-
PDF
- Split View
-
Views
-
Cite
Cite
Maria V. Grau, John A. Baron, Robert S. Sandler, Kristin Wallace, Robert W. Haile, Timothy R. Church, Gerald J. Beck, Robert W. Summers, Elizabeth L. Barry, Bernard F. Cole, Dale C. Snover, Richard Rothstein, Jack S. Mandel, Prolonged Effect of Calcium Supplementation on Risk of Colorectal Adenomas in a Randomized Trial, JNCI: Journal of the National Cancer Institute, Volume 99, Issue 2, 17 January 2007, Pages 129–136, https://doi.org/10.1093/jnci/djk016
Close - Share Icon Share
Abstract
Calcium supplementation has been shown to decrease the risk of recurrence of colorectal adenomas in randomized trials. However, the duration of this protective effect after cessation of active supplementation is not known.
In the Calcium Polyp Prevention Study, 930 subjects with a previous colorectal adenoma were randomly assigned from November 1988 through April 1992 to receive placebo or 1200 mg of elemental calcium daily for 4 years. The Calcium Follow-up Study was an observational phase of the trial that tracked adenoma occurrence for an average of 7 years after the end of randomized treatment and gathered information regarding the use of medications, vitamins, and supplements during that time. We obtained follow-up information for 822 subjects, 597 of whom underwent at least one colonoscopy after the end of study treatment and are included in this analysis. Generalized linear models were used to compute relative risks (RRs) and 95% confidence intervals (CIs) for the effect of randomized calcium treatment on risk of adenoma recurrence during the first 5 years after study treatment ended and during the subsequent 5 years. Statistical tests were two-sided.
During the first 5 years after randomized treatment ended, subjects in the calcium group still had a substantially and statistically significantly lower risk of any adenoma than those in the placebo group (31.5% versus 43.2%; adjusted RR = 0.63, 95% CI = 0.46 to 0.87, P = .005) and a smaller and not statistically significant reduction in risk of advanced adenomas (adjusted RR = 0.85, 95% CI = 0.43 to 1.69, P = .65). However, the randomized treatment was not associated with the risk of any type of polyp during the next 5 years. The findings were broadly similar when the analysis was restricted to subjects who did not report use of any calcium supplements after the treatment phase of the trial ended.
The protective effect of calcium supplementation on risk of colorectal adenoma recurrence extends up to 5 years after cessation of active treatment, even in the absence of continued supplementation.
In randomized trials, calcium supplementation has been found to reduce the risk of colorectal adenomas in individuals with a previous adenoma. However, the duration of the protective effect was not determined.
Participants in a randomized trial of calcium supplementation for colorectal adenoma prevention were followed for an average of 7 years after the end of randomized treatment.
During the first 5 years after randomized treatment ended, subjects in the calcium group continued to have a lower risk of adenomas (31.5%) than those in the placebo group (43.2%). However, after 5 years, no difference between groups was evident. The result was similar when the analysis was restricted to subjects who did not take calcium supplements.
The protective effect of calcium treatment on colorectal adenomas in persons at high risk of these tumors extends for up to 5 years beyond active treatment.
Data from follow-up colonoscopies were not available for all subjects, and the interval between the end of treatment and the follow-up colonoscopy varied. Information on dietary calcium intake during the follow-up period was not available.
To date, calcium is the only dietary substance that has been found to be chemopreventive against large bowel neoplasia in large clinical trials ( 1 , 2 ). In the Calcium Polyp Prevention Study, subjects with a recent history of colorectal adenomas were randomly assigned to calcium supplementation or placebo. Subjects in the calcium group had a statistically significant 17% lower risk of one or more colorectal adenomas and an even larger reduction in risk of advanced adenomas ( 2 , 3 ). However, it is not known whether continued calcium supplementation is required for ongoing suppression of carcinogenesis; nor is it known if there is a “rebound” increase in risk after cessation of use.
With these issues in mind, we conducted the Calcium Follow-up Study as an observational phase of the Calcium Polyp Prevention Study. The goal was to explore the effect of calcium supplementation on the occurrence of adenomas after the end of randomized treatment.
Subjects and Methods
Study Design
A detailed description of the Calcium Polyp Prevention Study design and its findings has been published previously ( 2 ). Briefly, the Calcium Polyp Prevention Study was a double-blind, placebo-controlled, randomized trial of calcium carbonate as a chemopreventive agent for colorectal neoplasia in subjects with a recent history of colorectal adenomas. The study involved six centers: the Cleveland Clinic Foundation, Dartmouth–Hitchcock Medical Center (also the Coordinating Center for the trial), the University of Southern California–Southern California Kaiser Permanente Medical Group, the University of Iowa, the University of Minnesota (also the Pathology Center for the trial), and the University of North Carolina. Human Subjects Committees at each participating institution approved the research, and all subjects provided written informed consent.
Eligible subjects had at least one histologically confirmed adenoma within 3 months before enrollment, with the large bowel mucosa entirely examined and judged free of remaining polyps. Information regarding demographic characteristics, medical history, and lifestyle habits was collected at enrollment. Diet was assessed with a validated semiquantitative food-frequency questionnaire ( 4 ). Participants were asked to forgo the use of calcium supplements or any multivitamin containing calcium while on study treatment.
Following eligibility screening and a 3-month placebo run-in period, 930 eligible subjects were randomly assigned during November 1988 through April 1992 to receive either placebo or 1200 mg of elemental calcium (3 g of calcium carbonate) daily. Participants underwent two follow-up colonoscopies, at 1 and 4 years after study entry. Every 6 months during the trial, subjects completed questionnaires regarding health problems, use of over-the-counter products and prescribed medications, and compliance with the study regimen. Recruitment began in November 1988, and randomized treatment ended in December 1996. Subjects were told their randomized treatment assignments in August 1997.
