-
PDF
- Split View
-
Views
-
Cite
Cite
Neil B Chilton, Philip S Curry, L Robbin Lindsay, Kateryn Rochon, Timothy J Lysyk, Shaun J Dergousoff, Passive and Active Surveillance for Ixodes scapularis (Acari: Ixodidae) in Saskatchewan, Canada, Journal of Medical Entomology, Volume 57, Issue 1, January 2020, Pages 156–163, https://doi.org/10.1093/jme/tjz155
Close - Share Icon Share
Abstract
Passive and active surveillance for the blacklegged tick, Ixodes scapularis Say, in the Canadian province of Saskatchewan was conducted over a 9-yr period (2009–2017). More than 26,000 ixodid ticks, representing 10 species, were submitted through passive surveillance. Most (97%) of these were the American dog tick, Dermacentor variabilis (Say). Of the 65 I. scapularis adults submitted, 75% were collected from dogs. Infection rates of Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti in I. scapularis were 12%, 8%, and 0%, respectively. Although the I. scapularis submitted by passive surveillance were collected from five of seven ecoregions in central and southern Saskatchewan, they were most frequent in the Moist Mixed Grassland and Aspen Parklands. In contrast, no I. scapularis were collected from the extensive field sampling conducted at multiple sites in different ecoregions across the province. Hence, there is no evidence of I. scapularis having established a breeding population in Saskatchewan. Nonetheless, continued surveillance for blacklegged ticks is warranted given their important role as a vector of medically and veterinary important pathogens, and because they have recently become established across much of the southern portions of the neighboring province of Manitoba.
The blacklegged tick, Ixodes scapularis Say (Acari: Ixodidae), is the principal vector of Borrelia burgdorferi, the causative agent of Lyme borreliosis (or Lyme disease), in eastern North America (Kurtenbach et al. 2006, Eisen et al. 2017). Over 275,000 cases of Lyme borreliosis were reported in the United States between 2008 and 2015, the majority of which occurred in the Midwest and the Northeast (Schwartz et al. 2017). These two geographical regions also represent the principal foci for cases of human granulocytic anaplasmosis (Dahlgren et al. 2011, Eisen et al. 2017), a disease caused by another bacterium, Anaplasma phagocytophilum (Bakken and Dumler 2015), which is also transmitted to humans by I. scapularis (Massung et al. 2002). Blacklegged ticks are also vectors of Powassan virus and Babesia microti, a protozoan parasite responsible for human babesiosis (Eisen et al. 2017).
The distributional range of I. scapularis continues to expand in some northern areas of the United States (e.g., Hamer et al. 2010, Russart et al. 2014, Hahn et al. 2016, Oliver et al. 2017), raising concern over the spread of tick-borne pathogens. Passerine birds, white-tailed deer, and other mammalian hosts are responsible for the dispersal of I. scapularis (Brinkerhoff et al. 2009); however, white-tailed deer are not involved in the spread of B. burgdorferi because they are incompetent reservoirs for this pathogen (Telford et al. 1988). In contrast, many species of passerine birds are competent hosts for B. burgdorferi (Rand et al. 1998, Richter et al. 2000, Ogden et al. 2008, Brinkerhoff et al. 2009) and are capable of dispersing infected ticks over large distances to nonendemic areas (Ogden et al. 2008, Scott et al. 2012, Brinkerhoff et al. 2009). For example, an estimated three billion migratory passerines transport between 50 and 170 million I. scapularis larvae and nymphs from the United States into Canada each year during their spring migration (Ogden et al. 2008).
The first resident population of I. scapularis in Canada was discovered at Long Point in southern Ontario during the 1970s (Watson and Anderson 1976). An established or resident (i.e., reproducing) population of I. scapularis is defined as the presence of all the three active life cycle stages (i.e., larva, nymph, and adult) on resident animals or in the environment at that locality for at least two consecutive years (Health Canada 1991). Despite the influx of large numbers of I. scapularis immatures on migratory birds from endemic areas in the United States (Ogden et al. 2008), Long Point was the only known locality in Canada with an established population of I. scapularis until 1997 (Barker et al. 1992, Barker and Lindsay 2000). By 2006, only seven established populations of I. scapularis were known in Canada; six in southern Ontario, and one in Nova Scotia (Ogden et al. 2006a). All of these geographically isolated populations were located in the warmest parts of these two provinces (Ogden et al. 2005). Given that off-host survival and development of I. scapularis are temperature-dependent, it was postulated that the cooler climatic conditions in Canada were not favorable for tick survival and development, thus restricting the establishment of new tick populations (Lindsay et al. 1995, Ogden et al. 2005). However, mathematical models, based on climatic changes associated with global warming and increased tick survival, predicted significant range expansion of I. scapularis by the 2020s into many areas in southern Canada; from Saskatchewan in the west, across to the Atlantic provinces in the east (Ogden et al. 2006a). Other mathematical models also predicted that range expansion by I. scapularis in eastern Canada would result in a significantly increased risk of Lyme borreliosis, with the number of persons living in endemic areas increasing from 18% in 2010 to 75% by 2025 (Leighton et al. 2012). Currently, there are many I. scapularis populations in the Canadian provinces of Ontario, Quebec, Nova Scotia, New Brunswick, and Manitoba (Ogden et al. 2009, 2014; Public Health Agency of Canada 2018). Results of recent passive and active surveillance in western Manitoba have indicated continued range expansion into areas near the Saskatchewan border (MHSAL 2018, Gasmi et al. 2019, K. Rochon et al., unpublished data). There has also been significant increase in the number of reported cases of human Lyme borreliosis in Canada (Ogden et al. 2009, 2014; Bouchard et al. 2015; Gasmi et al. 2017, 2019).
