-
PDF
- Split View
-
Views
-
Cite
Cite
Scott R. Freeman, Amanda L. Drake, Lauren F. Heilig, Marla Graber, Kristie McNealy, Lisa M. Schilling, Robert P. Dellavalle, Statins, Fibrates, and Melanoma Risk: a Systematic Review and Meta-analysis, JNCI: Journal of the National Cancer Institute, Volume 98, Issue 21, 1 November 2006, Pages 1538–1546, https://doi.org/10.1093/jnci/djj412
Close - Share Icon Share
Abstract
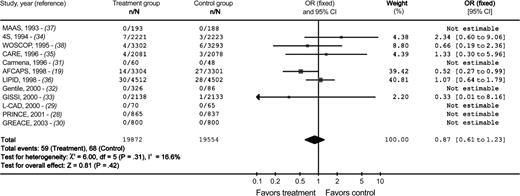
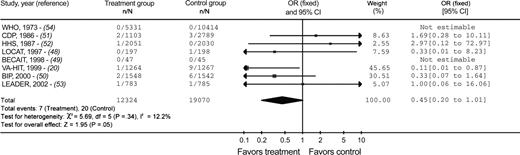
Background: Large randomized, controlled clinical trials of lovastatin and gemfibrozil for heart disease prevention have reported statistically significantly lower melanoma incidences in persons receiving these medications. Results of in vitro animal model and human case–control studies also suggest that statins and fibrates may reduce the risk of melanoma. Methods: We performed a systematic review of trials that randomly assigned participants to receive statins or fibrates versus an alternative therapy for a minimum of 6 months. Trials were identified by searching five electronic databases and the reference lists of eligible publications. Unpublished data were solicited from trial investigators and pharmaceutical companies. A meta-analysis was performed using a fixed-effects model, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to estimate pooled treatment effects. All statistical tests were two-sided. Results: We obtained data on incident melanomas from 20 of 36 qualifying randomized controlled trials (12 statin trials and eight fibrate trials), with a total of 70 820 participants. A total of 127 melanomas occurred among the 39 426 participants in the statin trials (59 among the 19 872 statin group participants and 68 among the 19 554 control group participants). A total of 27 melanomas occurred among the 31 394 participants enrolled in the fibrate trials (seven among the 12 324 fibrate group participants and 20 among the 19 070 control group participants). Overall, incidence of melanoma was not statistically significantly associated with the use of either statins (OR = 0.87, 95% CI = 0.61 to 1.23) or fibrates (OR = 0.45, 95% CI = 0.20 to 1.01). In a subgroup analysis by drug, only lovastatin use (in one trial) was statistically significantly associated with lower incidence of melanoma (OR = 0.52, 95% CI = 0.27 to 0.99). Conclusions: These findings do not validate the possibility that statins or fibrates prevent melanoma.
Many cancer cells, including melanoma cells, use cholesterol differently than noncancerous cells ( 1 – 3 ) . Such observations have fostered hypotheses that drugs altering cholesterol levels may slow or stop tumor growth, enhance the anticancer effects of chemotherapy, or possibly even prevent cancer ( 4 , 5 ) . Statins and fibrates, drugs commonly prescribed to people with hyperlipidemia, display antitumor activity in experimental models of cancer, including models of melanoma ( 6 – 9 ) .
Statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A reductase, the rate-limiting enzyme in the mevalonate synthesis pathway. Inhibition of this pathway by statins has antiproliferative, anti-invasive, and proapoptotic effects on malignant cell lines ( 10 , 11 ) . Statins induce apoptosis in vitro by inhibiting geranylgeranylation of several key intracellular signaling proteins ( 8 , 11 – 13 ) . Other molecular mechanisms proposed to explain the anticancer properties of statins include cell cycle arrest through stabilization of the cyclin-dependent kinases p21 and p27 and inhibition of cell migration due to RhoA inactivation and subsequent actin destabilization ( 14 , 15 ) . The molecular mechanisms underlying the anticancer activities of fibrates are not defined. However, statins and fibrates lower serum cholesterol via different pathways: statins inhibit cholesterol synthesis, whereas fibrates interact with the peroxisome proliferator–activated receptor alpha to alter lipid metabolism through several poorly understood mechanisms.
Multiple lines of evidence suggest that statins possess anticancer properties in humans. Several case–control studies have reported lower rates of cancer in persons taking statins ( 16 , 17 ) . In addition, two large randomized, double-blinded, placebo-controlled, multiyear clinical trials of lovastatin ( 18 ) and gemfibrozil ( 19 ) reported that participants who took either medication had statistically significantly fewer melanomas than those who did not. In these randomized controlled trials, the numbers of all other cancers, including lymphomas and prostate, colon, lung, bladder, and breast cancers, were not statistically significantly different between the two groups of participants.
Despite promising evidence from in vitro studies and animal models about the effectiveness of statins and fibrates for melanoma prevention, these results do not necessarily translate to a similar effect in humans. In addition, the ability of a statin or fibrate to slow the growth or spread of tumor cells may not correlate with a role in cancer prevention. Moreover, many malignant cell lines are not inhibited in vitro by the steady-state concentrations of these drugs that are achieved in patients undergoing hyperlipidemic therapy. For example, the hyperlipidemic treatment dose of lovastatin (approximately 1 mg/kg daily) yields a steady-state serum concentration of 0.15–0.3 μM ( 20 ) , whereas in vitro lovastatin concentrations greater than 1 μM are needed to promote melanoma cell death ( 8 ) . However, the in vitro evidence, together with the lower melanoma rates observed in some clinical trials ( 18 , 19 ) and the inadequacy of the currently available treatment for advanced melanoma, suggests that further evaluation of these drugs for chemoprevention is warranted.
Because lovastatin and gemfibrozil promote the development of liver cancer in rodents ( 21 ) , many human clinical trials of statins and fibrates have incorporated cancer surveillance ( 22 , 23 ) . Thus, we performed a systematic review of melanoma incidence from randomized controlled clinical trials of statins or fibrates to formally evaluate the relation between these drugs and melanoma. This meta-analysis is a briefer version of a Cochrane Review ( 24 ) and differs from that review by including trials with shorter duration (6 months or longer instead of 4 years or longer).
M ETHODS
Search Strategy
Comprehensive search algorithms ( 24 ) were used to search the following electronic databases for potentially relevant trials: MEDLINE (from January 1966 through March 2003), EMBASE (from January 1980 through September 2003), The Cochrane Central Register of Controlled Trials (through March 2003), CancerLit (from January 1975 through October 2002), and the Web of Science—Science Citation Index (from January 1970 through May 2003). In addition, we examined the reference lists of qualifying trial publications for additional relevant trials, and we requested information about unpublished ongoing trials and conference abstracts via correspondence with authors and pharmaceutical companies. No language restrictions were imposed. Non-English manuscripts were examined by readers fluent in the language of the article.
Selection Criteria
Trials were eligible for inclusion in the meta-analysis if 1) they had randomly allocated study participants to the experimental and placebo or non–placebo control group(s), 2) they had an experimental design in which at least one arm had therapy with a statin or therapy with a fibrate as the intervention, and 3) the mean length of trial participation was at least 6 months. The third inclusion criterion was used because the benefits of the intervention may require long-term exposure ( 25 ) . Participants of any eligible trials were included in the meta-analysis; the majority of participants had coronary artery disease and were enrolled in the trials to evaluate cardiovascular outcomes. Eligible interventions included statin or fibrate medications taken orally. Both placebo and non–placebo control arms were allowed for comparison. The primary outcome measure was the incidence of melanoma during trial participation (i.e., the number of people who were diagnosed with a new melanoma during the trial). Secondary outcome measures were 1) the incidence of melanoma with poor prognosis (i.e., those greater than 3 mm thick); 2) the incidence of dysplastic nevi (i.e., moles with atypical architecture or cellular features) confirmed by histologic report; 3) published overall cancer incidence (i.e., tumors of any organ); and 4) mortality due to melanoma as reported by the trial authors.
Data Extraction
Methods, interventions, outcomes, and results were extracted from qualifying trial publications by at least two reviewers who used a standardized data extraction form and discrepancies were resolved by consensus. Authors of the included studies were contacted and asked to provide additional data, including 1) a summary of melanoma outcomes (i.e., the number of person-years on intervention drug, the number of participants who dropped out, and the incidence of melanoma among participants); 2) all available information on the histology of the incident melanomas and ensuing workups, including Breslow's depth, Clark's level, histologic type of melanoma (i.e., lentigo maligna, acral lentiginous, superficial spreading, or nodular), presence or absence of ulceration, sentinel lymph node status, tumor stage, anatomic location of the tumor (not published here to protect participant confidentiality), treatment and disease course, participant vital status, and deaths due to melanoma; 3) participants' exposures to statins and fibrates before their initiation of trial participation, for those with incident melanoma; and 4) any additional information regarding incident melanomas. We offered the study authors a monetary incentive of US $50 for returning an information sheet summarizing melanoma outcomes for their study as well as US $50 for additional information on each unpublished melanoma incidence to offset costs associated with providing these data. Patient consent for participation was governed by the approved human subjects' protection protocol of each qualifying trial. The Colorado Multiple Institutional Review Board approved this study.
