-
PDF
- Split View
-
Views
-
Cite
Cite
Megumi Hori, Hirokazu Tanaka, Kenji Wakai, Shizuka Sasazuki, Kota Katanoda, Secondhand smoke exposure and risk of lung cancer in Japan: a systematic review and meta-analysis of epidemiologic studies, Japanese Journal of Clinical Oncology, Volume 46, Issue 10, October 2016, Pages 942–951, https://doi.org/10.1093/jjco/hyw091
Close - Share Icon Share
Abstract
Systematic evaluation of the association between secondhand smoke exposure and lung cancer in Japan has yet to be conducted. Here, we performed a systematic review and meta-analysis of the relationship between secondhand smoke and lung cancer in Japanese non-smokers.
Relevant studies were collected from the MEDLINE and Ichushi Web databases using a combination of search terms and Medical Subject Headings. Eligible studies were identified, and relative risks or odds ratios were extracted to calculate pooled risk estimates. This procedure was performed independently by at least two authors. Stratified analyses were carried out according to study design, publication year, and whether or not potential confounding variables were accounted for. The presence of publication bias was assessed via funnel plots.
We identified four cohort studies and five case-control studies. Quantitative synthesis was conducted only for secondhand smoke exposure in the home during adulthood. Of the 12 populations included in meta-analysis, positive secondhand smoke exposure-lung cancer associations were observed in 11, whereas an inverse association was found in the remaining 1. The pooled relative risk of lung cancer associated with secondhand smoke exposure was 1.28 (95% confidence interval: 1.10–1.48). We found no evidence of publication bias, and a significant association remained even when potentially missing studies were included (pooled relative risk: 1.26; 95% confidence interval: 1.09–1.46). The results were stable across different subgroup analyses, including by study design, publication year, and when adjusting for confounding variables.
Secondhand smoke exposure in the home during adulthood results in a statistically significant increase in the risk of lung cancer.
Introduction
In 1981, Hirayama reported for the first time that non-smoking wives of smokers had an increased risk of lung cancer mortality (1), a report which resulted in substantial controversy and prompted verification studies worldwide (2). In 1986, Blot and Fraumeni reported the results of a meta-analysis of the relationship between secondhand smoke (SHS) exposure and lung cancer in non-smoking women (2). According to the report, which covered 12 studies, including two involving Japanese subjects, the overall relative risk was 1.3 (95% confidence interval [CI]: 1.1–1.5). Since then, a total of 21 meta-analyses have been performed on this subject, the most recent being a report made by Taylor et al. in 2007. Almost all of these meta-analyses have reported an overall relative risk of 1.2–1.3 (3).
In response to accumulating data from epidemiological studies, international organizations and US government agencies conducted a comprehensive evaluation of the health effects of SHS exposure. In 1986, the International Agency for Research on Cancer (IARC) Monographs concluded that exposure to SHS increased the risk of cancer (4), and the US National Research Council and the Surgeon General's Report similarly concluded that SHS exposure increased the risk of lung cancer in non-smokers (5,6). These evaluation results have since been reinforced by subsequent epidemiological and experimental studies, and the IARC has also concluded SHS exposure as ‘carcinogenic to humans’ (Group 1) in its 2004 and 2010 monographs (7,8), as well as in the Surgeon General's Report in 2006 (9).
Preventive measures against SHS exposure are specified in Article 8 of the World Health Organization (WHO)’s Framework Convention on Tobacco Control (FCTC), which was ratified by the Japanese government in 2004. The first principle of Article 8 is that total elimination of smoking and tobacco smoke in a particular space or environment is required to create a 100% smoke-free environment (10). A WHO report from 2015 ranked Japan the lowest among the FCTC ratifying countries with regard to implementing preventive measures against SHS exposure (11).
The Research Group for Development and Evaluation of Cancer Prevention Strategies in Japan has spearheaded a comprehensive evaluation of risk factors for cancer in the Japanese population since 2003 (12,13). Their current evaluation of the association between SHS exposure and lung cancer is ‘probable’, not ‘convincing’ (12), partly because of the lack of comprehensive reviews of Japanese studies. Although Zhong et al. examined findings from five studies involving Japanese subjects and reported a pooled relative risk of 1.30 (95% CI: 1.06–1.59) (14), those five studies were published more than 25 years ago. Subsequent changes in the smoking environment may have resulted in a decrease in SHS exposure. Therefore, to evaluate the association between SHS exposure and lung cancer in Japan, we conducted a systematic review and meta-analysis of the relationship between SHS exposure and lung cancer in Japanese non-smokers.
Materials and methods
Protocol and registration
We followed the PRISMA Statement (15). Our protocol was registered and is available on PROSPERO, an international prospective register of systematic reviews (registration number: CRD42015027797).
Eligibility criteria
The target population was Japanese non-smoking individuals. The group of interest was individuals exposed to SHS, and the control group was non-smoking individuals not exposed to SHS. The outcome was lung cancer incidence or mortality. The types of study we included were cohort studies and case-control studies.
Information sources
We searched for studies using the MEDLINE (PubMed) and Ichushi Web (Japanese) databases with a search strategy combining search terms and Medical Subject Headings (MeSH). A search using the same text words was conducted through J-STAGE (Japanese) and Medical Online (Japanese). Searches were limited to studies published through 31 July 2015. We did not specify an earliest date of publication in our searches. Citation tracking and manual searching of references were also carried out.
Searches
The search strategy used for PubMed is detailed in the ***Appendix. Publication searches in PubMed was done using MeSH terms. We also conducted a publication search using search terms to identify recently-published studies, as newer studies may not have yet been provided with appropriate MeSHs.
Study selection
All published articles that reported on the relationship between SHS and lung cancer risk among Japanese people were identified. At least two out of three authors of this paper (MH, HT, KK) independently assessed the eligibility of studies using the title and abstract for initial screening, followed by a review of the full text. Inclusion criteria for quantitative meta-analysis were as follows: cohort study or case-control study, and reporting a risk estimate, i.e. relative risk or odds ratio of lung cancer incidence or mortality associated with SHS exposure. We excluded experimental, mechanistic and ecological studies. Articles that presented no original data, such as reviews, were also excluded. If more than one study was published using the same dataset, the report containing the most comprehensive information on the dataset was included. Any disagreement was resolved by a consensus among the three authors of this paper.
Data collection process
For the meta-analysis, two authors (MH, HT) independently extracted from the selected studies the data items described below. Extracted data were verified by a third author (KK). Any disagreement regarding the extracted data was resolved by consensus among the three authors of this paper.