The aim of the Calcium Follow-up Study, a posttreatment observational phase of the Calcium Polyp Prevention Study, was to investigate the persistence of the protective effect of the study agent after cessation of active supplementation. In January 1999, 3–7 years after the end of the active treatment for individual subjects, we began sending the study participants annual follow-up questionnaires that addressed medical events and medication use since the cessation of active calcium supplementation (for the first questionnaire) or since the previous questionnaire (for subsequent questionnaires). The questionnaires, which were sent through September 2003, were designed to track the occurrence of important medical events, including colonoscopies, and the use of medications, vitamins, and other dietary supplements, including calcium.
Study coordinators attempted to conduct a telephone interview for participants who did not respond to the mailed questionnaire within 21 days. Failing that, the coordinators contacted a friend or relative who had been previously identified by each subject as a source of follow-up information. In such cases, we requested information about health conditions and posttrial endoscopies only, and we ended participation of that subject in the study.
For each colonoscopy a participant reported, we obtained copies of the endoscopy report and relevant pathology reports. Slides of each lesion removed from the bowel were sent to a study pathologist for uniform review regarding diagnosis and, for neoplastic lesions, the degree of dysplasia. If the local and study pathologists disagreed, we accepted the study pathologist's diagnosis. Advanced lesions were defined as adenomas with at least 25% villous component, those containing advanced dysplasia or invasive cancer, or those with an estimated diameter of at least 1 cm (as assessed by the endoscopist).
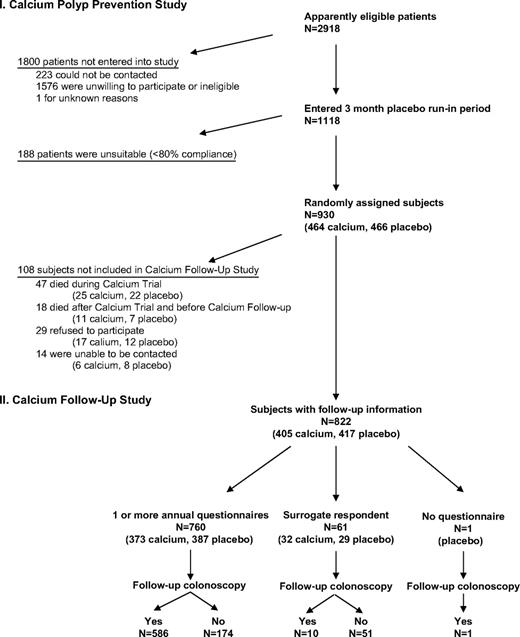
We attempted to contact all living participants in the Calcium Polyp Prevention Study ( Fig. 1 ). Of the 930 original participants, 47 had died during the treatment phase of the trial and 18 were found to have died after the end of treatment but before the follow-up study began. Of the remaining 865 subjects, 760 (88%) personally completed at least one follow-up questionnaire, 61 (7.1%) had a surrogate respondent, 29 (3.4%) declined participation in the follow-up, and 14 (1.6%) could not be contacted. Medical record review disclosed that one additional subject who had completed the treatment phase of the trial had had a subsequent colonoscopy but had died before completing any follow-up questionnaire. Of the 822 subjects for whom we obtained at least some posttreatment follow-up information, 597 (73%) underwent at least one colonoscopy after the end of randomized treatment. Study follow-up ended on September 30, 2003. The average time from the end of randomized treatment to the completion of the final questionnaire was 7.7 years (standard deviation [SD] = 1.8).
Trial flow diagram. The flow of subjects in the Calcium Polyp Prevention study is shown at top and that of subjects in the Calcium Follow-up Study is shown at bottom .
Statistical Analyses
Our primary objective was to explore the duration of the preventive effect of calcium supplementation on adenoma occurrence, using an intention-to-treat analysis based on the randomized treatment assignment for the active phase of the trial. Consequently, the 225 subjects without any colonoscopy after the end of treatment—and therefore for whom adenoma data were not available—were excluded, leaving 597 subjects for analysis. Because most surveillance colonoscopies are scheduled at 3- to 5-year intervals, we conducted separate and independent analyses for the first 5 years after cessation of randomized treatment and for the subsequent years of follow-up for a maximum of 5 additional years. All colonoscopies performed during each period were used to characterize polyp occurrence.
We used a contingency table and Pearson's chi-square test to compare the numbers of colonoscopies in the treatment groups and a two-sample t test to compare the mean times to first and last colonoscopies between the two treatment groups after the end of the treatment phase of the trial (excluding subjects without an examination). Overdispersed generalized linear models for the Poisson distribution as an approximation to the binomial family ( 5 ) were used to estimate crude and adjusted relative risks (RRs) of adenoma recurrence; covariates in the adjusted models were age, sex, study center, time after the end of the treatment phase up to the last colonoscopy in the posttreatment follow-up phase, and use of calcium supplements during any year after study treatment but before the first colonoscopy in the study period under analysis (first 5 years after cessation of treatment or subsequent years). In secondary analyses, we evaluated the risks of small tubular adenomas (<1 cm), advanced adenomas, and hyperplastic polyps. We determined the statistical significance of differences in the relative risks for small tubular adenomas and advanced adenomas by using generalized estimating equation methods ( 6 ), including both endpoints in a single model. All statistical tests were two-sided. Statistical computations were conducted using Stata 9 software (StataCorp, College Station, TX).
A subject's “regular use” of a medication, vitamin, or dietary supplement for a given year was defined as use for at least 75 days during the year (approximately twice a week for the entire year or once a day for 2.5 months). We were unable to assess accurately the doses of calcium supplements used during the follow-up period because it appeared that some subjects reported the amount of elemental calcium, whereas others reported the amount of the calcium salt. We therefore classified subjects as users of calcium supplements during the posttreatment phase of the trial if they reported regular use for at least 1 year during that period.
In addition to performing analyses stratified by follow-up period, i.e., before and after 5 years posttreatment, we also investigated relative risk trends over the entire observational phase and computed unadjusted and adjusted risk ratios independently for each year that was covered by follow-up questionnaires. The two sets of results were similar, and only the unadjusted findings are presented.