Given the predictions of Ogden et al. (2006a) that populations of I. scapularis may become established in southwestern Saskatchewan by the 2020s, we commenced a passive surveillance program for ticks in the province starting in 2009. In this paper, we report the findings of this 9-yr study, along with results of active sampling from 2008 to 2017. Included are the results of molecular analyses conducted to determine the prevalence of B. burgdorferi, A. phagocytophilum, and B. microti in blacklegged ticks from Saskatchewan.
Materials and Methods
Passive Surveillance
Ticks were submitted from veterinarians, veterinary technicians, physicians, and members of the public as part of a 2009–2017 passive surveillance program for I. scapularis in Saskatchewan. Submissions were solicited through the media, to veterinary clinics by fliers, and on websites of the University of Saskatchewan (Department of Biology) and the Saskatchewan Ministry of Health. Data presented in this paper are based on all ticks submitted to the University of Saskatchewan (U of S) between 2009 and 2017, and those submitted to the Roy Romanow Provincial Laboratory (RRPL) in 2012–2014 and 2017. Each tick was identified to the species based on morphological characters (Gregson 1956, Lindquist et al. 2016). Most of the ticks submitted to the U of S have been fixed in 70% ethanol and stored at 4°C. Also included are single records of I. scapularis submitted to RRPL in 2009, 2010, and 2011, and five I. scapularis submitted to the U of S and RRPL in 2008 (i.e., prior to commencement of the formal surveillance program). Submitters were asked to provide information on the locality where ticks were collected, the date of collection, source (e.g., human, dog, cat, domestic livestock, or vegetation), and if the host had travelled outside of the province in the previous 2 wk. Collection locations are therefore based on the information provided by the submitter and may not be the location where they were acquired. Only records of ticks collected in Saskatchewan are reported in this paper. The collection localities for I. scapularis were also examined in relation to the seven ecoregions in southern and central Saskatchewan (Fig. 1). These ecoregions range from the semiarid Mixed Grassland in the southern part of the province, to the moister and more wooded Moist Mixed Grassland, Aspen Parkland, and Boreal Transition, and Mid-Boreal Upland and Mid-Boreal Lowland ecoregions of the Boreal Forest. The Aspen Parkland and the Boreal Transition, which consist of a mixture of forest and farmland, are considered transitional between the Boreal Forest to the north, and the Mixed and Moist Mixed Grasslands to the south (Environment Canada 2008, Shorthouse 2010).
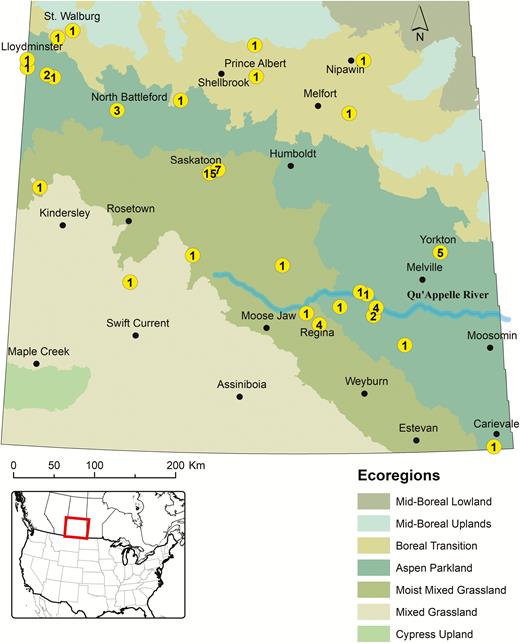
Map depicting the locations in different ecoregions of central and southern Saskatchewan from where I. scapularis adults were collected by passive surveillance (2008–2017). The number of individual ticks submitted from each location are indicated inside circles. The ecoregions are characterized based on soil types, elevation, rainfall, and the associated plant and animal communities. The Cypress Upland ranges from grasses to a mixed montane-type open forest composed of lodgepole pine, deciduous trees, and shrubs. The Mixed Grassland is semiarid and dominated by grasses (e.g., spear grass, wheat grass, and blue gramma grass), sagebrush, and other shrubs, while the Moist Mixed Grassland is dominated by spear grass, wheat grass, and shrubs (e.g., Saskatoon berry and chokecherry). The Aspen Parkland is mostly farmland and undisturbed areas characterized by trembling aspen and tall shrubs, with bur oak in drier areas, whereas the Boreal Transition represents a mix of farmland and forest characterized by tall trembling aspen with a thick understory. The Mid-Boreal Upland contains mixed coniferous and deciduous forest dominated by with stands of trembling aspen, balsam poplar, and spruce. The Mid-Boreal Lowland is a flat, low-lying region with an abundance of wetlands located in east central Saskatchewan. Common trees in the area include white spruce, balsam fir, aspen, American elm, green ash, and Manitoba maple (Environment Canada 2008, Shorthouse 2010).
Active Surveillance
Active sampling for I. scapularis was conducted between May and July (2013–2017) and between September and November (2016–2017) at sites in six ecoregions of central and southern Saskatchewan; however, sampling effort and technique (i.e., flagging or dragging vegetation for ticks) varied among sites and years. At some sites, at least two persons used flags (~1 m × 1 m flannel cloth attached to a pole) to search for ticks on vegetation during a period of at least 30 min. Drag sampling, using a 1 × 1 m flannel cloth attached to wooden dowels, was performed by at least two people at each site sampled. The protocol used for drag sampling followed that described in Rochon et al. (2012). A total area of 94–240 m2 (mean = 200 m2) was dragged during each sampling effort per site. Ticks were removed from flags and drags and identified using the same taxonomic keys mentioned previously.