Data items
We extracted the following data items from each study: characteristics of study participants (including sex and age), follow-up period and completeness of cohort studies, case and control definition in case-control studies, exposure (including place and source, measurement and category), risk estimates (including relative risk, odds ratio and CI) and adjusted confounding variables. We contacted authors of eligible studies to obtain any relevant information that could not be retrieved from the original reports.
Risk of bias in individual studies
The quality and risk of bias in the included studies were assessed by the three authors (MH, HT, KK) based on the perspective of selection (validity and representativeness of the selection of case/control/participants), comparability (relevant adjustment of potential confounding factors), exposure (ascertainment of exposure) and outcomes (assessment of outcome and adequacy of follow-up for cohort studies).
Statistical analyses
Relative risks or odds ratios were extracted from the selected studies, and their standard errors were calculated from the respective CIs. No distinction was made between risk parameters (odds ratio, relative risk). When multiple risk estimates were provided for different doses of exposure, we used the risk estimate as a representative value, in the following order of priority: ‘currently smoking’, ‘approximately 20 cigarettes per day’, or ‘almost every day’ for spouse's or household members’ smoking or frequency of exposure. When sex-specific estimates were available, we analyzed them separately.
Pooled relative risk and 95% CIs were calculated for the effect of SHS on lung cancer using a fixed effects model, or random effects model, depending on heterogeneity between studies. We tested heterogeneity with Cochran's Q statistic, with P < 0.10 indicating heterogeneity and assessed inconsistency using the I2 statistic. When significant inconsistency was determined (I2 ≥ 50%), the random effects model was used to calculate pooled relative risk. When inconsistency was not significant (I2 < 50%), the fixed effects model was used.
In addition, we performed subgroup analyses for type of study (cohort studies versus case-control studies), year of publication (early publications; 1984–90 versus recent publications; 2001–13), adjustment for at least one of the following factors (socioeconomic status (SES), medical examination history, green and yellow vegetable intake, fruit intake, air pollution exposure or indoor pollution exposure from heating). We also performed sensitivity analyses to confirm the robustness of our findings with respect to different doses of SHS exposure. We estimated pooled relative risk using the risk estimates in the lowest or highest SHS dose category instead of the representative exposure category, when available.
The presence of publication bias was assessed with funnel plots. To test the funnel plot asymmetry, Egger's regression test was used. The potential influence of unpublished studies on the pooled relative risk estimates was examined by trim and fill analysis.
All statistical analyses were performed using the R software (version 3.2.2, R Foundation for Statistical Computing, Vienna, Austria) (16).
Results
Literature search and study characteristics
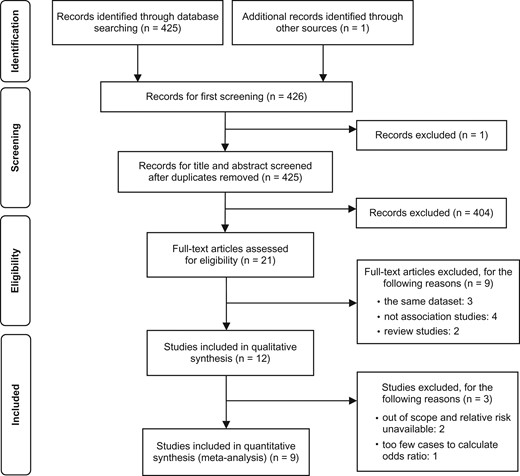
The search strategy collected 425 articles, and one additional article was identified through citation tracking (Fig. 1). Based on titles and abstracts, we identified 21 potentially relevant articles. Of these, three were excluded because they were multiple articles that used the same dataset (1,17,18), four were excluded because they were not association studies of SHS exposure (19–22) and two were excluded because they were review articles (2,23). The remaining 12 studies met the eligibility criteria for systematic review (24–35). Of these, three studies were excluded: two reported no relevant relative risks (27,29), and one had too few cases to calculate the odds ratio (28). Although we contacted the authors of two studies that lacked relevant information, we were unable to obtain any additional data. Thus, nine studies were included in our systematic review and meta-analysis, three of which (one cohort study and two case-control studies) reported results for males and females separately (a total of 12 populations) (24–26,30–35). With regard to place of exposure, all nine studies assessed SHS exposure at home. Two studies additionally assessed exposure at workplaces (30,34). With regard to time of exposure, all nine studies assessed exposure during adulthood, and two additionally assessed exposure during childhood (32,35). We therefore conducted a quantitative synthesis only for SHS exposure at home during adulthood.
PRISMA flow diagram and number of records identified for the association between secondhand smoke (SHS) exposure and lung cancer.
The full list of articles with their main characteristics and SHS data is shown in Table 1 (four cohort studies) and Table 2 (five case-control studies). These studies were published between 1984 and 2013.