We explored the possibility that posttreatment calcium supplement use modified the effect of randomized treatment during the follow-up period by conducting an additional analysis that was restricted to subjects who did not take any calcium supplements during the follow-up phase before the first colonoscopy in the posttreatment period under consideration.
Because calcium supplementation reduced the risk of adenoma recurrence at the end of the treatment period, subjects who had been randomly assigned to receive calcium may have preferentially been allocated longer subsequent surveillance intervals and thus been omitted from the first 5-year follow-up period. This selection might have distorted our comparison of posttreatment adenoma occurrence in either of the two follow-up intervals. Therefore, we also compared adenoma risk between treatment groups in the subgroup of subjects without adenomas at the year 4 examination, i.e., at the end of the active trial.
Results
The characteristics of the subjects in the calcium treatment (N = 405) and placebo (N = 417) arms in the Calcium Follow-up Study were similar ( Table 1 ). There were no material differences with regard to age, sex, total months on active treatment in the calcium trial, total months of at least 50% compliance with the study treatment, and length of follow-up in the observational phase. During the posttreatment follow-up period, the two treatment groups were similar with regard to numbers of colonoscopies, time to first and last colonoscopy, and use of calcium supplements after the end of randomized treatment ( Table 1 ). Of the 822 subjects, 597 (73%) had at least one colonoscopy and 251 (30.5%) had two or more colonoscopies. The average time from the end of study treatment to the last colonoscopy in the follow-up study was 5.9 years (SD = 1.9 years). Of the 760 subjects who personally completed one or more posttreatment follow-up questionnaires (183 in the placebo group and 181 in the calcium group), 364 (48%) reported use of calcium supplements in the observational phase on at least one questionnaire. However, only 196 (25%) of these subjects (98 in each treatment group) reported calcium use during at least half of the posttreatment follow-up years ( Table 1 ).
Characteristics of the 822 subjects in the Calcium Polyp Prevention Study for whom follow-up data were available, by original treatment group *
| Characteristic . | Placebo (N = 417) * . | Calcium (N = 405) * . |
|---|---|---|
| Age at baseline in y, mean ± SD | 60.6 ± 9.1 | 60.5 ± 8.9 |
| Sex, No. (%) | ||
| Male | 288 (69) | 301 (74) |
| Female | 129 (31) | 104 (26) |
| Baseline dietary calcium intake in milligrams, mean ± SD | 865 ± 423 | 889 ± 451 |
| Months on randomly assigned treatment, mean ± SD | 44.9 ± 5.1 | 45.5 ± 6.5 |
| No. of lifetime adenomas at baseline, mean ± SD | 2.6 ± 2.9 | 2.4 ± 2.5 |
| Total months in the follow-up phase, † mean ± SD | 86.0 ± 31.8 | 84.9 ± 32.9 |
| Follow-up colonoscopies, No. (%) † | ||
| 0 | 114 (27) | 111 (27) |
| 1 | 171 (41) | 176 (43) |
| ≥2 | 132 (32) | 119 (29) |
| Time to first colonoscopy during the follow-up period after the end of randomized treatment in y, mean ± SD ‡ | 4.3 ± 1.7 | 4.6 ± 1.7 |
| Time to last colonoscopy during the follow-up period after the end of randomized treatment in y, mean ± SD ‡ | 6.0 ± 1.9 | 5.9 ± 1.8 |
| Calcium use during follow-up phase, No. (%) § | ||
| No use | 204 (53) | 192 (51) |
| Any use ‖ | 183 (47) | 181 (49) |
| Calcium use for at least half of the follow-up years, No. (%) ¶ | 98 (25) | 98 (26) |
| Characteristic . | Placebo (N = 417) * . | Calcium (N = 405) * . |
|---|---|---|
| Age at baseline in y, mean ± SD | 60.6 ± 9.1 | 60.5 ± 8.9 |
| Sex, No. (%) | ||
| Male | 288 (69) | 301 (74) |
| Female | 129 (31) | 104 (26) |
| Baseline dietary calcium intake in milligrams, mean ± SD | 865 ± 423 | 889 ± 451 |
| Months on randomly assigned treatment, mean ± SD | 44.9 ± 5.1 | 45.5 ± 6.5 |
| No. of lifetime adenomas at baseline, mean ± SD | 2.6 ± 2.9 | 2.4 ± 2.5 |
| Total months in the follow-up phase, † mean ± SD | 86.0 ± 31.8 | 84.9 ± 32.9 |
| Follow-up colonoscopies, No. (%) † | ||
| 0 | 114 (27) | 111 (27) |
| 1 | 171 (41) | 176 (43) |
| ≥2 | 132 (32) | 119 (29) |
| Time to first colonoscopy during the follow-up period after the end of randomized treatment in y, mean ± SD ‡ | 4.3 ± 1.7 | 4.6 ± 1.7 |
| Time to last colonoscopy during the follow-up period after the end of randomized treatment in y, mean ± SD ‡ | 6.0 ± 1.9 | 5.9 ± 1.8 |
| Calcium use during follow-up phase, No. (%) § | ||
| No use | 204 (53) | 192 (51) |
| Any use ‖ | 183 (47) | 181 (49) |
| Calcium use for at least half of the follow-up years, No. (%) ¶ | 98 (25) | 98 (26) |
Includes all randomly assigned subjects for whom follow-up information was available. “Baseline” refers to baseline of the Calcium Polyp Prevention Study. SD = standard deviation.
Follow-up phase is defined as the period from the end of randomized treatment to the completion of the last annual follow-up questionnaire.
Among the 597 subjects with at least one colonoscopy in the follow-up period.
Among the 760 subjects who personally completed at least one annual follow-up questionnaire.
Any questionnaire reporting use of calcium supplements at least 1 year after the end of the Calcium Polyp Prevention Study.