Assessment of Methodologic Quality
At least two reviewers independently assessed the methodologic quality of qualifying trials. Articles were rated as A (adequate), B (unclear), or C (inadequate) in each of the following categories: randomization procedure, allocation concealment, intention to treat, blinding of participants, and blinding of outcomes assessors. Discrepancies were resolved by consensus.
Statistical Analysis
Associations between statins and fibrates and the risk of melanoma were analyzed separately. Meta-analyses were performed using a fixed-effects model. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to estimate pooled treatment effects. The results were expressed with respect to the presence or absence of a melanoma diagnosis. Because of the lack of participant-specific data from every trial, it was not possible to adjust for person-years of follow-up in the different study arms, and, thus, melanoma incidence was not adjusted for study dropouts. Melanoma incidence was calculated by using the following formula: the number of incident melanomas in the study arm divided by the number of persons in the study arm times the mean duration of the trial in years. Statistical heterogeneity among studies was measured by calculating the heterogeneity statistic I2 , which is the proportion of total variation contributed by between-study variation ( 26 ) . When statistical heterogeneity was observed (i.e., when I2 was greater than 50%), sensitivity analyses were used to examine the effects of excluding the following subgroups of studies: those with fewer than 500 participants, those with pharmaceutical industry funding, and those with low methodologic quality (defined as any study receiving the lowest grade possible in any measured category). All statistical tests were two-sided, and the cutoff for statistical significance was set at P ≤.05. Heterogeneity of studies was calculated using the Cochrane Q test, with predefined statistical significance level set at 0.10. Weights presented ( Tables 1 and 2 ) represent individual estimates of treatment effect (weighted averages) weighted by assessment of precision of the estimates. A more complete description of the statistical methodology used in this meta-analysis can be found in the Cochrane Handbook ( 27 ) .
Characteristics of included studies *
| Trial acronym or author, year of publication (reference) . | Study arm, dosage (No. of randomly assigned participants) . | . | . | . | % Total dropout † , S/F : O . | No. of incident melanomas ‡ , S/F : O . | Melanoma rate § , S/F : O . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | S . | F . | O . | Trial length . | . | . | . | ||
| 4S, 1994 ( 34 ) | Simvastatin, 10–40 mg qd (2221) | – | Placebo (2223) | 5.4 y ‖ | 5.0 : 7.0 | 7 : 3 | 0.58 : 0.25 | ||
| AFCAPS, 1998 ( 18 ) | Lovastatin, 20–40 mg qd (3304) | – | Placebo (3301) | 5.2 y ¶ | 16 : 23 | 14 : 27 | 0.81 : 1.57 | ||
| BECAIT, 1998 ( 49 ) | – | Bezafibrate, 200 mg tid ( 47 ) | Placebo ( 45 ) | 5.0 y # | 11 : 13 | 0 : 0 | 0 : 0 | ||
| BIP, 2000 ( 50 ) | Bezafibrate, 400 mg qd (1548) | Placebo (1542) | 6.2 y ¶ | 31 : 33 | 2 : 6 | 0.21 : 0.63 | |||
| CARE, 1996 ( 35 ) | Pravastatin, 40 mg qd (2081) | – | Placebo (2078) | 5.0 y ‖ | 15 : 28 | 4 : 3 | 0.38 : 0.29 | ||
| Carmena, 1996 ( 31 ) | Pravastatin, 40 mg qd ( 57 ) | – | Cholestyramine, 16 mg qd ( 57 ) | 1.0 y # | 7.0 : 16 | 0 : 0 | 0 : 0 | ||
| CDP, 1986 ( 51 ) | – | Clofibrate, 1.8 g qd (1103) | Placebo ** (2789) | 6.2 y ¶ | 6.0 : 6.0 | 2 : 3 | 0.29 : 0.17 | ||
| Gentile, 2000 ( 32 ) | Atorvastatin, 10 mg qd (85) | – | Placebo (86) | 24 wk # | 1.2 : 0 | 0 : 0 | 0 : 0 | ||
| Simvastatin, 10 mg qd (78) | 0 : 0 | 0 : 0 | 0 : 0 | ||||||
| Pravastatin, 20 mg qd (82) | 1.2 : 0 | 0 : 0 | 0 : 0 | ||||||
| Lovastatin, 20 mg qd (81) | 1.2 : 0 | 0 : 0 | 0 : 0 | ||||||
| GISSI, 2000 ( 33 ) | Pravastatin, 20–40 mg qd (2138) | – | No treatment (2133) | 1.9 y ¶ , 2.0 y ‖ | 19 : 14 | 0 : 1 | 0 : 0.25 | ||
| GREACE, 2002 ( 30 ) | Atorvastatin, 10–80 mg qd (800) | – | Usual care †† (800) | 3.0 y ¶ | 1.3 : 0 | 0 : 0 | 0 : 0 | ||
| HHS, 1987 ( 52 ) | Gemfibrozil, 600 mg tid (2051) | – | Placebo (2030) | 5.3 y ¶ | 18 : 18 | 1 : 0 | 0.1 : 0 | ||
| LEADER, 2002 ( 53 ) | – | Bezafibrate, 400 mg qd or qod if creatine level of 135–149 mmol/L (783) | Placebo (785) | 4.6 y ‖ | 49 : 52 | 1 : 1 | 0.28 : 0.28 | ||
| L-CAD, 2000 ( 29 ) | Pravastatin, 20–40 mg qd (70) | – | Usual care ‡‡ ( 65 ) | 2.0 # | 0 : 14 | 0 : 0 | 0 : 0 | ||
| LIPID, 1998 ( 36 ) | Pravastatin, 40mg qd (4512) | – | Placebo (4502) | 6.1y ¶ | 19 : 19 | 30 : 28 | 1.09 : 1.02 | ||
| LOCAT, 1997 ( 48 ) | – | Gemfibrozil, 1200 mg qd (197) | Placebo (198) | 2.5 y ¶ | 6.0 : 6.1 | 0 : 1 | 0 : 2 | ||
| MAAS, 1993 ( 37 ) | Simvastatin, 20 mg qd (193) | – | Placebo (188) | 4.0 y # | 11 : 5.0 | 0 : 0 | 0 : 0 | ||
| PRINCE, 2001 ( 28 ) | Pravastatin, 40 mg qd (1014) | – | Placebo (999) | 24 wk # | 34 : 33 | 0 : 0 | 0 : 0 | ||
| VA-HIT, 1999 ( 19 ) | – | Gemfibrozil, 1200 mg qd (1264) | Placebo (1267) | 5.1 y ‖ | 0.02 : 0 | 1 : 9 | 0.16 : 1.39 | ||
| WHO, 1973 ( 54 ) | – | Clofibrate, 1.6 g qd (5331) | Placebo (10 414) | 5.3 y ¶ | 15 : 16 | 0 : 0 | 0 : 0 | ||
| WOSCOP, 1995 ( 38 ) | Pravastatin, 40 mg qd (3302) | – | Placebo (3293) | 4.9 y ¶ | 16 : 15 | 4 : 6 | 0.25 : 0.37 | ||
| Trial acronym or author, year of publication (reference) . | Study arm, dosage (No. of randomly assigned participants) . | . | . | . | % Total dropout † , S/F : O . | No. of incident melanomas ‡ , S/F : O . | Melanoma rate § , S/F : O . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | S . | F . | O . | Trial length . | . | . | . | ||
| 4S, 1994 ( 34 ) | Simvastatin, 10–40 mg qd (2221) | – | Placebo (2223) | 5.4 y ‖ | 5.0 : 7.0 | 7 : 3 | 0.58 : 0.25 | ||