Characteristics of cohort studies included in the meta-analysis
| References . | Study period . | Study population . | Exposure . | Relative riska (95% CI) . | Adjustment . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Setting . | Number of nonsmoking subjects . | Event . | Number of incident cases or deaths . | Place/Source . | Category . | ||||
| Hirayama (25) | 1966–81 | Population-based, 29 public health center areas in 6 prefectures | 91 540 women | Death | 200 | Husband's smoking habit | Current, overall | 1.45 (0.98–2.15) | Husband's age |
| 1–14/day | 1.42 (0.94–2.14) | ||||||||
| 20+/day | 1.91 (1.29–2.91) | ||||||||
| Nishino (31) | 1984–92 | Population-based, a city and two towns in Miyagi Prefecture | 9675 women | Incidence | 24 | Household members’ smoking habit | Husband (+) | 1.80 (0.69–4.72) | Age, study area, alcohol, green and yellow vegetable intake, fruit intake, meat intake, past history of lung diseases |
| Ozasa (32) | 1988–90 | Population-based, 45 cities, towns, or villages in 18 prefectures | 420 201; women (person year) | Death | 109 | Home | Almost everyday | 1.06 (0.68–1.65) | Age, study area |
| Sometimes, 1–4/week | 0.84 (0.49–1.45) | ||||||||
| 3 hours or longer day | 1.12 (0.55–2.28) | ||||||||
| 67 997; men (person year) | Death | 24 | Home | Almost everyday | 0.45 (0.09–2.23) | Age, study area | |||
| Sometimes, 1–4/week | 1.48 (0.57–3.84) | ||||||||
| 3 hours or longer day | 5.29(1.03–27.18) | ||||||||
| Kurahashi (30) | 1990–2004 | Population-based, 5 public health center areas (Cohort I), and 6 public health center areas (Cohort II) | 28 414 women | Incidence | 109 | Husband's smoking habit | Current | 1.34 (0.81–2.21) | Age, study area, menopause, alcohol, family history of lung cancer |
| References . | Study period . | Study population . | Exposure . | Relative riska (95% CI) . | Adjustment . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Setting . | Number of nonsmoking subjects . | Event . | Number of incident cases or deaths . | Place/Source . | Category . | ||||
| Hirayama (25) | 1966–81 | Population-based, 29 public health center areas in 6 prefectures | 91 540 women | Death | 200 | Husband's smoking habit | Current, overall | 1.45 (0.98–2.15) | Husband's age |
| 1–14/day | 1.42 (0.94–2.14) | ||||||||
| 20+/day | 1.91 (1.29–2.91) | ||||||||
| Nishino (31) | 1984–92 | Population-based, a city and two towns in Miyagi Prefecture | 9675 women | Incidence | 24 | Household members’ smoking habit | Husband (+) | 1.80 (0.69–4.72) | Age, study area, alcohol, green and yellow vegetable intake, fruit intake, meat intake, past history of lung diseases |
| Ozasa (32) | 1988–90 | Population-based, 45 cities, towns, or villages in 18 prefectures | 420 201; women (person year) | Death | 109 | Home | Almost everyday | 1.06 (0.68–1.65) | Age, study area |
| Sometimes, 1–4/week | 0.84 (0.49–1.45) | ||||||||
| 3 hours or longer day | 1.12 (0.55–2.28) | ||||||||
| 67 997; men (person year) | Death | 24 | Home | Almost everyday | 0.45 (0.09–2.23) | Age, study area | |||
| Sometimes, 1–4/week | 1.48 (0.57–3.84) | ||||||||
| 3 hours or longer day | 5.29(1.03–27.18) | ||||||||
| Kurahashi (30) | 1990–2004 | Population-based, 5 public health center areas (Cohort I), and 6 public health center areas (Cohort II) | 28 414 women | Incidence | 109 | Husband's smoking habit | Current | 1.34 (0.81–2.21) | Age, study area, menopause, alcohol, family history of lung cancer |
CI, confidence interval.
aThe standard error and 95% CI were re-calculated from the reported relative risk and CI. Therefore, 95% CIs in this table do not always correspond to the CIs reported in each study.
Characteristics of cohort studies included in the meta-analysis
| References . | Study period . | Study population . | Exposure . | Relative riska (95% CI) . | Adjustment . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Setting . | Number of nonsmoking subjects . | Event . | Number of incident cases or deaths . | Place/Source . | Category . | ||||
| Hirayama (25) | 1966–81 | Population-based, 29 public health center areas in 6 prefectures | 91 540 women | Death | 200 | Husband's smoking habit | Current, overall | 1.45 (0.98–2.15) | Husband's age |
| 1–14/day | 1.42 (0.94–2.14) | ||||||||
| 20+/day | 1.91 (1.29–2.91) | ||||||||
| Nishino (31) | 1984–92 | Population-based, a city and two towns in Miyagi Prefecture | 9675 women | Incidence | 24 | Household members’ smoking habit | Husband (+) | 1.80 (0.69–4.72) | Age, study area, alcohol, green and yellow vegetable intake, fruit intake, meat intake, past history of lung diseases |
| Ozasa (32) | 1988–90 | Population-based, 45 cities, towns, or villages in 18 prefectures | 420 201; women (person year) | Death | 109 | Home | Almost everyday | 1.06 (0.68–1.65) | Age, study area |
| Sometimes, 1–4/week | 0.84 (0.49–1.45) | ||||||||
| 3 hours or longer day | 1.12 (0.55–2.28) | ||||||||
| 67 997; men (person year) | Death | 24 | Home | Almost everyday | 0.45 (0.09–2.23) | Age, study area | |||
| Sometimes, 1–4/week | 1.48 (0.57–3.84) | ||||||||
| 3 hours or longer day | 5.29(1.03–27.18) | ||||||||
| Kurahashi (30) | 1990–2004 | Population-based, 5 public health center areas (Cohort I), and 6 public health center areas (Cohort II) | 28 414 women | Incidence | 109 | Husband's smoking habit | Current | 1.34 (0.81–2.21) | Age, study area, menopause, alcohol, family history of lung cancer |
| References . | Study period . | Study population . | Exposure . | Relative riska (95% CI) . | Adjustment . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Setting . | Number of nonsmoking subjects . | Event . | Number of incident cases or deaths . | Place/Source . | Category . | ||||
| Hirayama (25) | 1966–81 | Population-based, 29 public health center areas in 6 prefectures | 91 540 women | Death | 200 | Husband's smoking habit | Current, overall | 1.45 (0.98–2.15) | Husband's age |
| 1–14/day | 1.42 (0.94–2.14) | ||||||||
| 20+/day | 1.91 (1.29–2.91) | ||||||||
| Nishino (31) | 1984–92 | Population-based, a city and two towns in Miyagi Prefecture | 9675 women | Incidence | 24 | Household members’ smoking habit | Husband (+) | 1.80 (0.69–4.72) | Age, study area, alcohol, green and yellow vegetable intake, fruit intake, meat intake, past history of lung diseases |
| Ozasa (32) | 1988–90 | Population-based, 45 cities, towns, or villages in 18 prefectures | 420 201; women (person year) | Death | 109 | Home | Almost everyday | 1.06 (0.68–1.65) | Age, study area |
| Sometimes, 1–4/week | 0.84 (0.49–1.45) | ||||||||
| 3 hours or longer day | 1.12 (0.55–2.28) | ||||||||
| 67 997; men (person year) | Death | 24 | Home | Almost everyday | 0.45 (0.09–2.23) | Age, study area | |||
| Sometimes, 1–4/week | 1.48 (0.57–3.84) | ||||||||
| 3 hours or longer day | 5.29(1.03–27.18) | ||||||||
| Kurahashi (30) | 1990–2004 | Population-based, 5 public health center areas (Cohort I), and 6 public health center areas (Cohort II) | 28 414 women | Incidence | 109 | Husband's smoking habit | Current | 1.34 (0.81–2.21) | Age, study area, menopause, alcohol, family history of lung cancer |
CI, confidence interval.
aThe standard error and 95% CI were re-calculated from the reported relative risk and CI. Therefore, 95% CIs in this table do not always correspond to the CIs reported in each study.
