-
PDF
- Split View
-
Views
-
Cite
Cite
Allison M Kohler, Erik R Olson, Jonathan G Martin, Paula Spaeth Anich, Ultraviolet fluorescence discovered in New World flying squirrels (Glaucomys), Journal of Mammalogy, Volume 100, Issue 1, 28 February 2019, Pages 21–30, https://doi.org/10.1093/jmammal/gyy177
Close - Share Icon Share
Abstract
Fluorescence of visible wavelengths under ultraviolet (UV) light has been previously detected in a wide range of birds, reptiles, and amphibians and a few marsupial mammals. Here, we report the discovery of vivid UV fluorescence of the pelage in Glaucomys, the New World flying squirrels. Fluorescence in varying intensities of pink was observed in females and males of all extant species (G. oregonensis, G. sabrinus, and G. volans) across all sampled geographic areas in North and Central America and a temporal range of 130 years. We observed fluorescence in museum specimens (n = 109) and wild individuals (n = 5) on both dorsal and ventral surfaces. Museum specimens of three co-occurring, diurnal sciurid species (Sciurus carolinensis, S. niger, and Tamiasciurus hudsonicus) were also examined but did not fluoresce. The ecological significance of this trait in the nocturnal–crepuscular flying squirrels warrants further investigation.
Research into ultraviolet (UV) fluorescence in biological substances has a long history. The phenomenon was first documented in plants (Herschel 1845; Lloyd 1924; Goodwin and Kavanagh 1948) and has subsequently been detected in many marine and terrestrial invertebrates (anthozoa—Miyawaki 2002; arachnids—Gaffin et al. 2012; and lepidopterans—Olofsson et al. 2010). Among the vertebrates, fish (Garcia and de Perera 2002; Michiels et al. 2008; Sparks et al. 2014), amphibians (Nowogrodzki 2017; Taboada et al. 2017), reptiles (Gruber and Sparks 2015; Prötzel et al. 2018), birds (Pearn et al. 2001; Weidensaul et al. 2011), and certain mammalian species within Didelphidae (Meisner 1983; Pine et al. 1985) fluoresce under UV light. Although fluorescence occurs across a range of lineages, body sizes, and habitat types, it is manifested variably across these scales.
In vertebrates, fluorescent compounds have been found in bone (Dooley and Moncrief 2012; Prötzel et al. 2018), plumage (Pearn et al. 2001; Arnold et al. 2002; McGraw et al. 2007; Weidensaul et al. 2011), carapace and skin (Gruber and Sparks 2015), and pelage (Meisner 1983; Pine et al. 1985). Furthermore, vertebrates fluoresce across a wide spectrum of visible colors including shades of red (Pine et al. 1985; Gruber and Sparks 2015), yellow (Arnold et al. 2002; McGraw et al. 2007), green (Pine et al. 1985; Gruber and Sparks 2015; Taboada et al. 2017), blue (Taboada et al. 2017; Prötzel et al. 2018), and pink (Meisner 1983; Pine et al. 1985; Weidensaul et al. 2011). Despite the fact that fluorescence spans a wide range of taxa, tissue types, and color patterns, its ecological significance is not clear. In some instances, compelling data suggest fluorescence has important ecological functions such as influencing mate choice (Arnold et al. 2002) or enhancing antipredator defenses (Olofsson et al. 2010). In other cases, the ecological significance of the trait is largely unknown (Weidensaul et al. 2011; Gruber and Sparks 2015).
Within Mammalia, fluorescence has yet to be widely documented or studied. Externally visible UV fluorescence has only been observed within Didelphidae. Meisner (1983) detected fluorescence of the skin and pelage of the Virginia opossum (Didelphis virginiana) under UV light. Pine et al. (1985) examined museum skins of 31 didelphid species and reported UV fluorescence in 23 species, without any notable patterns related to sex, age, or season. The fluorescing didelphids described by Pine et al. (1985) inhabited a broad range of New World ecosystem and microhabitat types, but all had similar crepuscular or nocturnal activity patterns. Diurnal eastern fox squirrels (Sciurus niger) have a high incidence of UV fluorescence in their crania and teeth (Dooley and Moncrief 2012), but this fluorescence has not been observed in any external features such as their skin or pelage.
