-
PDF
- Split View
-
Views
-
Cite
Cite
Joannie Lortet-Tieulent, Jacques Ferlay, Freddie Bray, Ahmedin Jemal, International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013, JNCI: Journal of the National Cancer Institute, Volume 110, Issue 4, April 2018, Pages 354–361, https://doi.org/10.1093/jnci/djx214
Close - Share Icon Share
Abstract
Cancers of the corpus uteri—primarily of the endometrium—rank as the sixth most common neoplasm in women worldwide. Analyses of the global patterns and trends of uterine cancer rates are needed in view of the ongoing obesity epidemic, a major risk factor for the disease.
Data on endometrial cancer (ICD-10 C54) incidence from population-based cancer registries in 43 populations, published in CI5plus or by registries, were extracted for 1978 to 2013. Age-standardized incidence rates were computed for all ages and for pre- (25–49 years) and postmenopausal ages (50 years and older). Temporal trends were assessed with Joinpoint analysis, and the effects of birth cohort and year of diagnosis on the overall trends were examined using age-period-cohort modeling.
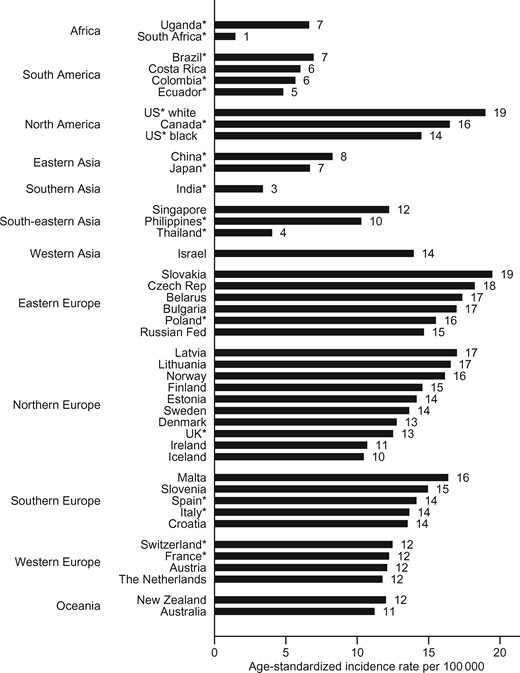
In 2006 to 2007, rates varied 10-fold across countries. The highest rates were in North America, Eastern and Northern Europe (19 cases per 100 000 among whites in the United States, 95% confidence interval [CI] = 18 to 20, and in Slovakia, 95% CI = 18 to 21), and the lowest rates were in middle-income countries (South Africa 1, 95% CI = 0 to 3, and India 3, 95% CI = 3 to 4). Rates during the most recent 10 data years increased in 26 of the 43 populations considered in this study, with South Africa and several countries in Asia showing the largest increase. The risk of endometrial cancer increased both in consecutive generations and over time in 11 of 23 populations, with the increases more pronounced in Japan, the Philippines, Belarus, Singapore, Costa Rica, and New Zealand.
Endometrial cancer incidence rates increased over time and in successive generations in several countries, especially in those countries with rapid socioeconomic transitions.
Cancers of the corpus uteri—hereafter denoted as endometrial cancer, given that the vast majority of these cancers are of adenocarcinomas arising from the endometrium (1,2)—is the sixth most commonly diagnosed cancer and the 14th leading cause of cancer death in women worldwide, with 320 000 estimated new cases and 76 000 deaths in 2012 (3). It occurs in women predominantly after menopause. The estimated age-standardized incidence rates (ASRs) vary from one to 30 cases per 100 000 women across countries globally, with the highest rates found in countries with a very high Human Development Index, where almost two-thirds of all cases occur. Low rates are observed in several Sub-Saharan African, Middle-Eastern, and South-Central Asian countries (3).
Hormones play an important role in the etiology of endometrial cancer (4), and unopposed estrogens are a key contributor to each of the established risk factors for the disease, including early menarche, late menopause, nulliparity, menopausal hormone use (MHU), and obesity (5). Other risk factors include diabetes, hypertension (6), and family history of endometrial cancer. Conversely, high parity, late age at first or last birth (7), combined estrogen-progesterone oral contraceptives (OC), and smoking (8) are associated with a reduction in risk. The prevalence of a number of the established risk factors appears to be rising in most parts of the world; obesity, especially, has doubled in less than 30 years globally (9). High body mass index (BMI) alone is estimated to account for more than one-third (34%) of the total endometrial cancer incidence worldwide, and nearly half (48%) of the cases in North America (10). Previous studies examining temporal trends in the incidence of endometrial cancer were limited to specific countries or regions, and based on old data (11). Herein, we examine contemporary worldwide trends in incidence of endometrial cancer rates using up-to-date incidence data as compiled by the International Agency for Research on Cancer (IARC).
Methods
Data Sources
Endometrial cancer incidence (ICD-10 C54) from 1978 to 2007 was obtained from the IARC CI5plus cancer registry database (12) for 42 countries, with a minimum of 15 consecutive data years ending in 2007. For several of these countries, the series were augmented with more recent data, as late as 2013, from cancer registries, extending the study period for these countries up to 35 years. Of the 42 countries, incidence data were based on national cancer registries for 24 countries, and regional or aggregate of regional registry data for the remaining countries (Supplementary Table 1, available online). For the United States, incidence rates were examined for blacks and whites, separately. Corresponding population data were obtained from IARC and additional sources.
Statistical Analysis
Age-standardized incidence rates per 100 000 were computed using the world standard population. To analyze temporal changes in incidence rates, Joinpoint regression (13) was used, fitting a series of joined straight lines to the trends in the ASRs. A Monte Carlo Permutation method is used to test the significance of the joinpoint models. A logarithmic transformation of the rates, the standard error calculated using the binomial approximation, and a maximum number of three joinpoints were specified as options in the analysis. To assess the magnitude and direction of the recent trend in each country, the average annual percent change (AAPC) in ASR over the last 10 available years (1998–2007 to 2004–2013, depending on the country) was used (14), by age group (25–49 and 50 years and older) and for all ages combined.