Use of calcium supplements for at least half of the follow-up years with questionnaire information.
Characteristics of the 822 subjects in the Calcium Polyp Prevention Study for whom follow-up data were available, by original treatment group *
| Characteristic . | Placebo (N = 417) * . | Calcium (N = 405) * . |
|---|---|---|
| Age at baseline in y, mean ± SD | 60.6 ± 9.1 | 60.5 ± 8.9 |
| Sex, No. (%) | ||
| Male | 288 (69) | 301 (74) |
| Female | 129 (31) | 104 (26) |
| Baseline dietary calcium intake in milligrams, mean ± SD | 865 ± 423 | 889 ± 451 |
| Months on randomly assigned treatment, mean ± SD | 44.9 ± 5.1 | 45.5 ± 6.5 |
| No. of lifetime adenomas at baseline, mean ± SD | 2.6 ± 2.9 | 2.4 ± 2.5 |
| Total months in the follow-up phase, † mean ± SD | 86.0 ± 31.8 | 84.9 ± 32.9 |
| Follow-up colonoscopies, No. (%) † | ||
| 0 | 114 (27) | 111 (27) |
| 1 | 171 (41) | 176 (43) |
| ≥2 | 132 (32) | 119 (29) |
| Time to first colonoscopy during the follow-up period after the end of randomized treatment in y, mean ± SD ‡ | 4.3 ± 1.7 | 4.6 ± 1.7 |
| Time to last colonoscopy during the follow-up period after the end of randomized treatment in y, mean ± SD ‡ | 6.0 ± 1.9 | 5.9 ± 1.8 |
| Calcium use during follow-up phase, No. (%) § | ||
| No use | 204 (53) | 192 (51) |
| Any use ‖ | 183 (47) | 181 (49) |
| Calcium use for at least half of the follow-up years, No. (%) ¶ | 98 (25) | 98 (26) |
| Characteristic . | Placebo (N = 417) * . | Calcium (N = 405) * . |
|---|---|---|
| Age at baseline in y, mean ± SD | 60.6 ± 9.1 | 60.5 ± 8.9 |
| Sex, No. (%) | ||
| Male | 288 (69) | 301 (74) |
| Female | 129 (31) | 104 (26) |
| Baseline dietary calcium intake in milligrams, mean ± SD | 865 ± 423 | 889 ± 451 |
| Months on randomly assigned treatment, mean ± SD | 44.9 ± 5.1 | 45.5 ± 6.5 |
| No. of lifetime adenomas at baseline, mean ± SD | 2.6 ± 2.9 | 2.4 ± 2.5 |
| Total months in the follow-up phase, † mean ± SD | 86.0 ± 31.8 | 84.9 ± 32.9 |
| Follow-up colonoscopies, No. (%) † | ||
| 0 | 114 (27) | 111 (27) |
| 1 | 171 (41) | 176 (43) |
| ≥2 | 132 (32) | 119 (29) |
| Time to first colonoscopy during the follow-up period after the end of randomized treatment in y, mean ± SD ‡ | 4.3 ± 1.7 | 4.6 ± 1.7 |
| Time to last colonoscopy during the follow-up period after the end of randomized treatment in y, mean ± SD ‡ | 6.0 ± 1.9 | 5.9 ± 1.8 |
| Calcium use during follow-up phase, No. (%) § | ||
| No use | 204 (53) | 192 (51) |
| Any use ‖ | 183 (47) | 181 (49) |
| Calcium use for at least half of the follow-up years, No. (%) ¶ | 98 (25) | 98 (26) |
Includes all randomly assigned subjects for whom follow-up information was available. “Baseline” refers to baseline of the Calcium Polyp Prevention Study. SD = standard deviation.
Follow-up phase is defined as the period from the end of randomized treatment to the completion of the last annual follow-up questionnaire.
Among the 597 subjects with at least one colonoscopy in the follow-up period.
Among the 760 subjects who personally completed at least one annual follow-up questionnaire.
Any questionnaire reporting use of calcium supplements at least 1 year after the end of the Calcium Polyp Prevention Study.
Use of calcium supplements for at least half of the follow-up years with questionnaire information.
The association of randomized calcium treatment assignment with the occurrence of neoplastic polyps after the end of study treatment was assessed for the first 5 years after active treatment and for the subsequent 5 years ( Table 2 ). During the first 5 years after active treatment, 347 subjects had at least one follow-up colonoscopy. Those who had been randomly assigned to calcium had a markedly reduced risk of all adenomas (31.5% versus 43.2%; adjusted RR = 0.63, 95% confidence interval [CI] = 0.46 to 0.87, P = .005). The risk of small tubular adenomas was similarly reduced (adjusted RR = 0.58, 95% CI = 0.41 to 0.84, P = .003), but the reduction in risk of advanced adenomas was smaller and not statistically significant (adjusted RR = 0.85, 95% CI = 0.43 to 1.69, P = .65). However, the difference between these two relative risks was compatible with chance ( P = .15). In the subsequent years of the observational follow-up—i.e., 5–10 years after study treatment—424 subjects had at least one colonoscopy. There was no effect of study treatment on the risk of all adenomas (adjusted RR = 1.09, 95% CI = 0.85 to 1.39, P = .511) or of advanced adenomas (adjusted RR = 1.10, 95% CI = 0.65 to 1.88, P = .717) during that period ( Table 2 ).
