-
PDF
- Split View
-
Views
-
Cite
Cite
Lisa A. Carey, Richard Metzger, E. Claire Dees, Frances Collichio, Carolyn I. Sartor, David W. Ollila, Nancy Klauber-DeMore, Jan Halle, Lynda Sawyer, Dominic T. Moore, Mark L. Graham, American Joint Committee on Cancer Tumor–Node–Metastasis Stage After Neoadjuvant Chemotherapy and Breast Cancer Outcome, JNCI: Journal of the National Cancer Institute, Volume 97, Issue 15, 3 August 2005, Pages 1137–1142, https://doi.org/10.1093/jnci/dji206
Close - Share Icon Share
Abstract
Background: Response to neoadjuvant chemotherapy is used as an intermediate endpoint for breast cancer relapse and survival. Most breast cancer response classification systems use pathologic complete response, either alone or in conjunction with clinical assessments, to categorize response. We examined the ability of the revised 2003 American Joint Committee on Cancer (AJCC) tumor–node–metastasis (TNM) staging system, which considers both the number of involved axillary lymph nodes and the extent of tumor in the breast to predict patient survival after neoadjuvant chemotherapy for breast cancer. Methods: We assessed the pathologic stage of residual tumor in 132 patients with nonmetastatic breast cancer after they had undergone neoadjuvant chemotherapy and examined the association between AJCC TNM stage and subsequent distant disease–free survival and overall survival. All statistical tests were two-sided. Results: At a median follow-up of 5 years, pathologic stage in the surgical specimens after neoadjuvant chemotherapy using the revised AJCC system was strongly associated with both distant disease–free survival and overall survival. A higher pathologic stage of residual tumor after neoadjuvant chemotherapy was associated with a statistically significant lower rate of distant disease–free survival (stage 0: 95%, stage I: 84%, stage II: 72%, and stage III: 47%; Ptrend <.001). The 5-year distant disease–free survival for patients with residual stage IIIC tumors was only 18% (95% CI = 0% to 36%). Conclusion: Classification of residual tumor in the breast and axillary surgical specimens after neoadjuvant chemotherapy using the revised AJCC TNM system is useful for predicting distant relapse and survival.
The extent of residual tumor after neoadjuvant chemotherapy is a well-established intermediate endpoint for breast cancer relapse and survival ( 1 – 3 ) . Different methods have been used to categorize residual tumor, but they generally provide only limited prognostic categories, often do not include assessments of both the primary breast tumor and axillary lymph nodes, or require both pathologic and clinical assessments. A revision of the American Joint Committee on Cancer tumor–node–metastasis (AJCC TNM) staging system was implemented in January of 2003 ( 4 ) . This revision included several important modifications to the breast cancer classification system, such as a designation for response after neoadjuvant treatment and incorporation of the number of involved axillary lymph nodes into the nodal categories.
In this study, we examined the ability of the revised AJCC TNM staging system to predict patient survival after neoadjuvant chemotherapy for breast cancer.
M ETHODS
All breast cancer patients diagnosed with clinical stage II or III disease according to the 1988 AJCC TNM system who were treated at the University of North Carolina at Chapel Hill with neoadjuvant chemotherapy from January 1, 1992 through December 31, 2000 were eligible for this study. Most of these patients were treated in one of two neoadjuvant chemotherapy trials that were open during that time. In one trial, patients were treated first with neoadjuvant doxorubicin, next with surgery and adjuvant cyclophosphamide, methotrexate, and fluorouracil (CMF), and finally with radiation ( 2 ) . In the other trial, patients were treated first with neoadjuvant doxorubicin plus cyclophosphamide, then with paclitaxel with or without trastuzumab, and finally with surgery followed by radiation ( 5 ) . We obtained clinical and outcome data from the protocol case report forms and medical charts (including pathology reports). We used the pathology reports to determine the 2003 revised AJCC TNM stage of the surgical specimens from patients who had undergone neoadjuvant chemotherapy ( Table 1 ). We determined the stage of each specimen according to the 1988 AJCC TNM and the revised 2003 AJCC TNM staging systems. Residual disease was defined as invasive carcinoma. Pathologic complete response included residual ductal carcinoma in situ. The revised 2003 AJCC TNM staging method (i.e., yTNM), in which a “y” designates the stage of the residual tumor after neoadjuvant therapy, defines tumor size (T) as follows: T1, tumor is 2 cm or less in the greatest dimension; T2, tumor is more than 2 cm but not more than 5 cm in the greatest dimension; T3, tumor is more than 5 cm in the greatest dimension; or T4, any tumor that involves local structures, such as the chest wall or skin. When tumor shrinkage from chemotherapy resulted in nests of residual tumors, these tumors were categorized by the distance over which the nests extended unless there were clearly defined multicentric tumor foci that could be distinguished pathologically. Axillary lymph node (N) categories include N0, no axillary lymph nodes contain metastases of at least 0.2 mm detectable by hematoxylin–eosin staining; N1, metastases detectable in one, two, or three axillary lymph nodes; N2, metastases detectable in at least four but fewer than 10, or matted or fixed axillary lymph nodes; and N3, metastases detectable in 10 or more axillary lymph nodes or any infraclavicular lymph node involvement.
Revised 2003 AJCC TNM staging system for breast cancer as applied to pathologic extent of disease after neoadjuvant chemotherapy *
| Tumor and nodal categories . | Stage . |
|---|---|
| T0N0 (including residual ductal carcinoma in situ) | 0 |
| T1N0 | I |
| T0-1N1; T2N0 | IIA |
| T2N1; T3N0 | IIB |
| T0-3N2; T3N1 | IIIA |
| Any T4 | IIIB |
| Any N3 | IIIC |
| Tumor and nodal categories . | Stage . |
|---|---|
| T0N0 (including residual ductal carcinoma in situ) | 0 |
| T1N0 | I |
| T0-1N1; T2N0 | IIA |
| T2N1; T3N0 | IIB |
| T0-3N2; T3N1 | IIIA |
| Any T4 | IIIB |
| Any N3 | IIIC |
Nodal metastases (N) reflect both the number and the presence of involved axillary lymph nodes. AJCC TNM = American Joint Committee on Cancer tumor–node–metastasis.