Assessment of the risk of endometrial cancer incidence was restricted to 23 populations with at least 25 years of consecutive data and more than 1 million women per year during 2006 to 2007, and they were examined using the age-period-cohort (APC) model. The APC model allows the assessment of the effects of cohort (year of birth) and period (year of diagnosis) on the overall incidence trends. It assumes that the incidence rates are constant within five-year age group a (age 30–79 years) and five-year period p (maximum span from 1978–1982 to 2008–2012). Birth cohorts c were derived from period and age such that c = p – a, ranging from 1903–1907 to 1978–1982. Further, the model assumes that new cancer cases follow a Poisson distribution (allowing for extra-Poisson variation) to estimate trends and deviations (parameters). The parameters are combined to produce functions that describe relationships between the observed rate and age, calendar period, and birth cohort. Cohort (and period) effects are presented as rate ratios —namely the age-specific rates in any given cohort (or period) relative to the referent cohort (or period), adjusted for age and nonlinear period (or cohort) effects. The reference period was set to 1993–1997—the central period for 19 populations and still a time of common MHU (or the closest)—and the reference age group was set to 50 to 54 years (perimenopause). Consequently, the reference cohort was 1943 to 1947. Cohort effects in risk of disease reflect generational changes in prevalence of risk factors, whereas period effects signal factors that affect all ages at the same time. The data management and statistical computations were performed using Stata (15) and the APC web tool (https://analysistools.nci.nih.gov/apc/) (16). All statistical tests were two-sided; a P value of less than .05 was considered statistically significant.
Results
Cross-Sectional Incidence
In general, the highest ASRs (all ages) in 2006 to 2007 were observed in North America and Europe, with rates as high as 19 per 100 000 in Slovakia (95% confidence interval [CI] = 18 to 21) and US whites (95% CI = 18 to 20) (Figure 1). In contrast, the lowest rates were found in middle-income countries (South Africa 1, 95% CI = 0 to 3, and India 3, 95% CI = 3 to 4). Within-country incidence was 4 to 20 times higher in postmenopausal women (age 50 years and older) compared with premenopausal women (age 25–49 years) (Supplementary Figure 1, available online). Rates varied 10-fold across countries, both in pre- and postmenopausal women.
Average annual age-standardized incidence rate of endometrial cancer, in 2006 to 2007, all ages. *Denotes regional registries.
Temporal Variations
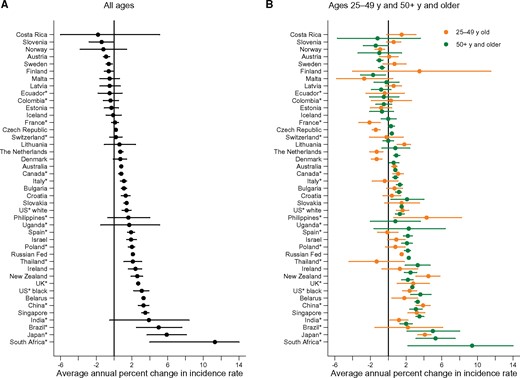
Figure 2 presents the change in ASR over the latest 10 years available (around 2001–2010) (details in Table 1). ASR for all ages statistically significantly increased in more than half of the populations (26 populations out of 43). The most rapid statistically significant increases occurred in countries with the lowest rates (11.3% per year, 95% CI = 4.0 to 19.0, in South Africa where incidence rates more than doubled in 10 years). Sixteen countries exhibited no change in incidence trends (mostly in Northern Europe), while only in three countries were statistically significant downward incidence trends observed (Slovenia –1.4%, 95% CI = –2.8 to 0.0; Austria –0.9%, 95% CI = –1.2 to –0.5; and Sweden –0.6%, 95% CI = –0.9 to –0.2 per year). In most countries, the recent trends in all ages were a continuation of earlier trends (Table 1; Supplementary Figure 2, available online). In the United States, the historically low rates among black women relative to white women underwent statistically significant rapid increases in the incidence (3.1%, 95% CI = 2.2 to 3.9, per year) since the late 1990s, and rates became similar by race by 2011 (Supplementary Figure 2, available online). Incidence trends among premenopausal women mirrored the upward trends in postmenopausal women (Figure 2; Supplementary Figure 2, available online). Yet, there were notable exceptions. In several countries, recent statistically significant increases among postmenopausal women contrasted with statistically significant decreases (Denmark, the Czech Republic, and the Netherlands) or stabilizations (Brazil, Thailand, Israel, Bulgaria, Poland, Croatia, Italy, Spain, and Ireland) in rates in premenopausal women. Trends in Norway, Finland, and France indicated statistically significant declining rates among premenopausal women but constant rates in older women.
Trends in endometrial cancer incidence rates, for 1978–2013, and over the last 10 years, for all ages