Pathogen Detection in I. scapularis
Total genomic DNA was extracted from each I. scapularis using the DNeasy Blood & Tissue Kit (Qiagen Inc., Mississauga, ON, Canada) using the manufacturer’s protocol, but with the modifications described previously (Dergousoff and Chilton 2007). The species identity of each blacklegged tick was confirmed by PCR and DNA sequencing of ~400 bp of the mitochondrial 16S rRNA gene (Krakowetz et al. 2011). This also provided a test of the DNA extraction efficiency for pathogen testing. Genomic DNA (30 µl) from each blacklegged tick was then sent to the National Microbiology Laboratory (NML) in Winnipeg (Manitoba) for pathogen detection using multiplex real-time PCR, targeting the 23S rRNA gene of B. burgdorferi and the msp2 gene of A. phagocytophilum (Ogden et al. 2008). DNA extracts that were positive for B. burgdorferi were confirmed using a real-time PCR targeting the ospA gene (Ogden et al. 2008). In addition, DNA extracts collected after 2012 (n = 45) were also tested for the presence of B. microti using a real-time PCR assay targeting the chaperonin-containing t-complex polypeptide l (CCTη) gene (Nakajima et al. 2009).
Results
Passive Surveillance
Over 26,000 ixodid ticks were submitted through passive surveillance between 2008 and 2017 (Table 1). The number of submissions varied among years, ranging from 212 (in 2009) to 580 (in 2016), with an average of eight ticks per submission. Ten tick species were identified: Dermacentor variabilis (Say), Dermacentor andersoni Stiles, Dermacentor albipictus (Packard), I. scapularis, Ixodes kingi Bishopp, Ixodes sculptus Neumann, Haemaphysalis leporispalustris (Packard), Haemaphysalis chordeilis (Packard), Amblyomma americanum Linneaus, and Rhipicephalus sanguineus (Latreille). Two argasid ticks were also submitted; one in 2012, the other in 2016 (data not shown). Most (97%) of the ixodid ticks were D. variabilis adults. The number of I. scapularis varied from 1 to 15 per year, representing less than 1% of the total number of ticks submitted annually (Table 1).
Annual numbers of ticks obtained by passive surveillance between 2008 and 2017 in Saskatchewan, Canada
| Tick species . | 2008 . | 2009 . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ixodes scapularis | 5a | 5 | 3 | 3 | 1 | 10 | 5 | 9 | 9 | 15 | 65 |
| Ixodes kingi | – | 0 | 7 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 11 |
| Ixodes sculptus | – | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 5 |
| Dermacentor variabilis | – | 1,467 | 1,089 | 636 | 2,677 | 1,556 | 2,943 | 5,065 | 5,244 | 5,067 | 25,744 |
| Dermacentor andersoni | – | 1 | 34 | 19 | 60 | 59 | 0 | 10 | 0 | 0 | 183 |
| Dermacentor albipictus | – | 4 | 3 | 76 | 156 | 99 | 221 | 15 | 43 | 10 | 627 |
| Haemaphysalis spp. | – | 1 | 1 | 0 | 0 | 1 | 0 | 3 | 2 | 19 | 27 |
| Rhipicephalus sanguineus | – | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 6 |
| Amblyomma americanum | – | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total no. of ticks | 5 | 1,478 | 1,139 | 736 | 2,895 | 1,726 | 3,176 | 5,103 | 5,299 | 5,112 | 26,669 |
| No. of submissions | 5 | 212 | 249 | 257 | 431 | 321 | 531 | 305 | 580 | 413 | 3,304 |
| Mean no. of ticks per submission | – | 7 | 4.6 | 2.9 | 6.7 | 5.4 | 6 | 16.7 | 9.1 | 12.4 | 8.1b |
| % I. scapularis | n/a | 0.3 | 0.3 | 0.4 | 0.03 | 0.6 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2b |
| Tick species . | 2008 . | 2009 . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ixodes scapularis | 5a | 5 | 3 | 3 | 1 | 10 | 5 | 9 | 9 | 15 | 65 |
| Ixodes kingi | – | 0 | 7 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 11 |
| Ixodes sculptus | – | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 5 |
| Dermacentor variabilis | – | 1,467 | 1,089 | 636 | 2,677 | 1,556 | 2,943 | 5,065 | 5,244 | 5,067 | 25,744 |
| Dermacentor andersoni | – | 1 | 34 | 19 | 60 | 59 | 0 | 10 | 0 | 0 | 183 |
| Dermacentor albipictus | – | 4 | 3 | 76 | 156 | 99 | 221 | 15 | 43 | 10 | 627 |
| Haemaphysalis spp. | – | 1 | 1 | 0 | 0 | 1 | 0 | 3 | 2 | 19 | 27 |
| Rhipicephalus sanguineus | – | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 6 |
| Amblyomma americanum | – | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total no. of ticks | 5 | 1,478 | 1,139 | 736 | 2,895 | 1,726 | 3,176 | 5,103 | 5,299 | 5,112 | 26,669 |
| No. of submissions | 5 | 212 | 249 | 257 | 431 | 321 | 531 | 305 | 580 | 413 | 3,304 |
| Mean no. of ticks per submission | – | 7 | 4.6 | 2.9 | 6.7 | 5.4 | 6 | 16.7 | 9.1 | 12.4 | 8.1b |
| % I. scapularis | n/a | 0.3 | 0.3 | 0.4 | 0.03 | 0.6 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2b |
aNo records for ticks in 2008 except for I. scapularis.
bExcludes data for 2008.
