-
PDF
- Split View
-
Views
-
Cite
Cite
Susan P Elias, Jack W Witham, Elizabeth F Schneider, Peter W Rand, Malcolm L Hunter, Charles Lubelczyk, Robert P Smith, Emergence of Ixodes scapularis (Acari: Ixodidae) in a Small Mammal Population in a Coastal Oak-Pine Forest, Maine, USA, Journal of Medical Entomology, Volume 59, Issue 2, March 2022, Pages 725–740, https://doi.org/10.1093/jme/tjab209
Close - Share Icon Share
Abstract
In the United States, surveillance has been key to tracking spatiotemporal emergence of blacklegged ticks [Ixodes scapularis Say (Ixodida:Ixodidae)] and their pathogens such as Borrelia burgdorferi Johnson, Schmid, Hyde, Steigerwalt & Brenner (Spirochaetales: Spirochaetaceae), the agent of Lyme disease. On the Holt Research Forest in midcoastal Maine, collection of feeding ticks from live-trapped small mammal hosts allowed us to track the emergence and establishment of I. scapularis, 1989–2019. From 1989–1995, we collected only I. angustus Neumann (Ixodida: Ixodidae)(vole tick), Dermacentor variabilis Say (Ixodida: Ixodidae) (American dog tick), and I. marxi Banks (Ixodida: Ixodidae) (squirrel tick) from seven species of small mammals. The most abundant tick host was the white-footed mouse [Peromyscus leucopus Rafinesque (Rodentia:Cricetidae)] followed by the red-backed vole (Myodes gapperi Vigors (Rodentia: Cricetidae)). Emergence of I. scapularis was signaled via the appearance of subadult I. scapularis in 1996. Emergence of B. burgdorferi was signaled through its appearance in I. scapularis feeding on mice in 2005. There was a substantial increase in I. scapularis prevalence (proportion of hosts parasitized) and burdens (ticks/host) on white-footed mice and red-backed voles in 2007. The ~11-yr time-to-establishment for I. scapularis was consistent with that seen in other studies. White-footed mice comprised 65.9% of all captures and hosted 94.1% of the total I. scapularis burden. The white-footed mouse population fluctuated interannually, but did not trend up as did I. scapularis prevalence and burdens. There were concurrent declines in I. angustus and D. variabilis. We discuss these results in the broader context of regional I. scapularis range expansion.
In the United States, surveillance has been key to tracking emergence of medically important ticks such as the blacklegged tick [Ixodes scapularis Say (Ixodida:Ixodidae)] and the pathogens they carry (Eisen and Paddock 2021). I. scapularis infected with the bacterial spirochete Borrelia burgdorferi sensu stricto (s.s.) Johnson, Schmid, Hyde, Steigerwalt & Brenner (Spirochaetales: Spirochaetaceae) can cause Lyme disease (Spielman et al. 1985, Mead et al. 2015, Rosenberg et al. 2018). High Lyme disease incidence states are in the Northeast, mid-Atlantic, and upper Midwest (Schwartz et al. 2017), reflecting the emergence and geographic expansion of I. scapularis (Eisen et al. 2016a). Phylogeographic analyses indicate I. scapularis has been expanding its range in the Northeast and Midwest US from southern refugia since the Last Glacial Maximum (Humphrey et al. 2010, Van Zee et al. 2015, Eisen and Eisen 2018, Xu et al. 2020). Various forms of tick surveillance have shown accelerated emergence and range expansion of I. scapularis over the past two decades, with northward expansion associated with climate change (e.g., Clow et al. 2017, Eisen and Eisen 2018, Talbot et al. 2019, Eisen and Paddock 2021). Climate, seasonal weather, habitat, and host conditions permitting, I. scapularis can cycle through its life stages of eggs, larvae, nymphs, and adults in 2–4 yr (Eisen et al. 2016b).
Ixodes scapularis subadults (larvae and nymphs) depend on blood meals from a range of vertebrate hosts, including birds, white-tailed deer (Odocoileus virginianus Zimmerman (Artiodactyla: Cervidae)), and small mammals (Eisen et al. 2016b). White-footed mice [Peromyscus leucopus Rafinesque (Rodentia:Cricetidae)] and some other small mammal species are important blood meal hosts of subadult I. scapularis, are competent reservoirs of B. burgdorferi s.s., (henceforth called B. burgdorferi), and help maintain and amplify enzootic transmission cycles (e.g., Mather et al. 1989, Brisson et al. 2008, Brunner et al. 2008, Barbour 2017, Johnson et al. 2017).
Small mammal trapping to collect feeding ticks is an important form of active tick surveillance (Machtinger and Williams 2020). Though more labor intensive than drag sampling to collect questing (host-seeking) ticks, mammal trapping provides information about pathogens in reservoir hosts (Machtinger and Williams 2020), and tracks the presence of nidicolous tick species which rarely quest outside the nests or burrows of their hosts (Sonenshine and Roe 2014). To track the emergence and establishment of I. scapularis and B. burgdorferi in a forest in Maine, we collected ticks from small mammals over a time span of three decades. We also examined the trend in I. angustus Neumann (Ixodida: Ixodidae) (vole tick), a nidicolous tick that seemed increasingly rare as time went on.
The Holt Research Forest is a forest in the midcoastal town of Arrowsic, Sagadahoc County, Maine, in the northeast corner of New England. Forest ecology research began in 1983, including a small mammal live trapping program. This program has shown that white-footed mice and red-backed voles (Myodes gapperi Vigors (Rodentia: Cricetidae)) exhibit approximately four-year population cycles with one or two peak years and one or two crash years occurring in each cycle (Elias et al. 2004, 2006a; Wang et al. 2009). Also, peak acorn mast years were not always followed by peaks in white-footed mouse abundance (Elias et al. 2004, Wang et al. 2009), contrary to popular assumption (e.g., Bever 2017).
In 1989 staff of the Lyme & Vector-borne Disease Laboratory at the Maine Medical Center Research Institute (MMCRI) began a collaboration with staff at the Holt Research Forest to surveil ticks using small mammals. The Holt Research Forest small mammal trapping program was designed to monitor small mammals rather than study the complex ecology of tick-borne disease risk in a forest setting as has been done in other programs (e.g., Ostfeld et al. 2018). Ticks were collected only during August trapping sessions, corresponding with the seasonal peak of I. scapularis larval questing. Nevertheless, the existing trapping program allowed compilation of a 31-yr tick surveillance data set allowing us to track the emergence and establishment of I. scapularis and B. burgdorferi.
Concurrently, MMCRI initiated a statewide, passive tick surveillance program during 1989–2013, following Maine’s first discovery of I. scapularis on white-tailed deer in 1985, and Maine’s first reported Lyme disease case in 1986 (Smith et al. 1992). Passive surveillance is the submission of ticks found on humans or pets and indexes tick abundance over time and space (Rand et al. 2007, Koffi et al. 2012, Ripoche et al. 2018, Gasmi et al. 2019). Specificity of passive surveillance data can be limited (submitted ticks could be adventitious ticks), however, early submissions of I. scapularis from a town, county, province may signal emergence and can trigger the use of active surveillance, which is better suited to pinpointing locations of emergent I. scapularis (e.g., Ogden et al. 2014, Clow et al. 2018). Maine’s passive surveillance program showed I. scapularis abundance in southern Maine increased during a 10–14 yr time span, followed by a fluctuating but stable pattern (Elias et al. 2020). This represented the trajectory from emergence (uptrend) through establishment (no trend) of I. scapularis as predicted by Ginsberg (1993) and Ogden et al. (2007).
In this study, we focus on long-term patterns of I. scapularis emergence and establishment with interest in time-to-establishment. An emergent I. scapularis population is signaled by the appearance and increasing abundance of I. scapularis, seen as an upward trend. Emergence transitions to the establishment, signaled by a leveling off in abundance (Ginsberg 1993, Ogden et al. 2007, Elias et al. 2020). The emergence-establishment temporal pattern is consistent with a sigmoid-shaped Gompertz growth curve, which has been used to describe growth in plant, animal, and bacterial populations (Tjørve and Tjørve 2017). Our emergence-establishment definition is different from the short-term, area-based assessment protocols used in the US and Canada used to classify areas as having emergent or established I. scapularis populations (Canada Health 1991, Dennis et al. 1998, Eisen et al. 2016a, USCDC 2021). The US and Canada classification criteria differ somewhat, but concur with our time-based premise that emergence precedes establishment and an established population consists of a reproducing population of I. scapularis.
Our aim was to quantify the length and timing of emergence and establishment of I. scapularis via active surveillance of tick prevalence in the small mammal community at the Holt Research Forest. Our hypotheses were that the temporal trend of emergence and establishment would mirror that seen in Maine’s passive surveillance data for Sagadahoc County, that populations of white-footed mice and red-backed voles would fluctuate interannually as previously documented, but without trends, and that white-footed mice would be key I. scapularis hosts by virtue of highest abundance and highest I. scapularis prevalence (proportion of hosts parasitized), by carrying most of the I. scapularis burden in the small mammal community and by hosting the highest proportion of B. burgdorferi-infected I. scapularis, consistent with patterns seen in the US Northeast (Halsey et al. 2018).
Materials and Methods
Study Area
The Holt Research Forest, Arrowsic, Maine, United States (43°52′N 69°46′W) (Fig. 1), is an oak-pine forest (Maine Natural Areas Program 2021) with mature trees 80–100 yr old, and lies between tidal portions of the Kennebec River and Back River in Sagadahoc County in the midcoast region of the state. Long-term forestry and wildlife research studies began in 1983 on a 40-ha portion of the forest. Following a selective harvest in winter of 1987–1988, the closed canopy forest was interspersed with natural gaps and harvest gaps (4 and 26% of total area, respectively) (Kimball et al. 1995). Common overstory tree species in terms of trees per hectare and basal area are red oak (Quercus rubra L.), white pine (Pinus strobus L.), red maple (Acer rubrum L.), red spruce (Picea rubens Sarg.), hemlock (Tsuga canadensis L.), yellow birch (Betula alleghaniensis Britton), and paper birch (Betula papyrifera Marshall). Native shrub species in the understory include huckleberry (Gaylussacia baccata Wangenh.) and high-bush blueberry (Vaccinium corymbosum L.). Due to aggressive management, there is no Japanese barberry (Berberis thunbergii DC), a nonnative, invasive shrub associated with higher I. scapularis density in southern Maine (Lubelczyk et al. 2004, Elias et al. 2006b). White-footed mice and red-backed voles are the most common small mammal species on the forest, are key prey species, are seed predators and dispersers, and fluctuate partly in response to mast crops of acorns, pine, and birch (McKracken et al. 1999; Elias et al. 2004, 2006a; Wang et al. 2009; Ogawa et al. 2017). Small mammal predators observed at the Holt Research Forest include birds such as barred owl (Strix varia Barton), great horned owl (Bubo virginianus J. F. Gmelin), goshawk (Accipiter gentilis L.), and broad-winged hawk (Bubo platypterus Vieillot), and mammals such as red fox (Vulpes vulpes L.), coyote (Canis latrans Say), fisher (Pekania pennanti Erxleben), short-tailed weasel (Mustela erminea L.), and long-tailed weasel (Mustela frenata Lichtenstein).