| Region . | Population . | Trend 1 . | Trend 2 . | Trend 3 . | Trend 4 . | Last 10 years . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period . | APC . | Period . | APC . | Period . | APC . | Period . | APC . | Period . | AAPC (95% CI) . | ||
| Africa | South Africa* | 1998–2012 | 11.3† | − | − | − | − | − | − | 2003–2012 | 11.3† (4.0; 19.0) |
| Uganda* | 1991–2012 | 1.7 | − | − | − | − | − | − | 2003–2012 | 1.7 (–1.5; 5.1) | |
| South America | Brazil* | 1988–2007 | 5.0† | − | − | − | − | − | − | 1998–2007 | 5.0† (2.5; 7.6) |
| Colombia* | 1983–2007 | –0.4 | − | − | − | − | − | − | 1998–2007 | –0.4 (–1.2; 0.5) | |
| Costa Rica | 1980–1988 | –3.3 | 1988–2005 | 4.4† | 2005–2007 | –20.9 | − | − | 1998–2007 | –1.8 (–8.3; 5.1) | |
| Ecuador* | 1985–2007 | –0.5 | − | − | − | − | − | − | 1998–2007 | –0.5 (–1.8; 0.9) | |
| North America | Canada* | 1983–1990 | –1.9† | 1990–2007 | 0.8† | − | − | − | − | 1998–2007 | 0.8† (0.5; 1.0) |
| US* black | 1978–1997 | 0.3 | 1997–2012 | 3.1† | − | − | − | − | 2003–2012 | 3.1† (2.2; 3.9) | |
| US* white | 1978–1988 | –2.3† | 1988–1999 | 0.6† | 1999–2003 | –1.1 | 2003–2012 | 1.4† | 2003–2012 | 1.4† (0.9; 1.9) | |
| Eastern Asia | China* | 1988–2007 | 3.3† | − | − | − | − | − | − | 1998–2007 | 3.3† (2.7; 3.9) |
| Japan* | 1978–2003 | 2.9† | 2003–2007 | 9.8† | − | − | − | − | 1998–2007 | 5.9† (3.7; 8.1) | |
| Southern Asia | India* | 1983–2005 | 1.4† | 2005–2007 | 13.0 | − | − | − | − | 1998–2007 | 3.9 (–0.5; 8.4) |
| South-eastern Asia | Philippines* | 1983–1994 | –2.0 | 1994–1999 | 13.5† | 1999–2007 | 0.2 | − | − | 1998–2007 | 1.6 (–0.7; 4.0) |
| Singapore | 1978–2007 | 3.5† | − | − | − | − | − | − | 1998–2007 | 3.5† (3.1; 3.9) | |
| Thailand* | 1983–2007 | 2.1† | − | − | − | − | − | − | 1998–2007 | 2.1† (1.1; 3.1) | |
| Western Asia | Israel | 1983–2007 | 1.9† | − | − | − | − | − | − | 1998–2007 | 1.9† (1.4; 2.5) |
| Eastern Europe | Belarus | 1978–2007 | 3.3† | − | − | − | − | − | − | 1998–2007 | 3.3† (3.1; 3.5) |
| Bulgaria | 1993–2011 | 1.1† | − | − | − | − | − | − | 2002–2011 | 1.1† (0.8; 1.4) | |
| Czech Republic | 1983–2010 | 0.2† | − | − | − | − | − | − | 2001–2010 | 0.2† (0.0; 0.3) | |
| Poland* | 1988–2008 | 2.0† | − | − | − | − | − | − | 1999–2008 | 2.0† (1.6; 2.3) | |
| Russian Federation | 1993–1996 | 4.3† | 1996–2013 | 2.1† | − | − | − | − | 2004–2013 | 2.1† (2.0; 2.3) | |
| Slovakia | 1978–2008 | 1.4† | − | − | − | − | − | − | 1999–2008 | 1.4† (1.1; 1.6) | |
| Northern Europe | Denmark | 1978–1980 | 7.4 | 1980–2000 | –1.4† | 2000–2012 | 0.7 | − | − | 2003–2012 | 0.7 (–0.1; 1.4) |
| Estonia | 1978–1996 | 1.9† | 1996–2007 | –0.3 | − | − | − | − | 1998–2007 | –0.3 (–1.1; 0.5) | |
| Finland | 1978–1998 | 1.5† | 1998–2012 | –0.6 | − | − | − | − | 2003–2012 | –0.6 (–1.1; 0.0) | |
| Iceland | 1978–2012 | –0.1 | − | − | − | − | − | − | 2003–2012 | –0.1 (–0.9; 0.8) | |
| Ireland | 1994–2011 | 2.4† | − | − | − | − | − | − | 2002–2011 | 2.4† (1.6; 3.1) | |
| Latvia | 1983–1996 | 2.8† | 1996–2007 | –0.5 | − | − | − | − | 1998–2007 | –0.5 (–1.6; 0.7) | |
| Lithuania | 1978–1989 | 1.1 | 1989–2001 | 3.9† | 2001–2007 | –1.0 | − | − | 1998–2007 | 0.6 (–1.1; 2.4) | |
| Norway | 1978–1988 | 0.0 | 1988–2010 | 1.7† | 2010–2012 | –10.7 | − | − | 2003–2012 | –1.2 (–3.8; 1.4) | |
| Sweden | 1978–1986 | –1.1† | 1986–1998 | 1.4† | 1998–2012 | –0.6† | − | − | 2003–2012 | –0.6† (–0.9; –0.2) | |
| UK* | 1978–1980 | 24.2 | 1980–1994 | 0.4 | 1994–2012 | 2.7† | − | − | 2003–2012 | 2.7† (2.5; 2.9) | |
| Southern Europe | Croatia | 1988–2012 | 1.3† | − | − | − | − | − | − | 2003–2012 | 1.3† (0.8; 1.8) |
| Italy* | 1988–2007 | 1.1† | − | − | − | − | − | − | 1998–2007 | 1.1† (0.7; 1.4) | |
| Malta | 1992–2009 | –0.5 | − | − | − | − | − | − | 2000–2009 | –0.5 (–1.6; 0.6) | |
| Slovenia | 1978–2001 | 2.0† | 2001–2011 | –1.4† | − | − | − | − | 2002–2011 | –1.4† (–2.8; 0.0) | |
| Spain* | 1988–2007 | 1.9† | − | − | − | − | − | − | 1998–2007 | 1.9† (1.5; 2.3) | |
| Western Europe | Austria | 1990–2009 | –0.9† | − | − | − | − | − | − | 2000–2009 | –0.9† (–1.2; –0.5) |
| France* | 1988–2009 | 0.1 | − | − | − | − | − | − | 2000–2009 | 0.1 (–0.3; 0.5) | |
| Switzerland* | 1983–1988 | –5.9† | 1988–2008 | 0.2 | − | − | − | − | 1999–2008 | 0.2 (–0.5; 0.9) | |
| Netherlands | 1989–2008 | 0.7† | − | − | − | − | − | − | 1999–2008 | 0.7† (0.5; 1.0) | |
| Oceania | Australia | 1982–2009 | 0.8† | − | − | − | − | − | − | 2000–2009 | 0.8† (0.6; 0.9) |
| New Zealand | 1983–1993 | –1.3 | 1993–2010 | 2.6† | − | − | − | − | 2001–2010 | 2.6† (1.9; 3.2) | |
| Region . | Population . | Trend 1 . | Trend 2 . | Trend 3 . | Trend 4 . | Last 10 years . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period . | APC . | Period . | APC . | Period . | APC . | Period . | APC . | Period . | AAPC (95% CI) . | ||
