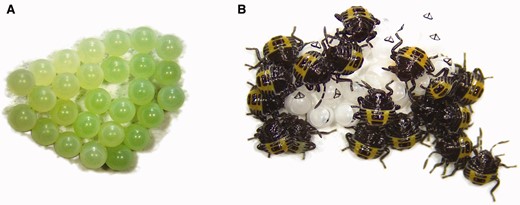
Stink bug (H. halys) egg mass (A) and first-instar nymphs on hatched egg mass (B) (photo credit: D. Pezzini).
Podisusmaculiventris (Spined Soldier Bug)
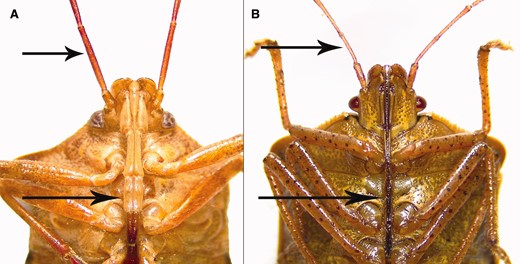
Mouthparts (i.e., rostra) of predatory (A) and herbivorous (B) stink bugs. Rostrum of predator is thick (about twice the thickness of the antenna), and rostrum of herbivore is thin (similar to thickness of antenna) (arrows indicate rostra and antennae; photo credit: D. Pezzini).
Biology
General
Stink bugs are herbivorous, predaceous, or occasionally omnivorous; generalists or specialists in feeding preference; and occur in a diversity of habitats ranging from natural to cultivated, and grassy or herbaceous to arboreal (McPherson 1982, De Clerq 2000). With such diversity, it is no surprise that this group contains both beneficial and pest species (McPherson 1982, Panizzi et al. 2000, De Clerq 2000). Members of the subfamily Asopinae are predaceous and some (e.g., Podisus maculiventris and Perillus bioculatus (F.)) are well-known predators of significant pests (De Clerq 2000). Members of the subfamilies Pentatominae and Podopinae are primarily herbivorous, but some have been reported to occasionally prey on other insects (McPherson 1982). Most species, such as E. servus, E. variolarius, C. hilaris, and H. halys, are generalists and feed on many hosts across several plant families (McPherson 1982, Rice et al. 2014).
Stink bugs of temperate regions generally overwinter as adults in protected locations (e.g., under leaf litter or other debris; Saulish and Musolin 2012). Adults of H. halys will also overwinter under loose tree bark or in buildings (Rice et al. 2014). Some species overwinter in other life stages. For example, Apoecilus cynicus (Say), a predatory species in the region, overwinters as eggs (Saulish and Musolin 2012). The winter is passed in a physiological state called diapause, which is associated with suppressed sexual development and behavior, active growth of the fat body, reduced oxygen consumption, and increased cold hardiness (Saulish and Musolin 2012). In most species of temperate stink bugs with diapause in the adult stage, diapause is induced by short day lengths experienced during the nymphal stage; however, temperature and food quality can also play a role in diapause induction (Saulish and Musolin 2012). Development resumes in spring with longer day lengths, increasing temperatures, and availability of food resources (Saulish and Musolin 2012). The surrounding landscape can play a role in population buildup of stink bug species, particularly those that are agricultural pests (Panizzi 1997). The generalist feeding habits and high mobility of these species allow them to move throughout the landscape utilizing different plant species (wild and cultivated) at different times, often depending on timing of fruit and seed development of the plants (Panizzi 1997, McPherson and McPherson 2000, Reisig 2011, Pilkay et al. 2015). Mating occurs in an end-to-end position (McPherson 1982). Females generally lay barrel-shaped eggs in clusters on plant tissues, such as the undersides of leaves (Panizzi et al. 2000, McPherson and McPherson 2000; Fig. 4). After egg hatch, stink bugs develop through five instars prior to becoming adults (Panizzi et al. 2000, McPherson and McPherson 2000). In the midwestern United States, stink bugs generally undergo one or two generations per year (i.e., univoltine or bivoltine, respectively; McPherson 1982).