Mammal Trapping
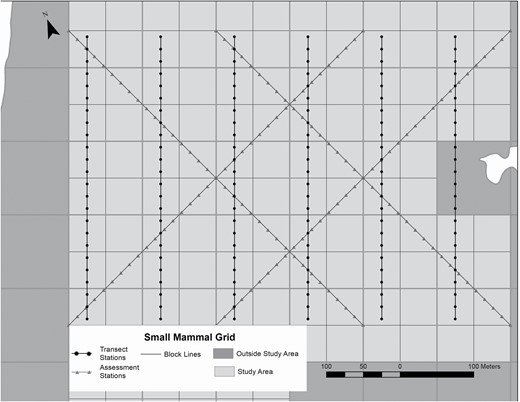
During two consecutive weeks in August, biologists live-trapped small mammals on a 600 m × 400 m grid using a census-assessment line method (O’Farrell and Austin 1978). August was ideal for tracking the emergence of I. scapularis and prevalence of B. burgdorferi in the small mammal host community, since in southern Maine late summer is the peak questing time for I. scapularis larvae, and since I. scapularis acquire B. burgdorferi from infected hosts (Rollend et al. 2013). During week one we trapped for 5 consecutive nights on 6 parallel, 400-m census lines with 24 trap stations per line spaced 17 m apart (144 stations, Fig. 2). During week two we trapped for 3 consecutive nights on two pairs of parallel, 600-m assessment lines running diagonally to the transect lines with 35 trap stations per line spaced 17 m apart (140 stations). Incorporating an 8.5 m boundary strip (half the distance between stations [Krebs 1966]), we considered the effectively sampled area to be ~24.8 ha (608.5 m ×408.5 m). At each station, we set two large (7.62 × 8.89 × 22.86 cm), aluminum Sherman traps (H. B. Sherman Traps, Inc., Tallahassee, FL) baited with a mixture of rolled oats and peanut butter, including a 5.1 × 5.1 cm pad of pressed cotton (Nestlets; Ancare Corporation, Bellmore, NY) for bedding and insulation. We checked traps daily between 0600 and 1400 hr. We ear-tagged animals (National Band and Tag Company, Newport, Kentucky) and recorded age, sex, and weight. Capture and handling procedures followed animal care and use guidelines of the American Society of Mammalogists (Sikes et al. 2016) and were approved by the University of Maine Institutional Animal Care and Use Committee (IACUC protocol A2020-05-05) and Maine Department of Inland Fisheries and Wildlife (permit 2020-562).
Layout of trap stations placed on north–south ‘transect’ lines and diagonal ‘assessment’ lines on a 600 m × 400 m small mammal trapping grid on the Holt Research Forest, Arrowsic, Maine, 1983–2019.
Tick Collection
Beginning in 1989 the principal ecologist (J.W.W.) at the Holt Research Forest collected ticks found attached to the bodies of captured animals (Schmidt et al. 1999, Machtinger and Williams 2020). Daily mammal processing was handled by one to two personnel and in peak years well over 100 animals were processed in a day (the highest daily capture was 209 individuals). Thus by design, tick collections were not exhaustive, but intended as an appraisal of minimum tick prevalence (proportion of hosts parasitized) and tick burden (ticks/host). Short-tailed shrews [Blarina brevicauda Say (Soricomorpha: Soricidae)] were not tagged or examined as closely for ticks as were the other species because the primary handler was adversely affected by the poisonous bites of this species (Maynard 1889, Krosch 1973, Kita et al. 2004). Forceps were used to remove ticks and place them in vials. Depending on the testing method (explained below), during 1989–2013 ticks were placed in vials with moistened plaster of Paris to keep ticks alive, and during 2016–2019 ticks were placed in vials containing 90% ethanol and stored at −80°C. Ticks were delivered to the MMCRI Vector-borne Disease Laboratory, identified to species and stage (larva, nymph, adult), and a subset tested. There were no tick collections in 2000 and 2004 and ticks collected in 2014 and 2015 were not tested. Tick collections and testing volume were limited in some years by white-footed mouse and/or red-backed vole population ‘crashes’ when host abundance was so low that few to no ticks were collected, or in the case of early years when there were no or few I. scapularis.
Tick Testing
During 1989–2013 ticks were tested for B. burgdorferi sensu lato by direct fluorescent antibody (DFA) microscopy (Donahue et al. 1987) and during 2016–2019 by polymerase chain reaction (PCR) (Graham et al. 2018). Individual I. scapularis (larvae and nymphs) and all stages of I. angustus from white-footed mice, red-backed voles, red squirrels [Tamiasciuris hudsonicus Erxleben (Rodentia: Sciuridae)], and chipmunks [Tamias striatus Linnaeus (Rodentia: Sciuridae)] were tested by DFA; about 80% of DFA tests were from I. scapularis larvae from white-footed mice. Ticks collected during 2016–2019 were tested in 2020 for B. burgdorferi sensu lato and Anaplasma phagocytophilum Foggie (Rickettsiales: Anaplasmataceae) via M1b/M3 paired-assay, real-time PCR (Graham et al. 2018). Testing for A. phagocytophilum was not a study aim but was part of the M1b/M3 assay. We did not use the Graham et al. (2018) M4 PCR assay that differentiates between B. burgdorferi s.s. and B. mayonii sp. nov. because B. mayonii is not yet known to occur outside the midwest US (Fleshman et al. 2021).
For PCR, larvae were pooled by individual small mammal host and DNA was extracted from each pool following the DNeasy Blood & Tissue Kit (Qiagen, Germantown, MD) insect protocol, except 80 μl of sterile, nuclease-free water (Growcells, Irvine, CA) was used for elution in place of buffer AE. Samples were run in a 10 µl reaction using 4.8 µl of sample DNA and 5.2 µl of master mix with primer/probe combinations and iQ Multiplex Powermix (Bio-Rad Laboratories, Inc., Hercules, CA). Negative control was sterile, nuclease-free water and positive controls were B. burgdorferi s.s. and A. phagocytophilum (BEI Resources, Manassas, VA). Samples not definitively positive were rerun and ambiguous results considered negative. We note that the DFA and PCR tests did not discriminate between B. burgdorferi and B. mayonii sp. nov., thus all tests were for B. burgdorferi sensu lato.
Analysis
Small Mammal Abundance. For the entire small mammal trapping period (1983–2019), we summarized the annual and overall number of individual small mammals captured by species, sex, and age. Studies variously report small mammal abundance as minimum number alive (MNA) (Krebs 1966) per 100 trap nights or per hectare, so we tabulated both and plotted the annual time series for the four most frequently trapped species: the white-footed mouse, the red-backed vole, the eastern chipmunk [Tamias striatus Linnaeus (Rodentia:Sciuridae)], and the short-tailed shrew. We examined each time series for fluctuations and tested for linear trend over time (linear regression, trends significant at P ≤ 0.05, with regression coefficient b1 expressing average annual change in MNA per 100 trap nights). There were no notable changes in small mammal abundance leading up to the period during which tick collection began.
Prevalence of Tick Species. For the entire tick collection period (1989–2019), for each small mammal species we calculated tick prevalence (proportion of hosts parasitized) and burden (ticks/host) by tick species and stage. For white-footed mice and red-backed voles (the most abundant species), we combined the stages and plotted the time series of annual tick prevalence and burdens. We tested for the presence of linear trends over time in I. scapularis prevalence and burdens.
Prevalence of B. burgdorferi. The basic sampling unit was the individual small mammal. PCR was run on pools per animal of I. scapularis larvae, so we pooled DFA results by individual animal. We reasoned that pooling of DFAs was acceptable given that B. burgdorferi prevalence (proportion of ticks positive) of individual versus pooled DFAs was highly correlated (n = 9 yr, r = 0.95, P < 0.0001), and that mean positivity did not differ (n = 19 yr, x̅individual = 53.6 [SE = 6.9] vs. x̅pooled = 59.2 [5.7], P = 0.53).
Testing volume was limited in some years by white-footed mouse and/or red-backed vole population ‘crashes’ when host abundance was so low that few to no ticks were collected, or in the case of early years when there were no or few I. scapularis. Thus we did not conduct a trend analysis for the prevalence of B. burgdorferi in ticks. However, for white-footed mice we calculated annual B. burgdorferi prevalence in larvae and nymphs in years where the number of test pools was at least five. Transovarial transmission of B. burgdorferi s.s. is considered negligible in I. scapularis (Barbour et al. 2017, Johnson et al. 2017) so B. burgdorferi prevalence in feeding larvae can serve as a proxy to that of the white-footed mouse host population. Xeno-diagnosis and vaccine studies show a close relationship between infection prevalence in white-footed mice and infection prevalence in the larvae that feed upon upon them in both laboratory and field settings (e.g., Donahue et al. 1987, Bunikis et al. 2004, Stafford et al. 2020). We calculated prevalence of B. burgdorferi in I. angustus and I. scapularis (all stages combined) for white-footed mice, red-backed voles, chipmunks, and red squirrels (I. scapularis only). For I. scapularis we tested for differences (χ 2 test for differences in proportions among host species, significant at P ≤ 0.05).
Comparison with passive surveillance. We referred to the data from our statewide, passive tick surveillance program (1989–2013) (Rand et al. 2007, Elias et al. 2020) to ascertain when I. scapularis first appeared in Maine and in Sagadahoc County, to plot annual Sagadahoc County tick submissions, and to visually assess the period of transition from emergence to establishment in Sagadahoc County and compare this with the active surveillance data from the Holt Research Forest. We used SAS/STAT (SAS 2021) for all analyses.
Results
Fluctuations and Trends in Small Mammal Abundance
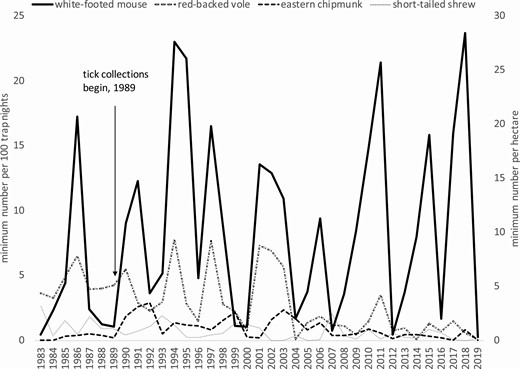
From 1983–2019, the entire 37-yr period of small mammal trapping, white-footed mouse abundance fluctuated over the years but without trend (Fig. 3). Species that fluctuated and exhibited downward trends were red-backed voles, (n = 37 yr, b1 = −0.12, Fig. 3), short-tailed shrews (n = 34 yr, b1 = −0.03, Fig. 3), and masked shrews (n = 24 yr, b1 = −0.02, not shown).
Population fluctuations of the four most commonly captured small mammal species on the Holt Research Forest, Arrowsic, Maine, 1983–2019: white-footed mouse, red-backed vole, eastern chipmunk, and short-tailed shrew.