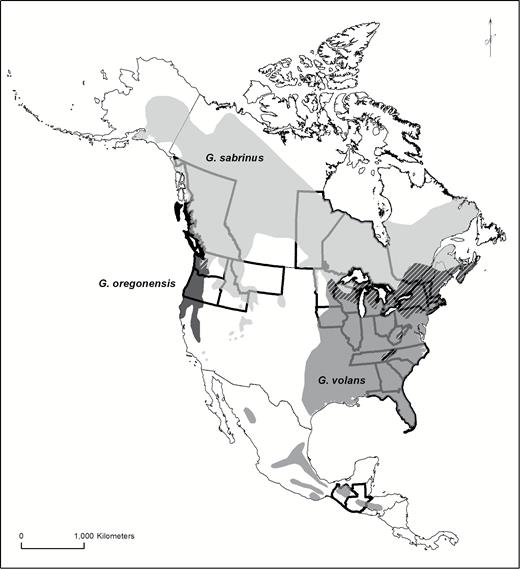
Here, we extend the list of visibly fluorescing mammals to include all three of the nocturnal–crepuscular New World flying squirrels (Glaucomys): Humboldt’s flying squirrel (G. oregonensis), northern flying squirrel (G. sabrinus), and southern flying squirrel (G. volans). The flying squirrels are small-bodied (50–150 g) and inhabit coniferous, hardwood, and mixed forests from the northern United States and Canada to low-latitude highlands in Mexico, Guatemala, and Honduras (Fig. 1; Dolan and Carter 1977; Wells-Gosling and Heaney 1984). Glaucomys oregonensis, a newly described “cryptic species,” has a relatively small geographic distribution along the forests of the Pacific coast and areas of overlap with G. sabrinus in Washington, United States, and British Columbia, Canada (Fig. 1; Arbogast et al. 2017). The geographic range of G. sabrinus includes northern forests across Canada and much of the northern United States (where it overlaps with G. volans) and sky islands in western and southwestern United States and Appalachia (another zone of co-occurrence with G. volans; Fig. 1). Glaucomys volans is found in eastern North American forests and highlands in Central America (Fig. 1). The omnivorous, arboreal flying squirrels are unique among New World sciurids due to the presence of furred patagia extending from their wrists to their ankles and furred, flattened tails that enable them to glide from tree to tree (Dolan and Carter 1977; Wells-Gosling and Heaney 1984). In visible light, their pelage is camouflaged via counter-shading: dark on the dorsal surface (brown or gray) and light on the ventral surface (white or cream). Sexual dimorphism of the pelage is minimal (Dolan and Carter 1977; Wells-Gosling and Heaney 1984).
Geographic distribution of New World flying squirrels: Glaucomys oregonensis (black), G. sabrinus (light gray), and G. volans (dark gray). Hatching indicates regions of overlap between species. We examined museum specimens from Guatemalan, Mexican, and United States states and Canadian provinces highlighted with bold boundaries. Geographic ranges modified from Arbogast et al. (2017).
Glaucomys spp. are nocturnal–crepuscular and active year-round. Bimodal activity patterns with peaks of movement and foraging around sunset and sunrise are typical under cooler conditions (Dolan and Carter 1977; Wells-Gosling and Heaney 1984). The hours immediately after sunset and before sunrise may be particularly significant for understanding UV fluorescence. Under certain celestial and meteorological conditions, the light environments of dawn and dusk are dominated by UV wavelengths (Johnsen et al. 2006; Cronin and Bok 2016). These UV wavelengths are also present during the daytime; however, visible wavelengths are dominant. Thus, UV wavelengths are relatively more abundant during twilight and may therefore be crucial for animal perception and communication at dawn, dusk, and overnight (Johnsen et al. 2006; Cronin and Bok 2016).
We studied the effects of UV wavelengths on the pelage characteristics of the nocturnal–crepuscular New World flying squirrels and compared them to three co-occurring diurnal sciurids. Using a combination of opportunistic observations of living, wild Glaucomys individuals and a systematic examination of museum specimens, we documented nearly ubiquitous fluorescence in the three Glaucomys species.
Materials and Methods
In May 2017, JGM made the initial opportunistic observation of fluorescence in G. volans in Bayfield County, Wisconsin, United States, during an exploratory forest survey of early spring flora and lichens using a handheld LED UV flashlight (iLumen8 100 LED). JGM observed a bright pink fluorescence of G. volans when exposed to UV light. Following this observation, we initiated our formal investigation into the prevalence of fluorescence in Glaucomys and other North American sciurids.
We examined skin specimens of the three New World flying squirrels (G. oregonensis, G. sabrinus, and G. volans; n = 109; Table 1; Supplementary Data SD1) and three diurnal sciurid species (Sciurus carolinensis, S. niger, and Tamiasciurus hudsonicus; n = 26; Table 1; Supplementary Data SD1) at the Field Museum of Natural History (FMNH, Chicago, Illinois) and the Science Museum of Minnesota (SMM, St. Paul, Minnesota). To distinguish cryptic G. oregonensis and G. sabrinus specimens, we relied on geographic range information from Arbogast et al. (2017) and only examined G. oregonensis specimens that were collected from areas that were allopatric to G. sabrinus (Arbogast et al. 2017; Fig. 1; Table 1). Glaucomys sabrinus specimens came from western, central, and eastern parts of the United States and Canada (Fig. 1; Table 1); and G. volans specimens spanned the United States, Mexico, and Guatemala (Fig. 1; Table 1). The specimens were collected between 1887 and 2007 (Table 1; Supplementary Data SD1).