Revised 2003 AJCC TNM staging system for breast cancer as applied to pathologic extent of disease after neoadjuvant chemotherapy *
| Tumor and nodal categories . | Stage . |
|---|---|
| T0N0 (including residual ductal carcinoma in situ) | 0 |
| T1N0 | I |
| T0-1N1; T2N0 | IIA |
| T2N1; T3N0 | IIB |
| T0-3N2; T3N1 | IIIA |
| Any T4 | IIIB |
| Any N3 | IIIC |
| Tumor and nodal categories . | Stage . |
|---|---|
| T0N0 (including residual ductal carcinoma in situ) | 0 |
| T1N0 | I |
| T0-1N1; T2N0 | IIA |
| T2N1; T3N0 | IIB |
| T0-3N2; T3N1 | IIIA |
| Any T4 | IIIB |
| Any N3 | IIIC |
Nodal metastases (N) reflect both the number and the presence of involved axillary lymph nodes. AJCC TNM = American Joint Committee on Cancer tumor–node–metastasis.
Supraclavicular lymph node metastases, although considered nonmetastatic in the revised AJCC system, were not included in the original database because they were considered metastatic in the older (1988) AJCC system. We did not include metastases that had been detected by immunohistochemical methods alone.
Distant disease–free survival was calculated as the time from the date of diagnosis of primary tumor to the date of the last follow-up, the date of development of distant or regional metastases (i.e., the date of radiologic or clinical documentation of metastatic disease), or the date of death from any cause, whichever occurred first. Distant disease–free survival did not include local recurrences in the conserved breast or the axilla or on the chest wall. Overall survival was calculated as the time from the date of diagnosis of invasive breast cancer to the date of the last follow-up or death from any cause, whichever occurred first. We used the Kaplan–Meier method to estimate distant disease–free survival and overall survival. We used an order-restricted version of the log-rank test (i.e., a trend test) to test for ordered differences between estimated time-to-event curves. We did an exploratory Cox regression was used to examine the impact of clinical covariates on distant disease–free survival and overall survival. However, the data did not conform to proportional hazards assumptions. Statistical analyses were performed using JMP Version 5 and SAS statistical software, Version 8.2 (both products of the SAS Institute, Inc., Cary, NC). All statistical tests were two-sided. This study was approved by the committee on the Protection of the Rights of Human Subjects of the University of North Carolina at Chapel Hill.
R ESULTS
Characteristics of the Primary Tumors and Treatments
Table 2 presents the patient demographics and tumor characteristics for the 132 patients who were included in this study. Most patients were treated on or according to one of two neoadjuvant regimens that reflected the institutional protocols in place at the University of North Carolina from 1992 through 2000: 84 patients (64%) received anthracycline-based neoadjuvant chemotherapy, and 47 patients (36%) received combined anthracycline/taxane–based neoadjuvant chemotherapy. All patients with estrogen receptor (ER) –positive tumors or tumors with unknown ER status were offered adjuvant tamoxifen. All patients underwent either breast-conserving surgery or modified radical mastectomy at the discretion of the treating physicians after multidisciplinary review, and all patients had axillary lymph node dissection. Seventy-two patients (54%) received additional adjuvant chemotherapy; of these, 59 received CMF as mandated by one of the institutional protocols. A total of 115 patients (87%) received adjuvant radiation therapy, and 60 (77%) of 78 patients with ER or progesterone receptor (PR)–positive tumors or tumors with unknown hormone receptor status received adjuvant tamoxifen.
Demographic and tumor characteristics of the study cohort (N = 132) *
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 48 (23–72) |
| Menopausal status, n (%) | |
| Pre/perimenopausal | 75 (57) |
| Postmenopausal | 57 (43) |
| Race, n (%) | |
| White | 82 (62) |
| African American | 43 (33) |
| Other | 7 (5) |
| Clinical stage at diagnosis † , n (%) | |
| IIA | 13 (10) |
| IIB | 25 (19) |
| IIIA | 61 (46) |
| IIIB | 33 (25) |
| ER status, n (%) | |
| Positive/borderline | 64 (48) |
| Negative | 54 (41) |
| Unknown | 14 (11) |
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 48 (23–72) |
| Menopausal status, n (%) | |
| Pre/perimenopausal | 75 (57) |
| Postmenopausal | 57 (43) |
| Race, n (%) | |
| White | 82 (62) |
| African American | 43 (33) |
| Other | 7 (5) |
| Clinical stage at diagnosis † , n (%) | |
| IIA | 13 (10) |
| IIB | 25 (19) |
| IIIA | 61 (46) |
| IIIB | 33 (25) |
| ER status, n (%) | |
| Positive/borderline | 64 (48) |
| Negative | 54 (41) |
| Unknown | 14 (11) |
ER = estrogen receptor.
Revised AJCC version 6 ( 4 ) .
Demographic and tumor characteristics of the study cohort (N = 132) *
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 48 (23–72) |
| Menopausal status, n (%) | |
| Pre/perimenopausal | 75 (57) |
| Postmenopausal | 57 (43) |
| Race, n (%) | |
| White | 82 (62) |
| African American | 43 (33) |
| Other | 7 (5) |
| Clinical stage at diagnosis † , n (%) | |
| IIA | 13 (10) |
| IIB | 25 (19) |
| IIIA | 61 (46) |
| IIIB | 33 (25) |
| ER status, n (%) | |
| Positive/borderline | 64 (48) |
| Negative | 54 (41) |
| Unknown | 14 (11) |
| Characteristic . | Value . |
|---|---|
| Median age, y (range) | 48 (23–72) |
| Menopausal status, n (%) | |
| Pre/perimenopausal | 75 (57) |
| Postmenopausal | 57 (43) |
| Race, n (%) | |
| White | 82 (62) |
| African American | 43 (33) |
| Other | 7 (5) |
| Clinical stage at diagnosis † , n (%) | |
| IIA | 13 (10) |
| IIB | 25 (19) |
| IIIA | 61 (46) |
| IIIB | 33 (25) |
| ER status, n (%) | |
| Positive/borderline | 64 (48) |
| Negative | 54 (41) |
| Unknown | 14 (11) |
ER = estrogen receptor.