From 1989–2019, the 29-yr period of tick collection (excluding 2000 and 2004), white-footed mice comprised 65.9% of all captures, followed by red-backed voles (18.6%), eastern chipmunks (6.3%), short-tailed shrews (3.7%), red squirrels (2.0%), and the remainder of species (Table 1). Thus, white-footed mice were three times more abundant than red-backed voles and ~10 and ~13 times more abundant than chipmunks and short-tailed shrews, respectively. Abundance (minimum number alive) of white-footed mice averaged 9.5/100 trap nights (range 0.3–23.7) and 7.7 per ha (range 0.2–20.6).
Number of individual small mammals captured during August trapping sessions, Holt Research Forest, Arrowsic, Maine, 1983–2019, and during the period of tick collection, 1989–2019
| Small Mammal Species . | 1983–2019a . | 1989–2019b . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Years caught . | n . | Years caught . | n . | Per 100 trap nights . | Per hectarec . | % of all individuals . | Sex/age known . | ||
| . | . | . | . | . | . | . | . | Females . | Males . | Juveniles . |
| White-footed mouse (Peromyscus leucopus Rafinesque) | 37 | 6,164 | 29 | 5551 | 9.46 | 7.72 | 65.9 | 1,862 | 2,410 | 1,133 |
| Red-backed vole (Myodes gapperi L. Vigors) | 37 | 2,074 | 29 | 1,566 | 2.67 | 2.18 | 18.6 | 514 | 618 | 357 |
| Eastern chipmunk (Tamias striatus) | 34 | 602 | 28 | 529 | 0.90 | 0.76 | 6.3 | 136 | 168 | 203 |
| Short-tailed shrew (Blarina brevicauda Say) | 33 | 471 | 25 | 308 | 0.52 | 0.50 | 3.7 | 9 | 27 | 3 |
| Red squirrel (Tamiasciuris hudsonicus Erxleben) | 31 | 230 | 24 | 172 | 0.29 | 0.29 | 2.0 | 69 | 34 | 56 |
| Southern flying squirrel (Glaucomys volans L.) | 27 | 176 | 23 | 163 | 0.28 | 0.29 | 1.9 | 46 | 50 | 59 |
| Masked shrew (Sorex cinereus Kerr) | 24 | 136 | 10 | 62 | 0.11 | 0.25 | 0.7 | 0 | 0 | 5 |
| Meadow vole (Microtus pennsylvanicus Ord) | 15 | 93 | 16 | 52 | 0.09 | 0.13 | 0.6 | 2 | 1 | 0 |
| Northern flying squirrel (Glaucomys sabrinus Shaw) | 12 | 72 | 6 | 12 | 0.02 | 0.08 | 0.1 | 5 | 2 | 3 |
| Weasel (Mustela sp) | 4 | 5 | 4 | 5 | 0.01 | 0.05 | 0.1 | 0 | 0 | 0 |
| Woodland jumping mouse (Napaeozapus insignis Miller) | 2 | 2 | 2 | 2 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Gray squirrel (Sciuris carolinensis Gmelin) | 2 | 2 | 2 | 2 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Meadow jumping mouse (Zapus hudsonius Zimmermann) | 1 | 1 | 1 | 1 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Total | 10,028 | 8,425 | ||||||||
| Small Mammal Species . | 1983–2019a . | 1989–2019b . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Years caught . | n . | Years caught . | n . | Per 100 trap nights . | Per hectarec . | % of all individuals . | Sex/age known . | ||
| . | . | . | . | . | . | . | . | Females . | Males . | Juveniles . |
| White-footed mouse (Peromyscus leucopus Rafinesque) | 37 | 6,164 | 29 | 5551 | 9.46 | 7.72 | 65.9 | 1,862 | 2,410 | 1,133 |
| Red-backed vole (Myodes gapperi L. Vigors) | 37 | 2,074 | 29 | 1,566 | 2.67 | 2.18 | 18.6 | 514 | 618 | 357 |
| Eastern chipmunk (Tamias striatus) | 34 | 602 | 28 | 529 | 0.90 | 0.76 | 6.3 | 136 | 168 | 203 |
| Short-tailed shrew (Blarina brevicauda Say) | 33 | 471 | 25 | 308 | 0.52 | 0.50 | 3.7 | 9 | 27 | 3 |
| Red squirrel (Tamiasciuris hudsonicus Erxleben) | 31 | 230 | 24 | 172 | 0.29 | 0.29 | 2.0 | 69 | 34 | 56 |
| Southern flying squirrel (Glaucomys volans L.) | 27 | 176 | 23 | 163 | 0.28 | 0.29 | 1.9 | 46 | 50 | 59 |
| Masked shrew (Sorex cinereus Kerr) | 24 | 136 | 10 | 62 | 0.11 | 0.25 | 0.7 | 0 | 0 | 5 |
| Meadow vole (Microtus pennsylvanicus Ord) | 15 | 93 | 16 | 52 | 0.09 | 0.13 | 0.6 | 2 | 1 | 0 |
| Northern flying squirrel (Glaucomys sabrinus Shaw) | 12 | 72 | 6 | 12 | 0.02 | 0.08 | 0.1 | 5 | 2 | 3 |
| Weasel (Mustela sp) | 4 | 5 | 4 | 5 | 0.01 | 0.05 | 0.1 | 0 | 0 | 0 |
| Woodland jumping mouse (Napaeozapus insignis Miller) | 2 | 2 | 2 | 2 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Gray squirrel (Sciuris carolinensis Gmelin) | 2 | 2 | 2 | 2 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Meadow jumping mouse (Zapus hudsonius Zimmermann) | 1 | 1 | 1 | 1 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Total | 10,028 | 8,425 | ||||||||
a74,180 trap nights across 37 yr.
b58,708 trap nights across 29 yr (ticks not collected in 2000 and 2004).
cAssumes 24.8 ha sampled.
Number of individual small mammals captured during August trapping sessions, Holt Research Forest, Arrowsic, Maine, 1983–2019, and during the period of tick collection, 1989–2019
| Small Mammal Species . | 1983–2019a . | 1989–2019b . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Years caught . | n . | Years caught . | n . | Per 100 trap nights . | Per hectarec . | % of all individuals . | Sex/age known . | ||
| . | . | . | . | . | . | . | . | Females . | Males . | Juveniles . |
| White-footed mouse (Peromyscus leucopus Rafinesque) | 37 | 6,164 | 29 | 5551 | 9.46 | 7.72 | 65.9 | 1,862 | 2,410 | 1,133 |
| Red-backed vole (Myodes gapperi L. Vigors) | 37 | 2,074 | 29 | 1,566 | 2.67 | 2.18 | 18.6 | 514 | 618 | 357 |
| Eastern chipmunk (Tamias striatus) | 34 | 602 | 28 | 529 | 0.90 | 0.76 | 6.3 | 136 | 168 | 203 |
| Short-tailed shrew (Blarina brevicauda Say) | 33 | 471 | 25 | 308 | 0.52 | 0.50 | 3.7 | 9 | 27 | 3 |
| Red squirrel (Tamiasciuris hudsonicus Erxleben) | 31 | 230 | 24 | 172 | 0.29 | 0.29 | 2.0 | 69 | 34 | 56 |
| Southern flying squirrel (Glaucomys volans L.) | 27 | 176 | 23 | 163 | 0.28 | 0.29 | 1.9 | 46 | 50 | 59 |
| Masked shrew (Sorex cinereus Kerr) | 24 | 136 | 10 | 62 | 0.11 | 0.25 | 0.7 | 0 | 0 | 5 |
| Meadow vole (Microtus pennsylvanicus Ord) | 15 | 93 | 16 | 52 | 0.09 | 0.13 | 0.6 | 2 | 1 | 0 |
| Northern flying squirrel (Glaucomys sabrinus Shaw) | 12 | 72 | 6 | 12 | 0.02 | 0.08 | 0.1 | 5 | 2 | 3 |
| Weasel (Mustela sp) | 4 | 5 | 4 | 5 | 0.01 | 0.05 | 0.1 | 0 | 0 | 0 |
| Woodland jumping mouse (Napaeozapus insignis Miller) | 2 | 2 | 2 | 2 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Gray squirrel (Sciuris carolinensis Gmelin) | 2 | 2 | 2 | 2 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Meadow jumping mouse (Zapus hudsonius Zimmermann) | 1 | 1 | 1 | 1 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Total | 10,028 | 8,425 | ||||||||
| Small Mammal Species . | 1983–2019a . | 1989–2019b . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Years caught . | n . | Years caught . | n . | Per 100 trap nights . | Per hectarec . | % of all individuals . | Sex/age known . | ||
| . | . | . | . | . | . | . | . | Females . | Males . | Juveniles . |
| White-footed mouse (Peromyscus leucopus Rafinesque) | 37 | 6,164 | 29 | 5551 | 9.46 | 7.72 | 65.9 | 1,862 | 2,410 | 1,133 |
| Red-backed vole (Myodes gapperi L. Vigors) | 37 | 2,074 | 29 | 1,566 | 2.67 | 2.18 | 18.6 | 514 | 618 | 357 |
| Eastern chipmunk (Tamias striatus) | 34 | 602 | 28 | 529 | 0.90 | 0.76 | 6.3 | 136 | 168 | 203 |
| Short-tailed shrew (Blarina brevicauda Say) | 33 | 471 | 25 | 308 | 0.52 | 0.50 | 3.7 | 9 | 27 | 3 |
| Red squirrel (Tamiasciuris hudsonicus Erxleben) | 31 | 230 | 24 | 172 | 0.29 | 0.29 | 2.0 | 69 | 34 | 56 |
| Southern flying squirrel (Glaucomys volans L.) | 27 | 176 | 23 | 163 | 0.28 | 0.29 | 1.9 | 46 | 50 | 59 |
| Masked shrew (Sorex cinereus Kerr) | 24 | 136 | 10 | 62 | 0.11 | 0.25 | 0.7 | 0 | 0 | 5 |
| Meadow vole (Microtus pennsylvanicus Ord) | 15 | 93 | 16 | 52 | 0.09 | 0.13 | 0.6 | 2 | 1 | 0 |
| Northern flying squirrel (Glaucomys sabrinus Shaw) | 12 | 72 | 6 | 12 | 0.02 | 0.08 | 0.1 | 5 | 2 | 3 |
| Weasel (Mustela sp) | 4 | 5 | 4 | 5 | 0.01 | 0.05 | 0.1 | 0 | 0 | 0 |
| Woodland jumping mouse (Napaeozapus insignis Miller) | 2 | 2 | 2 | 2 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Gray squirrel (Sciuris carolinensis Gmelin) | 2 | 2 | 2 | 2 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Meadow jumping mouse (Zapus hudsonius Zimmermann) | 1 | 1 | 1 | 1 | 0.00 | 0.04 | 0.0 | 0 | 0 | 1 |
| Total | 10,028 | 8,425 | ||||||||
a74,180 trap nights across 37 yr.
b58,708 trap nights across 29 yr (ticks not collected in 2000 and 2004).
cAssumes 24.8 ha sampled.