Sampling of North American sciurid specimens from museums (Field Museum of Natural History [FMNH] or Science Museum of Minnesota [SMM]) with mean dorsal fluorescence scores (± SE) and mean ventral body fluorescence scores (± SE).
| Species | Sex | n | States or provinces | Collection dates | Repositories | Dorsal fluorescence | Ventral body fluorescence |
| Glaucomys oregonensis | Female | 3 | Washington, Oregon, Unites States | 1898–1935a | FMNH, SMM | 1.11 ± 0.44 | 3.58 ± 0.52 |
| Male | 3 | ||||||
| Unknown | 2 | ||||||
| Glaucomys sabrinus | Female | 37 | British Columbia, Manitoba, Ontario, Canada; Idaho, Massachusetts, Minnesota, Montana, Vermont, United States | 1894–1998 | FMNH, SMM | 2.98 ± 0.17 | 3.21 ± 0.14 |
| Male | 34 | ||||||
| Unknown | 4 | ||||||
| Glaucomys volans | Female | 17 | Chimaltenango, Guatemala; Chiapas, Mexico; Florida, Georgia, Illinois, Iowa, Michigan, Minnesota, New York, North Carolina, Ohio, South Carolina, Tennessee, West Virginia, United States | 1887–2007 | FMNH, SMM | 2.83 ± 0.25 | 3.13 ± 0.26 |
| Male | 9 | ||||||
| Sciurus carolinensis | Female | 6 | Minnesota, United States | 1905–1992 | SMM | 0 | 0 |
| Male | 4 | ||||||
| Sciurus niger | Female | 3 | Minnesota, United States | 1968–2001 | SMM | 0 | 0 |
| Male | 2 | ||||||
| Tamiasciurus hudsonicus | Female | 5 | Alaska, Minnesota, Ohio, United States | 1974–1999 | SMM | 0 | 0 |
| Male | 6 |
| Species | Sex | n | States or provinces | Collection dates | Repositories | Dorsal fluorescence | Ventral body fluorescence |
| Glaucomys oregonensis | Female | 3 | Washington, Oregon, Unites States | 1898–1935a | FMNH, SMM | 1.11 ± 0.44 | 3.58 ± 0.52 |
| Male | 3 | ||||||
| Unknown | 2 | ||||||
| Glaucomys sabrinus | Female | 37 | British Columbia, Manitoba, Ontario, Canada; Idaho, Massachusetts, Minnesota, Montana, Vermont, United States | 1894–1998 | FMNH, SMM | 2.98 ± 0.17 | 3.21 ± 0.14 |
| Male | 34 | ||||||
| Unknown | 4 | ||||||
| Glaucomys volans | Female | 17 | Chimaltenango, Guatemala; Chiapas, Mexico; Florida, Georgia, Illinois, Iowa, Michigan, Minnesota, New York, North Carolina, Ohio, South Carolina, Tennessee, West Virginia, United States | 1887–2007 | FMNH, SMM | 2.83 ± 0.25 | 3.13 ± 0.26 |
| Male | 9 | ||||||
| Sciurus carolinensis | Female | 6 | Minnesota, United States | 1905–1992 | SMM | 0 | 0 |
| Male | 4 | ||||||
| Sciurus niger | Female | 3 | Minnesota, United States | 1968–2001 | SMM | 0 | 0 |
| Male | 2 | ||||||
| Tamiasciurus hudsonicus | Female | 5 | Alaska, Minnesota, Ohio, United States | 1974–1999 | SMM | 0 | 0 |
| Male | 6 |
aCollection year was not listed for all specimens.
Sampling of North American sciurid specimens from museums (Field Museum of Natural History [FMNH] or Science Museum of Minnesota [SMM]) with mean dorsal fluorescence scores (± SE) and mean ventral body fluorescence scores (± SE).
| Species | Sex | n | States or provinces | Collection dates | Repositories | Dorsal fluorescence | Ventral body fluorescence |
| Glaucomys oregonensis | Female | 3 | Washington, Oregon, Unites States | 1898–1935a | FMNH, SMM | 1.11 ± 0.44 | 3.58 ± 0.52 |
| Male | 3 | ||||||
| Unknown | 2 | ||||||
| Glaucomys sabrinus | Female | 37 | British Columbia, Manitoba, Ontario, Canada; Idaho, Massachusetts, Minnesota, Montana, Vermont, United States | 1894–1998 | FMNH, SMM | 2.98 ± 0.17 | 3.21 ± 0.14 |
| Male | 34 | ||||||
| Unknown | 4 | ||||||
| Glaucomys volans | Female | 17 | Chimaltenango, Guatemala; Chiapas, Mexico; Florida, Georgia, Illinois, Iowa, Michigan, Minnesota, New York, North Carolina, Ohio, South Carolina, Tennessee, West Virginia, United States | 1887–2007 | FMNH, SMM | 2.83 ± 0.25 | 3.13 ± 0.26 |
| Male | 9 | ||||||
| Sciurus carolinensis | Female | 6 | Minnesota, United States | 1905–1992 | SMM | 0 | 0 |
| Male | 4 | ||||||
| Sciurus niger | Female | 3 | Minnesota, United States | 1968–2001 | SMM | 0 | 0 |
| Male | 2 | ||||||
| Tamiasciurus hudsonicus | Female | 5 | Alaska, Minnesota, Ohio, United States | 1974–1999 | SMM | 0 | 0 |
| Male | 6 |
| Species | Sex | n | States or provinces | Collection dates | Repositories | Dorsal fluorescence | Ventral body fluorescence |
| Glaucomys oregonensis | Female | 3 | Washington, Oregon, Unites States | 1898–1935a | FMNH, SMM | 1.11 ± 0.44 | 3.58 ± 0.52 |
| Male | 3 | ||||||
| Unknown | 2 | ||||||
| Glaucomys sabrinus | Female | 37 | British Columbia, Manitoba, Ontario, Canada; Idaho, Massachusetts, Minnesota, Montana, Vermont, United States | 1894–1998 | FMNH, SMM | 2.98 ± 0.17 | 3.21 ± 0.14 |
| Male | 34 | ||||||
| Unknown | 4 | ||||||
| Glaucomys volans | Female | 17 | Chimaltenango, Guatemala; Chiapas, Mexico; Florida, Georgia, Illinois, Iowa, Michigan, Minnesota, New York, North Carolina, Ohio, South Carolina, Tennessee, West Virginia, United States | 1887–2007 | FMNH, SMM | 2.83 ± 0.25 | 3.13 ± 0.26 |
| Male | 9 | ||||||
| Sciurus carolinensis | Female | 6 | Minnesota, United States | 1905–1992 | SMM | 0 | 0 |
| Male | 4 | ||||||
| Sciurus niger | Female | 3 | Minnesota, United States | 1968–2001 | SMM | 0 | 0 |
| Male | 2 | ||||||
| Tamiasciurus hudsonicus | Female | 5 | Alaska, Minnesota, Ohio, United States | 1974–1999 | SMM | 0 | 0 |
| Male | 6 |
aCollection year was not listed for all specimens.