Characteristics of case-control studies included in the meta-analysis
| References . | Study period . | Study subjects . | Exposure . | Odds ratio a (95% CI) . | Adjustment . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Setting . | Definition . | Number of nonsmoking cases . | Number of nonsmoking controls . | Place/Source . | Category . | ||||
| Akiba (24) | Hiroshima Nagasaki atomic bomb survivors cohort (nested case-control) | Case: Newly diagnosed cases of primary lung cancer | 94 women | 270 women | Spouse's smoking habit | Husband smoked | 1.50 (0.87–2.59) | Year of birth, sex, city of residence, participation in biennial medical examination, vital statistics | |
| Control: Cohort members without lung cancer | 19 men | 110 men | Spouse's smoking habit | Wife smoked | 1.80 (0.43–7.59) | ||||
| Inoue (26) | 1980–83 | Population-based, two cities in Kanagawa Prefecture | Case: Women lung cancer deaths | 83 women | 166 women | Husband ‘s smoking | <20 cigarettes/day | 1.39 (0.26–7.50) | Age, year of deaths, district. |
| 1973–81 | Control: Women cerebrovascular deaths | ≥20 cigarettes/day | 3.09 (0.73–13.14) | ||||||
| Shimizu (34) | 1982–85 | Hospital-based, 4 hospitals in Nagoya City | Case: Female in-patients with lung cancer | 90 women | 163 women | The presence of a smoking family member | Husband | 1.08 (0.64–1.82) | Age, hospital, date of admission. |
| Control: Female in-patients other than with lung cancer | |||||||||
| Sobue (35) | 1986–88 | Hospital-based, 8 hospitals in Osaka Prefecture | Case: Newly-admitted patients in wards for lung cancer | 144 women | 731 women | Smoking status of household members | Husband smoked | 1.13 (0.78–1.63) | Age, years of education |
| Control: Newly-admitted patients in one or two wards for other diseases. | |||||||||
| Seki (33) | 1997–2009 | Hospital-based, a hospital in Miyagi City | Case: Lung cancer patients | 292 women | 1810 women | Spouse's smoking habit | Husband smoked | 1.31 (0.99–1.73) | Age, year of recruitment, area of residence, referral status (screening or not), occupation, alcohol drinking, family history of lung cancer |
| Control: Non-cancer patients | 70 men | 600 men | Spouse's smoking habit | Wife smoked | 1.29 (0.34–4.90) | ||||
| References . | Study period . | Study subjects . | Exposure . | Odds ratio a (95% CI) . | Adjustment . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Setting . | Definition . | Number of nonsmoking cases . | Number of nonsmoking controls . | Place/Source . | Category . | ||||
| Akiba (24) | Hiroshima Nagasaki atomic bomb survivors cohort (nested case-control) | Case: Newly diagnosed cases of primary lung cancer | 94 women | 270 women | Spouse's smoking habit | Husband smoked | 1.50 (0.87–2.59) | Year of birth, sex, city of residence, participation in biennial medical examination, vital statistics | |
| Control: Cohort members without lung cancer | 19 men | 110 men | Spouse's smoking habit | Wife smoked | 1.80 (0.43–7.59) | ||||
| Inoue (26) | 1980–83 | Population-based, two cities in Kanagawa Prefecture | Case: Women lung cancer deaths | 83 women | 166 women | Husband ‘s smoking | <20 cigarettes/day | 1.39 (0.26–7.50) | Age, year of deaths, district. |
| 1973–81 | Control: Women cerebrovascular deaths | ≥20 cigarettes/day | 3.09 (0.73–13.14) | ||||||
| Shimizu (34) | 1982–85 | Hospital-based, 4 hospitals in Nagoya City | Case: Female in-patients with lung cancer | 90 women | 163 women | The presence of a smoking family member | Husband | 1.08 (0.64–1.82) | Age, hospital, date of admission. |
| Control: Female in-patients other than with lung cancer | |||||||||
| Sobue (35) | 1986–88 | Hospital-based, 8 hospitals in Osaka Prefecture | Case: Newly-admitted patients in wards for lung cancer | 144 women | 731 women | Smoking status of household members | Husband smoked | 1.13 (0.78–1.63) | Age, years of education |
| Control: Newly-admitted patients in one or two wards for other diseases. | |||||||||
| Seki (33) | 1997–2009 | Hospital-based, a hospital in Miyagi City | Case: Lung cancer patients | 292 women | 1810 women | Spouse's smoking habit | Husband smoked | 1.31 (0.99–1.73) | Age, year of recruitment, area of residence, referral status (screening or not), occupation, alcohol drinking, family history of lung cancer |
| Control: Non-cancer patients | 70 men | 600 men | Spouse's smoking habit | Wife smoked | 1.29 (0.34–4.90) | ||||
aThe standard error and 95% CI were re-calculated from the reported odds ratios and CIs. Therefore, 95% CIs in this table do not always correspond to the CIs reported in each study.