Tick Species and Tick Hosts
Laboratory staff identified 3,052 ticks to species on 1,301 infested small mammals (Table 2). From most to least abundant the tick species were I. scapularis, I. angustus (vole tick), Dermacentor variabilis Say (American dog tick), and I. marxi Banks (squirrel tick) (Cooley and Kohls 1945, Brinton et al. 1965). White-footed mice hosted 94.1% of I. scapularis, 64.6% of I. angustus, and 75.4% of D. variabilis (Table 3).
Tick species, small mammal hosts, and tick prevalence (proportion of hosts parasitized) on the Holt Research Forest, Arrowsic, Maine, 1989–2019 (excluding 2000 and 2004 when ticks were not collected)
| Tick species . | Mammal species . | No. years host species captured . | No. years host species infested . | No.Hosts . | No. hosts infested . | % hosts infestedb . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Feeding singly and/or cofeeding . | Only cofeeding . | ||||||
| . | . | . | . | . | All stages . | Larvae . | Nymphs . | Adultsa . | Lar/nym . | Lar/ad . | Nym/ad . | . |
| Ixodes scapularis Say | White-footed mouse | 29 | 19 | 5,551 | 830 | 755 | 171 | 0 | 96 | 0 | 0 | 15.0 |
| Red-backed vole | 29 | 9 | 1,566 | 20 | 10 | 11 | 0 | 1 | 0 | 0 | 1.3 | |
| Eastern chipmunk | 28 | 12 | 529 | 26 | 18 | 10 | 0 | 2 | 0 | 0 | 4.9 | |
| Short-tailed shrew | 25 | 1 | 308 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.3 | |
| Red squirrel | 24 | 9 | 172 | 22 | 11 | 17 | 0 | 6 | 0 | 0 | 12.8 | |
| Southern flying squirrel | 23 | 2 | 163 | 2 | 1 | 2 | 0 | 1 | 0 | 0 | 1.2 | |
| 901 | 796 | 211 | 0 | 106 | 0 | 0 | ||||||
| Ixodes angustus Neumann | White-footed mouse | 29 | 23 | 5,551 | 165 | 25 | 112 | 40 | 6 | 1 | 5 | 3.0 |
| Red-backed vole | 29 | 18 | 1,566 | 67 | 3 | 54 | 19 | 2 | 0 | 6 | 4.3 | |
| Eastern chipmunk | 28 | 7 | 529 | 7 | 0 | 5 | 4 | 0 | 0 | 1 | 1.3 | |
| Red squirrel | 24 | 2 | 172 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1.2 | |
| Meadow vole | 16 | 1 | 52 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1.9 | |
| 242 | 28 | 173 | 64 | 8 | 1 | 12 | ||||||
| Dermacentor variabilis Say | White-footed mouse | 29 | 19 | 5,551 | 114 | 13 | 103 | 0 | 2 | 0 | 0 | 2.1 |
| Red-backed vole | 29 | 13 | 1,566 | 36 | 3 | 33 | 1 | 0 | 1 | 0 | 2.3 | |
| Red squirrel | 24 | 1 | 172 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0.6 | |
| 151 | 16 | 137 | 1 | 2 | 1 | 0 | ||||||
| Ixodes marxi Banks | White-footed mouse | 29 | 1 | 5,551 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| Red-backed vole | 29 | 1 | 1,566 | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 0.1 | |
| Red squirrel | 24 | 2 | 172 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1.2 | |
| Southern flying squirrel | 23 | 1 | 163 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0.6 | |
| Northern flying squirrel | 29 | 1 | 12 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 8.3 | |
| Total | 7 | 1 | 3 | 5 | 0 | 0 | 1 | |||||
| 1,301 | 841 | 524 | 70 | 116 | 2 | 13 | ||||||
| Tick species . | Mammal species . | No. years host species captured . | No. years host species infested . | No.Hosts . | No. hosts infested . | % hosts infestedb . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Feeding singly and/or cofeeding . | Only cofeeding . | ||||||
| . | . | . | . | . | All stages . | Larvae . | Nymphs . | Adultsa . | Lar/nym . | Lar/ad . | Nym/ad . | . |
| Ixodes scapularis Say | White-footed mouse | 29 | 19 | 5,551 | 830 | 755 | 171 | 0 | 96 | 0 | 0 | 15.0 |
| Red-backed vole | 29 | 9 | 1,566 | 20 | 10 | 11 | 0 | 1 | 0 | 0 | 1.3 | |
| Eastern chipmunk | 28 | 12 | 529 | 26 | 18 | 10 | 0 | 2 | 0 | 0 | 4.9 | |
| Short-tailed shrew | 25 | 1 | 308 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.3 | |
| Red squirrel | 24 | 9 | 172 | 22 | 11 | 17 | 0 | 6 | 0 | 0 | 12.8 | |
| Southern flying squirrel | 23 | 2 | 163 | 2 | 1 | 2 | 0 | 1 | 0 | 0 | 1.2 | |
| 901 | 796 | 211 | 0 | 106 | 0 | 0 | ||||||
| Ixodes angustus Neumann | White-footed mouse | 29 | 23 | 5,551 | 165 | 25 | 112 | 40 | 6 | 1 | 5 | 3.0 |
| Red-backed vole | 29 | 18 | 1,566 | 67 | 3 | 54 | 19 | 2 | 0 | 6 | 4.3 | |
| Eastern chipmunk | 28 | 7 | 529 | 7 | 0 | 5 | 4 | 0 | 0 | 1 | 1.3 | |
| Red squirrel | 24 | 2 | 172 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1.2 | |
| Meadow vole | 16 | 1 | 52 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1.9 | |
| 242 | 28 | 173 | 64 | 8 | 1 | 12 | ||||||
| Dermacentor variabilis Say | White-footed mouse | 29 | 19 | 5,551 | 114 | 13 | 103 | 0 | 2 | 0 | 0 | 2.1 |
| Red-backed vole | 29 | 13 | 1,566 | 36 | 3 | 33 | 1 | 0 | 1 | 0 | 2.3 | |
| Red squirrel | 24 | 1 | 172 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0.6 | |
| 151 | 16 | 137 | 1 | 2 | 1 | 0 | ||||||
| Ixodes marxi Banks | White-footed mouse | 29 | 1 | 5,551 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| Red-backed vole | 29 | 1 | 1,566 | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 0.1 | |
| Red squirrel | 24 | 2 | 172 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1.2 | |
| Southern flying squirrel | 23 | 1 | 163 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0.6 | |
| Northern flying squirrel | 29 | 1 | 12 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 8.3 | |
| Total | 7 | 1 | 3 | 5 | 0 | 0 | 1 | |||||
| 1,301 | 841 | 524 | 70 | 116 | 2 | 13 | ||||||
aOne male I. angustus each on one red-backed vole and one red squirrel, one male I. marxi on one red-backed vole.
bCoinfestation by more than one tick species: white-footed mouse I. scapularis/I. angustus n =28, I. scapularis/D. variabilis n = 4, I. angustus/D. variabilis n =2; red-backed vole I. scapularis/I. angustus n = 2; I. angustus/I. marxi/D. variabilis n = 1.
Tick species, small mammal hosts, and tick prevalence (proportion of hosts parasitized) on the Holt Research Forest, Arrowsic, Maine, 1989–2019 (excluding 2000 and 2004 when ticks were not collected)
| Tick species . | Mammal species . | No. years host species captured . | No. years host species infested . | No.Hosts . | No. hosts infested . | % hosts infestedb . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Feeding singly and/or cofeeding . | Only cofeeding . | ||||||
| . | . | . | . | . | All stages . | Larvae . | Nymphs . | Adultsa . | Lar/nym . | Lar/ad . | Nym/ad . | . |
| Ixodes scapularis Say | White-footed mouse | 29 | 19 | 5,551 | 830 | 755 | 171 | 0 | 96 | 0 | 0 | 15.0 |
| Red-backed vole | 29 | 9 | 1,566 | 20 | 10 | 11 | 0 | 1 | 0 | 0 | 1.3 | |
| Eastern chipmunk | 28 | 12 | 529 | 26 | 18 | 10 | 0 | 2 | 0 | 0 | 4.9 | |
| Short-tailed shrew | 25 | 1 | 308 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.3 | |
| Red squirrel | 24 | 9 | 172 | 22 | 11 | 17 | 0 | 6 | 0 | 0 | 12.8 | |
| Southern flying squirrel | 23 | 2 | 163 | 2 | 1 | 2 | 0 | 1 | 0 | 0 | 1.2 | |
| 901 | 796 | 211 | 0 | 106 | 0 | 0 | ||||||
| Ixodes angustus Neumann | White-footed mouse | 29 | 23 | 5,551 | 165 | 25 | 112 | 40 | 6 | 1 | 5 | 3.0 |
| Red-backed vole | 29 | 18 | 1,566 | 67 | 3 | 54 | 19 | 2 | 0 | 6 | 4.3 | |
| Eastern chipmunk | 28 | 7 | 529 | 7 | 0 | 5 | 4 | 0 | 0 | 1 | 1.3 | |
| Red squirrel | 24 | 2 | 172 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1.2 | |
| Meadow vole | 16 | 1 | 52 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1.9 | |
| 242 | 28 | 173 | 64 | 8 | 1 | 12 | ||||||
| Dermacentor variabilis Say | White-footed mouse | 29 | 19 | 5,551 | 114 | 13 | 103 | 0 | 2 | 0 | 0 | 2.1 |
| Red-backed vole | 29 | 13 | 1,566 | 36 | 3 | 33 | 1 | 0 | 1 | 0 | 2.3 | |
| Red squirrel | 24 | 1 | 172 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0.6 | |
| 151 | 16 | 137 | 1 | 2 | 1 | 0 | ||||||
| Ixodes marxi Banks | White-footed mouse | 29 | 1 | 5,551 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| Red-backed vole | 29 | 1 | 1,566 | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 0.1 | |
| Red squirrel | 24 | 2 | 172 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1.2 | |
| Southern flying squirrel | 23 | 1 | 163 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0.6 | |
| Northern flying squirrel | 29 | 1 | 12 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 8.3 | |
| Total | 7 | 1 | 3 | 5 | 0 | 0 | 1 | |||||
| 1,301 | 841 | 524 | 70 | 116 | 2 | 13 | ||||||
| Tick species . | Mammal species . | No. years host species captured . | No. years host species infested . | No.Hosts . | No. hosts infested . | % hosts infestedb . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | Feeding singly and/or cofeeding . | Only cofeeding . | ||||||
| . | . | . | . | . | All stages . | Larvae . | Nymphs . | Adultsa . | Lar/nym . | Lar/ad . | Nym/ad . | . |
| Ixodes scapularis Say | White-footed mouse | 29 | 19 | 5,551 | 830 | 755 | 171 | 0 | 96 | 0 | 0 | 15.0 |
| Red-backed vole | 29 | 9 | 1,566 | 20 | 10 | 11 | 0 | 1 | 0 | 0 | 1.3 | |
| Eastern chipmunk | 28 | 12 | 529 | 26 | 18 | 10 | 0 | 2 | 0 | 0 | 4.9 | |
| Short-tailed shrew | 25 | 1 | 308 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.3 | |
| Red squirrel | 24 | 9 | 172 | 22 | 11 | 17 | 0 | 6 | 0 | 0 | 12.8 | |
| Southern flying squirrel | 23 | 2 | 163 | 2 | 1 | 2 | 0 | 1 | 0 | 0 | 1.2 | |
| 901 | 796 | 211 | 0 | 106 | 0 | 0 | ||||||
| Ixodes angustus Neumann | White-footed mouse | 29 | 23 | 5,551 | 165 | 25 | 112 | 40 | 6 | 1 | 5 | 3.0 |
| Red-backed vole | 29 | 18 | 1,566 | 67 | 3 | 54 | 19 | 2 | 0 | 6 | 4.3 | |
| Eastern chipmunk | 28 | 7 | 529 | 7 | 0 | 5 | 4 | 0 | 0 | 1 | 1.3 | |
| Red squirrel | 24 | 2 | 172 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1.2 | |
| Meadow vole | 16 | 1 | 52 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1.9 | |
| 242 | 28 | 173 | 64 | 8 | 1 | 12 | ||||||
| Dermacentor variabilis Say | White-footed mouse | 29 | 19 | 5,551 | 114 | 13 | 103 | 0 | 2 | 0 | 0 | 2.1 |
| Red-backed vole | 29 | 13 | 1,566 | 36 | 3 | 33 | 1 | 0 | 1 | 0 | 2.3 | |
| Red squirrel | 24 | 1 | 172 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0.6 | |
| 151 | 16 | 137 | 1 | 2 | 1 | 0 | ||||||
| Ixodes marxi Banks | White-footed mouse | 29 | 1 | 5,551 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| Red-backed vole | 29 | 1 | 1,566 | 2 | 0 | 1 | 3 | 0 | 0 | 1 | 0.1 | |
| Red squirrel | 24 | 2 | 172 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 1.2 | |
| Southern flying squirrel | 23 | 1 | 163 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0.6 | |
| Northern flying squirrel | 29 | 1 | 12 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 8.3 | |
| Total | 7 | 1 | 3 | 5 | 0 | 0 | 1 | |||||
| 1,301 | 841 | 524 | 70 | 116 | 2 | 13 | ||||||
aOne male I. angustus each on one red-backed vole and one red squirrel, one male I. marxi on one red-backed vole.