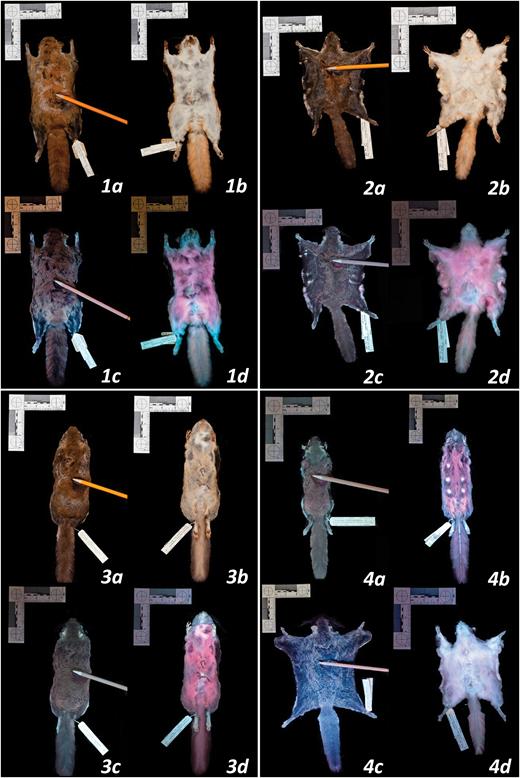
We photographed the dorsal and ventral side of each specimen (Canon EOS 50D, Canon USA Inc., Melville, New York; Sigma 17–70 mm f2.8–4 DC Macro) under visible light (Canon Speedlite 430EX) and then separately under 395 nm UV light (LED UV flashlight, iLumen8 100 LED). We used a yellow filter (K&F Concept, Guangdong Sheng, China) for photographs taken under UV light to absorb any residual light in the blue wavelengths. For each specimen, we parted a portion of fur on the dorsal surface to display the underfur. Images were captured using camera RAW files and post-processed with Adobe Lightroom (ver. 3.6; Adobe, San Jose, California), where white balance was adjusted from the neutral gray on the measurement scale references next to the specimens. Exposure was increased for dark images and the images presented here (Fig. 2) had dust and debris removed from the background. All other post-processing settings were left untouched, including color saturation and intensity.
Photographs of New World flying squirrels under visible light and 395 nm ultraviolet (UV) light. Panel 1: dorsal and ventral surfaces illuminated by visible light (a, b) and UV light (c, d) for Glaucomys sabrinus (FMNH 6482; dorsal fluorescence score = 2.3, chin and neck = 2.5, ventral body = 4.8, tail = 1.9). Panel 2: dorsal and ventral surfaces illuminated by visible light (a, b) and UV light (c, d) for G. volans (FMNH 64181; dorsal fluorescence score = 4.3, chin and neck = 2.4, ventral body = 4.0, tail = 2.3). Panel 3: dorsal and ventral surfaces illuminated by visible light (a, b) and UV light (c, d) for G. oregonensis (FMNH 51510; dorsal fluorescence score = 3.5, chin and neck = 4.3, ventral body = 5.0, tail = 4.5). Panel 4: variation in fluorescence on dorsal and ventral surfaces: (a) G. volans (FMNH 7726) fluoresced intensely on the dorsal surface (dorsal score = 3.6); (b) G. oregonensis (FMNH 51509) fluoresced intensely on the ventral surface (ventral body score = 4.4); (c) G. sabrinus (FMNH 5905) showed weak dorsal fluorescence (dorsal score = 0.5); (d) G. volans (FMNH 64183) showed weak ventral fluorescence (ventral body score = 1.3).
To rank the intensity of fluorescence for each photographed specimen, we used a qualitative visual fluorescence rank that ranged between 0 (no fluorescence) and 5 (vibrant fluorescence). Each specimen was ranked for five body areas (dorsal surface, ventral body, ventral chin and neck, ventral patagia [if applicable], and ventral tail) by four independent observers (PSA, AMK, JGM, ERO). Observers also estimated the percent cover of fluorescence for each body area. Individual observer rankings were then averaged to yield a mean fluorescence score and percent fluorescence cover for each body area for each individual.