Annual numbers of ticks obtained by passive surveillance between 2008 and 2017 in Saskatchewan, Canada
| Tick species . | 2008 . | 2009 . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ixodes scapularis | 5a | 5 | 3 | 3 | 1 | 10 | 5 | 9 | 9 | 15 | 65 |
| Ixodes kingi | – | 0 | 7 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 11 |
| Ixodes sculptus | – | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 5 |
| Dermacentor variabilis | – | 1,467 | 1,089 | 636 | 2,677 | 1,556 | 2,943 | 5,065 | 5,244 | 5,067 | 25,744 |
| Dermacentor andersoni | – | 1 | 34 | 19 | 60 | 59 | 0 | 10 | 0 | 0 | 183 |
| Dermacentor albipictus | – | 4 | 3 | 76 | 156 | 99 | 221 | 15 | 43 | 10 | 627 |
| Haemaphysalis spp. | – | 1 | 1 | 0 | 0 | 1 | 0 | 3 | 2 | 19 | 27 |
| Rhipicephalus sanguineus | – | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 6 |
| Amblyomma americanum | – | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total no. of ticks | 5 | 1,478 | 1,139 | 736 | 2,895 | 1,726 | 3,176 | 5,103 | 5,299 | 5,112 | 26,669 |
| No. of submissions | 5 | 212 | 249 | 257 | 431 | 321 | 531 | 305 | 580 | 413 | 3,304 |
| Mean no. of ticks per submission | – | 7 | 4.6 | 2.9 | 6.7 | 5.4 | 6 | 16.7 | 9.1 | 12.4 | 8.1b |
| % I. scapularis | n/a | 0.3 | 0.3 | 0.4 | 0.03 | 0.6 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2b |
| Tick species . | 2008 . | 2009 . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ixodes scapularis | 5a | 5 | 3 | 3 | 1 | 10 | 5 | 9 | 9 | 15 | 65 |
| Ixodes kingi | – | 0 | 7 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 11 |
| Ixodes sculptus | – | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 5 |
| Dermacentor variabilis | – | 1,467 | 1,089 | 636 | 2,677 | 1,556 | 2,943 | 5,065 | 5,244 | 5,067 | 25,744 |
| Dermacentor andersoni | – | 1 | 34 | 19 | 60 | 59 | 0 | 10 | 0 | 0 | 183 |
| Dermacentor albipictus | – | 4 | 3 | 76 | 156 | 99 | 221 | 15 | 43 | 10 | 627 |
| Haemaphysalis spp. | – | 1 | 1 | 0 | 0 | 1 | 0 | 3 | 2 | 19 | 27 |
| Rhipicephalus sanguineus | – | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 6 |
| Amblyomma americanum | – | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Total no. of ticks | 5 | 1,478 | 1,139 | 736 | 2,895 | 1,726 | 3,176 | 5,103 | 5,299 | 5,112 | 26,669 |
| No. of submissions | 5 | 212 | 249 | 257 | 431 | 321 | 531 | 305 | 580 | 413 | 3,304 |
| Mean no. of ticks per submission | – | 7 | 4.6 | 2.9 | 6.7 | 5.4 | 6 | 16.7 | 9.1 | 12.4 | 8.1b |
| % I. scapularis | n/a | 0.3 | 0.3 | 0.4 | 0.03 | 0.6 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2b |
aNo records for ticks in 2008 except for I. scapularis.
bExcludes data for 2008.
Sixty-five I. scapularis adults, comprising 64 females and 1 male, were submitted from 2008 to 2017. A majority (75%) of these blacklegged ticks were collected from dogs (Table 2). The other I. scapularis were found on cats, horses, and humans. Multiple submissions of individual I. scapularis were obtained in or around Saskatoon, Yorkton, Regina, North Battleford, and the Qu’Appelle Valley. Blacklegged ticks were collected from five of seven ecoregions in the province (Fig. 1); however, they were most frequent in the Moist Mixed Grassland (47%) and Aspen Parkland (40%). The other ecoregions from which blacklegged ticks were collected were the Mixed Grassland (3%), the Boreal Transition (8%), and the Mid-Boreal Upland (2%). The most northern record for I. scapularis in the province was from St. Walburg (53.634, −109.200), while the two westernmost records were from near Lloydminster (53.280, −110.005), situated on the Saskatchewan–Alberta border (Fig. 1). The southernmost record for I. scapularis in the province was south of the village of Carievale (49.173, −101.625).