bCoinfestation by more than one tick species: white-footed mouse I. scapularis/I. angustus n =28, I. scapularis/D. variabilis n = 4, I. angustus/D. variabilis n =2; red-backed vole I. scapularis/I. angustus n = 2; I. angustus/I. marxi/D. variabilis n = 1.
Counts of ticks and burdens (ticks/host) collected from small mammals, by tick species and life stage, Holt Research Forest, Arrowsic, Maine, 1989–2019, excluding 2000 and 2004 when ticks were not collected
| Tick species . | Mammal species . | No. hosts infested . | No. ticks identified . | Burdens (ticks/host) . | % of total tick burden . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | . | Larvae . | Nymphs . | Adults . | Total . | ||
| Ixodes scapularis | White-footed mouse | 830 | 2,103 | 211 | 0 | 2,314 | 2.8 | 94.1 |
| Eastern chipmunk | 26 | 40 | 11 | 0 | 51 | 2.0 | 2.1 | |
| Red squirrel | 22 | 32 | 25 | 0 | 57 | 2.6 | 2.3 | |
| Red-backed vole | 20 | 13 | 13 | 0 | 26 | 1.3 | 1.1 | |
| Southern flying squirrel | 2 | 1 | 2 | 0 | 3 | 0.1 | ||
| Short-tailed shrew | 1 | 7 | 0 | 0 | 7 | 0.3 | ||
| 901 | 2,196 | 262 | 0 | 2,458 | ||||
| Ixodes angustus | White-footed mouse | 165 | 39 | 158 | 48 | 245 | 1.5 | 64.6 |
| Red-backed vole | 67 | 12 | 89 | 21 | 122 | 1.8 | 32.2 | |
| Eastern chipmunk | 7 | 0 | 5 | 4 | 9 | 1.3 | 2.4 | |
| Red squirrel | 2 | 0 | 1 | 1 | 2 | 0.5 | ||
| Meadow vole | 1 | 0 | 1 | 0 | 1 | 0.3 | ||
| 242 | 51 | 254 | 74 | 379 | ||||
| Dermacentor variabilis | White-footed mouse | 114 | 22 | 128 | 0 | 150 | 1.3 | 75.4 |
| Red-backed vole | 36 | 4 | 42 | 2 | 48 | 1.3 | 24.1 | |
| Red squirrel | 1 | 0 | 1 | 0 | 1 | 0.5 | ||
| 151 | 26 | 171 | 2 | 199 | ||||
| Ixodes marxi | Red-backed vole | 2 | 0 | 2 | 5 | 7 | 43.8 | |
| Red squirrel | 2 | 0 | 5 | 1 | 6 | 37.5 | ||
| White-footed mouse | 1 | 1 | 0 | 0 | 1 | 6.3 | ||
| Northern flying squirrel | 1 | 0 | 1 | 0 | 1 | 6.3 | ||
| Southern flying squirrel | 1 | 0 | 0 | 1 | 1 | 6.3 | ||
| Total | 7 | 1 | 8 | 7 | 16 | |||
| 1,301 | 2,274 | 695 | 83 | 3,052 | ||||
| Tick species . | Mammal species . | No. hosts infested . | No. ticks identified . | Burdens (ticks/host) . | % of total tick burden . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | . | Larvae . | Nymphs . | Adults . | Total . | ||
| Ixodes scapularis | White-footed mouse | 830 | 2,103 | 211 | 0 | 2,314 | 2.8 | 94.1 |
| Eastern chipmunk | 26 | 40 | 11 | 0 | 51 | 2.0 | 2.1 | |
| Red squirrel | 22 | 32 | 25 | 0 | 57 | 2.6 | 2.3 | |
| Red-backed vole | 20 | 13 | 13 | 0 | 26 | 1.3 | 1.1 | |
| Southern flying squirrel | 2 | 1 | 2 | 0 | 3 | 0.1 | ||
| Short-tailed shrew | 1 | 7 | 0 | 0 | 7 | 0.3 | ||
| 901 | 2,196 | 262 | 0 | 2,458 | ||||
| Ixodes angustus | White-footed mouse | 165 | 39 | 158 | 48 | 245 | 1.5 | 64.6 |
| Red-backed vole | 67 | 12 | 89 | 21 | 122 | 1.8 | 32.2 | |
| Eastern chipmunk | 7 | 0 | 5 | 4 | 9 | 1.3 | 2.4 | |
| Red squirrel | 2 | 0 | 1 | 1 | 2 | 0.5 | ||
| Meadow vole | 1 | 0 | 1 | 0 | 1 | 0.3 | ||
| 242 | 51 | 254 | 74 | 379 | ||||
| Dermacentor variabilis | White-footed mouse | 114 | 22 | 128 | 0 | 150 | 1.3 | 75.4 |
| Red-backed vole | 36 | 4 | 42 | 2 | 48 | 1.3 | 24.1 | |
| Red squirrel | 1 | 0 | 1 | 0 | 1 | 0.5 | ||
| 151 | 26 | 171 | 2 | 199 | ||||
| Ixodes marxi | Red-backed vole | 2 | 0 | 2 | 5 | 7 | 43.8 | |
| Red squirrel | 2 | 0 | 5 | 1 | 6 | 37.5 | ||
| White-footed mouse | 1 | 1 | 0 | 0 | 1 | 6.3 | ||
| Northern flying squirrel | 1 | 0 | 1 | 0 | 1 | 6.3 | ||
| Southern flying squirrel | 1 | 0 | 0 | 1 | 1 | 6.3 | ||
| Total | 7 | 1 | 8 | 7 | 16 | |||
| 1,301 | 2,274 | 695 | 83 | 3,052 | ||||
Counts of ticks and burdens (ticks/host) collected from small mammals, by tick species and life stage, Holt Research Forest, Arrowsic, Maine, 1989–2019, excluding 2000 and 2004 when ticks were not collected
| Tick species . | Mammal species . | No. hosts infested . | No. ticks identified . | Burdens (ticks/host) . | % of total tick burden . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | . | Larvae . | Nymphs . | Adults . | Total . | ||
| Ixodes scapularis | White-footed mouse | 830 | 2,103 | 211 | 0 | 2,314 | 2.8 | 94.1 |
| Eastern chipmunk | 26 | 40 | 11 | 0 | 51 | 2.0 | 2.1 | |
| Red squirrel | 22 | 32 | 25 | 0 | 57 | 2.6 | 2.3 | |
| Red-backed vole | 20 | 13 | 13 | 0 | 26 | 1.3 | 1.1 | |
| Southern flying squirrel | 2 | 1 | 2 | 0 | 3 | 0.1 | ||
| Short-tailed shrew | 1 | 7 | 0 | 0 | 7 | 0.3 | ||
| 901 | 2,196 | 262 | 0 | 2,458 | ||||
| Ixodes angustus | White-footed mouse | 165 | 39 | 158 | 48 | 245 | 1.5 | 64.6 |
| Red-backed vole | 67 | 12 | 89 | 21 | 122 | 1.8 | 32.2 | |
| Eastern chipmunk | 7 | 0 | 5 | 4 | 9 | 1.3 | 2.4 | |
| Red squirrel | 2 | 0 | 1 | 1 | 2 | 0.5 | ||
| Meadow vole | 1 | 0 | 1 | 0 | 1 | 0.3 | ||
| 242 | 51 | 254 | 74 | 379 | ||||
| Dermacentor variabilis | White-footed mouse | 114 | 22 | 128 | 0 | 150 | 1.3 | 75.4 |
| Red-backed vole | 36 | 4 | 42 | 2 | 48 | 1.3 | 24.1 | |
| Red squirrel | 1 | 0 | 1 | 0 | 1 | 0.5 | ||
| 151 | 26 | 171 | 2 | 199 | ||||
| Ixodes marxi | Red-backed vole | 2 | 0 | 2 | 5 | 7 | 43.8 | |
| Red squirrel | 2 | 0 | 5 | 1 | 6 | 37.5 | ||
| White-footed mouse | 1 | 1 | 0 | 0 | 1 | 6.3 | ||
| Northern flying squirrel | 1 | 0 | 1 | 0 | 1 | 6.3 | ||
| Southern flying squirrel | 1 | 0 | 0 | 1 | 1 | 6.3 | ||
| Total | 7 | 1 | 8 | 7 | 16 | |||
| 1,301 | 2,274 | 695 | 83 | 3,052 | ||||
| Tick species . | Mammal species . | No. hosts infested . | No. ticks identified . | Burdens (ticks/host) . | % of total tick burden . | |||
|---|---|---|---|---|---|---|---|---|
| . | . | . | Larvae . | Nymphs . | Adults . | Total . | ||
| Ixodes scapularis | White-footed mouse | 830 | 2,103 | 211 | 0 | 2,314 | 2.8 | 94.1 |
| Eastern chipmunk | 26 | 40 | 11 | 0 | 51 | 2.0 | 2.1 | |
| Red squirrel | 22 | 32 | 25 | 0 | 57 | 2.6 | 2.3 | |
| Red-backed vole | 20 | 13 | 13 | 0 | 26 | 1.3 | 1.1 | |
| Southern flying squirrel | 2 | 1 | 2 | 0 | 3 | 0.1 | ||
| Short-tailed shrew | 1 | 7 | 0 | 0 | 7 | 0.3 | ||
| 901 | 2,196 | 262 | 0 | 2,458 | ||||
| Ixodes angustus | White-footed mouse | 165 | 39 | 158 | 48 | 245 | 1.5 | 64.6 |
| Red-backed vole | 67 | 12 | 89 | 21 | 122 | 1.8 | 32.2 | |
| Eastern chipmunk | 7 | 0 | 5 | 4 | 9 | 1.3 | 2.4 | |
| Red squirrel | 2 | 0 | 1 | 1 | 2 | 0.5 | ||
| Meadow vole | 1 | 0 | 1 | 0 | 1 | 0.3 | ||
| 242 | 51 | 254 | 74 | 379 | ||||
| Dermacentor variabilis | White-footed mouse | 114 | 22 | 128 | 0 | 150 | 1.3 | 75.4 |
| Red-backed vole | 36 | 4 | 42 | 2 | 48 | 1.3 | 24.1 | |
| Red squirrel | 1 | 0 | 1 | 0 | 1 | 0.5 | ||
| 151 | 26 | 171 | 2 | 199 | ||||
| Ixodes marxi | Red-backed vole | 2 | 0 | 2 | 5 | 7 | 43.8 | |
| Red squirrel | 2 | 0 | 5 | 1 | 6 | 37.5 | ||
| White-footed mouse | 1 | 1 | 0 | 0 | 1 | 6.3 | ||
| Northern flying squirrel | 1 | 0 | 1 | 0 | 1 | 6.3 | ||
| Southern flying squirrel | 1 | 0 | 0 | 1 | 1 | 6.3 | ||
| Total | 7 | 1 | 8 | 7 | 16 | |||
| 1,301 | 2,274 | 695 | 83 | 3,052 | ||||
Six species of small mammals hosted 2,458 I. scapularis, all of which were subadults and the majority of which (2,196; 89%) were larvae, with most (2,103; 82.5%,) on white-footed mice (Table 2). Five species of small mammals hosted adult as well as subadult I. angustus. Three small mammal species hosted D. variabilis, nearly all of which were subadult ticks, other than two female ticks. Five small mammal species hosted I. marxi (Table 2), a species found only sporadically across the years. Thirty-four white-footed mice (0.6% of all white-footed mice) and three red-backed voles (0.2% of all red-backed voles) were infested by two or three tick species at the same time.