Revised AJCC version 6 ( 4 ) .
Survival
The median follow-up for the cohort was 5 years (range = 1.2–11.1 years); 5-year distant disease–free survival was 68% (95% confidence interval [CI] = 59% to 77%), and 5-year overall survival was 74% (95% CI = 65% to 82%). A total of 42 patients (32%) experienced a distant relapse.
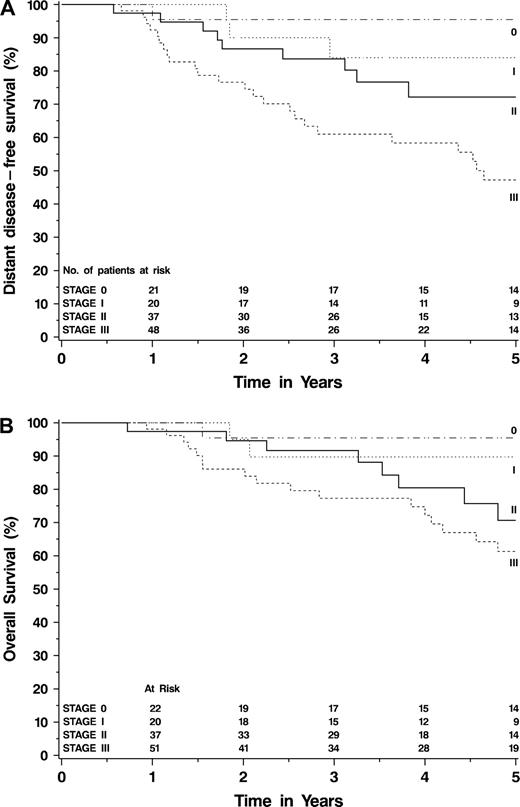
The pathologic stage of surgical specimens from patients who had undergone neoadjuvant chemotherapy was strongly associated with both distant disease–free ( Fig. 1, A ) and overall ( Fig. 1, B ) survival. The surgical specimens were fairly well distributed across all pathologic stages, except for an underrepresentation of those at stage IIIB ( Table 3 ). Patients who had pathologic complete response (i.e., their residual tumor after neoadjuvant chemotherapy was stage 0, with no evidence of invasive cancer) had a 5-year distant disease–free survival of 95% (95% CI = 87% to 100%). Patients whose residual tumors were stage I had a 5-year distant disease–free survival of 84% (95% CI = 67% to 100%). Patients whose residual tumors were stage IIA, stage IIB, or stage IIIA had similar 5-year distant disease–free survival, namely, 75% (95% CI = 55% to 96%), 65% (95% CI = 37% to 93%), and 68% (95% CI = 51% to 86%), respectively, although the small numbers of patients in each category limit generalizability. Only three patients had residual stage IIIB tumors, one of whom has relapsed. The 5-year distant disease–free survival for patients with residual stage IIIC tumors was only 18% (95% CI = 0% to 36%). All 18 of the patients in the stage IIIC group had 10 or more involved axillary lymph nodes; none had residual supraclavicular or infraclavicular lymph node involvement. No patients in the study developed metastatic disease during neoadjuvant chemotherapy. There were statistically significant differences in both distant disease–free survival ( Ptrend <.001) and overall survival ( Ptrend <.001) across all pathologic stages. Other variables that were statistically significantly associated with distant disease–free survival included the presence of inflammatory disease ( P <.001), the presence of lymphovascular invasion in the surgical specimen ( P <.001), pretreatment stage ( P <.001), tumor size ( P = .04), clinical nodal status ( P <.001), and posttreatment tumor size ( P =.02) (data not shown). Distant disease–free survival was not associated with patient age, race, menopausal status, clinical response rate, or the ER or PR status of the tumor (data not shown). Distant disease–free survival was also not associated with subsequent receipt of adjuvant chemotherapy ( P = .18) or adjuvant tamoxifen ( P = .48). Exploratory Cox regression modeling suggested that the relationship of distant disease–free survival with posttreatment AJCC stage may be more closely associated with number of involved nodes (N) than with extent of residual breast tumor (T) in this high-risk population.
Kaplan–Meier curves demonstrating relationships between the 2003 revised AJCC TNM pathologic stage after neoadjuvant chemotherapy and the survival endpoints. A ) Distant disease–free survival. B ) Overall survival.
Distant disease–free survival (DDFS) and overall survival (OS) as a function of revised 2003 AJCC TNM stage among patients who completed neoadjuvant chemotherapy (N = 132) *
| . | AJCC TNM stage . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | I . | IIA . | IIB . | IIIA . | IIIB . | IIIC . | . | ||||||
| No. of patients (%) | 22 (17) | 20 (15) | 25 (19) | 13 (10) | 31 (23) | 3 (2) | 18 (14) | |||||||
| No. patients with distant relapses (%) | 3 (14) | 3 (15) | 6 (24) | 4 (31) | 11 (35) | 1 (33) | 14 (78) | |||||||
| 5-year DDFS (95% CI) | 95% (87% to 100%) | 84% (67% to 100%) | 75% (55% to 96%) | 65% (37% to 93%) | 68% (51% to 86%) | 50% (0% to 100%) | 18% (0% to 36%) | Ptrend <.001 | ||||||
| 5-year OS (95% CI) | 95% (87% to 100%) | 90% (76% to 100%) | 70% (47% to 94%) | 73% (46% to 99%) | 69% (51% to 87%) | 100% † | 46% (22% to 70%) | Ptrend <.001 | ||||||
| . | AJCC TNM stage . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | I . | IIA . | IIB . | IIIA . | IIIB . | IIIC . | . | ||||||
| No. of patients (%) | 22 (17) | 20 (15) | 25 (19) | 13 (10) | 31 (23) | 3 (2) | 18 (14) | |||||||
| No. patients with distant relapses (%) | 3 (14) | 3 (15) | 6 (24) | 4 (31) | 11 (35) | 1 (33) | 14 (78) | |||||||
| 5-year DDFS (95% CI) | 95% (87% to 100%) | 84% (67% to 100%) | 75% (55% to 96%) | 65% (37% to 93%) | 68% (51% to 86%) | 50% (0% to 100%) | 18% (0% to 36%) | Ptrend <.001 | ||||||
| 5-year OS (95% CI) | 95% (87% to 100%) | 90% (76% to 100%) | 70% (47% to 94%) | 73% (46% to 99%) | 69% (51% to 87%) | 100% † | 46% (22% to 70%) | Ptrend <.001 | ||||||
AJCC TNM = American Joint Committee on Cancer tumor–node–metastasis; CI = confidence interval.