| Africa | South Africa* | 1998–2012 | 11.3† | − | − | − | − | − | − | 2003–2012 | 11.3† (4.0; 19.0) |
| Uganda* | 1991–2012 | 1.7 | − | − | − | − | − | − | 2003–2012 | 1.7 (–1.5; 5.1) | |
| South America | Brazil* | 1988–2007 | 5.0† | − | − | − | − | − | − | 1998–2007 | 5.0† (2.5; 7.6) |
| Colombia* | 1983–2007 | –0.4 | − | − | − | − | − | − | 1998–2007 | –0.4 (–1.2; 0.5) | |
| Costa Rica | 1980–1988 | –3.3 | 1988–2005 | 4.4† | 2005–2007 | –20.9 | − | − | 1998–2007 | –1.8 (–8.3; 5.1) | |
| Ecuador* | 1985–2007 | –0.5 | − | − | − | − | − | − | 1998–2007 | –0.5 (–1.8; 0.9) | |
| North America | Canada* | 1983–1990 | –1.9† | 1990–2007 | 0.8† | − | − | − | − | 1998–2007 | 0.8† (0.5; 1.0) |
| US* black | 1978–1997 | 0.3 | 1997–2012 | 3.1† | − | − | − | − | 2003–2012 | 3.1† (2.2; 3.9) | |
| US* white | 1978–1988 | –2.3† | 1988–1999 | 0.6† | 1999–2003 | –1.1 | 2003–2012 | 1.4† | 2003–2012 | 1.4† (0.9; 1.9) | |
| Eastern Asia | China* | 1988–2007 | 3.3† | − | − | − | − | − | − | 1998–2007 | 3.3† (2.7; 3.9) |
| Japan* | 1978–2003 | 2.9† | 2003–2007 | 9.8† | − | − | − | − | 1998–2007 | 5.9† (3.7; 8.1) | |
| Southern Asia | India* | 1983–2005 | 1.4† | 2005–2007 | 13.0 | − | − | − | − | 1998–2007 | 3.9 (–0.5; 8.4) |
| South-eastern Asia | Philippines* | 1983–1994 | –2.0 | 1994–1999 | 13.5† | 1999–2007 | 0.2 | − | − | 1998–2007 | 1.6 (–0.7; 4.0) |
| Singapore | 1978–2007 | 3.5† | − | − | − | − | − | − | 1998–2007 | 3.5† (3.1; 3.9) | |
| Thailand* | 1983–2007 | 2.1† | − | − | − | − | − | − | 1998–2007 | 2.1† (1.1; 3.1) | |
| Western Asia | Israel | 1983–2007 | 1.9† | − | − | − | − | − | − | 1998–2007 | 1.9† (1.4; 2.5) |
| Eastern Europe | Belarus | 1978–2007 | 3.3† | − | − | − | − | − | − | 1998–2007 | 3.3† (3.1; 3.5) |
| Bulgaria | 1993–2011 | 1.1† | − | − | − | − | − | − | 2002–2011 | 1.1† (0.8; 1.4) | |
| Czech Republic | 1983–2010 | 0.2† | − | − | − | − | − | − | 2001–2010 | 0.2† (0.0; 0.3) | |
| Poland* | 1988–2008 | 2.0† | − | − | − | − | − | − | 1999–2008 | 2.0† (1.6; 2.3) | |
| Russian Federation | 1993–1996 | 4.3† | 1996–2013 | 2.1† | − | − | − | − | 2004–2013 | 2.1† (2.0; 2.3) | |
| Slovakia | 1978–2008 | 1.4† | − | − | − | − | − | − | 1999–2008 | 1.4† (1.1; 1.6) | |
| Northern Europe | Denmark | 1978–1980 | 7.4 | 1980–2000 | –1.4† | 2000–2012 | 0.7 | − | − | 2003–2012 | 0.7 (–0.1; 1.4) |
| Estonia | 1978–1996 | 1.9† | 1996–2007 | –0.3 | − | − | − | − | 1998–2007 | –0.3 (–1.1; 0.5) | |
| Finland | 1978–1998 | 1.5† | 1998–2012 | –0.6 | − | − | − | − | 2003–2012 | –0.6 (–1.1; 0.0) | |
| Iceland | 1978–2012 | –0.1 | − | − | − | − | − | − | 2003–2012 | –0.1 (–0.9; 0.8) | |
| Ireland | 1994–2011 | 2.4† | − | − | − | − | − | − | 2002–2011 | 2.4† (1.6; 3.1) | |
| Latvia | 1983–1996 | 2.8† | 1996–2007 | –0.5 | − | − | − | − | 1998–2007 | –0.5 (–1.6; 0.7) | |
| Lithuania | 1978–1989 | 1.1 | 1989–2001 | 3.9† | 2001–2007 | –1.0 | − | − | 1998–2007 | 0.6 (–1.1; 2.4) | |
| Norway | 1978–1988 | 0.0 | 1988–2010 | 1.7† | 2010–2012 | –10.7 | − | − | 2003–2012 | –1.2 (–3.8; 1.4) | |
| Sweden | 1978–1986 | –1.1† | 1986–1998 | 1.4† | 1998–2012 | –0.6† | − | − | 2003–2012 | –0.6† (–0.9; –0.2) | |
| UK* | 1978–1980 | 24.2 | 1980–1994 | 0.4 | 1994–2012 | 2.7† | − | − | 2003–2012 | 2.7† (2.5; 2.9) | |
| Southern Europe | Croatia | 1988–2012 | 1.3† | − | − | − | − | − | − | 2003–2012 | 1.3† (0.8; 1.8) |
| Italy* | 1988–2007 | 1.1† | − | − | − | − | − | − | 1998–2007 | 1.1† (0.7; 1.4) | |
| Malta | 1992–2009 | –0.5 | − | − | − | − | − | − | 2000–2009 | –0.5 (–1.6; 0.6) | |
| Slovenia | 1978–2001 | 2.0† | 2001–2011 | –1.4† | − | − | − | − | 2002–2011 | –1.4† (–2.8; 0.0) | |
| Spain* | 1988–2007 | 1.9† | − | − | − | − | − | − | 1998–2007 | 1.9† (1.5; 2.3) | |
| Western Europe | Austria | 1990–2009 | –0.9† | − | − | − | − | − | − | 2000–2009 | –0.9† (–1.2; –0.5) |
| France* | 1988–2009 | 0.1 | − | − | − | − | − | − | 2000–2009 | 0.1 (–0.3; 0.5) | |
| Switzerland* | 1983–1988 | –5.9† | 1988–2008 | 0.2 | − | − | − | − | 1999–2008 | 0.2 (–0.5; 0.9) | |
| Netherlands | 1989–2008 | 0.7† | − | − | − | − | − | − | 1999–2008 | 0.7† (0.5; 1.0) | |
| Oceania | Australia | 1982–2009 | 0.8† | − | − | − | − | − | − | 2000–2009 | 0.8† (0.6; 0.9) |
| New Zealand | 1983–1993 | –1.3 | 1993–2010 | 2.6† | − | − | − | − | 2001–2010 | 2.6† (1.9; 3.2) | |
Regional registries. APC = annual percent change; AAPC = average annual percent change.
Change in rate is statistically significantly different from zero at the 5% level. The tests of significance use a Monte Carlo Permutation method. Only the model that best fits the observed incidence is presented, with up to four trends (three Joinpoints). Therefore, some cells are left blank.