Prevalence of Tick Species
On an annual average basis, I. scapularis prevalence in small mammals was 15.0% for white-footed mice, 12.8% for red squirrels, and 0.3–4.9% for the remainder of hosts. I. angustus and D. variabilis prevalence was lower than that of I. scapularis, and more evenly distributed among small mammal species (Table 3). On an individual host basis, tick burdens ranged from 1 to a maximum of 22 (22 I. scapularis larvae on one white-footed mouse in 2018). On an annual average basis, tick burdens (ticks/individual) did not vary substantially among host mammals (annual averages ranging from 1.3–2.8 per host species, Table 3).
Timeline of Emergence and Establishment of I. scapularis
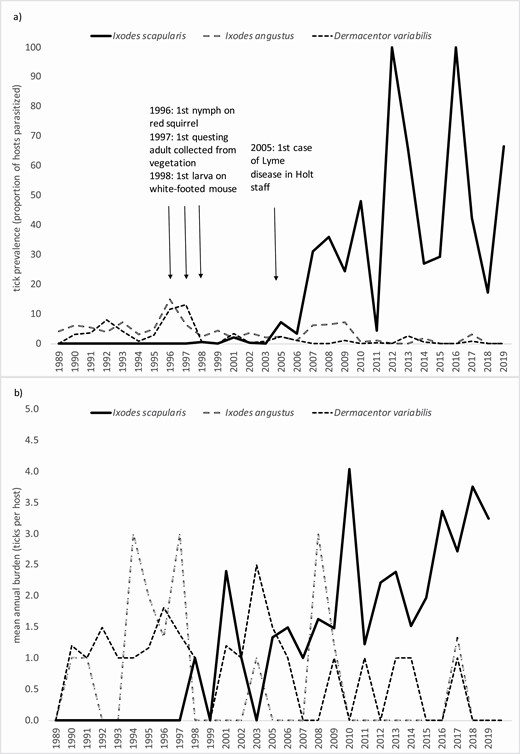
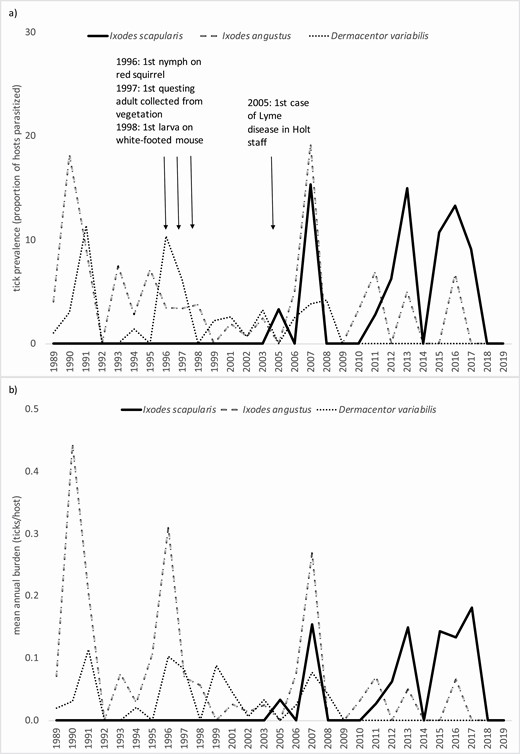
During 1989–2019, white-footed mice and red-backed vole tick infestation illustrated the emergence and establishment of I. scapularis. Whereas I. angustus, D. variabilis, and I. marxi were collected throughout the study beginning in 1989, the first I. scapularis was a nymph collected from a red squirrel in 1996. The second was a larva collected from a white-footed mouse in 1998 (Fig. 4a). Thus nearly all I. scapularis were found in the latter two of the three-decade study.
Prevalence (a) and burdens (b) of I. scapularis, I. angustus, and D. variabilis on white-footed mice, Holt Research Forest, Arrowsic, Maine, 1989–2019 (data not available for 2000 and 2004).
Prevalence of I. scapularis in white-footed mice increased approximately linearly through 1996–2006 (n = 9 yr, b1 = 0.49), followed by a noticeable surge in 2007 (Fig. 4a). Higher prevalence of I. scapularis was sustained during 2007–2019 across wide interannual fluctuations without trend (n = 13 yr, Fig. 4a). Concurrently, the prevalence of I. angustus and D. variabilis on white-footed mice declined. Burdens (ticks/host) of I. scapularis on white-footed provided a less clear signal of emergence than did prevalence, however, it can be seen that burdens increased during 2007–2019 (n = 13 yr, b1 = 0.49, Fig. 4b). Similar to patterns seen for white-footed mice, red-backed vole I. scapularis prevalence and burdens increased concurrently with declines in I. angustus and declines in D. variabilis (Fig. 5a and b).
Prevalence (a) and burdens (b) of I. scapularis, I. angustus, and D. variabilis on red-backed voles, Holt Research Forest, Arrowsic, Maine, 1989–2019 (data not available for 2000 and 2004).
Emergence and Establishment of B. burgdorferi
From 1989–2013, we tested 240 pools (380 individual ticks) of I. scapularis and 34 pools (38 individual ticks) of I. angustus for B. burgdorferi by DFA and from 2016–2019 we tested 98 pools (351 individual ticks) of I. scapularis larvae for B. burgdorferi by PCR.
B. burgdorferi was first detected in 2005 in I. scapularis larvae from 2 white-footed mice and one I. scapularis nymph from a red-backed vole (Table 4). This detection occurred nine years after the first I. scapularis larva was found in the small mammal community (red squirrel, 1996). Prevalence of B. burgdorferi in I. scapularis did not differ among hosts (white-footed mice, red-backed voles, red squirrels, and chipmunks 47.1–58.4%, χ 2 test, P = 0.77, Table 4), however, the wide 95% confidence intervals for red-backed voles, chipmunks, and red squirrels reflect low sample sizes and thus low confidence in knowing true infection prevalence of I. scapularis feeding on these species. The only B. burgdorferi positives in subadult I. angustus came from white-footed mice, with the first appearance in 2009. B. burgdorferi prevalence in mouse-derived I. scapularis was eight times greater than in mouse-derived I. angustus (58.4%, [179/293] vs. 7.3%, [3/41], P < 0.0001). We note that by PCR, 22.4% of larval pools (22/98) of I. scapularis were positive for A. phagocytophilum.