| AFCAPS, 1998 ( 18 ) | Lovastatin, 20–40 mg qd (3304) | – | Placebo (3301) | 5.2 y ¶ | 16 : 23 | 14 : 27 | 0.81 : 1.57 | ||
| BECAIT, 1998 ( 49 ) | – | Bezafibrate, 200 mg tid ( 47 ) | Placebo ( 45 ) | 5.0 y # | 11 : 13 | 0 : 0 | 0 : 0 | ||
| BIP, 2000 ( 50 ) | Bezafibrate, 400 mg qd (1548) | Placebo (1542) | 6.2 y ¶ | 31 : 33 | 2 : 6 | 0.21 : 0.63 | |||
| CARE, 1996 ( 35 ) | Pravastatin, 40 mg qd (2081) | – | Placebo (2078) | 5.0 y ‖ | 15 : 28 | 4 : 3 | 0.38 : 0.29 | ||
| Carmena, 1996 ( 31 ) | Pravastatin, 40 mg qd ( 57 ) | – | Cholestyramine, 16 mg qd ( 57 ) | 1.0 y # | 7.0 : 16 | 0 : 0 | 0 : 0 | ||
| CDP, 1986 ( 51 ) | – | Clofibrate, 1.8 g qd (1103) | Placebo ** (2789) | 6.2 y ¶ | 6.0 : 6.0 | 2 : 3 | 0.29 : 0.17 | ||
| Gentile, 2000 ( 32 ) | Atorvastatin, 10 mg qd (85) | – | Placebo (86) | 24 wk # | 1.2 : 0 | 0 : 0 | 0 : 0 | ||
| Simvastatin, 10 mg qd (78) | 0 : 0 | 0 : 0 | 0 : 0 | ||||||
| Pravastatin, 20 mg qd (82) | 1.2 : 0 | 0 : 0 | 0 : 0 | ||||||
| Lovastatin, 20 mg qd (81) | 1.2 : 0 | 0 : 0 | 0 : 0 | ||||||
| GISSI, 2000 ( 33 ) | Pravastatin, 20–40 mg qd (2138) | – | No treatment (2133) | 1.9 y ¶ , 2.0 y ‖ | 19 : 14 | 0 : 1 | 0 : 0.25 | ||
| GREACE, 2002 ( 30 ) | Atorvastatin, 10–80 mg qd (800) | – | Usual care †† (800) | 3.0 y ¶ | 1.3 : 0 | 0 : 0 | 0 : 0 | ||
| HHS, 1987 ( 52 ) | Gemfibrozil, 600 mg tid (2051) | – | Placebo (2030) | 5.3 y ¶ | 18 : 18 | 1 : 0 | 0.1 : 0 | ||
| LEADER, 2002 ( 53 ) | – | Bezafibrate, 400 mg qd or qod if creatine level of 135–149 mmol/L (783) | Placebo (785) | 4.6 y ‖ | 49 : 52 | 1 : 1 | 0.28 : 0.28 | ||
| L-CAD, 2000 ( 29 ) | Pravastatin, 20–40 mg qd (70) | – | Usual care ‡‡ ( 65 ) | 2.0 # | 0 : 14 | 0 : 0 | 0 : 0 | ||
| LIPID, 1998 ( 36 ) | Pravastatin, 40mg qd (4512) | – | Placebo (4502) | 6.1y ¶ | 19 : 19 | 30 : 28 | 1.09 : 1.02 | ||
| LOCAT, 1997 ( 48 ) | – | Gemfibrozil, 1200 mg qd (197) | Placebo (198) | 2.5 y ¶ | 6.0 : 6.1 | 0 : 1 | 0 : 2 | ||
| MAAS, 1993 ( 37 ) | Simvastatin, 20 mg qd (193) | – | Placebo (188) | 4.0 y # | 11 : 5.0 | 0 : 0 | 0 : 0 | ||
| PRINCE, 2001 ( 28 ) | Pravastatin, 40 mg qd (1014) | – | Placebo (999) | 24 wk # | 34 : 33 | 0 : 0 | 0 : 0 | ||
| VA-HIT, 1999 ( 19 ) | – | Gemfibrozil, 1200 mg qd (1264) | Placebo (1267) | 5.1 y ‖ | 0.02 : 0 | 1 : 9 | 0.16 : 1.39 | ||
| WHO, 1973 ( 54 ) | – | Clofibrate, 1.6 g qd (5331) | Placebo (10 414) | 5.3 y ¶ | 15 : 16 | 0 : 0 | 0 : 0 | ||
| WOSCOP, 1995 ( 38 ) | Pravastatin, 40 mg qd (3302) | – | Placebo (3293) | 4.9 y ¶ | 16 : 15 | 4 : 6 | 0.25 : 0.37 | ||
S = statin arm; F = fibrate arm; O = other/control arm; 4S = Scandanavian Simvastatin Survival Study; AFCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study; BECAIT = Bezafibrate Coronary Atherosclerosis Intervention Trial; BIP = Bezafibrate Infarction Prevention Study; CARE = Cholesterol and Recurrent Events Trial; CDP = Coronary Drug Project; GISSI = Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico; GREACE = Greek Atorvastatin and Coronary-heart-disease Evaluation; HHS = Helskinki Heart Study; LEADER = Lower Extremity Arterial Event Reduction Trial; L-CAD = Randomized Lipid-Coronary Artery Disease Study; LIPID = Long-Term Intervention With Pravastatin in Ischemic Disease; LOCAT = Lipid Coronary Angiography Trial; MAAS = Multicenter Anti-Atheroma Study; PRINCE = Pravastatin Inflammation/CRP Evaluation; VA-HIT = Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; WHO = World Health Organization Study; WOSCOP = West of Scotland Coronary Prevention Study; qd = every day; – = not applicable; tid = three times a day; qod = every other day; CRP = C-reactive protein.
Patients who were lost to follow-up and who prematurely stopped their trial arm participation.
Occurring during trial participation.
Melanoma incidence/1000 person-years.
Median trial duration.
Mean trial duration.
Trial duration, mean/median not specified.
Lactose.
Includes lifestyle changes plus all necessary drug treatment. A total of 113 participants in the other arm received statins or fibrates for the duration of the trial.
Includes lifestyle changes plus all necessary drug treatment. A total of 13 participants in the other arm received antilipidemic drugs: “8 were on statin therapy, and 5 were on miscellaneous medications” ( 29 )
Characteristics of included studies *
| Trial acronym or author, year of publication (reference) . | Study arm, dosage (No. of randomly assigned participants) . | . | . | . | % Total dropout † , S/F : O . | No. of incident melanomas ‡ , S/F : O . | Melanoma rate § , S/F : O . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | S . | F . | O . | Trial length . | . | . | . | ||
| 4S, 1994 ( 34 ) | Simvastatin, 10–40 mg qd (2221) | – | Placebo (2223) | 5.4 y ‖ | 5.0 : 7.0 | 7 : 3 | 0.58 : 0.25 | ||
| AFCAPS, 1998 ( 18 ) | Lovastatin, 20–40 mg qd (3304) | – | Placebo (3301) | 5.2 y ¶ | 16 : 23 | 14 : 27 | 0.81 : 1.57 | ||
| BECAIT, 1998 ( 49 ) | – | Bezafibrate, 200 mg tid ( 47 ) | Placebo ( 45 ) | 5.0 y # | 11 : 13 | 0 : 0 | 0 : 0 | ||
| BIP, 2000 ( 50 ) | Bezafibrate, 400 mg qd (1548) | Placebo (1542) | 6.2 y ¶ | 31 : 33 | 2 : 6 | 0.21 : 0.63 | |||
| CARE, 1996 ( 35 ) | Pravastatin, 40 mg qd (2081) | – | Placebo (2078) | 5.0 y ‖ | 15 : 28 | 4 : 3 | 0.38 : 0.29 | ||
| Carmena, 1996 ( 31 ) | Pravastatin, 40 mg qd ( 57 ) | – | Cholestyramine, 16 mg qd ( 57 ) | 1.0 y # | 7.0 : 16 | 0 : 0 | 0 : 0 | ||
| CDP, 1986 ( 51 ) | – | Clofibrate, 1.8 g qd (1103) | Placebo ** (2789) | 6.2 y ¶ | 6.0 : 6.0 | 2 : 3 | 0.29 : 0.17 | ||
| Gentile, 2000 ( 32 ) | Atorvastatin, 10 mg qd (85) | – | Placebo (86) | 24 wk # | 1.2 : 0 | 0 : 0 | 0 : 0 | ||
| Simvastatin, 10 mg qd (78) | 0 : 0 | 0 : 0 | 0 : 0 | ||||||
| Pravastatin, 20 mg qd (82) | 1.2 : 0 | 0 : 0 | 0 : 0 | ||||||
| Lovastatin, 20 mg qd (81) | 1.2 : 0 | 0 : 0 | 0 : 0 | ||||||