Characteristics of case-control studies included in the meta-analysis
| References . | Study period . | Study subjects . | Exposure . | Odds ratio a (95% CI) . | Adjustment . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Setting . | Definition . | Number of nonsmoking cases . | Number of nonsmoking controls . | Place/Source . | Category . | ||||
| Akiba (24) | Hiroshima Nagasaki atomic bomb survivors cohort (nested case-control) | Case: Newly diagnosed cases of primary lung cancer | 94 women | 270 women | Spouse's smoking habit | Husband smoked | 1.50 (0.87–2.59) | Year of birth, sex, city of residence, participation in biennial medical examination, vital statistics | |
| Control: Cohort members without lung cancer | 19 men | 110 men | Spouse's smoking habit | Wife smoked | 1.80 (0.43–7.59) | ||||
| Inoue (26) | 1980–83 | Population-based, two cities in Kanagawa Prefecture | Case: Women lung cancer deaths | 83 women | 166 women | Husband ‘s smoking | <20 cigarettes/day | 1.39 (0.26–7.50) | Age, year of deaths, district. |
| 1973–81 | Control: Women cerebrovascular deaths | ≥20 cigarettes/day | 3.09 (0.73–13.14) | ||||||
| Shimizu (34) | 1982–85 | Hospital-based, 4 hospitals in Nagoya City | Case: Female in-patients with lung cancer | 90 women | 163 women | The presence of a smoking family member | Husband | 1.08 (0.64–1.82) | Age, hospital, date of admission. |
| Control: Female in-patients other than with lung cancer | |||||||||
| Sobue (35) | 1986–88 | Hospital-based, 8 hospitals in Osaka Prefecture | Case: Newly-admitted patients in wards for lung cancer | 144 women | 731 women | Smoking status of household members | Husband smoked | 1.13 (0.78–1.63) | Age, years of education |
| Control: Newly-admitted patients in one or two wards for other diseases. | |||||||||
| Seki (33) | 1997–2009 | Hospital-based, a hospital in Miyagi City | Case: Lung cancer patients | 292 women | 1810 women | Spouse's smoking habit | Husband smoked | 1.31 (0.99–1.73) | Age, year of recruitment, area of residence, referral status (screening or not), occupation, alcohol drinking, family history of lung cancer |
| Control: Non-cancer patients | 70 men | 600 men | Spouse's smoking habit | Wife smoked | 1.29 (0.34–4.90) | ||||
| References . | Study period . | Study subjects . | Exposure . | Odds ratio a (95% CI) . | Adjustment . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Setting . | Definition . | Number of nonsmoking cases . | Number of nonsmoking controls . | Place/Source . | Category . | ||||
| Akiba (24) | Hiroshima Nagasaki atomic bomb survivors cohort (nested case-control) | Case: Newly diagnosed cases of primary lung cancer | 94 women | 270 women | Spouse's smoking habit | Husband smoked | 1.50 (0.87–2.59) | Year of birth, sex, city of residence, participation in biennial medical examination, vital statistics | |
| Control: Cohort members without lung cancer | 19 men | 110 men | Spouse's smoking habit | Wife smoked | 1.80 (0.43–7.59) | ||||
| Inoue (26) | 1980–83 | Population-based, two cities in Kanagawa Prefecture | Case: Women lung cancer deaths | 83 women | 166 women | Husband ‘s smoking | <20 cigarettes/day | 1.39 (0.26–7.50) | Age, year of deaths, district. |
| 1973–81 | Control: Women cerebrovascular deaths | ≥20 cigarettes/day | 3.09 (0.73–13.14) | ||||||
| Shimizu (34) | 1982–85 | Hospital-based, 4 hospitals in Nagoya City | Case: Female in-patients with lung cancer | 90 women | 163 women | The presence of a smoking family member | Husband | 1.08 (0.64–1.82) | Age, hospital, date of admission. |
| Control: Female in-patients other than with lung cancer | |||||||||
| Sobue (35) | 1986–88 | Hospital-based, 8 hospitals in Osaka Prefecture | Case: Newly-admitted patients in wards for lung cancer | 144 women | 731 women | Smoking status of household members | Husband smoked | 1.13 (0.78–1.63) | Age, years of education |
| Control: Newly-admitted patients in one or two wards for other diseases. | |||||||||
| Seki (33) | 1997–2009 | Hospital-based, a hospital in Miyagi City | Case: Lung cancer patients | 292 women | 1810 women | Spouse's smoking habit | Husband smoked | 1.31 (0.99–1.73) | Age, year of recruitment, area of residence, referral status (screening or not), occupation, alcohol drinking, family history of lung cancer |
| Control: Non-cancer patients | 70 men | 600 men | Spouse's smoking habit | Wife smoked | 1.29 (0.34–4.90) | ||||
aThe standard error and 95% CI were re-calculated from the reported odds ratios and CIs. Therefore, 95% CIs in this table do not always correspond to the CIs reported in each study.
Risk of bias within studies
Of the four cohort studies, two defined death due to lung cancer as the outcome, ascertained by death certificates, while the other two defined lung cancer incidence as the outcome, ascertained by population-based cancer registry (one study additionally used hospital records). For all cohort studies, SHS exposure was assessed using a self-reported questionnaire or interview. Whether participants were SHS exposed or non-exposed at home was determined by the smoking habits of subjects’ husbands in two studies, the presence of a smoking household member in one study, and the frequency of SHS exposure in one study. In the calculation of risk estimates, age was controlled for in all four cohort studies, and study area was further controlled for in three. Two cohort studies controlled for other potentially confounding factors, such as fruit and vegetable intake or menopausal status.
Among five case-control studies, one used death due to lung cancer for cases and cerebrovascular deaths for controls in the population-based framework. One was the nested case-control study which used newly diagnosed primary lung cancer for cases and original cohort members without lung cancer for controls. The other three studies were hospital-based case-control studies in which cases were defined as admission to a hospital for lung cancer. Among these studies, controls were defined as patients without lung cancer in two studies and as patients without cancer in the other study. SHS exposure was assessed using a self-reported questionnaire or interview in all case-control studies. One case control study which used death for subjects obtained SHS exposure by interview. In the published article of this study, there was no description about interviewee. Matching or adjustment was performed for age in all studies, and three studies performed additional adjustment for other confounding factors, including fruit and vegetable intake, educational status, occupation or referral status (screening or not).
Results of individual studies and synthesis of results
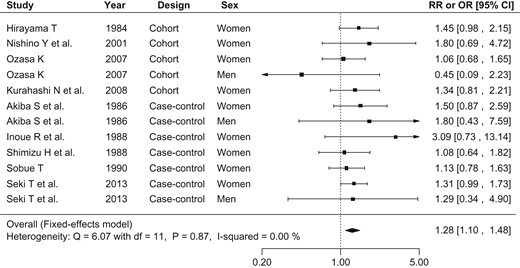
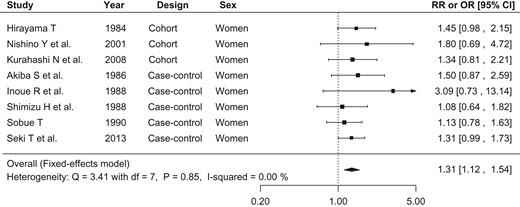
Figure 2 shows the relative risk or odds ratio of lung cancer associated with SHS in each study and pooled for all studies. Of the 12 populations, 11 showed positive associations, and one showed an inverse association, none of which were statistically significant. The fixed effects model was selected for the meta-analysis because heterogeneity tested for via Q-statistics was not significant (Q: 6.07, P value: 0.87) and no inconsistency was found (I2: 0.00%). The pooled relative risk of lung cancer associated with SHS exposure at home was 1.28 (95% CI: 1.10–1.48) for both sexes together. When we limited the analyses to SHS exposure from husband's smoking among females (eight populations), the pooled relative risk was 1.31 (95% CI: 1.12–1.54) (Fig. 3). The heterogeneity and inconsistency of this sub-dataset was not significant (Q: 3.41, P value: 0.85, I2: 0.00%).
Forest plot of relative risks and odds ratios of lung cancer associated with SHS exposure at home. The risk estimate and 95% CI from each study are represented by a square and segments, respectively. The pooled risk estimate is represented by a rhombus. SHS, secondhand smoke; RR, relative risk; OR, odds ratio; CI, confidence interval.
Forest plot of relative risks and odds ratios of lung cancer associated with SHS exposure at home, limiting to studies of females’ exposure to spouse's smoking. The risk estimate and 95% CI from each study are represented by a square and segments, respectively. The overall estimate is represented by a rhombus.