We used generalized linear models to explore patterns of fluorescence among flying squirrels. We examined the effects of species and of seasonality by using month as a factor in our models. In other taxa (e.g., Arnold et al. 2002), UV fluorescence appears to be related to sexual selection; therefore, we examined the influence of sex and the interactions between sex and species on squirrel fluorescence scores. We also considered latitude of collection as a factor to investigate whether the trait varied systematically across the considerable latitudinal range of the New World flying squirrels (Fig. 1). Collection locality was reported only at the state or province level for many specimens, so we placed specimens in 10° latitude bins for our analysis. All models were run in R (ver. 3.5.1—R Core Team 2018) using the MuMIn package to summarize and rank models according to second-order AICc (Akaike’s information criterion corrected for small sample size) scores. We also examined patterns of fluorescence among individuals’ body regions through simple linear regressions using Statistical JMP (ver. 14—SAS 2018).
In addition to the museum specimens, we also made a number of opportunistic observations of fluorescence in living, wild Glaucomys in 2017 and 2018. These opportunistic observations of live Glaucomys allowed us to determine if this phenomenon could also be observed in individuals within their natural habitat.
Results
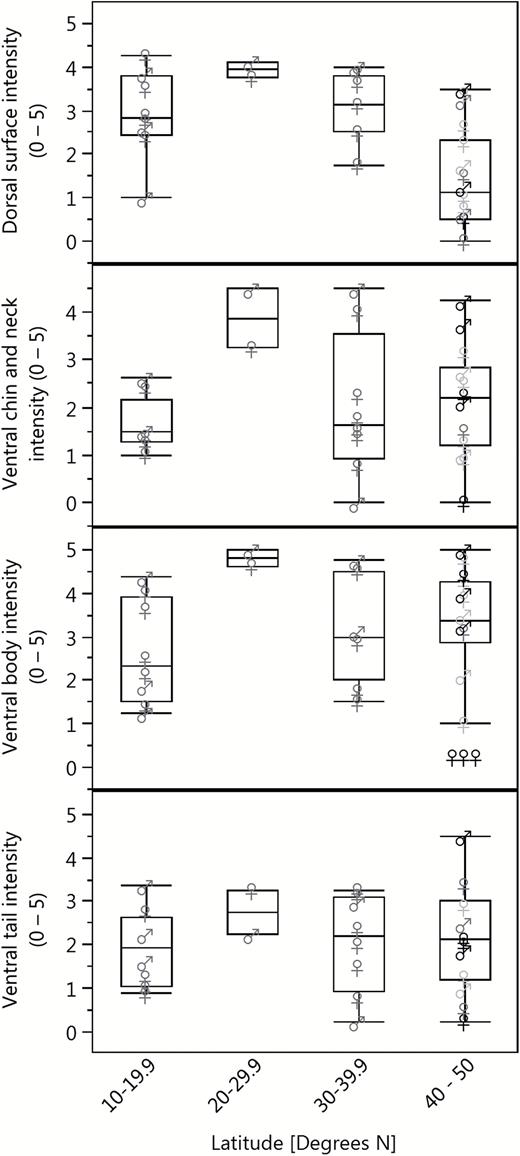
Pink UV fluorescence was seen in 108 of 109 Glaucomys museum specimens and was not observed in any Sciurus or Tamiasciurus specimens (Table 1; Figs. 2 and 3). Glaucomys fluorescence was generally most pronounced on the ventral body surface (Table 1; Figs. 2 and 3), an area that appears white or cream-colored in visible light. Hence, we used the ventral body fluorescence score as the dependent variable in our linear models. Of the resulting models, the intercept-only null model was the best fit to the data (Table 2). While there was substantial variation among individuals, there were no meaningful relationships between species, month, sex, year, or latitude and the intensity of fluorescence (Table 2; Figs. 4 and 5).
Linear regression models predicting Glaucomys spp. ventral body fluorescence scores. ∆AICc = difference in Akaike’s information criteria scores between focal model and top-ranked model; k = number of estimable parameters; ω = Akaike model weight; deviance = measure of model fit.
| Model | ΔAICc | k | ω | Deviance |
| Null | 0 | 1 | 0.37 | 150.5 |
| Month | 1.72 | 2 | 0.16 | 150.3 |
| Sexa | 2.00 | 2 | 0.14 | 149.6 |
| Latitude binb | 2.03 | 2 | 0.13 | 150.3 |
| Year | 2.05 | 2 | 0.13 | 149.9 |
| Speciesa | 3.68 | 3 | 0.06 | 150.4 |
| Sex * species | 7.85 | 6 | 0.01 | 145.6 |
| Model | ΔAICc | k | ω | Deviance |
| Null | 0 | 1 | 0.37 | 150.5 |
| Month | 1.72 | 2 | 0.16 | 150.3 |
| Sexa | 2.00 | 2 | 0.14 | 149.6 |
| Latitude binb | 2.03 | 2 | 0.13 | 150.3 |
| Year | 2.05 | 2 | 0.13 | 149.9 |
| Speciesa | 3.68 | 3 | 0.06 | 150.4 |
| Sex * species | 7.85 | 6 | 0.01 | 145.6 |
aCategorical variable.
b10° bins of latitude.