Effect of calcium treatment assignment in the Calcium Polyp Prevention Study on the risk of adenomas and advanced adenomas during the first 5 years of the follow-up study and during subsequent years, for all subjects and for subjects who did not take calcium supplements before the first colonoscopy in the follow-up period *
| . | First 5 years after treatment . | . | . | Subsequent 5–10 years after treatment . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenoma type † . | No. of subjects in placebo group . | No. of subjects in calcium group . | Adjusted RR ‡ (95% CI) . | No. of subjects in placebo group . | No. of subjects in calcium group . | Adjusted RR ‡ (95% CI) . | ||||||
| All subjects | ||||||||||||
| All adenomas | 80 | 51 | 0.63 (0.46 to 0.87) | 82 | 82 | 1.09 (0.85 to 1.39) | ||||||
| Small tubular adenomas | 71 | 44 | 0.58 (0.41 to 0.84) | 69 | 69 | 1.11 (0.83 to 1.47) | ||||||
| Advanced adenomas | 23 | 21 | 0.85 (0.43 to 1.69) | 26 | 26 | 1.10 (0.65 to 1.88) | ||||||
| Total No. of subjects | 185 | 162 | 216 | 208 | ||||||||
| Subjects who did not take calcium supplements before the first colonoscopy in the follow-up period | ||||||||||||
| All adenomas | 48 | 28 | 0.59 (0.40 to 0.90) | 50 | 53 | 1.05 (0.77 to 1.43) | ||||||
| Small tubular adenomas | 43 | 26 | 0.62 (0.41 to 0.94) | 45 | 44 | 0.98 (0.69 to 1.38) | ||||||
| Advanced adenomas | 14 | 10 | 0.62 (0.29 to 1.33) | 15 | 18 | 1.18 (0.57 to 2.43) | ||||||
| Total No. of subjects | 114 | 102 | 123 | 121 | ||||||||
| . | First 5 years after treatment . | . | . | Subsequent 5–10 years after treatment . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenoma type † . | No. of subjects in placebo group . | No. of subjects in calcium group . | Adjusted RR ‡ (95% CI) . | No. of subjects in placebo group . | No. of subjects in calcium group . | Adjusted RR ‡ (95% CI) . | ||||||
| All subjects | ||||||||||||
| All adenomas | 80 | 51 | 0.63 (0.46 to 0.87) | 82 | 82 | 1.09 (0.85 to 1.39) | ||||||
| Small tubular adenomas | 71 | 44 | 0.58 (0.41 to 0.84) | 69 | 69 | 1.11 (0.83 to 1.47) | ||||||
| Advanced adenomas | 23 | 21 | 0.85 (0.43 to 1.69) | 26 | 26 | 1.10 (0.65 to 1.88) | ||||||
| Total No. of subjects | 185 | 162 | 216 | 208 | ||||||||
| Subjects who did not take calcium supplements before the first colonoscopy in the follow-up period | ||||||||||||
| All adenomas | 48 | 28 | 0.59 (0.40 to 0.90) | 50 | 53 | 1.05 (0.77 to 1.43) | ||||||
| Small tubular adenomas | 43 | 26 | 0.62 (0.41 to 0.94) | 45 | 44 | 0.98 (0.69 to 1.38) | ||||||
| Advanced adenomas | 14 | 10 | 0.62 (0.29 to 1.33) | 15 | 18 | 1.18 (0.57 to 2.43) | ||||||
| Total No. of subjects | 114 | 102 | 123 | 121 | ||||||||
RR = relative risk; CI = confidence interval.
Some subjects had both small tubular adenomas and advanced adenomas.
For analysis of all subjects, relative risks were adjusted for age, sex, center, follow-up time, number of colonoscopies from the end of the active trial up to the last colonoscopy in the follow-up period under consideration, and calcium supplement use before the first colonoscopy in the follow-up period under consideration. For analysis of subjects who did not take calcium supplements before the first colonoscopy in the follow-up period, relative risks were adjusted for age, sex, center, follow-up time, and number of colonoscopies from the end of the active trial up to the last colonoscopy in the follow-up period under consideration.
Effect of calcium treatment assignment in the Calcium Polyp Prevention Study on the risk of adenomas and advanced adenomas during the first 5 years of the follow-up study and during subsequent years, for all subjects and for subjects who did not take calcium supplements before the first colonoscopy in the follow-up period *
| . | First 5 years after treatment . | . | . | Subsequent 5–10 years after treatment . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenoma type † . | No. of subjects in placebo group . | No. of subjects in calcium group . | Adjusted RR ‡ (95% CI) . | No. of subjects in placebo group . | No. of subjects in calcium group . | Adjusted RR ‡ (95% CI) . | ||||||
| All subjects | ||||||||||||
| All adenomas | 80 | 51 | 0.63 (0.46 to 0.87) | 82 | 82 | 1.09 (0.85 to 1.39) | ||||||
| Small tubular adenomas | 71 | 44 | 0.58 (0.41 to 0.84) | 69 | 69 | 1.11 (0.83 to 1.47) | ||||||
| Advanced adenomas | 23 | 21 | 0.85 (0.43 to 1.69) | 26 | 26 | 1.10 (0.65 to 1.88) | ||||||
| Total No. of subjects | 185 | 162 | 216 | 208 | ||||||||
| Subjects who did not take calcium supplements before the first colonoscopy in the follow-up period | ||||||||||||
| All adenomas | 48 | 28 | 0.59 (0.40 to 0.90) | 50 | 53 | 1.05 (0.77 to 1.43) | ||||||
| Small tubular adenomas | 43 | 26 | 0.62 (0.41 to 0.94) | 45 | 44 | 0.98 (0.69 to 1.38) | ||||||
| Advanced adenomas | 14 | 10 | 0.62 (0.29 to 1.33) | 15 | 18 | 1.18 (0.57 to 2.43) | ||||||
| Total No. of subjects | 114 | 102 | 123 | 121 | ||||||||
| . | First 5 years after treatment . | . | . | Subsequent 5–10 years after treatment . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenoma type † . | No. of subjects in placebo group . | No. of subjects in calcium group . | Adjusted RR ‡ (95% CI) . | No. of subjects in placebo group . | No. of subjects in calcium group . | Adjusted RR ‡ (95% CI) . | ||||||
| All subjects | ||||||||||||
| All adenomas | 80 | 51 | 0.63 (0.46 to 0.87) | 82 | 82 | 1.09 (0.85 to 1.39) | ||||||
| Small tubular adenomas | 71 | 44 | 0.58 (0.41 to 0.84) | 69 | 69 | 1.11 (0.83 to 1.47) | ||||||
| Advanced adenomas | 23 | 21 | 0.85 (0.43 to 1.69) | 26 | 26 | 1.10 (0.65 to 1.88) | ||||||
| Total No. of subjects | 185 | 162 | 216 | 208 | ||||||||
| Subjects who did not take calcium supplements before the first colonoscopy in the follow-up period | ||||||||||||
| All adenomas | 48 | 28 | 0.59 (0.40 to 0.90) | 50 | 53 | 1.05 (0.77 to 1.43) | ||||||
| Small tubular adenomas | 43 | 26 | 0.62 (0.41 to 0.94) | 45 | 44 | 0.98 (0.69 to 1.38) | ||||||
| Advanced adenomas | 14 | 10 | 0.62 (0.29 to 1.33) | 15 | 18 | 1.18 (0.57 to 2.43) | ||||||
| Total No. of subjects | 114 | 102 | 123 | 121 | ||||||||
RR = relative risk; CI = confidence interval.