Trends in endometrial cancer incidence rates, for 1978–2013, and over the last 10 years, for all ages
| Region . | Population . | Trend 1 . | Trend 2 . | Trend 3 . | Trend 4 . | Last 10 years . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period . | APC . | Period . | APC . | Period . | APC . | Period . | APC . | Period . | AAPC (95% CI) . | ||
| Africa | South Africa* | 1998–2012 | 11.3† | − | − | − | − | − | − | 2003–2012 | 11.3† (4.0; 19.0) |
| Uganda* | 1991–2012 | 1.7 | − | − | − | − | − | − | 2003–2012 | 1.7 (–1.5; 5.1) | |
| South America | Brazil* | 1988–2007 | 5.0† | − | − | − | − | − | − | 1998–2007 | 5.0† (2.5; 7.6) |
| Colombia* | 1983–2007 | –0.4 | − | − | − | − | − | − | 1998–2007 | –0.4 (–1.2; 0.5) | |
| Costa Rica | 1980–1988 | –3.3 | 1988–2005 | 4.4† | 2005–2007 | –20.9 | − | − | 1998–2007 | –1.8 (–8.3; 5.1) | |
| Ecuador* | 1985–2007 | –0.5 | − | − | − | − | − | − | 1998–2007 | –0.5 (–1.8; 0.9) | |
| North America | Canada* | 1983–1990 | –1.9† | 1990–2007 | 0.8† | − | − | − | − | 1998–2007 | 0.8† (0.5; 1.0) |
| US* black | 1978–1997 | 0.3 | 1997–2012 | 3.1† | − | − | − | − | 2003–2012 | 3.1† (2.2; 3.9) | |
| US* white | 1978–1988 | –2.3† | 1988–1999 | 0.6† | 1999–2003 | –1.1 | 2003–2012 | 1.4† | 2003–2012 | 1.4† (0.9; 1.9) | |
| Eastern Asia | China* | 1988–2007 | 3.3† | − | − | − | − | − | − | 1998–2007 | 3.3† (2.7; 3.9) |
| Japan* | 1978–2003 | 2.9† | 2003–2007 | 9.8† | − | − | − | − | 1998–2007 | 5.9† (3.7; 8.1) | |
| Southern Asia | India* | 1983–2005 | 1.4† | 2005–2007 | 13.0 | − | − | − | − | 1998–2007 | 3.9 (–0.5; 8.4) |
| South-eastern Asia | Philippines* | 1983–1994 | –2.0 | 1994–1999 | 13.5† | 1999–2007 | 0.2 | − | − | 1998–2007 | 1.6 (–0.7; 4.0) |
| Singapore | 1978–2007 | 3.5† | − | − | − | − | − | − | 1998–2007 | 3.5† (3.1; 3.9) | |
| Thailand* | 1983–2007 | 2.1† | − | − | − | − | − | − | 1998–2007 | 2.1† (1.1; 3.1) | |
| Western Asia | Israel | 1983–2007 | 1.9† | − | − | − | − | − | − | 1998–2007 | 1.9† (1.4; 2.5) |
| Eastern Europe | Belarus | 1978–2007 | 3.3† | − | − | − | − | − | − | 1998–2007 | 3.3† (3.1; 3.5) |
| Bulgaria | 1993–2011 | 1.1† | − | − | − | − | − | − | 2002–2011 | 1.1† (0.8; 1.4) | |
| Czech Republic | 1983–2010 | 0.2† | − | − | − | − | − | − | 2001–2010 | 0.2† (0.0; 0.3) | |
| Poland* | 1988–2008 | 2.0† | − | − | − | − | − | − | 1999–2008 | 2.0† (1.6; 2.3) | |
| Russian Federation | 1993–1996 | 4.3† | 1996–2013 | 2.1† | − | − | − | − | 2004–2013 | 2.1† (2.0; 2.3) | |
| Slovakia | 1978–2008 | 1.4† | − | − | − | − | − | − | 1999–2008 | 1.4† (1.1; 1.6) | |
| Northern Europe | Denmark | 1978–1980 | 7.4 | 1980–2000 | –1.4† | 2000–2012 | 0.7 | − | − | 2003–2012 | 0.7 (–0.1; 1.4) |
| Estonia | 1978–1996 | 1.9† | 1996–2007 | –0.3 | − | − | − | − | 1998–2007 | –0.3 (–1.1; 0.5) | |
| Finland | 1978–1998 | 1.5† | 1998–2012 | –0.6 | − | − | − | − | 2003–2012 | –0.6 (–1.1; 0.0) | |
| Iceland | 1978–2012 | –0.1 | − | − | − | − | − | − | 2003–2012 | –0.1 (–0.9; 0.8) | |
| Ireland | 1994–2011 | 2.4† | − | − | − | − | − | − | 2002–2011 | 2.4† (1.6; 3.1) | |
| Latvia | 1983–1996 | 2.8† | 1996–2007 | –0.5 | − | − | − | − | 1998–2007 | –0.5 (–1.6; 0.7) | |
| Lithuania | 1978–1989 | 1.1 | 1989–2001 | 3.9† | 2001–2007 | –1.0 | − | − | 1998–2007 | 0.6 (–1.1; 2.4) | |
| Norway | 1978–1988 | 0.0 | 1988–2010 | 1.7† | 2010–2012 | –10.7 | − | − | 2003–2012 | –1.2 (–3.8; 1.4) | |
| Sweden | 1978–1986 | –1.1† | 1986–1998 | 1.4† | 1998–2012 | –0.6† | − | − | 2003–2012 | –0.6† (–0.9; –0.2) | |
| UK* | 1978–1980 | 24.2 | 1980–1994 | 0.4 | 1994–2012 | 2.7† | − | − | 2003–2012 | 2.7† (2.5; 2.9) | |
| Southern Europe | Croatia | 1988–2012 | 1.3† | − | − | − | − | − | − | 2003–2012 | 1.3† (0.8; 1.8) |
| Italy* | 1988–2007 | 1.1† | − | − | − | − | − | − | 1998–2007 | 1.1† (0.7; 1.4) | |
| Malta | 1992–2009 | –0.5 | − | − | − | − | − | − | 2000–2009 | –0.5 (–1.6; 0.6) | |
| Slovenia | 1978–2001 | 2.0† | 2001–2011 | –1.4† | − | − | − | − | 2002–2011 | –1.4† (–2.8; 0.0) | |
| Spain* | 1988–2007 | 1.9† | − | − | − | − | − | − | 1998–2007 | 1.9† (1.5; 2.3) | |
| Western Europe | Austria | 1990–2009 | –0.9† | − | − | − | − | − | − | 2000–2009 | –0.9† (–1.2; –0.5) |
| France* | 1988–2009 | 0.1 | − | − | − | − | − | − | 2000–2009 | 0.1 (–0.3; 0.5) | |
| Switzerland* | 1983–1988 | –5.9† | 1988–2008 | 0.2 | − | − | − | − | 1999–2008 | 0.2 (–0.5; 0.9) | |
| Netherlands | 1989–2008 | 0.7† | − | − | − | − | − | − | 1999–2008 | 0.7† (0.5; 1.0) | |
| Oceania | Australia | 1982–2009 | 0.8† | − | − | − | − | − | − | 2000–2009 | 0.8† (0.6; 0.9) |
| New Zealand | 1983–1993 | –1.3 | 1993–2010 | 2.6† | − | − | − | − | 2001–2010 | 2.6† (1.9; 3.2) | |