Number of Ixodes scapularis collected from various hosts in Saskatchewan, Canada
| Year . | Humans . | Dogs . | Cats . | Horses . | Unknown . | Total . |
|---|---|---|---|---|---|---|
| 2008 | 1 | 4 | 0 | 0 | 0 | 5 |
| 2009 | 0 | 5 | 0 | 0 | 0 | 5 |
| 2010 | 0 | 3 | 0 | 0 | 0 | 3 |
| 2011 | 0 | 2 | 0 | 0 | 1 | 3 |
| 2012 | 0 | 1 | 0 | 0 | 0 | 1 |
| 2013 | 0 | 10 | 0 | 0 | 0 | 10 |
| 2014 | 1 | 3 | 1 | 0 | 0 | 5 |
| 2015 | 1 | 5 | 2 | 1 | 0 | 9 |
| 2016 | 0 | 9 | 0 | 0 | 0 | 9 |
| 2017 | 3 | 7 | 3 | 1 | 1 | 15 |
| Total (% of total) | 6 (9) | 49 (75) | 6 (9) | 2 (3) | 2 (3) | 65 |
| Year . | Humans . | Dogs . | Cats . | Horses . | Unknown . | Total . |
|---|---|---|---|---|---|---|
| 2008 | 1 | 4 | 0 | 0 | 0 | 5 |
| 2009 | 0 | 5 | 0 | 0 | 0 | 5 |
| 2010 | 0 | 3 | 0 | 0 | 0 | 3 |
| 2011 | 0 | 2 | 0 | 0 | 1 | 3 |
| 2012 | 0 | 1 | 0 | 0 | 0 | 1 |
| 2013 | 0 | 10 | 0 | 0 | 0 | 10 |
| 2014 | 1 | 3 | 1 | 0 | 0 | 5 |
| 2015 | 1 | 5 | 2 | 1 | 0 | 9 |
| 2016 | 0 | 9 | 0 | 0 | 0 | 9 |
| 2017 | 3 | 7 | 3 | 1 | 1 | 15 |
| Total (% of total) | 6 (9) | 49 (75) | 6 (9) | 2 (3) | 2 (3) | 65 |
Number of Ixodes scapularis collected from various hosts in Saskatchewan, Canada
| Year . | Humans . | Dogs . | Cats . | Horses . | Unknown . | Total . |
|---|---|---|---|---|---|---|
| 2008 | 1 | 4 | 0 | 0 | 0 | 5 |
| 2009 | 0 | 5 | 0 | 0 | 0 | 5 |
| 2010 | 0 | 3 | 0 | 0 | 0 | 3 |
| 2011 | 0 | 2 | 0 | 0 | 1 | 3 |
| 2012 | 0 | 1 | 0 | 0 | 0 | 1 |
| 2013 | 0 | 10 | 0 | 0 | 0 | 10 |
| 2014 | 1 | 3 | 1 | 0 | 0 | 5 |
| 2015 | 1 | 5 | 2 | 1 | 0 | 9 |
| 2016 | 0 | 9 | 0 | 0 | 0 | 9 |
| 2017 | 3 | 7 | 3 | 1 | 1 | 15 |
| Total (% of total) | 6 (9) | 49 (75) | 6 (9) | 2 (3) | 2 (3) | 65 |
| Year . | Humans . | Dogs . | Cats . | Horses . | Unknown . | Total . |
|---|---|---|---|---|---|---|
| 2008 | 1 | 4 | 0 | 0 | 0 | 5 |
| 2009 | 0 | 5 | 0 | 0 | 0 | 5 |
| 2010 | 0 | 3 | 0 | 0 | 0 | 3 |
| 2011 | 0 | 2 | 0 | 0 | 1 | 3 |
| 2012 | 0 | 1 | 0 | 0 | 0 | 1 |
| 2013 | 0 | 10 | 0 | 0 | 0 | 10 |
| 2014 | 1 | 3 | 1 | 0 | 0 | 5 |
| 2015 | 1 | 5 | 2 | 1 | 0 | 9 |
| 2016 | 0 | 9 | 0 | 0 | 0 | 9 |
| 2017 | 3 | 7 | 3 | 1 | 1 | 15 |
| Total (% of total) | 6 (9) | 49 (75) | 6 (9) | 2 (3) | 2 (3) | 65 |
Active Surveillance
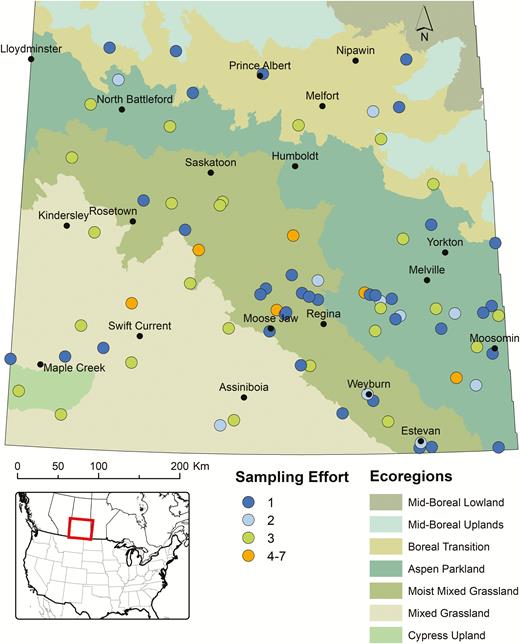
Seventy-eight sites (Fig. 2) were actively sampled for ticks by dragging (n = 35 sites) and/or flagging (n = 48 sites) vegetation during spring-summer and fall over a 5-yr period (2013–2017). Forty-one of these sites were sampled on multiple occasions; five of which were sampled at least once each year between 2014 and 2017. Although Dermacentor spp. were collected at many sites, particularly in central and southern Saskatchewan, no I. scapularis were collected from any site.
Map depicting the locations in different ecoregions of central and southern Saskatchewan from where active sampling (dragging or flagging of vegetation) for questing I. scapularis was conducted during the spring and summer (2013–2017). The different circles indicate the number of times active surveillance was performed at a locality.
Pathogen Detection in I. scapularis
Of 65 I. scapularis submitted, eight (12.3%) were PCR-positive for B. burgdorferi and five (7.7%) were positive for A. phagocytophilum (Table 3). Three blacklegged ticks were co-infected with B. burgdorferi and A. phagocytophilum. There was no evidence of infection with B. microti in any of the 45 ticks tested.