Risk of bias across studies
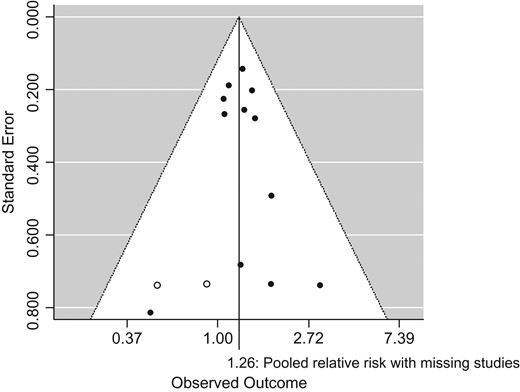
Although the funnel plot showed some asymmetry, no significant publication bias was detected for the full datasets included in the meta-analysis (Egger's test, P value: 0.71). According to trim and fill analysis, two studies may have been missing. Even when those potentially missing studies were added to the meta-analysis, the pooled relative risk of lung cancer remained significant: 1.26 (95% CI: 1.09–1.46) (Fig. 4).
Funnel plot with missing studies identified by the trim and fill method.
Open circles indicate filled missing studies. With missing studies, the overall relative risk is 1.26 (95% CI: 1.09, 1.46).
Additional analyses
Analyses stratified by type of study, year of publication and whether or not adjusting confounding variables revealed consistent results, with pooled relative risk ranging from 1.24 to 1.30 (Table 3). The pooled relative risk calculated from early publications (1984–90; 1.30 [95% CI: 1.05–1.61]) was higher than that from recent publications (2001–13; 1.25 [95% CI: 1.02–1.53]). Studies controlling for potential confounding factors tended to produce higher pooled relative risks.
Stratified and sensitivity meta-analysis of the association between lung cancer and secondhand smoke exposure at home
| Studies . | Number of populations . | Pooled estimate [95% CI] . | Heterogeneity . | ||
|---|---|---|---|---|---|
| Q-statistics . | P value . | I2 (%) . | |||
| Type of study | |||||
| Cohort | 5 | 1.28 [1.00–1.63] | 3.23 (df = 4) | 0.52 | 0.00 |
| Case-control | 7 | 1.27 [1.06–1.54] | 2.83 (df = 6) | 0.83 | 0.00 |
| Year of publication | |||||
| 1984–90 | 6 | 1.30 [1.05–1.61] | 3.17 (df = 5) | 0.67 | 0.00 |
| 2001–13 | 6 | 1.25 [1.02–1.53] | 2.84 (df = 5) | 0.73 | 0.00 |
| Adjusted confounding variable | |||||
| Only age or study area | 6 | 1.24 [0.99–1.55] | 4.51 (df = 5) | 0.48 | 0.00 |
| Age or study area, and at least one additional factora | 6 | 1.30 [1.07–1.59] | 1.46 (df = 5) | 0.92 | 0.00 |
| Category of exposure | |||||
| Included lowest category | 12 | 1.26 [1.09–1.47] | 4.53 (df = 11) | 0.95 | 0.00 |
| Included highest category | 12 | 1.37 [1.18–1.60] | 9.06 (df = 11) | 0.62 | 0.00 |
| Studies . | Number of populations . | Pooled estimate [95% CI] . | Heterogeneity . | ||
|---|---|---|---|---|---|
| Q-statistics . | P value . | I2 (%) . | |||
| Type of study | |||||
| Cohort | 5 | 1.28 [1.00–1.63] | 3.23 (df = 4) | 0.52 | 0.00 |
| Case-control | 7 | 1.27 [1.06–1.54] | 2.83 (df = 6) | 0.83 | 0.00 |
| Year of publication | |||||
| 1984–90 | 6 | 1.30 [1.05–1.61] | 3.17 (df = 5) | 0.67 | 0.00 |
| 2001–13 | 6 | 1.25 [1.02–1.53] | 2.84 (df = 5) | 0.73 | 0.00 |
| Adjusted confounding variable | |||||
| Only age or study area | 6 | 1.24 [0.99–1.55] | 4.51 (df = 5) | 0.48 | 0.00 |
| Age or study area, and at least one additional factora | 6 | 1.30 [1.07–1.59] | 1.46 (df = 5) | 0.92 | 0.00 |
| Category of exposure | |||||
| Included lowest category | 12 | 1.26 [1.09–1.47] | 4.53 (df = 11) | 0.95 | 0.00 |
| Included highest category | 12 | 1.37 [1.18–1.60] | 9.06 (df = 11) | 0.62 | 0.00 |
df, degrees of freedom.
aAdditional factors; socioeconomic status (including occupation and educational status), medical examination history, green and yellow vegetable intake, fruit intake, air pollution exposure or indoor pollution exposure from heating
Stratified and sensitivity meta-analysis of the association between lung cancer and secondhand smoke exposure at home
| Studies . | Number of populations . | Pooled estimate [95% CI] . | Heterogeneity . | ||
|---|---|---|---|---|---|
| Q-statistics . | P value . | I2 (%) . | |||
| Type of study | |||||
| Cohort | 5 | 1.28 [1.00–1.63] | 3.23 (df = 4) | 0.52 | 0.00 |
| Case-control | 7 | 1.27 [1.06–1.54] | 2.83 (df = 6) | 0.83 | 0.00 |
| Year of publication | |||||
| 1984–90 | 6 | 1.30 [1.05–1.61] | 3.17 (df = 5) | 0.67 | 0.00 |
| 2001–13 | 6 | 1.25 [1.02–1.53] | 2.84 (df = 5) | 0.73 | 0.00 |
| Adjusted confounding variable | |||||
| Only age or study area | 6 | 1.24 [0.99–1.55] | 4.51 (df = 5) | 0.48 | 0.00 |
| Age or study area, and at least one additional factora | 6 | 1.30 [1.07–1.59] | 1.46 (df = 5) | 0.92 | 0.00 |
| Category of exposure | |||||
| Included lowest category | 12 | 1.26 [1.09–1.47] | 4.53 (df = 11) | 0.95 | 0.00 |
| Included highest category | 12 | 1.37 [1.18–1.60] | 9.06 (df = 11) | 0.62 | 0.00 |
| Studies . | Number of populations . | Pooled estimate [95% CI] . | Heterogeneity . | ||
|---|---|---|---|---|---|
| Q-statistics . | P value . | I2 (%) . | |||
| Type of study | |||||
| Cohort | 5 | 1.28 [1.00–1.63] | 3.23 (df = 4) | 0.52 | 0.00 |
| Case-control | 7 | 1.27 [1.06–1.54] | 2.83 (df = 6) | 0.83 | 0.00 |
| Year of publication | |||||
| 1984–90 | 6 | 1.30 [1.05–1.61] | 3.17 (df = 5) | 0.67 | 0.00 |
| 2001–13 | 6 | 1.25 [1.02–1.53] | 2.84 (df = 5) | 0.73 | 0.00 |
| Adjusted confounding variable | |||||
| Only age or study area | 6 | 1.24 [0.99–1.55] | 4.51 (df = 5) | 0.48 | 0.00 |
| Age or study area, and at least one additional factora | 6 | 1.30 [1.07–1.59] | 1.46 (df = 5) | 0.92 | 0.00 |
| Category of exposure | |||||
| Included lowest category | 12 | 1.26 [1.09–1.47] | 4.53 (df = 11) | 0.95 | 0.00 |
| Included highest category | 12 | 1.37 [1.18–1.60] | 9.06 (df = 11) | 0.62 | 0.00 |
df, degrees of freedom.