For herbivorous stink bugs, adults and nymphs, except first instars, actively feed on plant tissues. The first instars are generally considered a nonfeeding stage and metabolize internal nutrient reserves, and acquire important microbial symbionts from the egg mass (McPherson and McPherson 2000, Panizzi et al. 2000; Fig. 4). The adults and fifth instars often cause more injury than the earlier stages (McPherson 1982). Stink bugs feed on all above-ground plant parts, including stems, petioles, leaves, flowers, fruits, and seeds, but they generally prefer developing shoots, fruits, and seeds (Todd and Herzog 1980, McPherson and McPherson 2000). Stink bugs feed by inserting their piercing–sucking mouthparts into plant tissues, injecting digestive enzymes, and sucking up nutrients from plant tissues (McPherson and McPherson 2000). The act of inserting the mouthparts into the tissue causes mechanical injury and tissues are chemically injured by the enzymes injected by the insects (Hori 2000). Feeding injury can result in reduced plant turgor pressure via removal of plant fluids, abnormal plant growth, deformation and discoloration of seeds and fruit, abortion of seeds and fruit, delayed plant maturity (e.g., stay-green syndrome in soybean), transmission of pathogens, or plant death (McPherson and McPherson 2000, Panizzi et al. 2000, Vyavhare et al. 2015b). Injury to fruit and seed is often greater when stink bugs feed earlier in the development of these plant structures (Panizzi et al. 2000). In crops, these various injuries can translate to reductions in quality and yield (McPherson and McPherson 2000, Panizzi et al. 2000).
Finally, as their common name implies, stink bugs produce odorous secretions from scent glands (Aldrich 1988). These secretions serve as defense against natural enemies or as aggregation-, sex-, or alarm pheromones (Aldrich 1988, McPherson and McPherson 2000).
Euschistus servus (Brown Stink Bug)
Euschistus servus can be found on a diversity of wild and cultivated plant species (McPherson 1982, McPherson and McPherson 2000, Panizzi et al. 2000). For example, a combined list of crop plants from which E. servus subspecies or their hybrid have been collected includes corn, soybean, wheat, oats, sunflower, sugar beet, alfalfa, clover, tobacco, cotton, tomato, cabbage, bean, pepper, squash, pea, okra, cantaloupe, blueberry, raspberry, grape, cherry, blackberry, apple, pear, peach, citrus, and pecan (McPherson 1982, McPherson and McPherson 2000). This species is considered the most economically important Euschistus species in the United States and Canada (Panizzi et al. 2000). However, the broad host range of E. servus may contribute to it not being an even more significant pest of crops such as soybean (McPherson and McPherson 2000). Because its host range includes numerous cultivated and wild plants with temporally overlapping reproductive (fruiting) growth stages, populations of E. servus may spread across several plant species on the landscape and not necessarily be concentrated in any one crop (Jones and Sullivan 1982, McPherson and McPherson 2000). Adults of E. servus overwinter under objects such as crop debris, leaves, and grass, and prefer to overwinter in open fields rather than wooded areas (McPherson 1982). In Iowa and Illinois, E. servus has been reported as being bivoltine (McPherson 1982). For example, in southern Illinois, peaks of adult activity were observed in early April to mid-May (overwintered adults), early July to late August (first-generation adults), and mid-September to late October (second-generation adults; Munyaneza and McPherson 1994). This species may be univoltine farther north. Eggs are laid in masses of 8–41 eggs (mean = 17.6; Munyaneza and McPherson 1994). When reared on green beans in a growth chamber at 23 °C, mean development time of E. servus from egg to adult was 44.3 d (egg = 5.8 d, first instar = 5.0 d, second instar = 6.0 d, third instar = 6.7 d, fourth instar = 9.3 d, and fifth instar = 11.5 d; Munyaneza and McPherson 1994).