| Region . | Population . | Trend 1 . | Trend 2 . | Trend 3 . | Trend 4 . | Last 10 years . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period . | APC . | Period . | APC . | Period . | APC . | Period . | APC . | Period . | AAPC (95% CI) . | ||
| Africa | South Africa* | 1998–2012 | 11.3† | − | − | − | − | − | − | 2003–2012 | 11.3† (4.0; 19.0) |
| Uganda* | 1991–2012 | 1.7 | − | − | − | − | − | − | 2003–2012 | 1.7 (–1.5; 5.1) | |
| South America | Brazil* | 1988–2007 | 5.0† | − | − | − | − | − | − | 1998–2007 | 5.0† (2.5; 7.6) |
| Colombia* | 1983–2007 | –0.4 | − | − | − | − | − | − | 1998–2007 | –0.4 (–1.2; 0.5) | |
| Costa Rica | 1980–1988 | –3.3 | 1988–2005 | 4.4† | 2005–2007 | –20.9 | − | − | 1998–2007 | –1.8 (–8.3; 5.1) | |
| Ecuador* | 1985–2007 | –0.5 | − | − | − | − | − | − | 1998–2007 | –0.5 (–1.8; 0.9) | |
| North America | Canada* | 1983–1990 | –1.9† | 1990–2007 | 0.8† | − | − | − | − | 1998–2007 | 0.8† (0.5; 1.0) |
| US* black | 1978–1997 | 0.3 | 1997–2012 | 3.1† | − | − | − | − | 2003–2012 | 3.1† (2.2; 3.9) | |
| US* white | 1978–1988 | –2.3† | 1988–1999 | 0.6† | 1999–2003 | –1.1 | 2003–2012 | 1.4† | 2003–2012 | 1.4† (0.9; 1.9) | |
| Eastern Asia | China* | 1988–2007 | 3.3† | − | − | − | − | − | − | 1998–2007 | 3.3† (2.7; 3.9) |
| Japan* | 1978–2003 | 2.9† | 2003–2007 | 9.8† | − | − | − | − | 1998–2007 | 5.9† (3.7; 8.1) | |
| Southern Asia | India* | 1983–2005 | 1.4† | 2005–2007 | 13.0 | − | − | − | − | 1998–2007 | 3.9 (–0.5; 8.4) |
| South-eastern Asia | Philippines* | 1983–1994 | –2.0 | 1994–1999 | 13.5† | 1999–2007 | 0.2 | − | − | 1998–2007 | 1.6 (–0.7; 4.0) |
| Singapore | 1978–2007 | 3.5† | − | − | − | − | − | − | 1998–2007 | 3.5† (3.1; 3.9) | |
| Thailand* | 1983–2007 | 2.1† | − | − | − | − | − | − | 1998–2007 | 2.1† (1.1; 3.1) | |
| Western Asia | Israel | 1983–2007 | 1.9† | − | − | − | − | − | − | 1998–2007 | 1.9† (1.4; 2.5) |
| Eastern Europe | Belarus | 1978–2007 | 3.3† | − | − | − | − | − | − | 1998–2007 | 3.3† (3.1; 3.5) |
| Bulgaria | 1993–2011 | 1.1† | − | − | − | − | − | − | 2002–2011 | 1.1† (0.8; 1.4) | |
| Czech Republic | 1983–2010 | 0.2† | − | − | − | − | − | − | 2001–2010 | 0.2† (0.0; 0.3) | |
| Poland* | 1988–2008 | 2.0† | − | − | − | − | − | − | 1999–2008 | 2.0† (1.6; 2.3) | |
| Russian Federation | 1993–1996 | 4.3† | 1996–2013 | 2.1† | − | − | − | − | 2004–2013 | 2.1† (2.0; 2.3) | |
| Slovakia | 1978–2008 | 1.4† | − | − | − | − | − | − | 1999–2008 | 1.4† (1.1; 1.6) | |
| Northern Europe | Denmark | 1978–1980 | 7.4 | 1980–2000 | –1.4† | 2000–2012 | 0.7 | − | − | 2003–2012 | 0.7 (–0.1; 1.4) |
| Estonia | 1978–1996 | 1.9† | 1996–2007 | –0.3 | − | − | − | − | 1998–2007 | –0.3 (–1.1; 0.5) | |
| Finland | 1978–1998 | 1.5† | 1998–2012 | –0.6 | − | − | − | − | 2003–2012 | –0.6 (–1.1; 0.0) | |
| Iceland | 1978–2012 | –0.1 | − | − | − | − | − | − | 2003–2012 | –0.1 (–0.9; 0.8) | |
| Ireland | 1994–2011 | 2.4† | − | − | − | − | − | − | 2002–2011 | 2.4† (1.6; 3.1) | |
| Latvia | 1983–1996 | 2.8† | 1996–2007 | –0.5 | − | − | − | − | 1998–2007 | –0.5 (–1.6; 0.7) | |
| Lithuania | 1978–1989 | 1.1 | 1989–2001 | 3.9† | 2001–2007 | –1.0 | − | − | 1998–2007 | 0.6 (–1.1; 2.4) | |
| Norway | 1978–1988 | 0.0 | 1988–2010 | 1.7† | 2010–2012 | –10.7 | − | − | 2003–2012 | –1.2 (–3.8; 1.4) | |
| Sweden | 1978–1986 | –1.1† | 1986–1998 | 1.4† | 1998–2012 | –0.6† | − | − | 2003–2012 | –0.6† (–0.9; –0.2) | |
| UK* | 1978–1980 | 24.2 | 1980–1994 | 0.4 | 1994–2012 | 2.7† | − | − | 2003–2012 | 2.7† (2.5; 2.9) | |
| Southern Europe | Croatia | 1988–2012 | 1.3† | − | − | − | − | − | − | 2003–2012 | 1.3† (0.8; 1.8) |
| Italy* | 1988–2007 | 1.1† | − | − | − | − | − | − | 1998–2007 | 1.1† (0.7; 1.4) | |
| Malta | 1992–2009 | –0.5 | − | − | − | − | − | − | 2000–2009 | –0.5 (–1.6; 0.6) | |
| Slovenia | 1978–2001 | 2.0† | 2001–2011 | –1.4† | − | − | − | − | 2002–2011 | –1.4† (–2.8; 0.0) | |
| Spain* | 1988–2007 | 1.9† | − | − | − | − | − | − | 1998–2007 | 1.9† (1.5; 2.3) | |
| Western Europe | Austria | 1990–2009 | –0.9† | − | − | − | − | − | − | 2000–2009 | –0.9† (–1.2; –0.5) |
| France* | 1988–2009 | 0.1 | − | − | − | − | − | − | 2000–2009 | 0.1 (–0.3; 0.5) | |
| Switzerland* | 1983–1988 | –5.9† | 1988–2008 | 0.2 | − | − | − | − | 1999–2008 | 0.2 (–0.5; 0.9) | |
| Netherlands | 1989–2008 | 0.7† | − | − | − | − | − | − | 1999–2008 | 0.7† (0.5; 1.0) | |
| Oceania | Australia | 1982–2009 | 0.8† | − | − | − | − | − | − | 2000–2009 | 0.8† (0.6; 0.9) |
| New Zealand | 1983–1993 | –1.3 | 1993–2010 | 2.6† | − | − | − | − | 2001–2010 | 2.6† (1.9; 3.2) | |
Regional registries. APC = annual percent change; AAPC = average annual percent change.