Prevalence of Borrelia burgdorferi s.l. in Ixodes angustus and I. scapularis feeding on small mammals, Holt Research Forest, Arrowsic, Maine, 1989–2019. Ticks were tested via direct fluorescent antibody (DFA) through 2013 and by PCR thereafter
| Mammal Species . | Tick Stage . | I.angustus . | I. scapularis . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pools (hosts) . | Pools (hosts) . | |||||||
| . | . | Year . | + . | neg . | Total . | % . | + . | neg . | Total . | % . |
| White-footed mouse | Larvae | 1990 | 0 | 1 | 1 | |||||
| 1997 | 0 | 1 | 1 | |||||||
| 2001 | 0 | 2 | 2 | |||||||
| 2005 | 2 | 1 | 3 | |||||||
| 2007 | 0 | 1 | 1 | |||||||
| 2008 | 2 | 2 | 4 | |||||||
| 2009 | 1 | 0 | 1 | 15 | 7 | 22 | 68.2 | |||
| 2010 | 56 | 41 | 97 | 57.7 | ||||||
| 2011 | 5 | 4 | 9 | 55.6 | ||||||
| 2012 | 2 | 3 | 5 | 40.0 | ||||||
| 2013 | 11 | 18 | 29 | 37.9 | ||||||
| 2016 | 1 | 0 | 1 | |||||||
| 2017 | 3 | 2 | 5 | 60.0 | ||||||
| 2018 | 51 | 28 | 79 | 64.6 | ||||||
| 2019 | 2 | 0 | 2 | |||||||
| 1 | 3 | 150 | 259 | 57.9 | ||||||
| Nymphs | 1989 | 0 | 1 | 1 | ||||||
| 1990 | 0 | 9 | 9 | 0.0 | ||||||
| 1997 | 0 | 12 | 12 | 0.0 | ||||||
| 2005 | 0 | 1 | 1 | |||||||
| 2007 | 0 | 1 | 1 | |||||||
| 2008 | 0 | 2 | 2 | 4 | 5 | 9 | 44.4 | |||
| 2009 | 2 | 4 | 6 | 33.3 | 5 | 0 | 5 | 100.0 | ||
| 2010 | 1 | 1 | 2 | |||||||
| 2011 | 8 | 2 | 10 | 80.0 | ||||||
| 2013 | 3 | 4 | 7 | 42.9 | ||||||
| 2 | 31 | 6.5 | 21 | 34 | 61.8 | |||||
| Adults | 1989–2009 | 0 | 31 | 7 | 0.0 | |||||
| White-footed mouse | All | 1989–2019 | 3 | 41 | 7.3 | 171 | 293 | 58.4 (52,64)b | ||
| Red-backed volea | All | 1989–2013 | 0 | 39 | 39 | 0.0 | 3 | 3 | 6 | 50.0 (12,88) |
| Chipmunka | All | 1990–2018 | 0 | 2 | 3 | 0.0 | 8 | 9 | 17 | 47.1 (23,72) |
| Red squirrela | All | 2009–2018 | 9 | 8 | 17 | 52.9 (28,77) | ||||
| Mammal Species . | Tick Stage . | I.angustus . | I. scapularis . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pools (hosts) . | Pools (hosts) . | |||||||
| . | . | Year . | + . | neg . | Total . | % . | + . | neg . | Total . | % . |
| White-footed mouse | Larvae | 1990 | 0 | 1 | 1 | |||||
| 1997 | 0 | 1 | 1 | |||||||
| 2001 | 0 | 2 | 2 | |||||||
| 2005 | 2 | 1 | 3 | |||||||
| 2007 | 0 | 1 | 1 | |||||||
| 2008 | 2 | 2 | 4 | |||||||
| 2009 | 1 | 0 | 1 | 15 | 7 | 22 | 68.2 | |||
| 2010 | 56 | 41 | 97 | 57.7 | ||||||
| 2011 | 5 | 4 | 9 | 55.6 | ||||||
| 2012 | 2 | 3 | 5 | 40.0 | ||||||
| 2013 | 11 | 18 | 29 | 37.9 | ||||||
| 2016 | 1 | 0 | 1 | |||||||
| 2017 | 3 | 2 | 5 | 60.0 | ||||||
| 2018 | 51 | 28 | 79 | 64.6 | ||||||
| 2019 | 2 | 0 | 2 | |||||||
| 1 | 3 | 150 | 259 | 57.9 | ||||||
| Nymphs | 1989 | 0 | 1 | 1 | ||||||
| 1990 | 0 | 9 | 9 | 0.0 | ||||||
| 1997 | 0 | 12 | 12 | 0.0 | ||||||
| 2005 | 0 | 1 | 1 | |||||||
| 2007 | 0 | 1 | 1 | |||||||
| 2008 | 0 | 2 | 2 | 4 | 5 | 9 | 44.4 | |||
| 2009 | 2 | 4 | 6 | 33.3 | 5 | 0 | 5 | 100.0 | ||
| 2010 | 1 | 1 | 2 | |||||||
| 2011 | 8 | 2 | 10 | 80.0 | ||||||
| 2013 | 3 | 4 | 7 | 42.9 | ||||||
| 2 | 31 | 6.5 | 21 | 34 | 61.8 | |||||
| Adults | 1989–2009 | 0 | 31 | 7 | 0.0 | |||||
| White-footed mouse | All | 1989–2019 | 3 | 41 | 7.3 | 171 | 293 | 58.4 (52,64)b | ||
| Red-backed volea | All | 1989–2013 | 0 | 39 | 39 | 0.0 | 3 | 3 | 6 | 50.0 (12,88) |
| Chipmunka | All | 1990–2018 | 0 | 2 | 3 | 0.0 | 8 | 9 | 17 | 47.1 (23,72) |
| Red squirrela | All | 2009–2018 | 9 | 8 | 17 | 52.9 (28,77) | ||||
aFirst B. burgdorferi-positive I. scapularis in red-backed vole 2005 (nymph), in chipmunk and red squirrel 2009 (larvae).
b95% confidence limits for binomial proportions (lower, upper).
Prevalence of Borrelia burgdorferi s.l. in Ixodes angustus and I. scapularis feeding on small mammals, Holt Research Forest, Arrowsic, Maine, 1989–2019. Ticks were tested via direct fluorescent antibody (DFA) through 2013 and by PCR thereafter
| Mammal Species . | Tick Stage . | I.angustus . | I. scapularis . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pools (hosts) . | Pools (hosts) . | |||||||
| . | . | Year . | + . | neg . | Total . | % . | + . | neg . | Total . | % . |
| White-footed mouse | Larvae | 1990 | 0 | 1 | 1 | |||||
| 1997 | 0 | 1 | 1 | |||||||
| 2001 | 0 | 2 | 2 | |||||||
| 2005 | 2 | 1 | 3 | |||||||
| 2007 | 0 | 1 | 1 | |||||||
| 2008 | 2 | 2 | 4 | |||||||
| 2009 | 1 | 0 | 1 | 15 | 7 | 22 | 68.2 | |||
| 2010 | 56 | 41 | 97 | 57.7 | ||||||
| 2011 | 5 | 4 | 9 | 55.6 | ||||||
| 2012 | 2 | 3 | 5 | 40.0 | ||||||
| 2013 | 11 | 18 | 29 | 37.9 | ||||||
| 2016 | 1 | 0 | 1 | |||||||
| 2017 | 3 | 2 | 5 | 60.0 | ||||||
| 2018 | 51 | 28 | 79 | 64.6 | ||||||
| 2019 | 2 | 0 | 2 | |||||||
| 1 | 3 | 150 | 259 | 57.9 | ||||||
| Nymphs | 1989 | 0 | 1 | 1 | ||||||
| 1990 | 0 | 9 | 9 | 0.0 | ||||||
| 1997 | 0 | 12 | 12 | 0.0 | ||||||
| 2005 | 0 | 1 | 1 | |||||||
| 2007 | 0 | 1 | 1 | |||||||
| 2008 | 0 | 2 | 2 | 4 | 5 | 9 | 44.4 | |||
| 2009 | 2 | 4 | 6 | 33.3 | 5 | 0 | 5 | 100.0 | ||
| 2010 | 1 | 1 | 2 | |||||||
| 2011 | 8 | 2 | 10 | 80.0 | ||||||
| 2013 | 3 | 4 | 7 | 42.9 | ||||||
| 2 | 31 | 6.5 | 21 | 34 | 61.8 | |||||
| Adults | 1989–2009 | 0 | 31 | 7 | 0.0 | |||||
| White-footed mouse | All | 1989–2019 | 3 | 41 | 7.3 | 171 | 293 | 58.4 (52,64)b | ||
| Red-backed volea | All | 1989–2013 | 0 | 39 | 39 | 0.0 | 3 | 3 | 6 | 50.0 (12,88) |
| Chipmunka | All | 1990–2018 | 0 | 2 | 3 | 0.0 | 8 | 9 | 17 | 47.1 (23,72) |
| Red squirrela | All | 2009–2018 | 9 | 8 | 17 | 52.9 (28,77) | ||||
| Mammal Species . | Tick Stage . | I.angustus . | I. scapularis . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pools (hosts) . | Pools (hosts) . | |||||||
| . | . | Year . | + . | neg . | Total . | % . | + . | neg . | Total . | % . |
| White-footed mouse | Larvae | 1990 | 0 | 1 | 1 | |||||
| 1997 | 0 | 1 | 1 | |||||||
| 2001 | 0 | 2 | 2 | |||||||
| 2005 | 2 | 1 | 3 | |||||||
| 2007 | 0 | 1 | 1 | |||||||
| 2008 | 2 | 2 | 4 | |||||||
| 2009 | 1 | 0 | 1 | 15 | 7 | 22 | 68.2 | |||
| 2010 | 56 | 41 | 97 | 57.7 | ||||||
| 2011 | 5 | 4 | 9 | 55.6 | ||||||
| 2012 | 2 | 3 | 5 | 40.0 | ||||||
| 2013 | 11 | 18 | 29 | 37.9 | ||||||
| 2016 | 1 | 0 | 1 | |||||||
| 2017 | 3 | 2 | 5 | 60.0 | ||||||
| 2018 | 51 | 28 | 79 | 64.6 | ||||||
| 2019 | 2 | 0 | 2 | |||||||
| 1 | 3 | 150 | 259 | 57.9 | ||||||
| Nymphs | 1989 | 0 | 1 | 1 | ||||||
| 1990 | 0 | 9 | 9 | 0.0 | ||||||
| 1997 | 0 | 12 | 12 | 0.0 | ||||||
| 2005 | 0 | 1 | 1 | |||||||
| 2007 | 0 | 1 | 1 | |||||||
| 2008 | 0 | 2 | 2 | 4 | 5 | 9 | 44.4 | |||
| 2009 | 2 | 4 | 6 | 33.3 | 5 | 0 | 5 | 100.0 | ||
| 2010 | 1 | 1 | 2 | |||||||
| 2011 | 8 | 2 | 10 | 80.0 | ||||||
| 2013 | 3 | 4 | 7 | 42.9 | ||||||
| 2 | 31 | 6.5 | 21 | 34 | 61.8 | |||||
| Adults | 1989–2009 | 0 | 31 | 7 | 0.0 | |||||
| White-footed mouse | All | 1989–2019 | 3 | 41 | 7.3 | 171 | 293 | 58.4 (52,64)b | ||
| Red-backed volea | All | 1989–2013 | 0 | 39 | 39 | 0.0 | 3 | 3 | 6 | 50.0 (12,88) |
| Chipmunka | All | 1990–2018 | 0 | 2 | 3 | 0.0 | 8 | 9 | 17 | 47.1 (23,72) |
| Red squirrela | All | 2009–2018 | 9 | 8 | 17 | 52.9 (28,77) | ||||
aFirst B. burgdorferi-positive I. scapularis in red-backed vole 2005 (nymph), in chipmunk and red squirrel 2009 (larvae).
b95% confidence limits for binomial proportions (lower, upper).
Discussion
Emergence and Establishment of Ixodes scapularis
Small mammal surveillance during 1989–2019 revealed the emergence and establishment of I. scapularis in a research forest on Maine’s Midcoast. Time-to-establishment of I. scapularis was ~11 yr, measuring from the collection of the first I. scapularis subadult in 1996 until the 2007 rise in I. scapularis prevalence in white-footed mice. This was consistent with the 10–14 yr time-to-establishment observed collectively in Maine’s southern 10 counties using passive surveillance data (Elias et al. 2020), and with the ~10–16 yr time-to-establishment observed in Maine’s neighboring Canadian maritime provinces, New Brunswick and Nova Scotia (Leighton et al. 2012).