Euschistus variolarius (Onespotted Stink Bug)
Euschistus variolarius can be found on a diversity of wild and cultivated plant species, including crops such as corn, soybean, wheat, rye, oats, sugar beet, alfalfa, clover, cotton, tobacco, bean, asparagus, tomato, potato, onion, squash, cantaloupe, strawberry, grape, raspberry, cherry, peach, and pear (McPherson 1982). Adults overwinter in protected locations, such as under dry leaves, logs, and dead grass in fence rows (Parish 1934). This species has been reported as univoltine or bivoltine (McPherson 1982), with univoltine populations occurring north of 40° latitude (Panizzi et al. 2000). For example, E. variolarius was observed to be univoltine in southern Illinois, with peak adult activity in mid-April to mid-June (overwintered adults), followed by appearance of first-generation adults in late June to late August (Munyaneza and McPherson 1994). Eggs are laid in masses of 6–27 (mean = 16.2) eggs (Munyaneza and McPherson 1994). When reared on green beans in a growth chamber at 23 °C, mean development time of E. variolarius from egg to adult was 46.8 d (egg = 5.4 d, first instar = 4.9 d, second instar = 5.7 d, third instar = 7.8 d, fourth instar = 9.7 d, and fifth instar = 13.3 d; Munyaneza and McPherson 1994).
Chinavia hilaris (Green Stink Bug)
The biology of C. hilaris was recently reviewed by Kamminga et al. (2012). Chinavia hilaris prefers woody plants (McPherson 1982, Kamminga et al. 2012). However, this species can be found on a variety of wild and cultivated plants, including crops such as corn, soybean, sugar beet, cotton, alfalfa, clover, asparagus, cabbage, eggplant, green bean, lima bean, pea, pepper, tomato, turnip, mustard, okra, strawberry, raspberry, black berry, grape, apple, apricot, cherry, orange, peach, pear, plum, and pecan (McPherson 1982). Adults of C. hilaris overwinter under leaf litter in deciduous wooded areas (McPherson 1982, Kamminga et al. 2012). This species is generally considered univoltine, particularly in the northern United States (McPherson 1982, Kamminga et al. 2012). Bivoltine populations of C. hilaris occur in the Gulf States and extend as far north as Kansas, Arkansas, and southern Illinois (McPherson 1982). Photoperiod (i.e., day length) is an important determinant of the number of generations for this species (Kamminga et al. 2012). This species is considered semimigratory (Panizzi et al. 2000), and migration from southern areas may contribute to populations in northern areas (http://www.ent.iastate.edu/soybeaninsects/node/145). Eggs are laid in masses of 1–72 eggs (Underhill 1934), with a mean of 32 eggs per cluster (Miner 1966). The lower developmental threshold for C. hilaris is 15 °C, and development from egg to adult requires the accumulation of 482.7 degree days (Simmons and Yeargan 1988). When reared on soybean seeds in a growth chamber at 24 °C, mean development time of C. hilaris from egg to adult was 48.3 d (egg = 9.9 d, first instar = 5.0 d, second instar = 8.9 d, third instar = 5.8 d, fourth instar = 7.2 d, and fifth instar = 11.5 d; Simmons and Yeargan 1988). However, Da Silva and Daane (2014) reported values considerably different (i.e., lower developmental threshold 12.3 °C and 588 degree days) and suggested the difference may be owing to genetics between populations in Kentucky and California or diet used in experiments.
Thyanta custator accerra (Redshouldered Stink Bug)
The biology of T.custator accerra has not been studied in detail compared with other common stink bugs. This species has been collected from numerous wild and cultivated plants, including crops such as corn, soybean, wheat, oats, sorghum, alfalfa, clover, bean, eggplant, lima bean, asparagus, and peach (McPherson 1982). This species overwinters as adults (McPherson 1982). Thyanta custator accerra is bivoltine in the southern United States (Panizzi et al. 2000), may be only partially bivoltine in north-central Illinois (McPherson 1982), and is likely univoltine in northern states.