Change in rate is statistically significantly different from zero at the 5% level. The tests of significance use a Monte Carlo Permutation method. Only the model that best fits the observed incidence is presented, with up to four trends (three Joinpoints). Therefore, some cells are left blank.
Average annual percent change in age-standardized endometrial cancer incidence rates for (A) all ages and (B) age 25 to 49 years and age 50 years and older, over the last 10 years available, and 95% confidence intervals. The last 10 years vary by population from 1998–2007 to 2004–2013. The average annual percent change was not computed for South Africa for age 25 to 49 years due to several years with no new cases. Confidence interval bars truncated on the plots for Costa Rica and South Africa for all ages, and age 50 years and older. *Denotes regional registries.
Age-Period-Cohort Analysis and Trends in Rates by Age
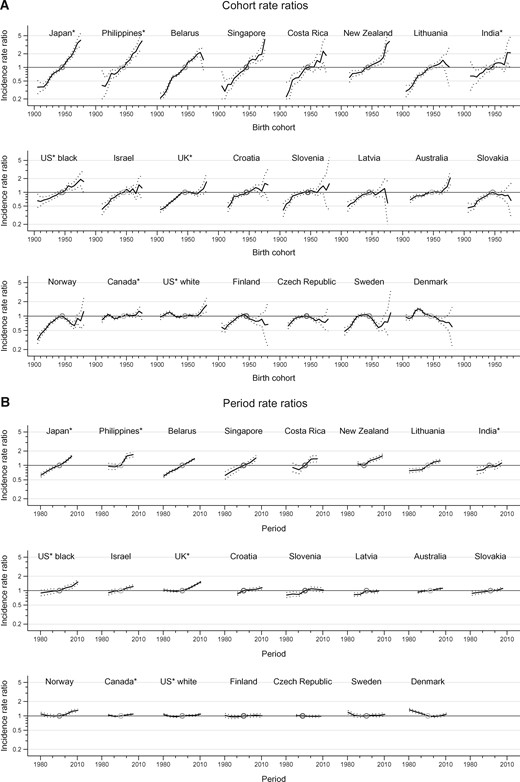
Figure 3 displays the changes in risk in successive cohorts and periods based on incidence rate ratios. The risk increased both in consecutive generations and over time in 11 populations out of 23. The increase was most evident in Japan, the Philippines, Belarus, Singapore, Costa Rica, and New Zealand. However, the cohort rate ratio curve revealed a decline in endometrial cancer incidence in the Nordic and two Eastern European countries among women born after the 1920s in Denmark, and in subsequent generations in the Czech Republic, Slovakia, Finland, Sweden, and Norway, followed by increases in birth cohorts born after 1965 in Norway and Sweden.
Incidence rate ratios of endometrial cancer by birth cohort and by period, 95% confidence intervals, in age 30 to 79 years in select countries. A) Cohort rate ratios. B) Period rate ratios. Countries are ordered by decreasing value of the net drift (the annual percentage change of the expected age-adjusted rates over time). In three countries, the upper bound of one confidence interval of birth cohort effects was higher than 6 (Japan 6.5, the Philippines 6.3, and Singapore 8.1) and truncated at 5 on the plots. *Denotes regional registries.
Supplementary Figure 3 (available online) presents age-specific incidence rates by birth cohort and by period. The absence of quasi-parallelism between age-specific curves in the majority of the populations indicates the combined influence of cohort and period effects, or their lack of influence in the case of stable trends. Only for Canadian, white American, and Danish women did cohort effects appear to drive the incidence trends, with the highest risk of endometrial cancer found in women born around the 1920s. In Northern Europe, there seems to be a differing period effect before and after menopause. In particular, there appear to be period-specific increases since 2000 after long declines in incidence in the United Kingdom, Norway, and Sweden in women younger than age 50 years, while the age-specific rates have continuously increased in older women since the 1980s. Marked period effects, with the risk of cancer increasing over time in all age groups, were visible in Asia (Japan, the Philippines, and Singapore) and in the geographically unrelated countries Belarus and New Zealand.
Discussion
Recent rates (2006–2007) for all ages varied 20-fold between countries, with the highest rates found in Europe and North America and the lowest rates in developing countries. Rates in all ages have been statistically significantly increasing in 26 out of 43 populations, as well as in 15 premenopausal and 27 postmenopausal populations. However, in France, the Netherlands, Denmark, Norway, and the Czech Republic, rates statistically significantly declined in women younger than age 50 years. The age-period-cohort analysis revealed that, in half of the populations, women were at ever-increasing risk of endometrial cancer, over time and in successive generations, and most evidently in some Asian countries (Japan, the Philippines, Singapore, and India), Belarus, Lithuania, Costa Rica, and New Zealand.
More than 80% of endometrial cancers (called type 1 endometrial cancers) are estrogen-related (5). Therefore, increasing trends in endometrial cancer incidence may be largely explained by the increasing trends in exogenous estrogen use—in peri- and postmenopausal women only—and endogenous estrogen exposure (nulliparity, fewer pregnancies, early age at menarche, and obesity) (5). Large increases in the use of hormones in postmenopausal women have been reported in the 1980s and early 1990s. For instance, in the 1990s, more than 30% of postmenopausal women age 45 to 64 years used hormones in parts of Australia, Canada, Finland, France, Iceland, and the United States, as opposed to less than 5% in Lithuania, Poland, and Russia (17). Menopausal hormone sales dropped following the publication of the results of the Women’s Health Initiative study in 2002 (18) and the Million Women Study in 2003 demonstrating the adverse health effects of MHU, including increased risk for breast cancer. It was later determined that estrogen/progestogen/androgen and estrogen-only therapies increase the risk of endometrial cancer, while continuous estrogen-progestogen therapies decrease it (19). Although sharp declines in other hormone-related cancer incidence (ie, ovarian and breast) (20) have been observed after the drastic reduction in MHU in some countries, our analysis did not reveal similar obvious positive effects on endometrial cancer incidence in the studied populations.