95% CI not calculable because only one patient in this category was still at risk at 5 years.
Distant disease–free survival (DDFS) and overall survival (OS) as a function of revised 2003 AJCC TNM stage among patients who completed neoadjuvant chemotherapy (N = 132) *
| . | AJCC TNM stage . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | I . | IIA . | IIB . | IIIA . | IIIB . | IIIC . | . | ||||||
| No. of patients (%) | 22 (17) | 20 (15) | 25 (19) | 13 (10) | 31 (23) | 3 (2) | 18 (14) | |||||||
| No. patients with distant relapses (%) | 3 (14) | 3 (15) | 6 (24) | 4 (31) | 11 (35) | 1 (33) | 14 (78) | |||||||
| 5-year DDFS (95% CI) | 95% (87% to 100%) | 84% (67% to 100%) | 75% (55% to 96%) | 65% (37% to 93%) | 68% (51% to 86%) | 50% (0% to 100%) | 18% (0% to 36%) | Ptrend <.001 | ||||||
| 5-year OS (95% CI) | 95% (87% to 100%) | 90% (76% to 100%) | 70% (47% to 94%) | 73% (46% to 99%) | 69% (51% to 87%) | 100% † | 46% (22% to 70%) | Ptrend <.001 | ||||||
| . | AJCC TNM stage . | . | . | . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 0 . | I . | IIA . | IIB . | IIIA . | IIIB . | IIIC . | . | ||||||
| No. of patients (%) | 22 (17) | 20 (15) | 25 (19) | 13 (10) | 31 (23) | 3 (2) | 18 (14) | |||||||
| No. patients with distant relapses (%) | 3 (14) | 3 (15) | 6 (24) | 4 (31) | 11 (35) | 1 (33) | 14 (78) | |||||||
| 5-year DDFS (95% CI) | 95% (87% to 100%) | 84% (67% to 100%) | 75% (55% to 96%) | 65% (37% to 93%) | 68% (51% to 86%) | 50% (0% to 100%) | 18% (0% to 36%) | Ptrend <.001 | ||||||
| 5-year OS (95% CI) | 95% (87% to 100%) | 90% (76% to 100%) | 70% (47% to 94%) | 73% (46% to 99%) | 69% (51% to 87%) | 100% † | 46% (22% to 70%) | Ptrend <.001 | ||||||
AJCC TNM = American Joint Committee on Cancer tumor–node–metastasis; CI = confidence interval.
95% CI not calculable because only one patient in this category was still at risk at 5 years.
Other Response Classification Systems and Survival
We found that the revised 2003 AJCC TNM system provided better prognostic discrimination for both distant disease–free survival ( P <.001) and overall survival ( P <.001) than the 1988 system it replaced. Table 4 lists several commonly used tumor response classification systems. We applied each system to our data set to evaluate their usefulness for detecting associations between response to therapy and survival. The National Surgical Adjuvant Breast and Bowel Project (NSABP) system ( 1 ) , which was developed to classify a large group of operable breast cancers, evaluates response in only the breast tumor. This classification system has four categories: pathologic complete response; clinical complete response with pathologic residual tumor; clinical partial response; and clinical nonresponse. The NSABP pathologic complete response category differs from the stage 0 category of the 2003 revised AJCC TNM in that the former but not the latter includes patients who have residual nodal disease. Patients whose residual tumors were classified as pathologic complete response with the NSABP system had 5-year distant disease–free and overall survival rates of 95% (95% CI = 72% to 99%) and 95% (95% CI =72% to 99%), respectively, rates that were identical to the rates we observed using the revised 2003 AJCC TNM system. However, the other NSABP categories and the NSABP classification method overall did not provide prognostic discrimination when applied to our patient group for either distant disease–free survival ( P = .33) or overall survival ( P = .57).