Some subjects had both small tubular adenomas and advanced adenomas.
For analysis of all subjects, relative risks were adjusted for age, sex, center, follow-up time, number of colonoscopies from the end of the active trial up to the last colonoscopy in the follow-up period under consideration, and calcium supplement use before the first colonoscopy in the follow-up period under consideration. For analysis of subjects who did not take calcium supplements before the first colonoscopy in the follow-up period, relative risks were adjusted for age, sex, center, follow-up time, and number of colonoscopies from the end of the active trial up to the last colonoscopy in the follow-up period under consideration.
To evaluate the possibility that preferential allocation of subjects in the calcium group to longer surveillance intervals could have distorted our findings, we repeated the analyses among the 308 subjects with no adenomas at the last study colonoscopy in the active phase of the trial (140 in the placebo group and 168 in the calcium group). The reduction in risk of recurrence of all adenomas in the first 5 years after treatment among subjects randomly assigned to calcium was very similar to that in the overall analysis (adjusted RR = 0.65, 95% CI = 0.37 to 1.16, P = .15).
The effect of randomized calcium supplementation on the risk of hyperplastic polyps after the active intervention period ended was similar to that for all adenomas. There was a non–statistically significant 40% reduction in risk of hyperplastic polyps in the first 5 years after treatment (adjusted RR = 0.60, 95% CI = 0.35 to 1.03, P = .07) but no reduction in the subsequent years (adjusted RR = 1.16, 95% CI = 0.76 to 1.77, P = .49).
In another analysis, we isolated the effect of randomized calcium treatment from that of calcium supplements taken after the end of the treatment phase by restricting the analysis to the 216 subjects (114 placebo, 102 calcium) who did not take calcium during the follow-up period from the end of active treatment up to their first colonoscopy in the first posttreatment period and the 244 participants who did not take any supplements up to the first colonoscopy in the subsequent period (123 in the placebo arm and 121 in the calcium arm) ( Table 2 ). Among these subjects, randomized calcium treatment during the trial caused a marked and statistically significant reduction in risk of all adenomas (adjusted RR = 0.59, 95% CI = 0.40 to 0.90) and of small tubular adenomas (adjusted RR = 0.62, 95% CI = 0.41 to 0.94) during the first 5 years after treatment. There was also a strong but not statistically significant reduction in risk of advanced adenomas (adjusted RR = 0.62, 95% CI = 0.29 to 1.33) during the first 5 years. As was the case for all subjects, however, there was no risk reduction from calcium in the subsequent years.
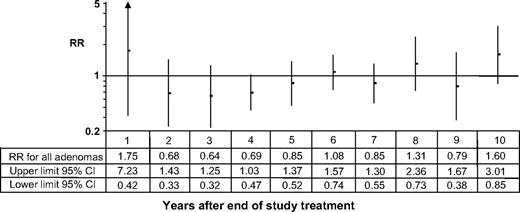
The relative risks for adenoma occurrence in each year of the follow-up study are consistent with the results for the two aggregated follow-up periods (i.e., the first and second 5 years after the active phase) ( Fig. 2 ). Calcium reduced the risk of all adenomas in 4 of the first 5 years after the end of randomized treatment, although with the small numbers of endpoints the individual relative risks are not statistically significant. (For example, only 13 subjects underwent colonoscopy during the first year after active treatment.) In each of the next 5 years, there tended to be no substantial reduction in risk.
Relative risk of adenoma recurrence by year of follow-up after the cessation of randomized treatment. The relative risks (RRs) and 95% confidence intervals (CIs) are illustrated on the graph by points and lines , respectively. The point estimates and upper and lower limits of the 95% confidence intervals are shown beneath the graph.
Discussion
In this posttreatment follow-up of the Calcium Polyp Prevention Study ( 2 ), we found that the chemopreventive effect of calcium on colorectal adenoma occurrence continued for 5 years after the cessation of active supplementation. Indeed, the risk reduction in calcium-treated subjects was more pronounced during the 5 years after active treatment than it was during active treatment, a pattern that was also seen for hyperplastic polyps. Active calcium treatment did not have a statistically significant effect on the risk of advanced adenomas during this time period, although the confidence interval for the reduction in risk was consistent with that for small tubular adenomas, and the effect was more marked when subjects who took calcium supplements during follow-up were omitted from the analysis. These results suggest that the benefits of calcium extend for several years after the agent is stopped, although they do not last indefinitely.
Extensive experimental and observational data have generally indicated that high calcium intake exerts protective effects on colorectal neoplasia ( 7 – 21 ). The strongest support for a protective effect has come from randomized chemoprevention trials in subjects with a previous adenoma. As we reported previously ( 2 , 3 ), during the treatment phase of our trial, calcium treatment reduced the risk of any incident adenoma by 17% (95% CI = 2% to 33%), and there was a more pronounced effect on risk of lesions with advanced features (35% risk reduction, 95% CI = 7% to 54%). Very similar findings regarding all adenomas were reported from a smaller European trial ( 1 ), in which calcium supplementation (2 g elemental calcium/day) conferred a 25% (95% CI = −29% to 57%) reduction in risk of adenomas.