Halyomorpha halys (Brown Marmorated Stink Bug)
The biology of H. halys in Asia and North America was recently reviewed by Lee et al. (2013) and Rice et al. (2014), respectively. In Asia, H. halys has been collected from 106 species of plants across 45 plant families (Lee et al. 2013). In the United States, H. halys has also been collected from numerous wild and cultivated plants, including crops such as corn, soybean, sunflower, cereal rye, wheat, garden cucumber, field pumpkin (summer squash), horseradish, Swiss chard, cabbage, collards, cayenne pepper, eggplant, garden tomato, filbert, hazelnut, common hop, bean, apricot, peach, raspberry, blackberry edible fig, highbush blueberry, wine grape, apple, cherry, pear, and pecan (Rice et al. 2014; http://www.stopbmsb.org/where-is-bmsb/host-plants/#host_plants_table). Adults overwinter under debris, in tree holes or under bark, in human-made structures, or in dry areas on mountains (Lee et al. 2013, 2014). This species is likely univoltine to bivoltine in the midwestern United States, as it is in the mid-Atlantic region (Rice et al. 2014). In southern Asia, it can have as many as four to six generations per year (Lee et al. 2013). Eggs are laid in masses of 20–30 eggs, and females lay about 244 egg clusters in a lifetime (Hoebeke and Carter 2003, Nielsen et al. 2008a). The lower and upper developmental thresholds for H. halys are 14°C and 35 °C, respectively, and development from egg to adult requires the accumulation of 538 degree days (Nielsen et al. 2008a). When reared on green beans and Spanish peanuts in a growth chamber at 25 °C, mean development time of H. halys from egg to adult was 44.9 d (egg = 6.1 d, first instar = 4.8 d, second instar = 9.6 d, third instar = 7.1 d, fourth instar = 7.4 d, and fifth instar = 10.4 d; Nielsen et al. 2008a).
Podisus maculiventris (Spined Soldier Bug)
Unlike the previously described species, P. maculiventris is predatory. This predator shows a preference for lepidopteran larvae (i.e., caterpillars), but is known to feed on >90 species of insects spanning eight insect orders occurring on a diversity of wild and cultivated plants (McPherson 1982, De Clerq 2000). Podisus maculiventris and other predatory bugs often prefer prey that are large relative to their body size (Cohen 2000). Like the plant-feeding stink bugs, P. maculiventris feeds with piercing–sucking mouthparts. Cohen (2000) describes the feeding of P. maculiventris and other predatory bugs as solid-to-liquid feeding. The predators use their mouthparts to pierce the body wall of their prey and inject saliva (Cohen 2000). Enzymes and mechanical action of the mouthparts liquefy the tissues of the prey, and the predator then sucks up the liquefied nutrients from inside the prey (Cohen 2000). Podisus maculiventris can feed on plants to acquire moisture and additional nutrients when prey is scarce, but this feeding is not known to cause crop injury (De Clerq 2000, Lambert 2007). Adults overwinter in protected locations, such as in leaf litter or under stones or bark of trees (De Clerq 2000). In much of the midwestern United States and southern Canada, P. maculiventris is univoltine to trivoltine, but more generations are likely in the southern United States (McPherson 1982, De Clerq 2000). Eggs are laid in masses of 15–30 eggs (De Clerq 2000). When reared at 23 °C, development time of P. maculiventris from egg to adult was 33.5–36.5 d for a population for the northeastern United States (De Clerq 2000).
Injury to Crops
Injury to soybean resulting from stink bug feeding (increasing stink bug feeding from left to right; photo credit: A. Michel).
Injury to corn resulting from stink bug feeding (photo credit: P. Thomison).