Comparison of different response classification systems in predicting outcome in the data set used in this study *
| Method (reference) . | Stage category . | No. of patients (%) . | 5-year DDFS (95% CI) . | 5-year OS (95% CI) . |
|---|---|---|---|---|
| 2003 AJCC ( 4 ) | 0 | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| I | 20 (15) | 84% (58% to 95%) | 90% (65% to 97%) | |
| II | 38 (29) | 72% (52% to 85%) | 71% (49% to 85%) | |
| III | 52 (39) | 47% (32% to 61%) | 61% (45% to 74%) | |
| Ptrend <.001 | Ptrend =.006 | |||
| NSABP ( 1 ) | Pathologic complete response: no invasive tumor in the breast | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Pathologic microscopic invasive tumor, no clinical tumor | 19 (15) | 51% (27% to 71%) | 52% (26% to 73%) | |
| Clinical partial response | 14 (11) | 34% (10% to 61%) | 47% (16% to 73%) | |
| No clinical response | 72 (57) | 70% (56% to 80%) | 80% (67% to 88%) | |
| Ptrend = .33 | Ptrend = .57 | |||
| Milan Cancer Institute ( 6 ) | Pathologic complete response: no invasive tumor in the breast | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Good partial response: >50% reduction in bidimensional tumor size | 91 (72) | 66% (54% to 75%) | 73% (61% to 82%) | |
| Minor/nonresponse: <50% reduction in bidimensional tumor size | 14 (11) | 34% (10% to 61%) | 47% (16% to 73%) | |
| Ptrend = .003 | Ptrend = .02 | |||
| M.D. Anderson ( 3 ) | Pathologic complete response: no invasive tumor in breast or axilla | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Less than pathologic complete response | 105 (83) | 62% (50% to 71%) | 70% (58% to 78%) | |
| P = .04 | P = .10 | |||
| UNC Surgical System 2002 † ( 2 ) | Candidate for breast conservation | 58 (52) | 88% (76% to 95%) | 94% (83% to 98%) |
| Not a candidate for breast conservation | 53 (48) | 53% (35% to 68%) | 52% (34% to 68%) | |
| P <.001 | P <.001 |
| Method (reference) . | Stage category . | No. of patients (%) . | 5-year DDFS (95% CI) . | 5-year OS (95% CI) . |
|---|---|---|---|---|
| 2003 AJCC ( 4 ) | 0 | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| I | 20 (15) | 84% (58% to 95%) | 90% (65% to 97%) | |
| II | 38 (29) | 72% (52% to 85%) | 71% (49% to 85%) | |
| III | 52 (39) | 47% (32% to 61%) | 61% (45% to 74%) | |
| Ptrend <.001 | Ptrend =.006 | |||
| NSABP ( 1 ) | Pathologic complete response: no invasive tumor in the breast | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Pathologic microscopic invasive tumor, no clinical tumor | 19 (15) | 51% (27% to 71%) | 52% (26% to 73%) | |
| Clinical partial response | 14 (11) | 34% (10% to 61%) | 47% (16% to 73%) | |
| No clinical response | 72 (57) | 70% (56% to 80%) | 80% (67% to 88%) | |
| Ptrend = .33 | Ptrend = .57 | |||
| Milan Cancer Institute ( 6 ) | Pathologic complete response: no invasive tumor in the breast | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Good partial response: >50% reduction in bidimensional tumor size | 91 (72) | 66% (54% to 75%) | 73% (61% to 82%) | |
| Minor/nonresponse: <50% reduction in bidimensional tumor size | 14 (11) | 34% (10% to 61%) | 47% (16% to 73%) | |
| Ptrend = .003 | Ptrend = .02 | |||
| M.D. Anderson ( 3 ) | Pathologic complete response: no invasive tumor in breast or axilla | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Less than pathologic complete response | 105 (83) | 62% (50% to 71%) | 70% (58% to 78%) | |
| P = .04 | P = .10 | |||
| UNC Surgical System 2002 † ( 2 ) | Candidate for breast conservation | 58 (52) | 88% (76% to 95%) | 94% (83% to 98%) |
| Not a candidate for breast conservation | 53 (48) | 53% (35% to 68%) | 52% (34% to 68%) | |
| P <.001 | P <.001 |
The total number of patients in each category may not total 132 because serial clinical measurements were not recorded for five patients and because the UNC system excluded inflammatory breast cancer. All P values are two-sided (order-restricted version of the log-rank test; i.e., trend test). DDFS = distant disease–free survival; OS = overall survival; CI = confidence interval; AJCC TNM = American Joint Committee on Cancer tumor–node–metastasis; NSABP = National Surgical Adjuvant Breast and Bowel Project; UNC = University of North Carolina.
Excludes patients with inflammatory breast cancer.
Comparison of different response classification systems in predicting outcome in the data set used in this study *
| Method (reference) . | Stage category . | No. of patients (%) . | 5-year DDFS (95% CI) . | 5-year OS (95% CI) . |
|---|---|---|---|---|
| 2003 AJCC ( 4 ) | 0 | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| I | 20 (15) | 84% (58% to 95%) | 90% (65% to 97%) | |
| II | 38 (29) | 72% (52% to 85%) | 71% (49% to 85%) | |
| III | 52 (39) | 47% (32% to 61%) | 61% (45% to 74%) | |
| Ptrend <.001 | Ptrend =.006 | |||
| NSABP ( 1 ) | Pathologic complete response: no invasive tumor in the breast | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Pathologic microscopic invasive tumor, no clinical tumor | 19 (15) | 51% (27% to 71%) | 52% (26% to 73%) | |
| Clinical partial response | 14 (11) | 34% (10% to 61%) | 47% (16% to 73%) | |
| No clinical response | 72 (57) | 70% (56% to 80%) | 80% (67% to 88%) | |
| Ptrend = .33 | Ptrend = .57 | |||
| Milan Cancer Institute ( 6 ) | Pathologic complete response: no invasive tumor in the breast | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Good partial response: >50% reduction in bidimensional tumor size | 91 (72) | 66% (54% to 75%) | 73% (61% to 82%) | |
| Minor/nonresponse: <50% reduction in bidimensional tumor size | 14 (11) | 34% (10% to 61%) | 47% (16% to 73%) | |
| Ptrend = .003 | Ptrend = .02 | |||
| M.D. Anderson ( 3 ) | Pathologic complete response: no invasive tumor in breast or axilla | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Less than pathologic complete response | 105 (83) | 62% (50% to 71%) | 70% (58% to 78%) | |
| P = .04 | P = .10 | |||
| UNC Surgical System 2002 † ( 2 ) | Candidate for breast conservation | 58 (52) | 88% (76% to 95%) | 94% (83% to 98%) |
| Not a candidate for breast conservation | 53 (48) | 53% (35% to 68%) | 52% (34% to 68%) | |
| P <.001 | P <.001 |
| Method (reference) . | Stage category . | No. of patients (%) . | 5-year DDFS (95% CI) . | 5-year OS (95% CI) . |
|---|---|---|---|---|
| 2003 AJCC ( 4 ) | 0 | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| I | 20 (15) | 84% (58% to 95%) | 90% (65% to 97%) | |
| II | 38 (29) | 72% (52% to 85%) | 71% (49% to 85%) | |
| III | 52 (39) | 47% (32% to 61%) | 61% (45% to 74%) | |
| Ptrend <.001 | Ptrend =.006 | |||
| NSABP ( 1 ) | Pathologic complete response: no invasive tumor in the breast | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Pathologic microscopic invasive tumor, no clinical tumor | 19 (15) | 51% (27% to 71%) | 52% (26% to 73%) | |