Linear regression models predicting Glaucomys spp. ventral body fluorescence scores. ∆AICc = difference in Akaike’s information criteria scores between focal model and top-ranked model; k = number of estimable parameters; ω = Akaike model weight; deviance = measure of model fit.
| Model | ΔAICc | k | ω | Deviance |
| Null | 0 | 1 | 0.37 | 150.5 |
| Month | 1.72 | 2 | 0.16 | 150.3 |
| Sexa | 2.00 | 2 | 0.14 | 149.6 |
| Latitude binb | 2.03 | 2 | 0.13 | 150.3 |
| Year | 2.05 | 2 | 0.13 | 149.9 |
| Speciesa | 3.68 | 3 | 0.06 | 150.4 |
| Sex * species | 7.85 | 6 | 0.01 | 145.6 |
| Model | ΔAICc | k | ω | Deviance |
| Null | 0 | 1 | 0.37 | 150.5 |
| Month | 1.72 | 2 | 0.16 | 150.3 |
| Sexa | 2.00 | 2 | 0.14 | 149.6 |
| Latitude binb | 2.03 | 2 | 0.13 | 150.3 |
| Year | 2.05 | 2 | 0.13 | 149.9 |
| Speciesa | 3.68 | 3 | 0.06 | 150.4 |
| Sex * species | 7.85 | 6 | 0.01 | 145.6 |
aCategorical variable.
b10° bins of latitude.
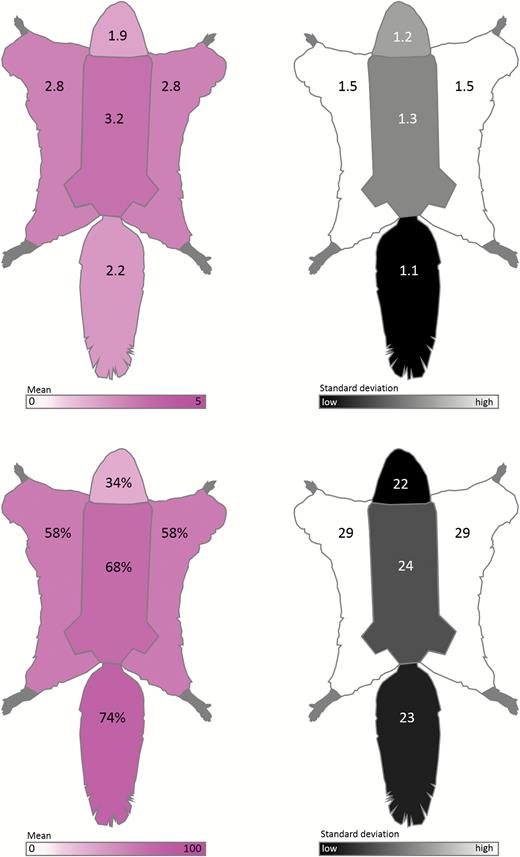
Schematic diagrams summarizing qualitative ventral body fluorescence scores and the percent cover of fluorescence of New World flying squirrel museum specimens (Glaucomys spp.; n = 109). Top left: average fluorescence scores of each ventral region (body, chin and neck, patagia, and tail) from 0 (no fluorescence) to 5 (vibrant fluorescence). Top right: SDs of fluorescence scores for each ventral body region. Bottom left: average percent cover of fluorescence for each ventral region (body, chin and neck, patagia, and tail). Bottom right: SDs of percent cover of fluorescence for each ventral body region.
Summary of the qualitative fluorescence scores for New World flying squirrel museum specimens by major body region. Fluorescence scores varied from 0 (no fluorescence) to 5 (vibrant fluorescence) and represent the average observer ranking for each body area.
Average fluorescence scores of the major body areas of museum specimens of Glaucomys oregonensis (black), G. sabrinus (light gray), and G. volans (dark gray) by latitude. Fluorescence scores vary from 0 (no fluorescence) to 5 (vibrant fluorescence) and represent the average observer ranking for each body area.