Number of blacklegged ticks PCR-positive for Borrelia burgdorferi and Anaplasma phagocytophilum in different years in Saskatchewan, Canada
| Year . | No. of I. scapularis . | No. of positive for B. burgdorferi . | No. of positive for A. phagocytophilum . | No. of positive for both pathogens . |
|---|---|---|---|---|
| 2008 | 5 | 0 | 1 | 0 |
| 2009 | 5 | 1 | 1 | 1 |
| 2010 | 3 | 0 | 0 | 0 |
| 2011 | 3 | 1 | 0 | 0 |
| 2012 | 1 | 0 | 0 | 0 |
| 2013 | 10 | 1 | 2 | 1 |
| 2014 | 5 | 0 | 0 | 0 |
| 2015 | 9 | 1 | 1 | 1 |
| 2016 | 9 | 0 | 0 | 0 |
| 2017 | 15 | 4 | 0 | 0 |
| Total (%) | 65 | 8 (12.3) | 5 (7.7) | 3 (4.6) |
| Year . | No. of I. scapularis . | No. of positive for B. burgdorferi . | No. of positive for A. phagocytophilum . | No. of positive for both pathogens . |
|---|---|---|---|---|
| 2008 | 5 | 0 | 1 | 0 |
| 2009 | 5 | 1 | 1 | 1 |
| 2010 | 3 | 0 | 0 | 0 |
| 2011 | 3 | 1 | 0 | 0 |
| 2012 | 1 | 0 | 0 | 0 |
| 2013 | 10 | 1 | 2 | 1 |
| 2014 | 5 | 0 | 0 | 0 |
| 2015 | 9 | 1 | 1 | 1 |
| 2016 | 9 | 0 | 0 | 0 |
| 2017 | 15 | 4 | 0 | 0 |
| Total (%) | 65 | 8 (12.3) | 5 (7.7) | 3 (4.6) |
Number of blacklegged ticks PCR-positive for Borrelia burgdorferi and Anaplasma phagocytophilum in different years in Saskatchewan, Canada
| Year . | No. of I. scapularis . | No. of positive for B. burgdorferi . | No. of positive for A. phagocytophilum . | No. of positive for both pathogens . |
|---|---|---|---|---|
| 2008 | 5 | 0 | 1 | 0 |
| 2009 | 5 | 1 | 1 | 1 |
| 2010 | 3 | 0 | 0 | 0 |
| 2011 | 3 | 1 | 0 | 0 |
| 2012 | 1 | 0 | 0 | 0 |
| 2013 | 10 | 1 | 2 | 1 |
| 2014 | 5 | 0 | 0 | 0 |
| 2015 | 9 | 1 | 1 | 1 |
| 2016 | 9 | 0 | 0 | 0 |
| 2017 | 15 | 4 | 0 | 0 |
| Total (%) | 65 | 8 (12.3) | 5 (7.7) | 3 (4.6) |
| Year . | No. of I. scapularis . | No. of positive for B. burgdorferi . | No. of positive for A. phagocytophilum . | No. of positive for both pathogens . |
|---|---|---|---|---|
| 2008 | 5 | 0 | 1 | 0 |
| 2009 | 5 | 1 | 1 | 1 |
| 2010 | 3 | 0 | 0 | 0 |
| 2011 | 3 | 1 | 0 | 0 |
| 2012 | 1 | 0 | 0 | 0 |
| 2013 | 10 | 1 | 2 | 1 |
| 2014 | 5 | 0 | 0 | 0 |
| 2015 | 9 | 1 | 1 | 1 |
| 2016 | 9 | 0 | 0 | 0 |
| 2017 | 15 | 4 | 0 | 0 |
| Total (%) | 65 | 8 (12.3) | 5 (7.7) | 3 (4.6) |
Discussion
Detailed knowledge of the geographical distribution, locations of established populations, relative abundance and seasonal activity of blacklegged ticks (I. scapularis), and the prevalence of the different pathogens they carry, is essential for estimating the risk of human infection with pathogens causing Lyme borreliosis (i.e., B. burgdorferi), granulocytic anaplasmosis (i.e., A. phagocytophilum), and babesiosis (i.e., B. microti). It has been predicted, based on mathematical modeling, that populations of I. scapularis will become established in southeastern Saskatchewan during the 2020s (Ogden et al. 2006a). In the present study, we assessed the presence/absence of established populations of I. scapularis in Saskatchewan by passive surveillance (2009–2017), and active sampling for ticks by flagging and/or dragging across 78 sites across the province (2013–2017). Active sampling is one of the most reliable and sensitive methods for identifying established tick populations (Koffi et al. 2012), but it failed to indicate questing I. scapularis. Other reliable methods include sampling ticks from trapped rodent hosts (Koffi et al. 2012), but this was not used because it was beyond the scope of this study.