| GISSI, 2000 ( 33 ) | Pravastatin, 20–40 mg qd (2138) | – | No treatment (2133) | 1.9 y ¶ , 2.0 y ‖ | 19 : 14 | 0 : 1 | 0 : 0.25 | ||
| GREACE, 2002 ( 30 ) | Atorvastatin, 10–80 mg qd (800) | – | Usual care †† (800) | 3.0 y ¶ | 1.3 : 0 | 0 : 0 | 0 : 0 | ||
| HHS, 1987 ( 52 ) | Gemfibrozil, 600 mg tid (2051) | – | Placebo (2030) | 5.3 y ¶ | 18 : 18 | 1 : 0 | 0.1 : 0 | ||
| LEADER, 2002 ( 53 ) | – | Bezafibrate, 400 mg qd or qod if creatine level of 135–149 mmol/L (783) | Placebo (785) | 4.6 y ‖ | 49 : 52 | 1 : 1 | 0.28 : 0.28 | ||
| L-CAD, 2000 ( 29 ) | Pravastatin, 20–40 mg qd (70) | – | Usual care ‡‡ ( 65 ) | 2.0 # | 0 : 14 | 0 : 0 | 0 : 0 | ||
| LIPID, 1998 ( 36 ) | Pravastatin, 40mg qd (4512) | – | Placebo (4502) | 6.1y ¶ | 19 : 19 | 30 : 28 | 1.09 : 1.02 | ||
| LOCAT, 1997 ( 48 ) | – | Gemfibrozil, 1200 mg qd (197) | Placebo (198) | 2.5 y ¶ | 6.0 : 6.1 | 0 : 1 | 0 : 2 | ||
| MAAS, 1993 ( 37 ) | Simvastatin, 20 mg qd (193) | – | Placebo (188) | 4.0 y # | 11 : 5.0 | 0 : 0 | 0 : 0 | ||
| PRINCE, 2001 ( 28 ) | Pravastatin, 40 mg qd (1014) | – | Placebo (999) | 24 wk # | 34 : 33 | 0 : 0 | 0 : 0 | ||
| VA-HIT, 1999 ( 19 ) | – | Gemfibrozil, 1200 mg qd (1264) | Placebo (1267) | 5.1 y ‖ | 0.02 : 0 | 1 : 9 | 0.16 : 1.39 | ||
| WHO, 1973 ( 54 ) | – | Clofibrate, 1.6 g qd (5331) | Placebo (10 414) | 5.3 y ¶ | 15 : 16 | 0 : 0 | 0 : 0 | ||
| WOSCOP, 1995 ( 38 ) | Pravastatin, 40 mg qd (3302) | – | Placebo (3293) | 4.9 y ¶ | 16 : 15 | 4 : 6 | 0.25 : 0.37 | ||
| Trial acronym or author, year of publication (reference) . | Study arm, dosage (No. of randomly assigned participants) . | . | . | . | % Total dropout † , S/F : O . | No. of incident melanomas ‡ , S/F : O . | Melanoma rate § , S/F : O . | ||
|---|---|---|---|---|---|---|---|---|---|
| . | S . | F . | O . | Trial length . | . | . | . | ||
| 4S, 1994 ( 34 ) | Simvastatin, 10–40 mg qd (2221) | – | Placebo (2223) | 5.4 y ‖ | 5.0 : 7.0 | 7 : 3 | 0.58 : 0.25 | ||
| AFCAPS, 1998 ( 18 ) | Lovastatin, 20–40 mg qd (3304) | – | Placebo (3301) | 5.2 y ¶ | 16 : 23 | 14 : 27 | 0.81 : 1.57 | ||
| BECAIT, 1998 ( 49 ) | – | Bezafibrate, 200 mg tid ( 47 ) | Placebo ( 45 ) | 5.0 y # | 11 : 13 | 0 : 0 | 0 : 0 | ||
| BIP, 2000 ( 50 ) | Bezafibrate, 400 mg qd (1548) | Placebo (1542) | 6.2 y ¶ | 31 : 33 | 2 : 6 | 0.21 : 0.63 | |||
| CARE, 1996 ( 35 ) | Pravastatin, 40 mg qd (2081) | – | Placebo (2078) | 5.0 y ‖ | 15 : 28 | 4 : 3 | 0.38 : 0.29 | ||
| Carmena, 1996 ( 31 ) | Pravastatin, 40 mg qd ( 57 ) | – | Cholestyramine, 16 mg qd ( 57 ) | 1.0 y # | 7.0 : 16 | 0 : 0 | 0 : 0 | ||
| CDP, 1986 ( 51 ) | – | Clofibrate, 1.8 g qd (1103) | Placebo ** (2789) | 6.2 y ¶ | 6.0 : 6.0 | 2 : 3 | 0.29 : 0.17 | ||
| Gentile, 2000 ( 32 ) | Atorvastatin, 10 mg qd (85) | – | Placebo (86) | 24 wk # | 1.2 : 0 | 0 : 0 | 0 : 0 | ||
| Simvastatin, 10 mg qd (78) | 0 : 0 | 0 : 0 | 0 : 0 | ||||||
| Pravastatin, 20 mg qd (82) | 1.2 : 0 | 0 : 0 | 0 : 0 | ||||||
| Lovastatin, 20 mg qd (81) | 1.2 : 0 | 0 : 0 | 0 : 0 | ||||||
| GISSI, 2000 ( 33 ) | Pravastatin, 20–40 mg qd (2138) | – | No treatment (2133) | 1.9 y ¶ , 2.0 y ‖ | 19 : 14 | 0 : 1 | 0 : 0.25 | ||
| GREACE, 2002 ( 30 ) | Atorvastatin, 10–80 mg qd (800) | – | Usual care †† (800) | 3.0 y ¶ | 1.3 : 0 | 0 : 0 | 0 : 0 | ||
| HHS, 1987 ( 52 ) | Gemfibrozil, 600 mg tid (2051) | – | Placebo (2030) | 5.3 y ¶ | 18 : 18 | 1 : 0 | 0.1 : 0 | ||
| LEADER, 2002 ( 53 ) | – | Bezafibrate, 400 mg qd or qod if creatine level of 135–149 mmol/L (783) | Placebo (785) | 4.6 y ‖ | 49 : 52 | 1 : 1 | 0.28 : 0.28 | ||
| L-CAD, 2000 ( 29 ) | Pravastatin, 20–40 mg qd (70) | – | Usual care ‡‡ ( 65 ) | 2.0 # | 0 : 14 | 0 : 0 | 0 : 0 | ||
| LIPID, 1998 ( 36 ) | Pravastatin, 40mg qd (4512) | – | Placebo (4502) | 6.1y ¶ | 19 : 19 | 30 : 28 | 1.09 : 1.02 | ||
| LOCAT, 1997 ( 48 ) | – | Gemfibrozil, 1200 mg qd (197) | Placebo (198) | 2.5 y ¶ | 6.0 : 6.1 | 0 : 1 | 0 : 2 | ||
| MAAS, 1993 ( 37 ) | Simvastatin, 20 mg qd (193) | – | Placebo (188) | 4.0 y # | 11 : 5.0 | 0 : 0 | 0 : 0 | ||
| PRINCE, 2001 ( 28 ) | Pravastatin, 40 mg qd (1014) | – | Placebo (999) | 24 wk # | 34 : 33 | 0 : 0 | 0 : 0 | ||
| VA-HIT, 1999 ( 19 ) | – | Gemfibrozil, 1200 mg qd (1264) | Placebo (1267) | 5.1 y ‖ | 0.02 : 0 | 1 : 9 | 0.16 : 1.39 | ||
| WHO, 1973 ( 54 ) | – | Clofibrate, 1.6 g qd (5331) | Placebo (10 414) | 5.3 y ¶ | 15 : 16 | 0 : 0 | 0 : 0 | ||
| WOSCOP, 1995 ( 38 ) | Pravastatin, 40 mg qd (3302) | – | Placebo (3293) | 4.9 y ¶ | 16 : 15 | 4 : 6 | 0.25 : 0.37 | ||
S = statin arm; F = fibrate arm; O = other/control arm; 4S = Scandanavian Simvastatin Survival Study; AFCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study; BECAIT = Bezafibrate Coronary Atherosclerosis Intervention Trial; BIP = Bezafibrate Infarction Prevention Study; CARE = Cholesterol and Recurrent Events Trial; CDP = Coronary Drug Project; GISSI = Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico; GREACE = Greek Atorvastatin and Coronary-heart-disease Evaluation; HHS = Helskinki Heart Study; LEADER = Lower Extremity Arterial Event Reduction Trial; L-CAD = Randomized Lipid-Coronary Artery Disease Study; LIPID = Long-Term Intervention With Pravastatin in Ischemic Disease; LOCAT = Lipid Coronary Angiography Trial; MAAS = Multicenter Anti-Atheroma Study; PRINCE = Pravastatin Inflammation/CRP Evaluation; VA-HIT = Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; WHO = World Health Organization Study; WOSCOP = West of Scotland Coronary Prevention Study; qd = every day; – = not applicable; tid = three times a day; qod = every other day; CRP = C-reactive protein.
Patients who were lost to follow-up and who prematurely stopped their trial arm participation.
Occurring during trial participation.
Melanoma incidence/1000 person-years.
Median trial duration.
Mean trial duration.
Trial duration, mean/median not specified.
Lactose.
Includes lifestyle changes plus all necessary drug treatment. A total of 113 participants in the other arm received statins or fibrates for the duration of the trial.
Includes lifestyle changes plus all necessary drug treatment. A total of 13 participants in the other arm received antilipidemic drugs: “8 were on statin therapy, and 5 were on miscellaneous medications” ( 29 )
Methodologic quality of included studies *
| Trial acronym or author, year of publication (reference) . | Randomization † . | Concealment ‡ . | Intention to treat § . | Blinding of participants ‖ . | Blinding of outcomes assessors ¶ . |
|---|---|---|---|---|---|
| 4S, 1994 ( 34 ) | A | A | A | A | A |
| AFCAPS, 1998 ( 18 ) | B | B | A | A | A |
| BECAIT, 1998 ( 49 ) | A | B | B | A | A |
| BIP, 2000 ( 50 ) | B | B | A | A | A |
| CARE, 1996 ( 35 ) | A | A | A | A | A |
| Carmena, 1996 ( 31 ) | B | B | B | A # | A # |
| CDP, 1986 ( 51 ) | A | A | B | A | A |
| Gentile, 2000 ( 32 ) | B | B | A | C | C |
| GISSI, 2000 ( 33 ) | B | B | A | C | C |
| GREACE, 2002 ( 30 ) | B | B | A | C | C |
| HHS, 1987 ( 52 ) | A | A | A | A | A |
| LEADER, 2002 ( 53 ) | B | B | A | A | A |
| L-CAD, 2000 ( 29 ) | B | B | A | B | B |
| LIPID, 1998 ( 36 ) | A | B | A | A | A |
| LOCAT, 1997 ( 48 ) | B | B | B | A | A |
| MAAS, 1993 ( 37 ) | B | B | A | A | A |
| PRINCE, 2001 ( 28 ) | B | B | A | A | A |
| VA-HIT, 1999 ( 19 ) | A | B | A | A | A |
| WHO, 1991 ( 54 ) | B | B | A | A | A |
| WOSCOP, 1995 ( 38 ) | A | B | A | A | A |
| Trial acronym or author, year of publication (reference) . | Randomization † . | Concealment ‡ . | Intention to treat § . | Blinding of participants ‖ . | Blinding of outcomes assessors ¶ . |
|---|---|---|---|---|---|
| 4S, 1994 ( 34 ) | A | A | A | A | A |
| AFCAPS, 1998 ( 18 ) | B | B | A | A | A |
| BECAIT, 1998 ( 49 ) | A | B | B | A | A |
| BIP, 2000 ( 50 ) | B | B | A | A | A |
| CARE, 1996 ( 35 ) | A | A | A | A | A |
| Carmena, 1996 ( 31 ) | B | B | B | A # | A # |
| CDP, 1986 ( 51 ) | A | A | B | A | A |
| Gentile, 2000 ( 32 ) | B | B | A | C | C |
| GISSI, 2000 ( 33 ) | B | B | A | C | C |
| GREACE, 2002 ( 30 ) | B | B | A | C | C |
| HHS, 1987 ( 52 ) | A | A | A | A | A |
| LEADER, 2002 ( 53 ) | B | B | A | A | A |
| L-CAD, 2000 ( 29 ) | B | B | A | B | B |
| LIPID, 1998 ( 36 ) | A | B | A | A | A |
| LOCAT, 1997 ( 48 ) | B | B | B | A | A |
| MAAS, 1993 ( 37 ) | B | B | A | A | A |
| PRINCE, 2001 ( 28 ) | B | B | A | A | A |
| VA-HIT, 1999 ( 19 ) | A | B | A | A | A |
| WHO, 1991 ( 54 ) | B | B | A | A | A |
| WOSCOP, 1995 ( 38 ) | A | B | A | A | A |
4S = Scandanavian Simvastatin Survival Study; AFCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study; BECAIT = Bezafibrate Coronary Atherosclerosis Intervention Trial ; BIP = Bezafibrate Infarction Prevention Study; CARE = Cholesterol and Recurrent Events Trial; CDP = Coronary Drug Project; GISSI = Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico; GREACE = Greek Atorvastatin and Coronary-heart-disease Evaluation; HHS = Helskinki Heart Study; LEADER = Lower Extremity Arterial Event Reduction Trial; L-CAD = Randomized Lipid-Coronary Artery Disease Study; LIPID = Long-Term Intervention With Pravastatin in Ischemic Disease; LOCAT = Lipid Coronary Angiography Trial; MAAS = Multicenter Anti-Atheroma Study; PRINCE = Pravastatin Inflammation/CRP Evaluation; VA-HIT = Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; WHO = World Health Organization Study; WOSCOP = West of Scotland Coronary Prevention Study.
Randomization: A = based on a clear description of how random numbers were generated; B = unclear; C = not based on validly generated random numbers.
Allocation concealment: A = third party or opaque sealed envelopes; B = unclear; C = open list, day of week or quasi-randomized.
Intention to treat: A = intention-to-treat analysis performed; B = unclear; C = intention-to-treat analysis not performed.
Blinding of participants: A = participant was blinded; B = unclear; C = participant was aware of allocation.
Blinding of outcomes assessors: A = assessor was blinded or independent; B = unclear; C = assessor was aware of allocation.
Double-blinded trial for first 3 months with 9-month open extension.
Methodologic quality of included studies *
| Trial acronym or author, year of publication (reference) . | Randomization † . | Concealment ‡ . | Intention to treat § . | Blinding of participants ‖ . | Blinding of outcomes assessors ¶ . |
|---|---|---|---|---|---|
| 4S, 1994 ( 34 ) | A | A | A | A | A |
| AFCAPS, 1998 ( 18 ) | B | B | A | A | A |
| BECAIT, 1998 ( 49 ) | A | B | B | A | A |
| BIP, 2000 ( 50 ) | B | B | A | A | A |
| CARE, 1996 ( 35 ) | A | A | A | A | A |
| Carmena, 1996 ( 31 ) | B | B | B | A # | A # |
| CDP, 1986 ( 51 ) | A | A | B | A | A |
| Gentile, 2000 ( 32 ) | B | B | A | C | C |
| GISSI, 2000 ( 33 ) | B | B | A | C | C |
| GREACE, 2002 ( 30 ) | B | B | A | C | C |
| HHS, 1987 ( 52 ) | A | A | A | A | A |
| LEADER, 2002 ( 53 ) | B | B | A | A | A |
| L-CAD, 2000 ( 29 ) | B | B | A | B | B |
| LIPID, 1998 ( 36 ) | A | B | A | A | A |
| LOCAT, 1997 ( 48 ) | B | B | B | A | A |
| MAAS, 1993 ( 37 ) | B | B | A | A | A |
| PRINCE, 2001 ( 28 ) | B | B | A | A | A |
| VA-HIT, 1999 ( 19 ) | A | B | A | A | A |
| WHO, 1991 ( 54 ) | B | B | A | A | A |
| WOSCOP, 1995 ( 38 ) | A | B | A | A | A |
| Trial acronym or author, year of publication (reference) . | Randomization † . | Concealment ‡ . | Intention to treat § . | Blinding of participants ‖ . | Blinding of outcomes assessors ¶ . |
|---|---|---|---|---|---|
| 4S, 1994 ( 34 ) | A | A | A | A | A |
| AFCAPS, 1998 ( 18 ) | B | B | A | A | A |
| BECAIT, 1998 ( 49 ) | A | B | B | A | A |
| BIP, 2000 ( 50 ) | B | B | A | A | A |
| CARE, 1996 ( 35 ) | A | A | A | A | A |
| Carmena, 1996 ( 31 ) | B | B | B | A # | A # |
| CDP, 1986 ( 51 ) | A | A | B | A | A |
| Gentile, 2000 ( 32 ) | B | B | A | C | C |
| GISSI, 2000 ( 33 ) | B | B | A | C | C |
| GREACE, 2002 ( 30 ) | B | B | A | C | C |
| HHS, 1987 ( 52 ) | A | A | A | A | A |
| LEADER, 2002 ( 53 ) | B | B | A | A | A |
| L-CAD, 2000 ( 29 ) | B | B | A | B | B |
| LIPID, 1998 ( 36 ) | A | B | A | A | A |
| LOCAT, 1997 ( 48 ) | B | B | B | A | A |
| MAAS, 1993 ( 37 ) | B | B | A | A | A |
| PRINCE, 2001 ( 28 ) | B | B | A | A | A |
| VA-HIT, 1999 ( 19 ) | A | B | A | A | A |
| WHO, 1991 ( 54 ) | B | B | A | A | A |
| WOSCOP, 1995 ( 38 ) | A | B | A | A | A |
4S = Scandanavian Simvastatin Survival Study; AFCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study; BECAIT = Bezafibrate Coronary Atherosclerosis Intervention Trial ; BIP = Bezafibrate Infarction Prevention Study; CARE = Cholesterol and Recurrent Events Trial; CDP = Coronary Drug Project; GISSI = Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico; GREACE = Greek Atorvastatin and Coronary-heart-disease Evaluation; HHS = Helskinki Heart Study; LEADER = Lower Extremity Arterial Event Reduction Trial; L-CAD = Randomized Lipid-Coronary Artery Disease Study; LIPID = Long-Term Intervention With Pravastatin in Ischemic Disease; LOCAT = Lipid Coronary Angiography Trial; MAAS = Multicenter Anti-Atheroma Study; PRINCE = Pravastatin Inflammation/CRP Evaluation; VA-HIT = Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; WHO = World Health Organization Study; WOSCOP = West of Scotland Coronary Prevention Study.
Randomization: A = based on a clear description of how random numbers were generated; B = unclear; C = not based on validly generated random numbers.
Allocation concealment: A = third party or opaque sealed envelopes; B = unclear; C = open list, day of week or quasi-randomized.
Intention to treat: A = intention-to-treat analysis performed; B = unclear; C = intention-to-treat analysis not performed.
Blinding of participants: A = participant was blinded; B = unclear; C = participant was aware of allocation.
Blinding of outcomes assessors: A = assessor was blinded or independent; B = unclear; C = assessor was aware of allocation.
Double-blinded trial for first 3 months with 9-month open extension.
R ESULTS
Automated searches of the databases yielded 4405 unique published articles whose titles indicated that they described randomized controlled trials that may have involved the use of statins or fibrates. Of these, 109 qualified for abstract assessment in that the titles described trials that might meet the inclusion criteria. Of these, 37 articles were excluded after abstract review, and 72 were examined by reviewing the full paper. Of the 72 full articles reviewed, a total of 36 were excluded because they failed to meet the inclusion criteria (i.e., no statin-free or fibrate-free arm [n = 16], no randomization [n = 4], a statin or fibrate was not used in isolation in the treatment arm [n = 9], or the mean treatment duration was less than 6 months [n = 7]). The corresponding author of each of the remaining 36 qualifying trials [21 statin trials ( 18 , 28 – 47 ) and 15 fibrate trials ( 19 , 48 – 61 ) ] was mailed up to three letters that described the aims of our study and included forms for providing additional unpublished data on participants who developed melanoma during the trial. The authors of 20 of the qualifying trials ( 18 , 19 , 28 – 38 , 48 – 54 ) provided unpublished melanoma incidence data ( Table 1 ), and authors of 10 of the 20 trials ( 18 , 19 , 35 , 36 , 38 , 48 – 51 , 53 ) provided participant-specific data. Eight trials enrolled only men ( 19 , 38 , 48 , 49 , 51 – 54 ) , and two trials excluded participants with a history of cancer ( 49 , 55 ) .
Six different statins were employed in the qualifying trials—lovastatin, pravastatin, simvastatin, atorvastatin, cerivastatin, and fluvastatin—at treatment dosages that ranged from 10 to 80 mg per day. The qualifying fibrate trials used four types of fibrates—bezafibrate, clofibrate, fenofibrate, and gemfibrozil—at dosages that ranged from 400 mg every other day to 1800 mg per day.
The methods used to validate melanoma diagnoses were frequently not reported. One trial ( 51 ) provided unpublished melanoma incidence data for participants in the fibrate treatment and placebo arms, but not for participants in other treatment arms, thus we used only these two arms for analysis. Table 2 shows the results of our quality assessment evaluation of each study according to the following assessment categories: descriptions of randomization, concealment, intention to treat, blinding of participants, and blinding of outcomes assessors.
Dropout rates ranged from 0% to 34% in the treatment arms and from 0% to 33% in the control arms of the statin trials. Dropout rates ranged from 0.02% to 49% in the treatment arms and from 0% to 52% in the control arms of the fibrate trials. Dropout rates for all trials except for the Coronary Drug Project (CDP) study ( 51 ) were extracted from publications. CDP dropout rates were provided by author correspondence.
A total of 154 incident melanomas occurred among 70 820 trial participants (127 among 39 426 statin trial participants [59 among the 19 872 statin arm participants and 68 among the 19 554 control arm participants] and 27 among 31 394 fibrate trial participants [seven among the 12 324 fibrate arm participants and 20 among the 19 070 control arm participants]). Ninety-five of 154 melanomas (62%) were unpublished (79 among statin trial participants and 16 among fibrate trial participants).
Two trials ( 18 , 19 ) had previously reported that participants in the intervention arm had statistically significantly fewer incident melanomas than participants in the placebo arm. However, none of the 20 studies for which we obtained unpublished melanoma incidence data ( 18 , 19 , 28 – 38 , 48 – 54 ) had statistically significantly fewer incident melanomas in the intervention arm than in the control or placebo arm. The publication of melanoma incidence data was associated with the report of a statistically significant difference between participants in different study arms. Two of two studies with a statistically significant association between the use of statins or fibrates and decreased melanoma incidence published this information ( 18 , 19 ) , and two ( 35 , 52 ) of 18 studies ( 28 – 38 , 48 – 54 ) without such a relationship (as documented by the unpublished data provided) published this information. Melanoma incidence rates varied among the studies from 0 to 1.57 melanomas per 1000 person-years.
When we used a fixed-effects model to pool trial results based on all of the acquired data, we found no statistically significant association between statin use and melanoma incidence (OR for statin versus control = 0.87, 95% CI = 0.61 to 1.23) ( Fig. 1 ) or fibrate use and melanoma incidence (OR for fibrate versus control = 0.45, 95% CI = 0.20 to 1.01) ( Fig. 2 ). The results did not differ statistically significantly when the analysis was performed by using a random-effects model to pool the trial results (data not shown).
Meta-analysis of the association between statin exposure and melanoma incidence. n = number of events; N = number of subjects in group. Squares to the left of the vertical line indicate decreased melanoma incidence in patients taking statins, whereas squares to the right of the vertical line indicate decreased melanoma incidence in patients in control groups. The horizontal line through each square represents the 95% confidence interval (CI). The size of each square reflects the relative weight of each study and the diamond represents the pooled effect ( width of the diamond indicates the 95% confidence interval). MAAS = Multicenter Anti-Atheroma Study; 4S = the Scandinavian Simvastatin Survival Study; WOSCOP = West of Scotland Coronary Prevention Study; CARE = Cholesterol and Recurrent Events Trial, the effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels; Carmena = the Spanish Multicenter Pravastatin Study; AFCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study, primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels; LIPID = Long-Term Intervention With Pravastatin in Ischemic Disease; Gentile = comparative efficacy study of atorvastatin vs. simvastatin, pravastatin, lovastatin, and placebo in type 2 diabetic patients with hypercholesterolemia; GISSI = Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico, results of the low-dose pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge; L-CAD = Randomized Lipid-Coronary Artery Disease study; PRINCE = Pravastatin Inflammation/CRP Evaluation; GREACE = Greek Atorvastatin and Coronary-heart-disease Evaluation.
Meta-analysis of the association between fibrate exposure and melanoma incidence. Squares to the left of the vertical line indicate decreased melanoma incidence in patients taking fibrates, whereas squares to the right of the vertical line indicate decreased melanoma incidence in patients in control groups. The horizontal line through each square represents the 95% confidence interval (CI). The size of each square reflects the relative weight of each study and the diamond represents the pooled effect ( width of the diamond indicates the 95% confidence interval). WHO = World Health Organization Study, a cooperative trial on the primary prevention of ischemic heart disease using clofibrate; CDP = Coronary Drug Project; HHS = Helsinki Heart Study; LOCAT = Lipid Coronary Angiography Trial; BECAIT = Bezafibrate Coronary Atherosclerosis Intervention Trial; VA-HIT = Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; BIP = Bezafibrate Infarction Prevention; LEADER = Lower Extremity Arterial Event Reduction Trial, bezafibrate in men with lower extremity arterial disease.
There was no statistically significant between-study variation (heterogeneity) among the trials included in the meta-analysis ( I2 = 12.2% [fibrates] and 16.6% [statins]). Analyses that were stratified by the type of statin or fibrate, by the sex of the participant, by the occurrence of melanoma during the study after 2 years of participation, or by trial funding source found no statistically significant differences between the intervention and control groups, with one exception. The lovastatin subgroup analysis, which included only one trial ( 18 ) , showed reduced melanoma incidence associated with lovastatin use (OR = 0.52, 95% CI = 0.27 to 0.99) ( Fig. 1 ). On the basis of the results of the lovastatin subgroup analysis, 244 people would need to be treated for 5 years to prevent one case of melanoma.
D ISCUSSION
In this meta-analysis, we examined the association between statin or fibrate therapy and an outcome unrelated to the primary outcome of the trial, melanoma incidence, among trials that randomly assigned participants to receive statins or fibrates versus placebo or other control for a minimum of 6 months. We found no statistically significant association between the use of statins or fibrates and melanoma incidence. Two of the 36 qualifying trials included in the meta-analysis had previously reported that participants in the intervention arm had statistically significantly fewer incident melanomas than participants in the control arm, two published the lack of a relationship, and the other 32 have not published melanoma outcomes. We found that additional unpublished data on melanoma incidence (provided by 20 of the 36 qualifying trials) did not support an association between statin or fibrate use and melanoma incidence. Another recent meta-analysis of published melanoma incidence in statin trials with therapeutic duration of at least 1 year reported similar results (OR for statin versus placebo = 0.84, 95% CI = 0.57 to 1.25, P = .30) ( 62 ) .
Published high-quality randomized controlled trials often selectively include and emphasize positive or unanticipated findings ( 63 ) . Two studies that found a statistically significant inverse association between melanoma incidence and drug therapy published those results, whereas only two of the 18 studies without data supporting an association published that result. This reporting discrepancy underscores the importance of obtaining unpublished data, especially data that are related to secondary or harm outcomes.
The performance of systematic reviews is often hindered by lack of access to unpublished data. To minimize this barrier, we contacted all qualifying study investigators who we thought might have potentially relevant unpublished data and offered them a monetary incentive to provide melanoma incidence data. Investigators affiliated with only two studies requested payment of the monetary incentive and provided the necessary tax numbers that enabled us to pay the incentive ( 38 , 51 ) . Investigators affiliated with one study ( 51 ) reported that the monetary incentive was essential for the retrieval of the melanoma outcomes data requested. It is reassuring for those interested in conducting research requiring unpublished data that financial support was not needed for the collection of the majority of unpublished data used in this meta-analysis.
Despite the lack of evidence from published studies to exclude the hypothesis that statins and fibrates prevent melanoma, evidence supporting the use of statins for melanoma prevention or treatment continues to build ( 6 , 64 – 69 ) . Thus, the potential use of statins for melanoma prevention and the utility of testing statins as therapy, especially in combination with chemotherapy, deserves further investigation ( 17 ) . It must also be recognized that the statin regimen of the clinical trials included in this study did not achieve steady-state concentrations proven to be antineoplastic in the preclinical in vitro studies. Therefore, it is possible the drug doses did not produce serum levels capable of affecting melanoma cells. Given the paucity of effective therapies for advanced melanoma, it may be appropriate to perform phase I and II clinical trials that combine statins with chemotherapy to test whether this drug combination is tolerable and, if it is, whether it is more effective than current chemotherapy alone.
This systematic review has several limitations. First, data were obtained for only 20 of 36 qualifying trials. Data from all studies would have led to a more precise estimate of the treatment effect. Second, because the clinical trials used 10 different drugs, treatment effects may be diluted if all drugs do not act similarly. Third, cancer was not the primary endpoint of any included trial. We were not able to obtain primary data regarding melanoma diagnosis for all study participants. Fourth, at least one item of histologic data (e.g., Breslow's depth, Clark's level, histologic subtype) was reported for only 32 of the 154 incident melanomas reported during trial participation, which prevented us from exploring the potential associations of the use of statins or fibrates with histologic variables at diagnosis. Fifth, stratified analyses based on participants' melanoma risk factors (e.g., dysplastic nevi) also were not possible because these data were not available. Sixth, this review specifically examined rare events; thus, the aggregated results presented here should be interpreted with caution. Although there was no statistically significant between-study variation (heterogeneity) among the trials included in this meta-analysis, calculated cumulative odds ratios and 95% confidence intervals can vary widely among systematic reviews that report rare events ( 26 ) .
The melanoma outcomes data collected in this meta-analysis of randomized controlled trials involving statins or fibrates provided no support for the hypothesis that these drugs prevent melanoma when taken at low doses for managing hypercholesterolemia. Until further evidence is established, the most effective way to reduce the risk of melanoma remains limiting one's exposure to ultraviolet radiation.
This study was supported by grant T32 ARO7411 from the National Institutes of Health, Bethesda, MD (S. R. Freeman); grant 5 D14HP00153 from the Health Services Research Award Faculty Development in Primary Care (L. M. Schilling); grant K-07 CA92550, and start-up funds provided by the National Cancer Institute, Bethesda (R. P. Dellavalle); a Department of Veterans Affairs Epidemiology Research and Information Center Seed Grant; and start-up funds provided by the University of Colorado Cancer Center. Study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
We thank the following investigators for providing data for this meta-analysis: Helen Pater, Project Manager, Long-Term Intervention With Pravastatin in Ischemic Disease Follow-Up Study; Michal Benderly, PhD, Bezafibrate Infarction Prevention Study Coordinating Center; Markolf Hanefeld, DSc, Diabetes Intervention Study Corresponding Author; Paul Canner, PhD, Coronary Drug Project Study Corresponding Author; Ian Ford, PhD, West of Scotland Coronary Protection Study Study Corresponding Author; Klaus Wenke, MD, PhD, SHT Study Corresponding Author; Hanna Bloomfield Rubins, MD, MPH, Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Corresponding Author; Frank Sacks, MD, Cholesterol and Recurrent Events Trial (CARE) Study Corresponding Author; Hans-Joachim Lanz and Chen-Sheng Lin, Bristol-Myers Squibb for CARE Study Information; Tom Meade and Jackie Cooper, Lower Extremity Arterial Event Reduction Trial Study Authors; H. A. Dewar, MD, Newcastle Study Corresponding Author; Terje R. Pedersen, MD, Scandanavian Simvastatin Survival Study (4S) Study, and Jonathan Tobert, MD, PhD, Merck Research Laboratories for 4S Study Information; Folkert W. Asselbergs, MD, Prevention of Renal Vascular Endstage Disease Intervention Trial Investigator; Jane Armitage, Heart Protection Study Clinical Coordinator; Michael Oliver, MD, World Health Organization Study and Scottish Society of Physicians Study Principal Author; Hallvard Holdaas, MD, Assessment of Lescol in Renal Transplantation Study Corresponding Author; Barry R. Davis, MD, PhD, Antihyperintensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Corresponding Author; Ulf de Faire, MD, PhD, Bezafibrate Coronary Atherosclerosis Intervention Trial Study Corresponding Author; Paul M. Ridker, MD, Pravastatin Inflammation/CRP Evaluation Corresponding Author; Hans-Richard Arntz, MD, Randomized Lipid-Coronary Artery Disease Study Corresponding Author; Vasilios Athyros, MD, Greek Atorvastatin and Coronary-heart-disease Evaluation Corresponding Author; R. S. Elkeles, MD, St Mary's, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention Corresponding Author; Thomas Luscher, MD, FRCP, FACC, Evaluation of Nifedipine and Cerivastatin on Recovery of Coronary Endothelial Function Corresponding Author; Professor Sandro Gentile, MD, PhD, Gentile Corresponding Author; Dr Roberto Marchioli, Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico Corresponding Author; Mikko Syvanne, MD, Lipid Coronary Angiography Trial Corresponding Author; and Dr Earl R. Feringa, Final Report of the Veterans Administration Cooperative Study of Atherosclerosis Corresponding Author. We thank Kathryn R. Johnson, MD, and Eric J. Hester, MD, for extracting data from the primary studies, and Kristy Lundahl, MBA, MS, for manuscript preparation. We thank Hywel Williams, MSc, PhD, FRCP, and Jo Leonardi-Bee, MSc, PhD, for statistical advice, and Finola Delamere, PhD, for search strategy advice.
R. P. Dellavalle has received an independent, peer-reviewed Atorvastatin Research Award funded by Pfizer Pharmaceuticals, which manufactures statins, to support research exploring gene expression in tumors from persons exposed and unexposed to statins. He also owns 100 common shares of Merck stock, another manufacturer of statins.
This paper is a copublication of a systematic review published in The Cochrane Library (2005) (see http://www.thecochranelibrary.com for information) (24) .
References
Fumagalli R, Grossi E, Paoletti P, Paoletti R. Studies on lipids in brain tumors.
Littman ML, Taguchi T, Mosbach EH. Effect of cholesterol free, fat free diet, and hypocholesterolemic agents on growth of transplantable animal tumors.
Versluis AJ, van Geel PJ, Oppelaar H, van Berkel TJ, Bijsterbosch MK. Receptor-mediated uptake of low-density lipoprotein by B16 melanoma cells in vitro and in vivo in mice.
Lenz M, Miehe WP, Vahrenwald F, Bruchelt G, Schweizer P, Girgert R. Cholesterol based antineoplastic strategies.
Jani JP, Specht S, Stemmler N, Blanock K, Singh SV, Gupta V, et al. Metastasis of B16F10 mouse melanoma inhibited by lovastatin, an inhibitor of cholesterol biosynthesis.
Prassanna P, Thibault A, Liu L, Samid D. Lipid metabolism as a target for brain cancer therapy: synergistic activity of lovastatin and phenylacetate against glioma cells.
Shellman YG, Kelly D, Ribble D, Miller L, Gendall J, VanBuskirk K, et al. Lovastatin-induced apoptosis in human melanoma cell lines.
Grabacka M, Placha W, Plonka PM, Pajak S, Urbanska K, Laidler P, et al. Inhibition of melanoma metastases by fenofibrate.
Wong WW-L, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis.
Marcelli M, Cunningham GR, Haidacher SJ, Padayatty SJ, Sturgis L, Kagan C, et al. Caspace-7 is activated during lovastatin-induced apoptosis of the prostate cancer cell line LNCaP.
Xia Z, Tann MM, Wong WW, Dimitroulakos J, Minden MD, Penn LZ. Blocking protein geranylgeranylation is essential for lovastatin-induced apoptosis of human acute myeloid leukemia cells.
Argawal B, Rao CV, Bhendwal S, Ramey WR, Shirin H, Reddy BS, et al. Lovastatin augments sulindac-induced apoptosis in colon cancer cells and potentiates chemopreventive effects of sulindac.
Rao S, Porter DC, Chen X, Herliczek T, Lowe M, Keyomarsi K. Lovastatin-mediated G1 arrest is through inhibition of the proteasome, independent of hydroxymethyl glutaryl-CoA reductase.
Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins.
Sleijfer S, van der Gaast A, Planting AS, Stoter G, Verweij J. The potential of statins as part of anti-cancer treatment.
Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study.
Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high density lipoprotein cholesterol. The Veterans Affairs High Density Lipoprotein Cholesterol Intervention Trial Study Group.
Thibault A, Samid D, Tompkins AC, Figg WD, Cooper MR, Hohl RJ, et al. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer.
Criqui MH. Cholesterol, primary and secondary prevention, and all-cause mortality.
Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials.
Dellavalle RP, Drake A, Graber M, Heilig LF, Hester EJ, Johnson KR, et al. Statins and fibrates for preventing melanoma.
Petitti DB. Meta-analysis, decision analysis and cost-effectiveness analysis. 2nd ed. Oxford (U.K.): Oxford University Press;
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis.
Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. Section 8. In: The Cochrane Library, Issue 3,
Albert MA, Danielson E, Rifai N, Ridker PM, PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study.
Arntz HR, Agrawal R, Wunderlich W, Schnitzer L, Stern R, Fischer F, et al. Beneficial effects of pravastatin (+/−colestyramine/niacin) initiated immediately after a coronary event (the randomized Lipid-Coronary Artery Disease [L-CAD] study).
Athyros VG, Papageorgiou AA, Mercouri BR, Athyros VV, Symeonidis AN, Basayannis EO, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study.
Carmena R, Deoya M, Gomezgerique J, Mata P, Serrano S, Franco M, et al. Pravastatin, cholestyramine, and bezafibrate in patients with heterozygous familial hypercholesterolemia: the Spanish Multicenter Pravastatin Study.
Gentile S, Turco S, Guarino G, Sasso CF, Amodio M, Magliano P, et al. Comparative efficacy study of atorvastatin vs simvastatin, pravastatin, lovastatin and placebo in type 2 diabetic patients with hypercholesterolaemia.
GISSI Study Group. Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico).
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S).
Sacks FM, Pfeffer MA, Moye LA, Rolleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels.
Marschner IC, Colquhoun D, Simes RJ, Glasziou P, Harris P, Singh BB, et al. Long-term risk stratification for survivors of acute coronary syndromes. Results from the Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Study. LIPID Study Investigators.
Dumont JM. Effect of cholesterol reduction by simvastatin on progression of coronary atherosclerosis: design, baseline characteristics, and progress of the Multicenter Anti-Atheroma Study (MAAS).
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group.
ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT).
Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial.
The ENCORE Investigators. Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease: the ENCORE I Study (Evaluation of Nifedipine and Cerivastatin On Recovery of coronary Endothelial function).
Kawaguchi A, Mitsudo K, Nobuyoshi M, Minamino R, Hayasaki K, Nakashima M, et al. Angiographic intervention trial using HMG CoA reductase inhibitor to evaluate retardation of obstructive multiple atheroma in West Japan (ATHEROMA study): rationale, design and baseline.
Santos AF, Keitel E, Bittar AE, Neumann J, Fuchs FD, Goldani JC, et al. Safety and efficacy of simvastatin for hyperlipidemia in renal transplant recipients: a double-blind, randomized, placebo-controlled study.
Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, et al. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST).
Ford I, Blauw GJ, Murphy MB, Shepherd J, Cobbe SM, Bollen EL, et al. Prospective Study of Pravastatin in the Elderly at Risk (PROSPER): screening experience and baseline characteristics.
Mercuri M, Bond MG, Sirtori CR, Veglia F, Crepaldi G, Feruglio FS, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic mediterranean population: the Carotid Atherosclerosis Italian Ultrasound Study.
West MS, Herd JA, Ballantyne CM, Pownall HJ, Simpson S, Gould L, et al. The Lipoprotein and Coronary Atherosclerosis Study (LCAS): design, methods, and baseline data of a trial of fluvastatin in patients without severe hypercholesterolemia.
Frick MH, Syvanne M, Nieminen MS, Kauma H, Majahalme S, Virtanen V, et al. Prevention of the angiographic progression of coronary and vein-graft atherosclerosis by gemfibrozil after coronary bypass surgery in men with low levels of HDL cholesterol. Lopid Coronary Angiography Trial (LOCAT) Study Group.
Ruotolo G, Ericsson CG, Tettamanti C, Karpe F, Grip L, Svane B, et al. Treatment effects on serum lipoprotein lipids, apolipoproteins and low density lipoprotein particle size and relationships of lipoprotein variables to progression of coronary artery disease in the Bezafibrate Coronary Atherosclerosis Intervention Trial (BECAIT).
The BIP Study Group. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study.
Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin.
Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease.
Meade T, Zuhrie R, Cook C, Cooper J. Bezafibrate in men with lower extremity arterial disease: randomised controlled trial.
Heady JA. A cooperative trial on the primary prevention of ischaemic heart disease using clofibrate: design, methods, and progress.
Hanefeld M, Fischer S, Schmechel H, Rothe G, Schulze J, Dude H, et al. Diabetes Intervention Study. Multi-intervention trial in newly diagnosed NIDDM.
Cesarone MR, Laurora G, De Sanctis MT, Pomante P, Belcaro G. Progression of lesions of the arterial wall evaluated by ultrasonic biopsy in asymptomatic subjects and in diabetic and hyperlipidemic patients treated with bezafibrate. A 4-year follow-up [in Italian].
Diabetes Atherosclerosis Intervention Study Investigators. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study [erratum in Lancet 2001;357:1890].
Elkeles RS, Diamond JR, Poulter C, Dhanjil S, Nicolaides AN, Mahmood S, et al. Cardiovascular outcomes in type 2 diabetes. A double-blind placebo-controlled study of bezafibrate: the St. Mary's, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention (SENDCAP) Study.
The Newcastle Clofibrate Study Group. Trial of clofibrate in the treatment of ischaemic heart disease. Five-year study by a group of physicians of the Newcastle upon Tyne region.
Scottish Society of Physicians. Ischaemic heart disease: a secondary prevention trial using clofibrate. Report by a research committee of the Scottish Society of Physicians.
The Veterans Administration Cooperative Study Group. The treatment of cerebrovascular disease with clofibrate. Final report of the Veterans Administration Cooperative Study of Atherosclerosis, Neurology Section.
Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis.
Smith R, Roberts I. Patient safety requires a new way to publish clinical trials.
Dellavalle RP, Nicholas MK, Schilling LM. Melanoma chemoprevention: a role for statins or fibrates?
Feleszko W, Zagozdzon R, Golab J, Jakobisiak M. Potentiated antitumor effects of cisplatin and lovastatin against MmB16 melanoma in mice.
Feleszko W, Mlynarczuk I, Olszewska D, Jalili A, Grzela T, Lasek W, et al. Lovastatin potentiates antitumor activity of doxorubicin in murine melanoma via an apoptosis-dependent mechanism.
Nordenberg J, Goldwasser I, Zoref-Shani E, Beery E, Sidi Y. Inhibition of B16 melanoma cell proliferation and alterations in p21 ras expression induced by interceptors of signal transduction pathways.
Francis SO, Mahlberg MJ, Johnson KR, Ming ME, Dellavalle RP. Melanoma chemoprevention. J Am Acad Dermatol. In press