aAdditional factors; socioeconomic status (including occupation and educational status), medical examination history, green and yellow vegetable intake, fruit intake, air pollution exposure or indoor pollution exposure from heating
The sensitivity analysis using relative risks for the lowest SHS dose category instead of the representative exposure category revealed a significant pooled relative risk of 1.26 (95% CI: 1.09–1.47). Pooled relative risk was slightly higher when using the values for highest dose than that using the representative exposure category (1.37; 95% CI: 1.18–1.60). Heterogeneity was not detected for any of the stratified or sensitivity analyses.
Discussion
Our meta-analysis revealed a statistically significant association between SHS exposure and lung cancer in Japanese non-smokers, with an overall relative risk of 1.28 (95% CI: 1.10–1.48). This association was consistent when data were stratified by study design, year of publication and consideration of potential confounding factors. This meta-analysis provides important scientific evidence for promoting tobacco control measures in Japan, including legislation for prevention of SHS exposure and warning labels on tobacco products.
To investigate the relationship between SHS exposure and lung cancer, Taylor et al. conducted a large systematic review of 55 studies in 2007 and reported that the relative risk of lung cancer from SHS in non-smoking women with smoking partners was 1.27 (95% CI: 1.17–1.37), with an overall relative risk in Asians of 1.31 (95% CI: 1.16–1.48) (3). Prior to this report, Zhong et al. reported a relative risk of 1.30 (95% CI: 1.06–1.59) based on data combined from five studies involving Japanese subjects (lung cancer from SHS in non-smoking women living with smoking partners) (14). Taylor et al. included six Japanese studies (including two cohort studies) in their review, and Zhong et al. included five Japanese studies (including one cohort study). The present meta-analysis involved a total of nine Japanese studies (four cohort studies and five case-control studies) by adding three studies published after the meta-analysis by Taylor et al. (30,32,33). Our meta-analysis including recently published studies revealed a similar significant association between SHS and lung cancer, which further strengthens the scientific evidence of the adverse health effects of SHS.
Regarding biases in individual studies, the first potential bias was the fact that smoking status and SHS exposure in study participants were collected through an interview or self-administered questionnaire. A fraction of smokers may proclaim that they are non-smokers and so be misclassified in these studies. Because smokers are more likely to develop lung cancer and tend to live with smokers, this misclassification will lead to an overestimation of the true risk of lung cancer (36,37). Hackshaow et al. evaluated the degree of this overestimation in detail and showed that potential misclassification would not lead to a substantial reduction of relative risk (from 1.24 to 1.18) (38). Considering that the relative risk in female smokers used by Hackshaow et al. was much higher than that in Japanese current smokers (12 vs. 2.8, compared with never smokers) (39), and that the percentage of misclassification they used was comparable to the reported value for Japanese people (7% vs. 8.8%) (40), the degree of potential overestimation in Japanese studies is probably minimal. Eight out of the selected nine studies used household members’ smoking status as the measure of SHS exposure. A previous study suggested a fair concordance between household members’ smoking status and self-reported SHS exposure (41).
The second potential bias was insufficient adjustment for potential confounders. Known risk factors for lung cancer other than tobacco smoke include air pollution, arsenic in drinking water, asbestos, indoor heaters, radon exposure and supplemental β-carotene. Low vegetable/fruit intake is also considered a highly probable or possible risk factor in the international (42) or domestic (12) evaluation of scientific evidence, respectively. SES or healthy lifestyle choices are also potential confounders, because SHS exposure has been reportedly associated with SES (43). Among the studies included in our meta-analysis, data were adjusted for the following confounders in addition to age and place of residence: educational status (35), participation in a medical examination (24), vegetable/fruit intake(31) and occupation and referral status (screening or not). (33). The pooled relative risk in these four studies alone was 1.29 (95% CI, 1.06–1.58), which was almost the same as that in all studies combined.
All case-control studies except one (24) were hospital controlled. Controls were defined as those having non-lung cancers (34,35), non-cancerous diseases (33) and cerebrovascular diseases (26). Breast cancer and stomach cancer were the most common non-lung cancers in these studies. According to the Surgeon General's Report in 2014, stroke is classified as a disease causally related to SHS, and breast cancer has also been suggested as causally related to SHS (44). As such, in studies that used these diseases as control, odds ratios may have been underestimated.
No statistically significant publication bias was detected in the present meta-analysis. However, given that many meta-analyses have reported a consistent association between SHS and lung cancer since the 1980 s, submitting or publishing conflicting results may have been suppressed. Indeed, the funnel plot analysis showed a possibility of two unreported studies with negative results. Nevertheless, a significant result was still observed even when these two potential studies were imputed in our analysis. Another two studies met the eligibility criteria but did not report numerical data that could be quantitatively combined to assess the relationship between SHS and lung cancer (an attempt to obtain the relevant data from the authors failed) (27,29). Although we were unable to determine how the outcome would be affected had these studies been included, it is unlikely that our primary results would be substantially changed by adding a small number of additional studies.
Concerning racial/ethnical differences in the relationship between SHS and lung cancer, consistent results have been reported across different races and ethnic groups (3,14,45), although a previous systematic review showed a slightly weaker association in North America than on other continents. In the present study, the overall relative risk of lung cancer, calculated only among non-smoking women with smoking partners, which was in line with the previous meta-analysis, was 1.31 (95% CI: 1.12–1.54) (eight studies). This value is slightly higher than in the North American population (1.15 [95% CI: 1.03–1.28]) and almost equal to that in the European population (1.31 [95% CI: 1.24–1.52]) (3), which may reflect a difference in residential environments.