Of the over 26,000 ixodid ticks submitted by passive surveillance, most were associated with humans and their pets (i.e., dogs, cats, and rabbits). Some ticks were also collected from farm animals (i.e., horses and cattle). Most (97%) ticks were D. variabilis (American dog tick). This finding was consistent with the results of the 1998 passive surveillance study conducted in Saskatchewan by Lindsay et al. (1999), where 99% of the 1,522 ticks submitted were D. variabilis. Most D. variabilis submitted were adults (males and females), as larvae and nymphs occur primarily on mice, shrews, voles, and chipmunks (Dergousoff et al. 2013). Questing D. variabilis adults are also the most common species collected by flagging or dragging vegetation (Dergousoff et al. 2013), and they are a common parasite of wildlife, humans, and domestic animals (dogs, cattle, and horses) in many parts of Saskatchewan (Dergousoff et al. 2013, Lindquist et al. 2016). This tick species has markedly expanded its distributional range in the province over the past 40 yr (Dergousoff et al. 2013). Another nine tick species, D. andersoni, D. albipictus, R. sanguineus, A. americanum, I. sculptus, I. kingi, I. scapularis, H. leporispalustris, and H. chordeilis, were also obtained by passive surveillance, six of which were reported by Lindsay et al. (1999). The winter or moose tick, D. albipictus, is most commonly found on moose; however, it is also a parasite of elk, deer, cattle, and horses (Lindquist et al. 2016). Most of the submitted specimens of D. albipictus originated from parasitized cattle and horses at localities in central and southern Saskatchewan. In contrast, the D. andersoni collected were from humans, dogs, and horses in the southwest, which is consistent with the distributional range for this tick species in the province (Dergousoff et al. 2013, Anstead and Chilton 2014, Dergousoff and Chilton 2016, Lindquist et al. 2016). The two species of Haemaphysalis, H. leporispalustris and H. chordeilis, which are parasites of lagomorphs and gallinaceous birds (respectively), have been recorded at several localities in Saskatchewan (Lindquist et al. 2016). The rotund tick, I. kingi, is a common parasite of Richardson’s ground squirrels (Urocitellus richardsonii) and northern pocket gophers (Thomomys talpoides) (Anstead and Chilton 2011, Anstead et al. 2014, Lindquist et al. 2016), and occasionally of dogs and cats in Saskatchewan (Lindquist et al. 2016). Ixodes sculptus is also a common parasite of Richardson’s ground squirrels in the province (Anstead and Chilton 2014, Anstead et al. 2014, Lindquist et al. 2016). Most specimens of I. sculptus and I. kingi obtained by passive surveillance were female ticks attached to dogs and cats. Six brown dog ticks (R. sanguineus) and one lone star tick (A. americanum) were obtained by passive surveillance. These records probably represent adventitious ticks as they are rarely detected in Saskatchewan and their presence in other Canadian provinces has been attributed to human/companion animal travel to endemic areas (Lindquist et al. 2016).
Ten (15%) of the 65 I. scapularis adults were PCR-positive for B. burgdorferi and/or A. phagocytophilum, while B. microti was not detected in any tick. Three (5%) blacklegged ticks were co-infected with B. burgdorferi and A. phagocytophilum. The rate of B. burgdorferi infection in blacklegged ticks from Saskatchewan (12%) is consistent with the prevalence reported from emerging areas of I. scapularis occurrence in other Canadian provinces: Manitoba (10–13%), Quebec (13–14%), New Brunswick (7%), Nova Scotia (11–15%), and Ontario (16%) (Ogden et al. 2006a,b; Dibernardo et al. 2014). The prevalence of A. phagocytophilum (8%) was also similar to that reported previously for Manitoba (6%), but higher than in Ontario, Quebec, and the Atlantic provinces (0.2–3%) (Krakowetz et al. 2014).
The number of I. scapularis submitted per year varied from 1 (in 2012) to 15 (in 2017), with a mean of 6.5 individuals per year, which represented on average only 0.2% of the total ticks submitted each year. These numbers are similar to those reported for the province in 1998, where 4 (0.3%) of 1,522 ticks were I. scapularis adults (Lindsay et al. 1999). Although no I. scapularis larvae or nymphs were detected by passive surveillance, this was consistent with results from other passive tick surveillance programs where most blacklegged ticks submitted are adults (Ogden et al. 2006b, Nelder et al. 2014, Gasmi et al. 2019). The adult blacklegged ticks collected by passive surveillance in the present study represent adventitious ticks because no I. scapularis were detected by active sampling for ticks conducted at a large number of sites over several years. These adventitious ticks were most likely transported into Saskatchewan as larvae or nymphs on passerine birds that travelled through endemic areas of the United States during their spring migration, and successfully developed through to the adults, which were fortuitously detected in the passive surveillance program. Anstead et al. (2011) did report the unexpected detection of six I. scapularis larvae parasitizing T. talpoides on an acreage near Clavet (i.e., ≈25 km southeast of Saskatoon). Molecular analyses revealed that these six individuals represented four different mitochondrial (mt) rRNA gene 16S haplotypes, indicating that these larvae were derived from at least four females, hence represented multiple colonizing events (Anstead et al. 2011). However, subsequent examination of the ectoparasites of T. talpoides and U. richardsonii, and flagging vegetation for ticks at this site, failed to detect the presence of I. scapularis (Anstead et al. 2011). Therefore, at this point in time, there is no evidence of an established population of I. scapularis in Saskatchewan. Nonetheless, it is important to continue surveillance efforts in the province to assess the potential risks for public health given that I. scapularis is expanding its distributional range in southern Canada at a rapid rate (Leighton et al. 2012). In addition, nine human cases of Lyme borreliosis have been reported in Saskatchewan since 2009, two of which may have been acquired within the province (Saskatchewan Ministry of Health 2019).
