-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Concetta Fargnoli, Tania Spica, Francesco Sera, Giovanni Pellacani, Alessandra Chiarugi, Stefania Seidenari, Paolo Carli, Sergio Chimenti, Ketty Peris, Re: MC1R, ASIP, and DNA Repair in Sporadic and Familial Melanoma in a Mediterranean Population, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 2, 18 January 2006, Pages 144–145, https://doi.org/10.1093/jnci/djj025
Close - Share Icon Share
We read with great interest the article by Landi et al. ( 1 ) reporting the association of melanocortin-1 receptor gene (MC1R) variants with melanoma risk and progression in sporadic and familial melanoma patients from northeastern Italy. They observed a two- to fourfold increase in risk of both sporadic and familial melanoma among individuals carrying MC1R variant alleles compared with those carrying wild-type MC1R.
We investigated whether MC1R variants were associated with the risk of sporadic cutaneous melanoma in a population from central and northeastern Italy. We recruited 165 sporadic melanoma patients at the Departments of Dermatology of the Universities of L'Aquila, Modena, and Florence, Italy. For each case patient, one control subject was matched by sex, age (within ± 1 year), and residential area (administrative province). The control subjects were recruited from patients who were treated for diseases unrelated to melanoma by the Surgery and Internal Medicine Departments of the same University Hospitals. Both groups included 82 men and 83 women with a median age of 49 years (range: 17–82 years). The study was approved by the local ethical committees, and written informed consent was obtained from all participants. Information on family and medical history, phenotypic risk factors for melanoma (skin type, hair and eye color, number of melanocytic nevi, and the presence of clinically atypical nevi), and UV exposure habits were collected through a standardized questionnaire and skin examination.
Genomic DNA was isolated from peripheral blood using the QIAamp Blood Kit (QIAgen GmbH, Hilden, Germany). All participants were genotyped for six single nucleotide polymorphisms (SNPs) of the MC1R gene (“red hair color” [RHC] variants: R151C, R160W and D294H; and “non–red hair color” [NRHC] variants: V60L, V92M, and R142H) ( 2 ) frequently detected in our population ( 3 , 4 ) , using the TaqMan SNP Genotyping Assays allelic discrimination method (Applied Biosystems, Foster City, CA, USA). To validate the SNP assay genotypes, 20 samples were randomly selected for sequencing. Results were consistent with genotypic data. Unadjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated from univariate conditional logistic regression models. All statistical tests were two-sided.
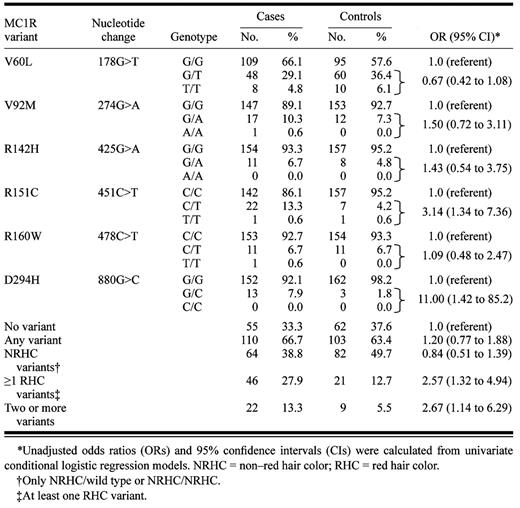
Genotype frequencies for the six MC1R variants analyzed are shown in Table 1 . Consistent with Landi et al.'s results, the V60L variant was the most common variant in our control population with an allele prevalence of 24.0%. Similarly, carriers of one or more RHC variants had a higher melanoma risk (OR = 2.57; 95% CI = 1.32 to 4.94) compared with carriers of the wild-type sequence. Interestingly, separate analysis of individual MC1R variants showed that the increase in melanoma risk was restricted to the R151C (OR = 3.14; 95% CI = 1.34 to 7.36) and D294H (OR = 11.00; 95% CI = 1.42 to 85.2) alleles.
Association between melanocortin-1 receptor (MC1R) variants and melanoma risk in an Italian population
 |
 |
Association between melanocortin-1 receptor (MC1R) variants and melanoma risk in an Italian population
 |
 |
Using multiple correspondence analysis we obtained optimal scale values for the following categorical variables: hair color, eye color, skin type, number of nevi, presence of clinically atypical nevi, and presence of actinic damage. Evaluation of the eigenvalues suggested the presence of two “latent factors”: the first was characterized by the grouping of the categories defining a light pigmentation phenotype (i.e., blond/red hair, blue eyes, skin type I–II); and the second was defined by a high nevus density phenotype (presence of clinically atypical nevi, >50 nevi). Given the optimal scores for each of the categorical variables, we calculated a rating for each subject on the two new variables, “pigmentation factor” and “nevi factor.” In our control population, the subjects with RHC variants had a higher pigmentation score (Mann–Whitney test, P = .009), with higher prevalence observed for skin type I–II (chi-square, P = .044) and light brown eye color (chi-square, P = .012). A higher prevalence was observed also for control subjects with a higher nevus score (chi-square, P = .040). An association with light pigmentation was observed for individuals with one or more NRHC variant alleles (Mann–Whitney test, P = .031).
Association between MC1R and melanoma thickness was also investigated in our group of melanoma patients. The odds ratios of having a melanoma ≥1 mm were 2.38 (95% CI = 1.01 to 5.60) for carriers of any MC1R variant, 2.80 (95% CI = 1.01 to 7.72) for carriers of one or more RHC variants, and 4.79 (95% CI = 1.43 to 16.03) for carriers of two or more variants compared with melanoma patients homozygous for the wild-type sequence. No multiplicative interaction between genotypes and nevi- or pigmentation-related variables was detected by the likelihood ratio test.
In conclusion, our case–control study supports results recently published by the Journal ( 1 ) . Our data suggest that individuals with MC1R variants are at increased melanoma risk and progression in a Mediterranean population from central and northeastern Italy.
This work was supported by grant 2001068929_004) from the Italian Ministry of University and Scientific Research.
References
Landi MT, Kanetsky PA, Tsang S, Gold B, Munroe D, Rebbeck T, et al. MC1R, ASIP, and DNA repair in sporadic and familial melanoma in a Mediterranean population.
Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, et al. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes.
Fargnoli MC, Chimenti S, Keller G, Höfler H. Identification of four novel melanocortin 1 receptor (MC1R) gene variants in a Mediterranean population.