| Clinical partial response | 14 (11) | 34% (10% to 61%) | 47% (16% to 73%) | |
| No clinical response | 72 (57) | 70% (56% to 80%) | 80% (67% to 88%) | |
| Ptrend = .33 | Ptrend = .57 | |||
| Milan Cancer Institute ( 6 ) | Pathologic complete response: no invasive tumor in the breast | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Good partial response: >50% reduction in bidimensional tumor size | 91 (72) | 66% (54% to 75%) | 73% (61% to 82%) | |
| Minor/nonresponse: <50% reduction in bidimensional tumor size | 14 (11) | 34% (10% to 61%) | 47% (16% to 73%) | |
| Ptrend = .003 | Ptrend = .02 | |||
| M.D. Anderson ( 3 ) | Pathologic complete response: no invasive tumor in breast or axilla | 22 (17) | 95% (72% to 99%) | 95% (72% to 99%) |
| Less than pathologic complete response | 105 (83) | 62% (50% to 71%) | 70% (58% to 78%) | |
| P = .04 | P = .10 | |||
| UNC Surgical System 2002 † ( 2 ) | Candidate for breast conservation | 58 (52) | 88% (76% to 95%) | 94% (83% to 98%) |
| Not a candidate for breast conservation | 53 (48) | 53% (35% to 68%) | 52% (34% to 68%) | |
| P <.001 | P <.001 |
The total number of patients in each category may not total 132 because serial clinical measurements were not recorded for five patients and because the UNC system excluded inflammatory breast cancer. All P values are two-sided (order-restricted version of the log-rank test; i.e., trend test). DDFS = distant disease–free survival; OS = overall survival; CI = confidence interval; AJCC TNM = American Joint Committee on Cancer tumor–node–metastasis; NSABP = National Surgical Adjuvant Breast and Bowel Project; UNC = University of North Carolina.
Excludes patients with inflammatory breast cancer.
The Milan Cancer Institute classification system described by Bonadonna et al. ( 6 ) also evaluates response in the breast tumor only, but it classifies response according to three categories: pathologic complete response; good partial response (i.e., greater than 50% reduction in bidimensional tumor size); and minor/non-response (i.e., no more than 50% reduction in bidimensional tumor size). We found that this method provided prognostic discrimination for both distant disease–free survival ( Ptrend = .003) and overall survival ( Ptrend = .02).
The M.D. Anderson classification system ( 3 ) discriminates between no invasive tumor in breast or axillary lymph nodes (i.e., pathologic complete response) and any residual disease in breast or axillary lymph nodes. Compared with patients who had any residual disease, those who were classified as having a pathologic complete response had statistically significantly better distant disease–free survival (95% versus 62%; P = .04) but not overall survival (95% versus 70%; P = .10).
The 2002 University of North Carolina surgical classification system ( 2 ) classifies response according to whether the patient was a candidate for breast conservation surgery; this system is not used to classify patients with inflammatory breast cancer because they are considered to be inappropriate candidates for breast conservation surgery regardless of their response to treatment. Compared with patients who were not considered candidates for breast conservation surgery, patients who were considered candidates for breast conservation surgery had statistically significantly better 5-year distant disease–free survival (88% versus 53%; P <.001) and 5-year overall survival (94% versus 52%; P <.001). Table 5 demonstrates how patients in each of the AJCC categories would be classified according to these other four systems.
Cross-classification of other response classification systems with the revised AJCC yTNM stages *
| . | . | . | Revised 2003 AJCC stage . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Method (reference) . | Stage category . | n . | 0 . | I . | II . | III . | |||
| NSABP ( 1 ) | Pathologic complete response: no invasive tumor in the breast | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Pathologic microscopic invasive tumor, no clinical tumor | 19 | 0 | 3 (16%) | 10 (53%) | 6 (31%) | ||||
| Clinical partial response | 14 | 0 | 1 (7%) | 2 (14%) | 11 (79%) | ||||
| No clinical response | 72 | 0 | 15 (20%) | 26 (36%) | 31 (43%) | ||||
| Milan Cancer Institute ( 6 ) | Pathologic complete response: no invasive tumor in the breast | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Good partial response: >50% reduction bidimensional tumor size | 91 | 0 | 18 (20%) | 36 (40%) | 37 (40%) | ||||
| Minor/nonresponse: ≤50% reduction bidimensional tumor size | 14 | 0 | 1 (7%) | 2 (14%) | 11 (79%) | ||||
| M.D. Anderson ( 3 ) | Pathologic complete response: no invasive tumor breast or axilla | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Less than pathologic complete response | 105 | 0 | 19 (18%) | 38 (36%) | 48 (47%) | ||||
| UNC Surgical System 2002 † ( 2 ) | Candidate for breast conservation | 58 | 13 (22%) | 14 (24%) | 20 (34%) | 11 (19%) | |||
| Not a candidate for breast conservation | 53 | 8 (15%) | 3 (6%) | 15 (28%) | 27 (51%) | ||||
| . | . | . | Revised 2003 AJCC stage . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Method (reference) . | Stage category . | n . | 0 . | I . | II . | III . | |||
| NSABP ( 1 ) | Pathologic complete response: no invasive tumor in the breast | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Pathologic microscopic invasive tumor, no clinical tumor | 19 | 0 | 3 (16%) | 10 (53%) | 6 (31%) | ||||
| Clinical partial response | 14 | 0 | 1 (7%) | 2 (14%) | 11 (79%) | ||||
| No clinical response | 72 | 0 | 15 (20%) | 26 (36%) | 31 (43%) | ||||
| Milan Cancer Institute ( 6 ) | Pathologic complete response: no invasive tumor in the breast | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Good partial response: >50% reduction bidimensional tumor size | 91 | 0 | 18 (20%) | 36 (40%) | 37 (40%) | ||||
| Minor/nonresponse: ≤50% reduction bidimensional tumor size | 14 | 0 | 1 (7%) | 2 (14%) | 11 (79%) | ||||
| M.D. Anderson ( 3 ) | Pathologic complete response: no invasive tumor breast or axilla | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Less than pathologic complete response | 105 | 0 | 19 (18%) | 38 (36%) | 48 (47%) | ||||
| UNC Surgical System 2002 † ( 2 ) | Candidate for breast conservation | 58 | 13 (22%) | 14 (24%) | 20 (34%) | 11 (19%) | |||
| Not a candidate for breast conservation | 53 | 8 (15%) | 3 (6%) | 15 (28%) | 27 (51%) | ||||
AJCC TNM = American Joint Committee on Cancer tumor–node–metastasis; NSABP = National Surgical Adjuvant Breast and Bowel Project; UNC = University of North Carolina. Denominators represented total patients in that response classification system category.