All nine studies included in our meta-analysis focused on SHS exposure at home during adulthood. SHS exposure in other situations was investigated in four studies: two assessing the exposure in the workplace (30,34) and two assessing exposure in childhood (32,35). While a combined relative risk could not be calculated for these different types of exposure, the relative risk and odds ratio in the two studies assessing SHS in the workplace were 1.32 and 1.2, respectively (30,34); these values are almost the same as that obtained in a meta-analysis of lung cancer and SHS in the workplace conducted primarily in Westerners (1.24 [95% CI, 1.18–1.29]) (46). Of the two studies assessing SHS in childhood, one suggested that SHS increased the risk of lung cancer (35), while the other study showed the opposite result (32). The evaluation by the IARC concluded that the association between lung cancer and SHS exposure in childhood was only suggestive (8). The conflicting findings may be due to attenuation of any effect or difficulty in exposure measurement over a long period of time between exposure and outcome.
With regard to the dose-response relationship, the relative risk in non-smoking wives of former smokers and current smokers was calculated in two cohort studies and found to be higher in wives of current smokers than in those married to former smokers (25,30). In addition, the relative risk was assessed according to smoking intensity of husbands of non-smoking women in three studies, and all showed that the relative risk of lung cancer (25,26) or lung adenocarcinoma (30) tended to increase with increasing smoking intensity. In contrast, no clear dose-response relationship was found in relation to the number of smokers who lived together or the frequency category of SHS exposure (31,32,34).
Differences in risk by histological type of lung cancer were reported in a cohort study by Kurahashi et al. in 2008 (30). In their study, the increase in the risk of lung cancer from SHS exposure in non-smoking wives of smokers was greater for adenocarcinoma (2.03; 95% CI: 1.07–3.86) than squamous cell cancer (1.34; 95% CI: 0.81–2.21). In a case-control study by Seki et al. in 2013, the odds ratio was statistically significant only for adenocarcinoma in non-smoking women (1.44; 95% CI: 1.06–1.95), but the point estimate was greater for squamous cell cancer (2.24; 95% CI: 0.60–8.38) (33). While consistent evidence supports that active smoking is more strongly associated with lung squamous cell carcinoma than with lung adenocarcinoma (39,47), difference in impact of SHS exposure between adenocarcinoma and squamous cell carcinoma was inconsistent among studies. A multi-national study in Europe showed that the impact of SHS exposure was large on squamous and small-cell carcinomas than on adenocarcinoma (48), whereas studies in the USA and Poland reported stronger or similar association with adenocarcinoma (49,50). A study in Hong Kong also showed a stronger association between SHS exposure and lung adenocarcinoma (51). A proposed mechanism of the differential effects of SHS and active smoke on lung is that sidestream smoke, which contains a tobacco-specific lung carcinogen, would be more likely to reach the peripheral portions of the lung than mainstream smoke, leading to relatively high risk of adenocarcinoma (52). Further studies are needed to clarify histological type-dependent effect of SHS exposure.
The fact that our meta-analysis confirmed a significant association between SHS and lung cancer has a critical implication for promoting legislation to prevent SHS exposure and enact comprehensive tobacco control policies in Japan. According to the National Health and Nutrition Survey in 2013, 20.6%, 42.4% and 50.9% of people were exposed to SHS at least once a month at home, in the workplace (those who had not been to work were excluded from the denominator) and at restaurants (those who had not been to a restaurant were excluded from the denominator), respectively (53). While our study assessed the relationship between SHS exposure at home in adulthood and lung cancer specifically, it is inappropriate to interpret this to mean that the adverse health effect is limited to the specific place or age of exposure. Evidence is sufficient to support an increased risk of lung cancer due to SHS exposure in the workplace during adulthood, as well as the association between SHS exposure and non-cancerous diseases such as stroke in adults and pulmonary function impairment in children (44). There is strong biologic support for a role of SHS in the etiology of lung cancer and other diseases (9). The Community Preventive Services Task Force in the USA recommends comprehensive tobacco control programs and smoke-free policies as measures to reduce SHS exposure (54). Smoke-free policies are defined as public-sector regulations and private-sector rules that prohibit smoking in indoor spaces and designated public areas. Smoking at home or in other places not directly covered by laws or rules should be targeted through public education campaigns (10). Taken together, these attempts are expected to promote awareness that indoor smoking is socially unacceptable in any and all locations.
In conclusion, a meta-analysis of data from epidemiological studies in Japanese populations showed that SHS exposure at home during adulthood results in a statistically significant increase in the risk of lung cancer. The overall relative risk of lung cancer from SHS exposure is ~1.3.
Supplementary data
Supplementary data are available at http://www.jjco.oxfordjournals.org.
Funding
This study was supported by a Health Labour Sciences Research Grant of the Ministry of Health, Labour and Welfare of Japan (Comprehensive Research on Life-Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus: H27-Junkankitou-Ippan-005).
Conflict of interest statement
Kota Katanoda received the third Kiyoko and Paul Bourdarie-Goto Scientific Prize in 2015.
References
The health consequences of involuntary smoking. In: Service USPH, ed. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health,
The health consequences of involuntary exposure to tobacco smoke A report of the surgeon general. In: Service USPH, ed. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health,
Guidelines for Implementation of Article 8 Guidelines on the Protection from Exposure to Tobacco Smoke: WHO Framework Convention on Tobacco Control. http://www.who.int/fctc/guidelines/adopted/article_8/en/ (17 December 2015, date last accessed).
WHO Report on the Global Tobacco Epidemic, 2015 Raising Taxes on Tobacco: World Health Organization. http://www.who.int/tobacco/global_report/2015/en/ (28 December 2015, date last accessed).
Development and Evaluation of Cancer Prevention Strategies in Janan. Epidemiology and Prevention Division, Research Center for Cancer Prevention and Screening, Natonal Cancer Center, Japan. http://epi.ncc.go.jp/en/can_prev/index.html (13 November 2015, date last accessed).
Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective: World Cancer Research Fund/American Institute for Cancer Research,
The health consequences of smoking - 50 years of progress. In: Service USPH, ed. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health,
The National Health and Nutrition Survey in Japan. 2013: Ministry of Health, Labour and Welfare, Japan. http://www.mhlw.go.jp/bunya/kenkou/eiyou/h25-houkoku.html (17 December 2015, date last accessed) [in Japanese].
Guide to Community Preventive Services. Reducing Tobacco Use and Secondhand Smoke Exposure. http://www.thecommunityguide.org/tobacco/index.html.