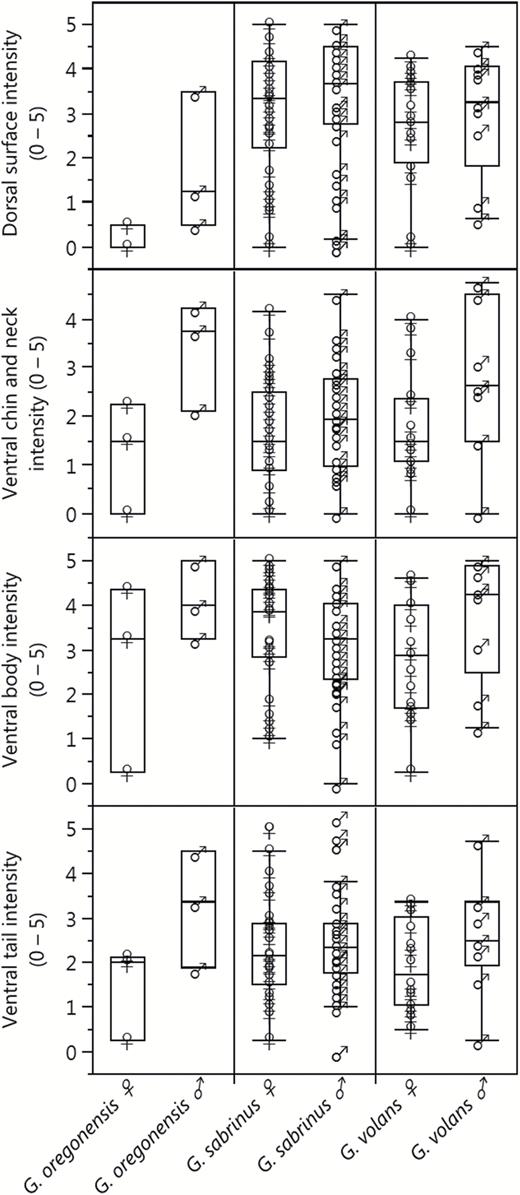
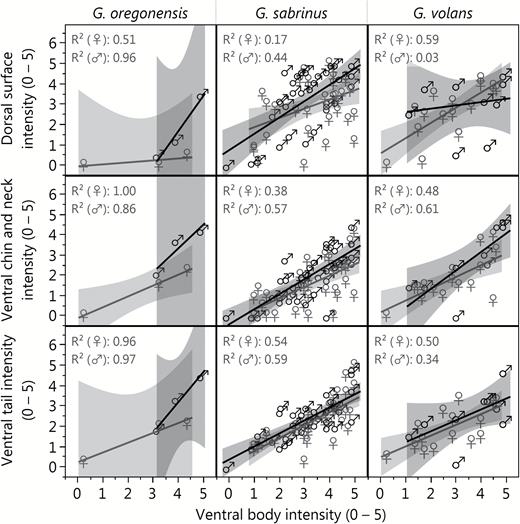
The percent cover of fluorescence was highest in the tail and ventral body and lowest on the ventral chin and neck (Fig. 3). Many specimens (n = 22) did not have exposed patagia; therefore, we excluded the patagia scores from subsequent analyses. The fluorescence scores of the dorsal surface, ventral chin and neck regions, and ventral tail showed positive associations with ventral body score (Fig. 6), although the strengths of these correlations varied. In G. sabrinus, all body regions for both sexes were strongly positively correlated with ventral body intensity (female dorsal surface F1,33 = 6.98, P = 0.01; female chin and neck F1,33 = 20.58, P < 0.0001; female ventral tail F1,33 = 38.35, P < 0.0001; male dorsal surface F1,32 = 25.01, P < 0.0001; male chin and neck F1,32 = 41.59, P < 0.0001; male ventral tail F1,32 = 45.69, P < 0.0001). Glaucomys volans females showed significant correlation between all body regions with ventral body intensity (dorsal surface F1,14 = 20.29, P = 0.0005; chin and neck F1,14 = 13.18, P = 0.003; ventral tail F1,14 = 13.88, P = 0.002), while males had significantly correlated chin and neck (F1,7 = 10.77, P = 0.01). In G. oregonensis, only female chin and neck fluorescence was strongly correlated with ventral body fluorescence (F1,1 = 225.33, P = 0.04); but sample sizes for this species were considerably lower. These positive relationships indicate that flying squirrels with vibrant fluorescence in one area are generally brightly fluorescent across their entire bodies. In some cases, the pattern of fluorescence on the ventral surface increased the contrast and visibility of the female mammary glands (e.g., Fig. 2 panel 4b).
Relationships between the ventral body fluorescence scores and fluorescence scores of the dorsal body, chin and neck, and tail for museum specimens of New World flying squirrels: Glaucomys oregonensis (left panel), G. sabrinus (center panel), and G. volans (right panel). Fluorescence scores vary from 0 (no fluorescence) to 5 (vibrant fluorescence) and represent the average observer ranking for each body area.
All opportunistic observations of living, wild Glaucomys were made in Bayfield County, Wisconsin, between August and September of 2017 and 2018 (n = 5). All three locations were in closed-canopy forests, with two observations occurring in a forested residential area in Washburn, Wisconsin; two observations in a mixed-forest area near a large, old white pine, Pinus strobus (Laughlin et al. 2017); and the remaining observation occurred in a mixed-hardwood area on the Bayfield Peninsula of Wisconsin. All individuals fluoresced similarly to the museum specimens when exposed to UV light.
Discussion
We report external UV fluorescence in a Eutherian mammal, Glaucomys spp. This trait was evident in nearly every individual sampled, and fluorescence was seen in all species, sexes, and localities, although the intensity varied in idiosyncratic ways. While it is possible that the UV fluorescence we observed is the result of an exogenous factor (e.g., consumption of fluorescent lichen) and not an intrinsic trait of the New World flying squirrels, it seems unlikely that a particular food item or other environmental factor could explain a pattern of pelage fluorescence that spans species, seasons, decades, countries, and habitat types to the extent that we document here.