Excludes patients with inflammatory breast cancer.
Cross-classification of other response classification systems with the revised AJCC yTNM stages *
| . | . | . | Revised 2003 AJCC stage . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Method (reference) . | Stage category . | n . | 0 . | I . | II . | III . | |||
| NSABP ( 1 ) | Pathologic complete response: no invasive tumor in the breast | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Pathologic microscopic invasive tumor, no clinical tumor | 19 | 0 | 3 (16%) | 10 (53%) | 6 (31%) | ||||
| Clinical partial response | 14 | 0 | 1 (7%) | 2 (14%) | 11 (79%) | ||||
| No clinical response | 72 | 0 | 15 (20%) | 26 (36%) | 31 (43%) | ||||
| Milan Cancer Institute ( 6 ) | Pathologic complete response: no invasive tumor in the breast | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Good partial response: >50% reduction bidimensional tumor size | 91 | 0 | 18 (20%) | 36 (40%) | 37 (40%) | ||||
| Minor/nonresponse: ≤50% reduction bidimensional tumor size | 14 | 0 | 1 (7%) | 2 (14%) | 11 (79%) | ||||
| M.D. Anderson ( 3 ) | Pathologic complete response: no invasive tumor breast or axilla | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Less than pathologic complete response | 105 | 0 | 19 (18%) | 38 (36%) | 48 (47%) | ||||
| UNC Surgical System 2002 † ( 2 ) | Candidate for breast conservation | 58 | 13 (22%) | 14 (24%) | 20 (34%) | 11 (19%) | |||
| Not a candidate for breast conservation | 53 | 8 (15%) | 3 (6%) | 15 (28%) | 27 (51%) | ||||
| . | . | . | Revised 2003 AJCC stage . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Method (reference) . | Stage category . | n . | 0 . | I . | II . | III . | |||
| NSABP ( 1 ) | Pathologic complete response: no invasive tumor in the breast | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Pathologic microscopic invasive tumor, no clinical tumor | 19 | 0 | 3 (16%) | 10 (53%) | 6 (31%) | ||||
| Clinical partial response | 14 | 0 | 1 (7%) | 2 (14%) | 11 (79%) | ||||
| No clinical response | 72 | 0 | 15 (20%) | 26 (36%) | 31 (43%) | ||||
| Milan Cancer Institute ( 6 ) | Pathologic complete response: no invasive tumor in the breast | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Good partial response: >50% reduction bidimensional tumor size | 91 | 0 | 18 (20%) | 36 (40%) | 37 (40%) | ||||
| Minor/nonresponse: ≤50% reduction bidimensional tumor size | 14 | 0 | 1 (7%) | 2 (14%) | 11 (79%) | ||||
| M.D. Anderson ( 3 ) | Pathologic complete response: no invasive tumor breast or axilla | 22 | 22 (100%) | 0 | 0 | 0 | |||
| Less than pathologic complete response | 105 | 0 | 19 (18%) | 38 (36%) | 48 (47%) | ||||
| UNC Surgical System 2002 † ( 2 ) | Candidate for breast conservation | 58 | 13 (22%) | 14 (24%) | 20 (34%) | 11 (19%) | |||
| Not a candidate for breast conservation | 53 | 8 (15%) | 3 (6%) | 15 (28%) | 27 (51%) | ||||
AJCC TNM = American Joint Committee on Cancer tumor–node–metastasis; NSABP = National Surgical Adjuvant Breast and Bowel Project; UNC = University of North Carolina. Denominators represented total patients in that response classification system category.
Excludes patients with inflammatory breast cancer.
D ISCUSSION
Although overall survival must remain the definitive endpoint for breast cancer trials, multiple studies have demonstrated that the degree of tumor response and the extent of residual disease after neoadjuvant chemotherapy are associated with relapse and survival ( 1 – 3 ) . Because of its value as a surrogate for survival, response to neoadjuvant chemotherapy is used as a primary endpoint in studies that examine the efficacy of new drugs and novel drug combinations. However, there is no standard classification system for reporting the response to neoadjuvant chemotherapy. Moreover, many of the response classification systems currently in use employ different combinations of pathologic results and clinical response assessment. Because of this lack of a standard, accurate, and reliable response classification system, most studies that have reported results of neoadjuvant regimens have used pathologic complete response as a surrogate for survival. However, pathologic complete response is a relatively uncommon phenomenon; for example, only 17% of the patients in our study had a pathologic complete response.