Soybean
The impact of stink bugs on soybean has been well studied and has been reviewed by Todd and Herzog (1980), Panizzi and Slansky (1985), and McPherson and McPherson (2000). Stink bugs can feed on all above-ground parts of soybean, but prefer pods and developing seeds (Todd and Herzog 1980, Lee et al. 2013). Fifth instars and adults cause more severe damage than early instars (Simmons and Yeargan 1988, McPherson and McPherson 2000). Species may vary in feeding duration and depth of injury to seed, which can result in different levels of damage (Corrêa-Ferreira and De Azevedo 2002, Depieri and Panizzi 2011). Initial colonization of soybean in the midwestern United States typically occurs during flowering (Koch and Pahs 2014, Koch and Rich 2015, Hunt, personal observation), as in other regions (Pilkay et al. 2015). Populations of stink bugs in soybean then increase and peak during pod and seed development stages (McPherson and McPherson 2000, Koch and Pahs 2014, Koch and Rich 2015, Hunt, personal observation, Michel, personal observation). Stink bug abundance is affected by planting date and maturity group of soybean (Gore et al. 2006, Owens et al. 2013, Temple et al. 2013). In addition, stink bug populations may be affected by other pest management tactics. For example, Rich and Koch (2016) found that H. halys preferred and survived better on aphid-resistant soybean than on aphid-susceptible soybean.
Stink bug injury to soybean can impact yield, seed quality, and germination rates (Todd and Herzog 1980, Panizzi and Slansky 1985, McPherson and McPherson 2000, Mesquita et. al. 2006). Although some studies report yield losses owing to stink bug injury (Boethel et. al. 2000; McPherson and McPherson 2000; Vyavhare et al. 2015a,b), others show no difference in yield owing to stink bug feeding (Corrêa-Ferreira and De Azevedo 2002, Owens 2012, Owens et al. 2013). The variation of results may be explained by several factors. The severity of damage caused by stink bugs can depend on soybean developmental stage, density of bugs, and duration of infestation (Young et. al. 2008, Owens 2012, Owens et al. 2013). Among these, soybean developmental stage is the main factor (Smith et al. 2009, Nielsen et al. 2011). In general, feeding during early pod and seed development can result in pod loss and seed abortion (flat pods); feeding during pod fill can result in shriveled, deformed, and smaller seeds; and feeding during seed maturation can result in slight deformation of seed and discolored puncture marks (Todd and Herzog 1980, Panizzi and Slansky 1985, McPherson and McPherson 2000, Mesquita et. al. 2006, Owens 2012, Koch and Rich 2015, Vyavhare et al. 2015a; Fig. 6). For example, in a caged experiment, infestation of soybean with E. servus and E. variolarius at different timings resulted in decreased injury with increasing plant reproductive growth stage (McPherson and McPherson 2000). Soybean has been shown to compensate for stink bug feeding by increasing the weight of unaffected seeds (Todd and Turnipseed 1974, Russin et al. 1987, Boethel et al. 2000, McPherson and McPherson 2000, Koch and Rich 2015).
In addition to impacts on yield, stink bug feeding can affect the quality and maturity of soybean. When seed fed upon by stink bugs is sown, reductions in germination, emergence, and survival of seedlings can be observed (Jensen and Newsom 1972). Germination of seed is more affected by the location of feeding punctures (e.g., punctures near the radicle–hypocotyl axis) than the overall number of feeding punctures (McPherson and McPherson 2000). Locally, stink bug feeding punctures form small brown or black spots in the pod (Kogan and Herzog 1980). Stink bug feeding can increase protein and decrease oil content of soybean seeds and alter the fatty acid composition of soybean oil (Todd and Herzog 1980, Panizzi and Slansky 1985, McPherson and McPherson 2000). However, such impacts to quality were not detected for H. halys feeding on soybean in Minnesota (Koch and Rich 2015). In addition, stink bug feeding, particularly during pod-set and pod-filling stages, can cause delayed plant maturity (i.e., “stay-green” syndrome; Todd and Herzog 1980, Panizzi and Slansky 1985, McPherson and McPherson 2000, Musser et. al 2011, Vyavhare et al. 2015b), which can adversely affect harvest of the crop (Musser et al. 2011). In the midwestern United States, delayed maturity has been observed in Ohio soybean with mixed infestation of stink bugs (Michel, personal observation) and in a cage study with H. halys in Minnesota (Koch and Rich 2015). Although some studies try to explain the mechanisms of delayed maturity of soybeans (Boethel et al. 2000, Egli and Bruening 2006), questions remain about the specific mechanism (Vyavhare et al. 2015b). Finally, feeding by stink bugs can transmit pathogens to soybean. For example, stink bugs transmit Nematospora coryli Peglion, which causes yeast-spot disease (Daugherty 1967, Ragsdale et al. 1979). Stink bugs can also transmit bacteria with potential plant pathogenicity to soybean (Ragsdale et al. 1979, Husseneder et al. 2016).