We therefore suggest that the observed fluorescence is an intrinsic, potentially ecologically significant trait of the Glaucomys flying squirrels. We posit four hypotheses to explain the presence of fluorescence in Glaucomys: 1) the trait is adaptive in the squirrels’ unique nocturnal–crepuscular light environment; 2) fluorescence is especially important on landscapes with snow cover; 3) fluorescence is used in intraspecific communication; and 4) fluorescence plays a role in antipredator behavior. These explanations are not necessarily mutually exclusive.
Among New World squirrels, only Glaucomys are active at twilight and night (Dolan and Carter 1977; Wells-Gosling and Heaney 1984). In addition, they do not hibernate and are active year-round at high latitudes (Dolan and Carter 1977; Wells-Gosling and Heaney 1984). As a result, they perpetually inhabit low-light environments, in contrast to the other diurnal and often-hibernating New World sciurids. UV light is important in these conditions (Johnsen et al. 2006; Cronin and Bok 2016), and prior research has shown that UV vision specifically appears to be important for nocturnal mammals (Zhao et al. 2009). The eye lenses of one New World flying squirrel, G. volans, have been examined and were found to be clear and capable of transmitting UV light, whereas the yellow lenses of all examined diurnal sciurids filter UV light (Yolton et al. 1974). It appears that vision may be stronger in low-light environments in Glaucomys than the other squirrels. Combined with the fluorescence we observed in the present study, this suggests that the vision, perception, and appearance of Glaucomys may constitute a suite of adaptations to twilight and night environments that are relatively rich in UV wavelengths.
Glaucomys’ fluorescence may be particularly adaptive during the winter season and in snowy conditions. Despite overall decreases in the intensity of sunlight, the amount of UV-B radiation may increase by a factor of two relative to summer levels (Klein 1978). Furthermore, snow cover reflects UV radiation that reaches the surface (McIntosh et al. 2011), amplifying the importance of the UV wavelength band in the winter over much of the geographic distribution of the New World flying squirrels.
Our third hypothesis is that fluorescence is beneficial to flying squirrels as a means of intraspecific communication. The prominent ventral fluorescence may allow Glaucomys to signal their movement patterns to one another as they glide through trees, flashing their ventral surfaces, and then concealing their fluorescent patches when latching onto or climbing trees. Some fluorescent reef fish may communicate their presence and activities to conspecifics in this way (Michiels et al. 2008). If fluorescence is indeed used for intraspecific signaling, it may also be possible that the trait plays a role in mate choice. In certain fish (Garcia and de Perera 2002) and bird species (Hunt et al. 1998; Arnold et al. 2002) UV fluorescence appears to indicate individual fitness and influences mate choice—individuals with the brightest fluorescence have a reproductive advantage (Hunt et al. 1998; Arnold et al. 2002; Garcia and de Perera 2002). It is possible that fluorescence in Glaucomys plays a role in intraspecific communication, possibly related to mate selection.
Finally, Glaucomys fluorescence may be ecologically significant for interspecific interactions, specifically predator avoidance. UV fluorescence has been observed in many organisms that share the twilight and nighttime landscape with flying squirrels. Co-occurring barn owls (Tyto alba), barred owls (Strix varia), and great horned owls (Bubo virginianus) are known predators of flying squirrels that also display bright pink or magenta UV fluorescence on their ventral surfaces (Weidensaul et al. 2011). Several other co-occurring owls that are not squirrel predators, including northern saw-whet owls (Aegolius acadicus) and screech-owls (Megascops spp.), also fluoresce pink ventrally. Flying squirrels using the same habitats at the same times of night as the owls show the same colors and patterns of fluorescence. This may be a form of Batesian mimicry, in which Glaucomys resemble predatory owls in order to escape avian predation. In addition, some plants and lichens contain UV fluorescent compounds (Lloyd 1924; Goodwin and Kavanagh 1948; Hale 1956; Klein 1978). In the same way that reef fishes exploit UV fluorescence (or UV reflectance) to blend in with the fluorescent corals and algae of their environments (Sparks et al. 2014), Glaucomys may appear camouflaged against fluorescing lichen-covered trees. In short, fluorescent flying squirrels may blend into to their UV-saturated, fluorescent environment.
New World flying squirrels, like several species of New World didelphids, demonstrate UV fluorescence of the pelage. In both groups, the fluorescence can be seen in museum specimens from different regions and habitats, after decades of preservation, and in both sexes. In the flying squirrels, we have also observed this trait in wild animals. These distantly related mammals share a major ecological trait—nocturnal–crepuscular activity patterns—that may be the key to understanding the ecological significance of their fluorescence. The discovery of fluorescence in Glaucomys prompts many new questions about how Eutherian mammals navigate the twilight and nocturnal realm.
Supplementary Data
Supplementary data are available at Journal of Mammalogy online.
Supplementary Data SD1.—List of museum specimens photographed and analyzed for UV fluorescence.
Acknowledgments
We thank the Field Museum of Natural History, especially L. Heaney, A. Ferguson, and L. Smith; the Science Museum of Minnesota, especially R. Oehlenschlager; N. Anich, J. Burman, M. Laughlin, T. Pichler, T. Van Deelen, and R. Zifko. This research was funded in part by Northland College, a Parsonage Fund awarded to AMK, the Risvedt Professorship awarded to JGM, the Sigurd Olson Professorship in Natural Sciences awarded to ERO, and a sabbatical leave awarded to PSA.