A large majority (75%) of I. scapularis adults collected by passive surveillance in the present study were from dogs; however, a small number were also collected from cats and horses (6% and 2%, respectively). Only six (9%) I. scapularis submitted were associated with humans. Therefore, dogs, and to a lesser extent horses, can be used as sentinels for the detection of blacklegged ticks and/or their pathogens because both hosts are susceptible to, and show clinical symptoms of, infection with B. burgdorferi and A. phagocytophilum. For example, granulocytic anaplasmosis was reported in three dogs and a horse in Saskatchewan (Cockwill et al. 2009, Burgess et al. 2012), while Schurer et al. (2014) reported that 2 (3%) of 77 dogs in southeastern Saskatchewan were seropositive for B. burgdorferi.
It is possible that I. scapularis populations may first become established in southeastern Saskatchewan given that several populations of I. scapularis have recently become established in Manitoba (Gasmi et al. 2019) and North Dakota (Russart et al. 2014). White-tailed deer moving along riparian habitats associated with the Qu’Appelle River (Saskatchewan), Assiniboine River (Manitoba), and Souris River (Manitoba) may transport ticks from resident populations in Manitoba and North Dakota into Saskatchewan. Several submissions of blacklegged ticks were obtained from localities in the Qu’Appelle River Valley and near Yorkton. Multiple submissions of blacklegged ticks were also obtained from localities in and around the cities of Saskatoon and Regina, but this may represent a sample bias given that these are areas of high human density. However, examination of the distributional patterns of I. scapularis collected by passive surveillance (Fig. 1) shows no clustering of samples in the southeast near the border with Manitoba. Blacklegged ticks were collected in five of the seven ecoregions; but primarily in the Moist Mixed Grassland, Aspen Parkland, and Boreal Transition. These ecoregions extend in a broad arc from southwestern Manitoba, northwestward through Saskatchewan to a northern apex in Central Alberta. This dispersal pattern of adventitious ticks is most likely associated with the behavior of their avian hosts. Large numbers of larval and nymphal ticks are transported from endemic areas of the United States into southern Canada by migratory passerines during their spring migration (Ogden et al. 2008). The geographical differences in the population genetic structure of I. scapularis in Canada indicate that ticks originated from different endemic areas in the United States as a consequence of the different flyways used by migratory passerines (Krakowetz et al. 2011, 2014; Mechai et al. 2013). For instance, a greater proportion of the mt 16S rRNA gene haplotypes reported for blacklegged ticks in the Canadian prairies (Manitoba and Saskatchewan) were found in ticks from the Midwest of the United States (Minnesota) than in other regions of Canada and northeastern United States (Krakowetz et al. 2014). This is consistent with the hypothesis that adventitious blacklegged ticks in the Canadian prairies may be translocated from populations in the Midwest (Minnesota and Wisconsin) of the United States by migratory passerines using the central and Mississippi flyways (Krakowetz et al. 2011, Scott et al. 2012).
Potential risk areas for the establishment of I. scapularis in Saskatchewan have been identified through development of a risk model for the Prairie Provinces (Gabriele-Rivet et al. 2017). This model integrates temperature, habitat as a combined geo-layer of forest cover and agricultural land use, and rainfall to produce a risk map for Saskatchewan, Manitoba, and Alberta. The map identifies areas of low to high potential (risk index 0–5) for I. scapularis, and has helped to further guide active tick surveillance efforts in Saskatchewan. Of the 64.6 million hectares of habitat classified in Saskatchewan, 1,463,322 ha have been identified as having some risk for establishment of I. scapularis, with 181,984 ha have been classified as having a high-risk potential (risk category 4–5) for establishment (Saskatchewan Ministry of Health, unpublished data). The majority of the high-risk areas are located in the Aspen Parkland and Boreal Transition ecoregions where there are sufficient deciduous tree areas and wooded riparian habitats that are highly suitable for the successful establishment of I. scapularis.
In conclusion, currently, there is no evidence of an established population of I. scapularis in Saskatchewan based on the results of active sampling for ticks across many sites in difference ecoregions across the province. Nonetheless, a small number of adventitious ticks have been collected by passive surveillance, a few (15%) of which were infected with B. burgdorferi and/or A. phagocytophilum. It is likely that I. scapularis will continue to be introduced into Saskatchewan from both established areas of the United States (e.g., Minnesota and Wisconsin; Hahn et al. 2016) and recently emerging areas (e.g., Manitoba and North Dakota; Russart et al. 2014, Gasmi et al. 2019) by the movements of migratory passerines and/or white-tailed deer. Therefore, continued enhanced active and passive surveillance is needed to determine when and where populations of I. scapularis, and their associated pathogens, become established, and the potential risks for human health.
Acknowledgments
Funding for this work was provided to N.B.C. from the Saskatchewan Ministry of Health. We also thank the Beef Cattle Research Council, the Saskatchewan Ministry of Agriculture, and the Public Health Agency of Canada who provided financial assistance for active tick sampling. Special thanks go to all those persons who submitted ticks for the passive surveillance program. We thank also Alexander Halpin, Prasobh Thampy, Jessica Thoroughgood, Mathew Yunik, Chulantha Diyes, Emily Sterling, Mathea Sanderson, Megan Bjordal, Allison Sproat, James Paul, James Armstrong, Diana Dunlop, Elisabeth Parker, Christine Himsl-Rayner, Jason Cousins, Branden Hilts, Brittany Heisler, and Marko Micovic for the valuable assistance in the field, and Brooks Waitt, Tyler Cote, and Antonia Dibernardo, who kindly provided diagnostic expertise for the tick testing performed at the NML. Rob Anderson is acknowledged for his assistance with Fig. 1. We also thank Jann Pelcat for providing potential ‘risk area’ data for Saskatchewan.
References Cited
Author notes
Retired