In this study, we examined the usefulness of the 2003 revised AJCC staging criteria for predicting distant relapse and survival. The revised AJCC system includes a designation (yTNM) for staging after a patient receives neoadjuvant chemotherapy. We found that the revised 2003 AJCC TNM staging system was an effective and easy way to measure the extent of pathologic residual disease in both the breast and the axillary lymph nodes after neoadjuvant chemotherapy. This staging method is simple to use because it requires only the pathology report. Furthermore, this method categorizes patients into more than two groups, allowing determination of prognostic groups with excellent, intermediate, and extremely poor outcomes. The revised 2003 AJCC TNM system predicts distant disease–free survival and overall survival better than does the 1988 version. The fact that approximately half of the patients in our study received further chemotherapy following surgery did not appear to substantially diminish the association we observed between pathologic complete response after neoadjuvant chemotherapy and outcome. This finding may reflect the small number of patients who received adjuvant as well as neoadjuvant chemotherapy or the fact that, even among the subset that received both neoadjuvant and adjuvant chemotherapy, the most effective agents (anthracyclines and taxanes) were administered in the neoadjuvant setting. The use of adjuvant tamoxifen was also not related to outcome. It is possible that this lack of association simply reflects the heterogeneous population of hormone receptor–negative and –positive tumors; it is also possible that 5 years of follow up suffices to measure the impact of chemotherapy but not that of endocrine therapy.
We also applied several other response classification methods to our data set. Only two methods—the method used at the Milan Cancer Institute ( 6 ) and the method used in our previous work ( 2 ) —provided statistically significant prognostic ability for both distant disease–free survival and overall survival. The method used at the Milan Cancer Institute incorporates pathologic results and a clinical assessment of response and, thus, is more complex and prone to interobserver variability than is the AJCC yTNM system. The method used at M.D. Anderson ( 3 ) , which distinguishes no residual tumor in breast or axilla from any residual tumor in either, provided statistically significant prognostic ability for distant disease–free survival but not for overall survival in this small data set. The method used previously at the University of North Carolina was easily performed and provided statistically significant prognostic ability for both distant disease–free and overall survival; however, it is limited in its usefulness because it cannot be applied to inflammatory breast cancers and provides only two prognostic categories. Although the pathologic complete response category was prognostic, the trend test for the method used by the NSABP ( 1 ) was not statistically significant for predicting either distant disease–free survival or overall survival, because there was poor prognostic ability among the three residual disease categories. For all of these methods, pathologic complete response was prognostic but prognostic ability was limited because of the small number of patients who achieved this endpoint.
The amount of residual disease in surgical specimens from patients who have received neoadjuvant chemotherapy is an excellent prognostic factor (5,7,8) and is often used as an intermediate endpoint in assessing the potential of new treatment regimens for nonmetastatic breast cancer. The identification of methods that are easily performed and provide prognostic discrimination will improve these efforts. Other theoretical directions for neoadjuvant therapy should also be considered. For example, the direct linkage of the extent of residual disease to the risk of subsequent relapse implies that achieving maximum tumor kill in the breast—either by defining the number of cycles of chemotherapy according to response or by defining the regimen and the use of non–cross-resistant regimens when response is insufficient—is a worthwhile and achievable goal. This latter approach has already been suggested by the reports from the NSABP B-27 ( 7 ) and Aberdeen ( 8 ) trials, in which patients who were randomly assigned to receive a non–cross-resistant regimen (anthracycline followed by taxane) had markedly better pathologic complete response than did patients who were randomly assigned to receive anthracycline alone. In the Aberdeen study, the improved pathologic complete response rate was associated with improved relapse–free and overall survival ( 8 ) . The pathologic extent of disease after neoadjuvant chemotherapy likely reflects the effect of the drugs administered as well as the extent of disease at diagnosis; response classification systems as intermediate endpoints for distant relapse and survival do not distinguish between the effects of these different elements.
The patients in our study who had residual stage IIIC tumors after conventional chemotherapy had a greater than 80% risk of disease recurrence or death and may therefore be candidates for novel therapeutic approaches. Although treatment to achieve minimal or no tumor burden may be an interesting approach for future neoadjuvant studies, such an approach is limited because pathologic staging systems are needed to define residual disease. Therapeutic exploitation of this approach will require improved clinical and radiographic methods to identify residual disease. Such noninvasive approaches may benefit from our finding that TNM staging of the breast and axilla after treatment is an easy and accurate response classification system.
This study is limited by several factors. The data set is small and derives from a heterogeneously treated and high-risk population treated with aggressive multimodality therapy. For this reason, our results may not be generalizable to a lower-risk population, particularly a node-negative population. The size and heterogeneity of both the patient population and treatment also make it difficult to evaluate the impact of additional adjuvant chemotherapy or tamoxifen.
Multiple studies have documented that response to neoadjuvant chemotherapy for breast cancer is an intermediate endpoint for relapse and survival. For this reason, regimens for early-stage breast cancer are increasingly being tested in neoadjuvant chemotherapy trials. The lack of an accurate, simple, and highly discriminating method to assess the intermediate endpoint of response has limited the data that can be derived from these studies. The AJCC system that we examined is simple and reproducible and, in our cohort, accurately predicted both good and poor distant disease–free survival and overall survival.
Supported by a UNC Breast Cancer SPORE award from the National Cancer Institute (CA58223), the Breast Cancer Research Foundation, and the National Institutes of Health (NIH) (M01RR00046). The authors thank Matthew G. Ewend, MD, for his critical review and Ms. Shanah Kirk for her assistance with the production of this manuscript. This work was presented in part at the 39th Annual Meeting of the American Society of Clinical Oncology, May 2003.
References
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer.
Cance WG, Carey LA, Calvo BF, Sartor C, Sawyer L, Moore DT, et al. Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical downstaging allows breast preservation and predicts outstanding local control and survival.
Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy.
Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6th ed. New York (NY):Springer;
Carey LA, Dees EC, Sawyer L, Moore DT, Dressler L, Cowan D, et al. Response to trastuzumab (Herceptin) given with paclitaxel (Taxol) immediately following 4AC as initial therapy for primary breast cancer.
Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Terenziani M, et al. Primary chemotherapy in operable breast cancer: eight-year experience at the Milan Cancer Institute.
Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27.