Corn
Stink bugs can colonize and feed on corn from emergence of the plants through maturity. The seedling and early reproductive stages of corn appear most susceptible to stink bug feeding. In the midwestern United States, injury to early growth stage corn has been reported from Indiana and Illinois (Edwards et al. 1985), Minnesota (B. Potter, personal communication), and Nebraska (Hunt, personal observation). Fields with increased risk of injury from stink bugs on early growth stages of corn are those with no-till or reduced-tillage, cover crop prior to planting, or corn following wheat (Edwards et al. 1985, Townsend and Sedlacek 1986, Sedlacek and Townsend 1988a).
Stink bugs feed on early vegetative corn by inserting their mouthparts into the bases of plants while their bodies rest on the soil surface or on the plants with heads oriented downward (Townsend and Sedlacek 1986). Feeding at the plant base causes mechanical and chemical injury to the growing point of the plant (Sedlacek and Townsend 1988a). Injury to early vegetative growth stages of corn by stink bugs can result in yield reduction (Annan and Bergman 1988), and symptoms include elongate holes surrounded by chlorotic or necrotic tissue on the leaves, twisting and bending of terminal leaves, tightly rolled or severed whorl leaves, wilting, stunting, tillering, and plant death (Annan and Bergman 1988, Sedlacek and Townsend 1988a). Feeding by E. servus and E. variolarius on corn seedlings can cause immediate termination of or delay in plant growth and result in decreased above- and below-ground biomass (Townsend and Sedlacek 1986, Sedlacek and Townsend 1988a). The most significant impacts from E. servus and E. variolarius feeding are tillering and plant mortality (Apriyanto et al. 1989a,b). Tillering of corn plants is caused by stink bugs feeding on lower portions of plants (Townsend and Sedlacek 1986). The type of tissue damaged and amount of tissue damaged are likely the most important factors contributing to injury, such as tillering (Apriyanto et al. 1989a). Plants that tiller in response to stink bug feeding are shorter, have delayed silking, and decreased yields compared with plants exposed to stink bugs that did not tiller and unexposed plants (Apriyanto et al. 1989b).
Susceptibility of early growth stages of corn to stink bug feeding varies with plant growth stage and stink bug life stage. In general, early corn growth stages (e.g., seedlings) are most susceptible and large nymphs and adults of stink bugs are most damaging (Sedlacek and Townsend 1988a).