Changes in reproductive factors also increase endometrial cancer cases. High parity protects from endometrial cancer, but it has been decreasing in most countries because of socioeconomic transition (21). Almost half of the 70 low-fertility countries (≤2.0 children per woman) are in Asia, Latin America, and the Caribbean (22). As examples, between 1950 to 1955 and 2000 to 2005, the fertility rates declined from more than 6.0 to 1.6 or fewer children per women in China, Singapore, and Thailand, from 3.0 to 1.3 in Japan, from 7.4 to 3.7 in the Philippines, and from 6.2 to 2.3 in Brazil. In parallel, nulliparity has more than doubled since 1994 in Austria, Japan, Spain, and Thailand (23), putting more women at greater risk of endometrial cancer. The highest rates of nulliparity are currently in low-fertility countries in Europe and Eastern Asia, with Singapore having the highest proportion of women with no children (23%). It is possible that these changing reproductive patterns may have in part contributed to the rapid increase in endometrial cancer incidence rates in Asian, Latin American, and other countries considered in this study.
The global increase in overweight and obesity in women between 1980 and 2013 from 30% to 38% (24) may have also contributed to the upward endometrial cancer incidence trends (25). The proportion of women with high BMI increased even faster than the global average in the high-income countries included in our study (except Japan); for instance, from 44% to 57% in the United Kingdom, to 60% in New Zealand, and to 62% in the United States. In the latter country, according to the World Cancer Research Fund (26), up to 59% of endometrial cancers could be prevented by appropriate levels of body fatness (median population BMI between 21 and 23) and physical activity (moderate physical activity for at least 30 minutes every day) (27). Globally, 107 000 new cases of endometrial cancer (34%) were due to high BMI (10) in 2012. Yet, while endometrial cancer incidence recently declined in Austria and Sweden, overweight also increased over 1980 to 2013 in these two countries from 27% to 43% and from 41% to 46%, respectively. Furthermore, in Japan, BMI has remained low and declined (from 19% to 18%), while endometrial cancer incidence surged upwards. Both findings indicate the combined influence of several other risk factors on endometrial cancer incidence. In addition, the prevalence of diabetes—known to be associated with endometrial cancer and closely related to high BMI—has been increasing globally in the recent decades, particularly in low- and middle-income countries (28), affecting 9% of the world population in 2014 (29).
In contrast to the trends in incidence rates in most countries considered for this analysis, incidence rates have decreased in more recent cohorts in several Northern European and two Eastern European countries. Reasons for this pattern remain unclear. As smoking decreases the risk of endometrial cancer among postmenopausal women by 29%, the large proportion of women who picked up the deleterious habit in some countries—the harmful effects of smoking outweigh by many orders of magnitude the potential benefit of preventing endometrial cancer—could explain the declines, but only to some extent. Denmark, Slovakia, and Finland all experienced declines in endometrial cancer incidence rate ratios, starting in cohorts born in the 1920s to 1940s onwards. Nevertheless, in 2006 to 2008, Danish female lung cancer incidence rates—an indicator of past smoking intensity—were triple compared with their Finnish and Slovak counterparts (30).
Oral contraceptive (OC) use may be another contributing factor for the declines observed in select European countries such as France. OC is a protective factor for endometrial cancer—every five years of usage is associated with a risk decline of 24%, and the risk reduction persists for more than 30 years after cessation (31). In general, in the 1990s, OC use was more common in Western Europe and Australia/New Zealand than in Central and Eastern Europe and North America (17). A meta-analysis estimated that OC use may have prevented about 400 000 cases of endometrial cancer over the past 50 years in developed countries (31). Of note, intrauterine devices, frequently used in Asian developing countries (32), also confer protection against endometrial cancer (33).
The remaining 10% to 20% of endometrial cancers (type 2) are estrogen independent but share some risk factors with type 1 endometrial cancers (low parity, early age at menarche, and diabetes) and the protective effects of OC use and smoking (34). The distinction between type 1 and 2 endometrial cancers is based on differences in histology and clinical outcomes (type 2 having a poor prognosis compared with type 1) (35), which suggests possible different biological pathways and could therefore also explain some of the differences in the observed global trends.
Finally, we cannot rule out the additional effect of an underlying linear period-based trend in some countries driven by a gradually increasing awareness of endometrial cancer among both the general public and health professionals, more intensive clinical investigation in current and past menopausal hormone users, and better access to care over the last decades.
A strength of our study is the use of high-quality incidence data from 43 populations across five continents spanning 36 years to provide contemporary global patterns of endometrial cancer incidence rates. Previous studies examined trends and patterns in incidence rates for specific regions (eg, in Europe [11,36–39]) or by country (eg, the United States [40], India [41,42], and Saudi Arabia [43]) based on older data. However, our study also has limitations. The lack of adjustment for hysterectomy in the population at risk, due to the lack of data by age group in each country over the study period, has the potential to bias the direction and magnitude of the incidence rates (44). For instance, between 2000 and 2014, hysterectomy rates in Denmark were low and remained around 35 procedures per 100 000 women (with a peak at 64 procedures per 100 000 in 2007), whereas rates in Austria and Australia were high and declined by 30% (from 320 to 216 procedures per 100 000) and 27% (from 357 to 262 procedures), respectively (45). In the United States and Finland—among countries with high historical hysterectomy rates—hysterectomy-corrected endometrial cancer incidence rates were estimated to be about 30% higher than uncorrected rates (46,47). Hence, the variations in the uncorrected incidence rates and trends across countries may in part reflect differences in hysterectomy rates and changes over time. Finally, interpretation of incidence patterns for 18 countries was based on regional rather than national data, particularly in low- and middle-income countries.
Endometrial cancer incidence rates increased over time and in successive generation in half of the populations, especially in those countries with rapid socioeconomic transitions. In particular, changes in reproductive factors (declines in fertility) combined with increases in overweight and diabetes may explain some of the increases in endometrial cancer incidence. Meanwhile, the rise in some countries was probably mitigated by the protective effect of OC use. Future studies should further examine factors contributing to the increasing trend.
Funding
This work was supported by the Intramural Department of the American Cancer Society.
Notes
The study sponsor played no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
We thank the cancer registries that are participating investigators for having contributed their data.