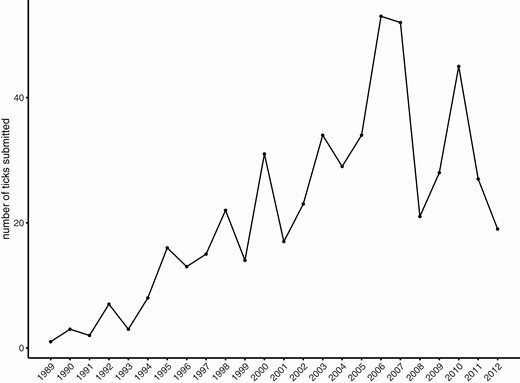
I. scapularis appeared earlier in the passive surveillance data than on small mammals in the Holt Research Forest (1996). The first I. scapularis submission to Maine’s passive surveillance program was a female tick found on a human in April 1989, in Cape Elizabeth, Cumberland County, which is south of Sagadahoc County. The first Sagadahoc County submission was in October 1989 (female tick/human host) from Bath, which is adjacent to Arrowisic. Submissions from Sagadahoc County continued from 1989 through the end of the program in (Fig. 6). Via visual assessment, the period of transition from emergence to establishment in the Sagadahoc County passive surveillance data occurred around 2005 (Fig. 6), similar to the ~2006–2007 period seen in small mammal infestation on the Holt Research Forest (Fig. 4a).
Number of I. scapularis submitted from Sagadahoc County, Maine, to the Maine Medical Center Research Institute’s passive tick surveillance program (Rand et al. 2007, Elias et al. 2020), 1989–2012.
Compared to the emergent period, the established state of I. scapularis (i.e., enzootic equilibrium) in the small mammal community was represented by higher, fluctuating, nontrending I. scapularis prevalence from 2007 through the end of the study. This is consistent with the prediction that I. scapularis populations will attain a stable cyclical equilibrium, assuming typical white-footed mouse densities and high white-tailed deer densities (Ogden et al. 2007). White-footed mouse abundance appeared typical (average 7.7 per ha, range 0.2–20.6) on our study area, a 100 ha, oak-pine, continuous (i.e., not fragmented) forest. In a continuous oak-pine forest in Millbrook, New York, mouse density ranged from <1 per ha to ~42 per ha (Ostfeld 1996). Nupp and Swihart (1996) estimated 10 mice per ha on average in forests ≥100 ha from studies in Illinois, Indiana, Ohio, Pennsylvania, and Virginia. Evidence for an increase in white-tailed deer density on the Holt Research Forest during the study is discussed in a subsequent section.
The emergence and establishment of I. scapularis on the Holt Research Forest occurred amidst a fluctuating but nontrending white-footed mouse population. Annual peaks in host-seeking I. scapularis correlate with lagged annual peaks in white-footed mouse abundance (e.g., Ostfeld et al. 2018), but to our knowledge, no study has shown a correlation between the increasing abundance of host-seeking I. scapularis and increasing abundance of white-footed mice at a multidecadal scale. This relationship has been implied, since white-footed mice appear to be expanding northward (Myers et al. 2009, Roy-Dufresne et al. 2013) but at our site we observed no increase in the white-footed mouse population during three decades.
Emergence and Establishment of B. burgdorferi
The testing data suggested a five- or six-year time-to-establishment period for B. burgdorferi. Even without a formal trend analysis for the prevalence of B. burgdorferi (due to few or ticks to test in some years), the data give the impression of little circulation of B. burgdorferi before 2005. This was followed by apparent amplification and establishment of B. burgdorferi in white-footed mice, chipmunks, red squirrels, and to a lesser extent, red-backed voles, by about 2008 or 2009. On Swans Island, Maine (an offshore island 155 linear km northeast of Arrowsic), testing for B. burgdorferi in questing adult I. scapularis started in 1994, spirochetes were detected in 2000, and prevalence increased from 4% in 2000 to 43% in 2007, a span of six years (MacQueen et al. 2012). In our study, overall prevalence of B. burgdorferi in I. scapularis did not differ among hosts (white-footed mice, red-backed voles, red squirrels, and chipmunks 47.1–58.4%, χ 2 test, P = 0.77, Table 4) consistent with the establishment of B. burgdorferi in the small mammal host community. However, it is reasonable to assume white-footed mice made the highest contribution to amplification of B. burgdorferi given they were most abundant (66% of individuals captured), had the highest prevalence of I. scapularis (15%), and carried 94% of the I. scapularis burden in the small mammal community we sampled.
It is possible that the establishment of B. burgdorferi in Maine lies on a longitudinal gradient between the mid-Atlantic states and the eastern Canadian provinces. At our study site, mammal and tick species captured and I. scapularis prevalence were similar to those seen during 2007–2008 in southern Quebec, where white-footed mice and deer mice (P. maniculatus Wagner) comprised 63% of captures and hosted 78% of I. scapularis (Bouchard et al. 2011). However, at our site the prevalence of B. burgdorferi in I. scapularis across the small mammal community was higher: 58.7% versus 4.6% (39/848) in Quebec (Bouchard et al. 2011). B. burgdorferi prevalence in I. scapularis larvae from white-footed mice in our study was 57.9%, similar to that seen in studies spanning the US Northeast (35–97%, Halsey et al. 2018).
Decline of Other Tick Species
I. scapularis became the dominant tick species in the small mammal community of the Holt Research Forest by the mid-2000s. We can only speculate on mechanisms by which I. angustus declined. Red-backed voles were more common hosts of I. angustus than Peromyscus mice in Alberta and Nova Scotia, Canada (Sorensen and Moses 1998, Martell et al. 1969), so on the Holt Research Forest the gradual decline in red-backed voles and shrews might have contributed to the decline in I. angustus. If I. scapularis subadults feed more aggressively than other resident tick species, they might exhaust host permissiveness, effectively displacing other ixodids. If I. scapularis and I. cookei hybridize as proposed by Patterson et al. (2017), perhaps the same could occur with I. scapularis and I. angustus resulting in a lower proportion of I. angustus identified in the tick community. Warmer, shorter winters in Maine, a manifestation of climate change (Elias et al. 2021), may benefit a tick that spends most of its life cycle off-host such as I. scapularis more than a nidicolous tick such as I. angustus.
The decline in D. variabilis may be inconsequential since the forest was marginal quality habitat for this tick species. D. variabilis is less associated with closed canopy forest than old field and ecotone habitats (Campbell and MacKay 1979, Mathisson et al. 2021). Currently, passive surveillance data indicate a robust D. variabilis population in Maine (University of Maine Cooperative Extension 2021).
Regional Context of I. scapularis Emergence and Establishment
Emergence of I. scapularis on the Holt Research Forest could be linked in part to climate change, as mentioned above, as well as to a regional increase in white-tailed deer. White-tailed deer are not reservoir-competent for B. burgdorferi (e.g., Brunner et al. 2008) but are focally prolific (e.g., Telford 2017), host subadult as well as adult I. scapularis, and provide a majority of female I. scapularis blood meals (Stafford 2007). Two lines of evidence suggest rising regional deer abundance. First, due to deer browsing pressure, hardwood tree regeneration on the Holt Research Forest has declined (Supp Fig. 1a [online only]) and oak seedlings, in particular, have low survival rates (Supp Fig. 1b [online only]). Second, buck kill data furnished by the Maine Department of Inland Fisheries and Wildlife (MDIFW) show rising trends beginning in 1997 in Arrowsic and adjacent Georgetown compared to neighboring Woolwich (Supp Fig. 2 [online only]). In 2018, estimated deer density was 23 per km2 (60 per mi2) in Arrowsic and Georgetown (Georgetown Conservation Commission 2021), four times greater than the wildlife management district goal of 6 per km2 (15 per mi2) (MDIFW 2017). Underlying causes of focal deer overabundance include land use restrictions that preclude hunting, and a diminished predator community (MDIFW 2017).
Limitations of the Study
We recognize a number of caveats. Trapping bias is a consideration since equal detection probability across species or within species is unlikely (Harkins et al. 2019). Sherman traps are biased against small mammals weighing under 15 grams (Torre et al. 2016) and those larger than mice and voles (e.g., Laudenslayer and Fargo 1997, Lambert et al. 2005, dos Santos-Filho et al. 2006, Hice and Velazco 2013). In our study the abundance of short-tailed shrews, red squirrels, and chipmunks was likely underestimated. However, Peromyscus may dominate many small mammal communities regardless of trap-bait combination used (e.g., Harkins et al. 2019) and in our study, the main focus was the use of white-footed mice to track the emergence and establishment of I. scapularis. In Maine the ranges of white-footed mice and deer mice overlap, with more deer mice in the northern part of state (Bennett 2020). Some of our white-footed mice could have been misidentified deer mice, but this would have been immaterial to the emergence of I. scapularis and B. burgdorferi on the Holt Research Forest. This is because deer mice are as competent as white-footed mice as subadult I. scapularis hosts and reservoirs of B. burgdorferi (Rand et al. 1993, Garman et al. 1994, Oliver et al. 2006). Our DFA test did not discriminate between B. burgdorferi and B. miyamotoi Fukunaga but we think this would not affect our inferences because the prevalence of B. miyamotoi in Maine is low. Only 3.6% of female I. scapularis collected in 2015 and 2016 from Maine were infected with B. miyamotoi (Han et al. 2019). This surveillance-based study took advantage of an existing small mammal trapping program and our ability to make inferences about tick ecology were limited given that 1) data were for August only so seasonal abundance of nymphal I. scapularis and all stages of other tick species was unknown, 2) we ascertained only minimum tick prevalence and burdens, 3) B. burgdorferi prevalence data were discontinuous, and 4) just one grid was sampled in one Maine forest. However, the goal was long-term surveillance, and the strength of a multidecadal time series is sampling consistency and longevity.
Conclusions
Long-term surveillance of small mammals and their tick burdens in a coastal Maine forest illustrated the emergence and establishment of I. scapularis and B. burgdorferi, and the decline of I. angustus. The emergence of Amblyomma americanum L. (lone star tick) is anticipated in Maine because the species is expanding its range northward and recently became established on Cape Cod, Massachusetts (Molaei et al. 2019) and can survive winters in southern coastal Maine (Linske et al. 2020). This species transmits the agents of diseases such as human monocytic ehrlichiosis and tularemia, and relies heavily on white-tailed deer for blood meals (Paddock and Yabsley 2007). Continued active tick surveillance at the Holt Research Forest would be a valuable contribution to the ongoing surveillance of ticks in Maine.
Acknowledgments
We thank many research assistants at the Holt Research Forest for small mammal live-trapping and collecting ticks over the years. In memory of and gratitude to Eleanor Lacombe (1932–2021), we thank her for her years at the Maine Medical Research Institute where she identified and tested thousands of ticks. We thank many research staff, including Danielle Cosenza, Jake Angelico, Molly Meagher, and Ina Guzja for identifying and processing ticks for PCR. We appreciate those who entered data, including Linda Siddons Turcotte, Natalie Fox, and Suzanne Appell. We thank the Maine Department of Inland Fisheries and Wildlife for furnishing deer data. This work was supported by the Holt Woodland Research Foundation, the Maine Tree Foundation, and the Maine Medical Center Research Institute. We thank our two anonymous reviewers for their thoughtful and constructive comments.
Dedication
We dedicate this study to Eleanor H. Lacombe (1932–2021). Her research career spanned 50 yr and included studies in cardiovascular physiology, environmental health, and vector-borne disease ecology. She was a founding member of the Maine Medical Center Research Institute’s Vector-Borne Disease Laboratory, was an expert on tick identification, coauthored over 60 research articles, presented at conferences in the United States and Europe, and was a mentor to dozens of young professionals. Her spirit and enthusiasm are missed.