In Minnesota, E. variolarius and E. servus euschistoides were the most abundant stink bug species found on corn during reproductive plant growth stages (Koch and Pahs 2015). During reproductive growth stages of corn, stink bugs will feed on developing ears and kernels and, depending on timing of infestation, can affect ear number, ear size, and kernel size and quality (Negrón and Riley 1987, Ni et al. 2010, Rice et al. 2014; Fig. 7). Corn plants appear most susceptible to stink bug feeding during early development of the corn ears, including late vegetative corn growth stages. Observations of ear abortion have been made for H. halys feeding on late vegetative stages of corn (Rice et al. 2014). Corn was more susceptible to E. servus feeding at the VT (tasseling) stage than the R1 (silking) or R2 (blister) stages (Ni et al. 2010). At the VT stage, three or more E. servus feeding for 9 d caused significant kernel damage and reduction in ear and kernel weight (Ni et al. 2010). As corn ear development progresses, feeding by stink bugs is more likely to affect grain quality. Halyomorpha halys will feed on developing kernels by piercing through corn husks and cause kernel shrinkage and discoloration (Rice et al. 2014, Cissel et al. 2015). Euschistus servus feeding at later reproductive growth stages caused greater effects on grain quality (kernel discoloration) than yield (Ni et al. 2010).
An additional concern related to stink bugs in corn production was the possibility that cattle fed H. halys-contaminated corn silage might produce milk tainted by odorous compounds from H. halys (Baldwin et al. 2014). However, H. halys contamination of silage did not affect feed consumption by cattle or milk production, and odorous compounds (i.e., E-2-decenal and tridecane) from H. halys were not detected in milk after cattle were fed contaminated silage nor after odor compounds were infused directly into the rumen of the cattle (Baldwin et al. 2014). The process of ensiling and metabolism of the cattle appear to mitigate the risk of milk being tainted by stink bug contamination in corn silage (Baldwin et al. 2014).
Management
Much of the information provided here is derived from literature on management of stink bugs in southern states. This information serves to inform the reader about management of these pests until more research can be performed on the management of stink bugs on soybean and corn in the midwestern United States. As state- and region-specific management recommendations are developed and refined, check the recommendations from Extension in your state.
Scouting and Thresholds in Soybean
In general, scouting for stink bugs in soybean should start as pods begin to develop and continue through seed development. Scouting can be performed with a sweep net or drop cloth. A sweep net is more often used with narrow-row (76.2-cm [30-inch] spacing or less) soybean and a drop cloth with wide-row (>76.2-cm [30-inch] spacing) soybean. Although both methods are similar in efficiency of catching stink bugs, using a sweep net is more convenient owing to ease of use and being less time consuming (Rudd and Jensen 1977, Todd and Herzog 1980). Scouting should include edge and interior areas of fields, because the abundance of stink bugs within fields can be greater on field edges (i.e., an edge effect; Todd and Herzog 1980, Leskey et al. 2012a, Koch and Pahs 2014, Venugopal et al. 2014) and areas of soybean adjacent to wooded habitats or early maturing crops (Leskey et al. 2012a, Venugopal et al. 2014). Recent research in cotton and soybean show that H. halys has a “startle-response” and readily drop off the plants (Kamminga et al. 2014, Herbert et al. 2015). However, Owens et al. (2013) show that sweep-net sampling is still an efficient method for the stink bug complex containing H. halys.
For stink bugs in soybean, treatment decisions are based on the combined count of nymphs (>0.64 cm [1/4 inch]) and adults of all herbivorous stink bug species. Economic thresholds for stink bugs in soybean in the midwestern United States depend on the end use of the soybean. For soybean grown for seed production, the economic threshold is presently 5 stink bugs per 25 sweeps or 1 stink bug per 0.3 m (1 ft) of row (Kogan 1976). For soybean grown for grain, the economic threshold is presently 10 stink bugs per 25 sweeps or 3 stink bugs per 0.3 m (1 ft) of row (Kogan 1976). These thresholds will need validation and refinement as stink bug infestations increase in the region. Owens et al. (2013) show the economic threshold for the invasive H. halys not differing from those recommended for native stink bugs.
Scouting and Thresholds in Corn
Scouting during the first 2 wk after corn emergence is critical to managing infestations of stink bugs early in the season. Check at least 10 consecutive plants in five or more locations per field for stink bug injury and stink bugs. In these early vegetative growth stages, examine the entire corn plant from near the base to within the whorl. For corn <61 cm (2 ft) tall, consider treatment if stink bugs are present on 10% or more of the plants. When injured plants are observed, consider treatment when 5% of the plants exhibit injury and stink bugs are present. Infestations of stink bugs on vegetative growth stages may be more likely to occur in late-planted fields and no-till fields. Fields planted during wet-field conditions may be particularly vulnerable if the seed furrow is not properly closed, allowing stink bug access to the below-ground growing point.
Corn is also vulnerable to injury from stink bugs during ear formation through ear fill. Scouting during this period is also performed by direct examination of plants, particularly in the ear zone. Action thresholds are based on counts of nymphs (>0.64 cm [1/4 inch]) and adults of herbivorous species. Check at least 10 consecutive plants in five or more locations in field for the presence of stink bugs. Insecticide sprays are recommended when stink bug density reaches one stink bug per four plants, from ear forming to beginning of pollen shed, and one stink bug per two plants, from end of pollen shed to the blister stage (Hunt et al. 2014).
Management Tactics
Panizzi and Slansky (1985) and McPherson and McPherson (2000) provide reviews of stink bug management, including tactics such as trap cropping, timing of planting, row spacing, resistant varieties, and biological control. Biological control of stink bugs is expounded upon by McPherson (1982), with a listing of natural enemies known to attack different species of stink bugs. A thorough review of management tactics is beyond the scope of this paper. However, we provide a brief review of more recent literature on chemical control for stink bugs, as this will be the most immediately implemented tactic against these emerging pests in soybean and corn in the midwestern United States.
Broad-spectrum insecticides are generally effective and commonly used for stink bug management (Willrich et al. 2003, Nielsen et al. 2008b, Kamminga et al. 2009, Leskey et al. 2012b, Rice et al. 2014). Stink bug susceptibility to insecticides varies by species, life stages, and sex. For example, E.servus has been shown to be less susceptible than C. hilaris to pyrethroid and organophosphate insecticides (Willrich et al. 2003, Snodgrass et al. 2005). Kamminga et al. (2009) showed differences in susceptibility of C. hilaris and E. servus to different neonicotinoid insecticides. The predator, P. maculiventris, is more susceptible than the herbivore, E. servus, to some insecticides (Tillman and Mullinix 2004). Nymphs of C. hilaris are more susceptible to insecticides than adults of C. hilaris (Kamminga et al. 2009). Organic insecticides can be more effective on early instars than older stages (Herbert et al. 2015). In addition, male stink bugs can be more susceptible to insecticides than females, owing to the smaller body size of males (Nielsen et al. 2008b). Residual activity of insecticides should be considered for mobile pests like H. halys, which can recolonize treated crops (Funayama 2012, Leskey et al. 2013). In addition to stink bugs, other insects can cause economic losses to these crops (e.g., soybean aphid in soybean); therefore, when economically significant infestations of multiple pests occur, products that can control multiple pests may be preferred.
Conclusion
In conclusion, the threat posed by new and emerging stink bug pests in corn and soybean in the midwestern United States is a challenge for growers and their crop advisors. Identification of these pests and knowledge of their biologies provides a foundation for management programs. Though much can be gained from review of literature, primarily from the southern states, on impacts of stink bugs to crops and management recommendations, further research on these topics is needed in the midwestern United States. In addition, sampling methods, treatment thresholds, and management tactics for stink bugs require further validation in the midwestern United States. Furthermore, future studies should examine the interaction between stink bugs and other pests and pest management tactics in soybean and corn of the midwestern United States (Rich and Koch 2016).
Acknowledgments
We thank Walter Rich, Dr. Christopher Philips, and two anonymous reviewers for providing reviews of earlier versions of this paper or section of this paper. This work was supported in part by the Minnesota Soybean Research and Promotion Council, Minnesota Environment and Natural Resources Trust Fund, Nebraska Soybean Board, Ohio Soybean Council, and North Central Soybean Research Program.
References Cited
Author notes
Subject Editor: Jeffrey Davis