In the Calcium Polyp Prevention Study, we found that during active supplementation, the protective effect of calcium was stronger for advanced adenomas than for all adenomas, although the difference was not statistically significant ( 3 ). It is not clear why, during the first 5 years after the end of treatment, the effect of calcium was stronger for all adenomas than for advanced adenomas. Lack of statistical power is one reasonable hypothesis: it is certainly possible that in reality the effect is similar for both types of lesions and that chance explains the apparent differences in the two analyses. However, other studies have found differences in the way in which early adenomas, advanced adenomas, and carcinomas respond to various environmental factors ( 22 – 24 ), and it is also possible that the extended effect of calcium is actually weaker for advanced lesions than for the earlier ones.
This study demonstrated a residual benefit from calcium supplementation that persisted for 5 years after subjects stopped treatment. The persistence of the effect is a provocative finding, but one that is difficult to explain. It suggests that a 4-year course of calcium treatment alters the colorectal mucosa in such a way that it can resist the development of new adenomas that would otherwise become apparent several years later. In any case, the mechanisms responsible for a protective effect of calcium are not clear. Two possible explanations have been hypothesized. One mechanism relates to calcium's ability to bind and precipitate bile acids and other free fatty acids in the lumen of the bowel ( 25 , 26 ), rendering them relatively inert and inhibiting the mucosal inflammation and proliferation that may be caused by these substances ( 26 – 30 ). Recently, another mechanism has been proposed that involves a direct effect of calcium through the calcium sensing receptor (CaSR), a cell surface receptor that is expressed on colonocytes ( 31 , 32 ). In vitro, activation of the CaSR has anticarcinogenic downstream effects ( 33 ), including increased expression of E-cadherin, p21, and p27 ( 34 , 35 ). Moreover, extracellular calcium itself seems to increase the expression of the CaSR ( 35 ). Because approximately 70% of the calcium in a supplement is typically excreted in the gastrointestinal tract ( 36 ), subjects who take calcium supplements daily for an extended period of time will inevitably have a steady increase of calcium concentration in the lumen that could stimulate the mucosal CaSRs and lead to a temporary suppression of proliferation as well as to other anticarcinogenic effects.
Hyperplastic polyps have recently been implicated in colorectal carcinogenesis ( 3 , 37 ), but relatively little is known about their epidemiology. It is now accepted that the serrated lesions often labeled “hyperplastic polyps” are actually heterogeneous, comprising hyperplastic polyps, serrated adenomas, sessile serrated polyps, and other variants, each of which probably has different implications for carcinogenesis ( 3 , 37 ). However, particularly during the treatment phase of the trial, we did not distinguish these lesions from each other, and in any case, we would not have had enough statistical power to analyze them individually. There are no prior observational data regarding the association of calcium supplement use with the development of hyperplastic polyps, although—consistent with the data from the treatment phase of our study ( 2 , 3 )—dietary calcium intake has been associated with a reduced risk of such lesions ( 38 ). The data we present here indicate that the suppressive effect of calcium on hyperplastic polyps resembles that for adenomas in showing a heightened reduction in risk in the first 5 years after treatment ended, with no reduction in risk thereafter.
Our study has several strengths. Most of our analyses were based on randomized treatments, and the self-reported adherence to study regimen was excellent, even in the last year of study treatment [80% of subjects reported taking the study agent 90%–100% of the time ( 2 )]. A total of 865 subjects were alive at the time the subjects were first contacted for this follow-up study, and we obtained some kind of posttreatment information for 822 (93%) of them. We tracked postintervention colonoscopies closely, and a single study pathologist provided a uniform blinded endpoint review. We also took into account the use of calcium supplements during the follow-up period. The main analysis was divided into two periods, the first 5 years after the end of the randomized treatment and the subsequent years. We chose 5 years as the cut point because most of the surveillance colonoscopies were scheduled within 3–5 years from the last examination. In addition, the pattern of relative risks over the follow-up period also supports this strategy in that it showed a difference between the relative risks before and after the 5-year threshold. The fact that our findings were similar among all subjects and those who did not have adenomas at the end of active treatment suggests that the allocation of surveillance intervals during the observational period did not introduce an important bias.
However, the study also has some limitations. Only 597 subjects in the Calcium Follow-up Study (73%) underwent at least one colonoscopy, and there was no uniform interval between the end of the active trial and the first posttreatment colonoscopy. We did not have accurate details about the frequency of use and dose of elemental calcium in the supplements taken during the observational postintervention phase; therefore, we were unable to evaluate comprehensively associations with the use of calcium supplements after the study treatment had ended. Moreover, we did not collect information regarding dietary calcium intake during follow-up, and any differences between the two randomly assigned groups in dietary calcium intake could have been a source of bias in our analysis. However, such differences are unlikely because the two treatment groups did not differ in baseline dietary calcium intake or in their use of calcium supplements during the follow-up period ( Table 1 ). Thus, the subjects' knowledge of their treatment status during the active phase of the trial seems to have had little effect on their subsequent behaviors.
To summarize, our study provides further evidence of the potential of calcium as a chemopreventive agent against colorectal adenomas among individuals with a history of these tumors. Our data indicate that, in these patients, the protective effect of calcium may extend for up to 5 years after the cessation of active treatment.
In addition to the authors, the Calcium Polyp Prevention Study Group also included the following: Investigators: L. A. Mott (Dartmouth Medical School), D. Howell (Maine Medical Center), J. Church (Cleveland Clinic Foundation), and J. Truszkowski (University of Iowa); Data and Safety Monitoring Committee: F. M. Giardiello (John Hopkins University), W. C. Willet (Harvard University), J. Lachin (George Washington University), L. J. Roberts (Vanderbilt University School of Medicine), and J. Grizzle (Fred Hutchinson Cancer Research Center); Polyp Prevention Studies Coordinating Center: L. H. Pearson, S. G. Ewell, D. Carmichael, J. E. Hebb, and S. Pearson; Study Coordinators: H. Hasson and J. Bauman (Cleveland Clinic); K Wood, L. E. Wettemann, and M. Hynes (Dartmouth Hitchcock Medical Center); R. Thompson and D. Finke (University of Iowa); J. Blomquist, D. Hostetler, and S. Waldemar (University of Minnesota); B. Schliebe (University of North Carolina); and L. Cherman-Gerstmann, P. Harmon, A. Montes, and N. Uk (University of Southern California).
Supported by grants 5 U01 CA46927 and R01 CA98286 from the National Institutes of Health (to J. A. Baron). Calcium and placebo tablets were provided by Lederle (now Wyeth), Pearl River, NY. John A. Baron served as a speaker supported by Wyeth in 2005. The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
We are indebted to the study subjects and their physicians for their cooperation and enthusiasm and to the study coordinators at the clinical sites.
References
Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group.
Baron J, Beach M, Mandel J, Van Stolk R, Haile R, Sandler R, et al. Calcium supplements for the prevention of colorectal adenomas.
Wallace K, Baron JA, Cole BF, Sandler RS, Karagas MR, Beach MA, et al. Effect of calcium supplementation on the risk of large bowel polyps.
Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing.
McCullagh P, Nelder JA. Generalized linear models. 2nd ed. London (U.K.): Chapman and Hall;
Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models.
Appleton GV, Davies PW, Bristol JB, Williamson RC. Inhibition of intestinal carcinogenesis by dietary supplementation with calcium.
Pence BC, Buddingh F. Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3.
Pence BC, Dunn DM, Zhao C, Landers M, Wargovich MJ. Chemopreventive effects of calcium but not aspirin supplementation in cholic acid-promoted colon carcinogenesis: correlation with intermediate endpoints.
Sitrin MD, Halline AG, Abrahams C, Brasitus TA. Dietary calcium and vitamin D modulate 1,2-dimethylhydrazine-induced colonic carcinogenesis in the rat.
Wargovich M, Allnutt D, Palmer C, Anaya P, Stephens L. Inhibition of the promotional phase of azoxymethane-induced colon carcinogenesis in the rat by calcium lactate: effect of simulating two human nutrient density levels.
Slattery ML, Sorenson AW, Ford MH. Dietary calcium intake as a mitigating factor in colon cancer.
Kampman E, Goldbohm R, Van Den Brandt P, Van't Veer P. Fermented dairy products, calcium, and colorectal cancer in the Netherlands Cohort Study.
Neugut A, Horvath K, Whelan R, Terry M, Garbowski G, Bertram A, et al. The effect of calcium and vitamin supplements on the incidence and recurrence of colorectal adenomatous polyps.
White E, Shannon JS, Patterson RE. Relationship between vitamin and calcium supplement use and colon cancer.
Whelan RL, Horvath KD, Gleason NR, Forde KA, Treat MD, Teitelbaum SL, et al. Vitamin and calcium supplement use is associated with decreased adenoma recurrence in patients with a previous history of neoplasia.
Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women's Health Study.
Kearney J, Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, et al. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men.
Martinez M, Giovannucci E, Colditz G, Stampfer M, Hunter D, Speizer F, et al. Calcium, vitamin D, and the occurrence of colorectal cancer among women.
Zheng W, Anderson KE, Kushi LH, Sellers TA, Greenstein J, Hong CP, et al. A prospective cohort study of intake of calcium, vitamin D, and other micronutrients in relation to incidence of rectal cancer among postmenopausal women.
Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men.
Boutron-Ruault MC, Senesse P, Meance S, Belghiti C, Faivre J. Energy intake, body mass index, physical activity, and the colorectal adenoma-carcinoma sequence.
Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men.
Terry MB, Neugut AI, Bostick RM, Sandler RS, Haile RW, Jacobson JS, et al. Risk factors for advanced colorectal adenomas: a pooled analysis.
Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis.
Van der Meer R, Lapre JA, Govers MJ, Kleibeuker JH. Mechanisms of the intestinal effects of dietary fats and milk products on colon carcinogenesis.
Nobre-Leitao C, Chaves P, Fidalgo P, Cravo M, Gouveia-Oliveira A, Ferra MA, et al. Calcium regulation of colonic crypt cell kinetics: evidence for a direct effect in mice.
Reshef R, Rozen P, Fireman Z, Fine N, Barzila M, Shasha S, et al. Effect of a calcium-enriched diet on the colonic epithelial hyperproliferation induced by N-methyl-N′-nitro-N-nitrosoguanidine in rats on a low calcium and fat diet.
Wargovich MJ, Eng VW, Newmark HL, Bruce WR. Calcium ameliorates the toxic effect of deoxycholic acid on colonic epithelium.
Bird RP, Schneider R, Stamp D, Bruce WR. Effect of dietary calcium and cholic acid on the proliferative indices of murine colonic epithelium.
Kallay E, Kifor O, Chattopadhyay N, Brown EM, Bischof MG, Peterlik M, et al. Calcium-dependent c-myc proto-oncogene expression and proliferation of Caco-2 cells: a role for a luminal extracellular calcium-sensing receptor.
Sheinin Y, Kallay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa.
Kallay E, Bajna E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Dietary calcium and growth modulation of human colon cancer cells: role of the extracellular calcium-sensing receptor.
Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation.
Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: interaction with Ca(2+) and 1,25-dihydroxyvitamin D(3).
Buset M, Lipkin M, Winawer S, Swaroop S, Friedman E. Inhibition of human colonic epithelial cell proliferation in vivo and in vitro by calcium.
Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept.