-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher G. McCusker, Nicola N. Doherty, Bernadette Molloy, Nichola Rooney, Connor Mulholland, Andrew Sands, Brian Craig, Moira Stewart, Frank Casey, A Randomized Controlled Trial of Interventions to Promote Adjustment in Children With Congenital Heart Disease Entering School and Their Families, Journal of Pediatric Psychology, Volume 37, Issue 10, November/December 2012, Pages 1089–1103, https://doi.org/10.1093/jpepsy/jss092
Close - Share Icon Share
Abstract
Objective To report on a randomized controlled trial of psychological interventions to promote adjustment in children with congenital heart disease and their families. Method Following baseline assessment, 90 children (aged 4–5 years) and their families were randomly assigned to an Intervention or Control group before entering school. 68 (76%) were retained at 10-month follow-up. Results Gains were observed on measures of maternal mental health and family functioning. Although no differences were found on measures of child behavior at home or school, children in the intervention group were perceived as “sick” less often by their mother and missed fewer days from school. A regression model, using baseline measures as predictors, highlighted the importance of maternal mental health, worry and child neurodevelopmental functioning for child behavioral outcomes almost a year later. Conclusions The intervention promoted clinically significant gains for the child and family. The program is of generalizable significance.
Accumulating evidence has highlighted that children with significant congenital heart disease (CHD) are at increased risk for problems with behavioral adjustment and cognitive functioning (Bellinger & Newburger, 2010). Problems in the domains of anxiety, depression, attention, social cognition, and relationships have been noted (Bellinger et al., 2009; Gupta, Mitchell, Giuffre, & Crawford 2001; Miatton, De Wolf, Francois, Thiery, & Vingerhoets, 2007; Shillingford et al., 2008), with some suggestions that difficulties increase with age (Karsdorp, Everaerd, Kindt, & Mulder, 2007) and remain manifest in young adulthood (Van Rijen et al., 2005). Although many children with CHD have a “normal” outcome, studies suggest that approximately 20% (more than twice that of healthy peers) fall into the clinically significant range on inventories of psychopathology (Bellinger & Newburger, 2010; Karsdorp, Everaerd, Kindt, & Mulder, 2007). Similarly, although most are within the average range in intellectual functioning, neurodevelopmental delays have been reliably reported, especially within the domains of sensorimotor, visuospatial and language competencies (Menahem, Poulakis, & Prior, 2008; Shillingford & Wernovsky, 2004).
Key risk factors have been postulated. Open heart surgery, together with cyanotic status (i.e., whether the heart defect has compromised lung oxygenation of the blood), have been predictive of neuropsychological deficits (Karsdorp, Everaerd, Kindt, & Mulder, 2007; Miatton, De Wolf, Francois, Thiery, & Vingerhoets, 2007; Shillingford & Wernovsky, 2004). However, some studies have suggested neurodevelopmental outcomes are compromised in children with significant CHD, regardless of cyanosis (Majnemer et al., 2009; Simons, Glidden, Sheslow, & Pizarro, 2010) and have remained evident across historical cohorts, despite advances in surgical techniques and neuroprotective strategies (Spijkerboer, Utens, Bogers, Helbing, & Verhulst, 2008). This may suggest underlying congenital etiologies are at play.
Such studies may also suggest that psychosocial processes, such as the response within families of having a child with a life-threatening illness, are important (Brosig, Mussatto, Kuhn, & Twedell, 2007; McCusker et al., 2007; Shillingford et al., 2008). Certainly in terms of behavioral outcomes, research increasingly suggests that family, and especially maternal, factors (e.g., worry, mental health, subjective perceptions of severity, and indices of family functioning) may be more important than illness severity or surgical factors in determining outcomes (Bellinger et al., 2009; Casey et al., 2010; De Maso et al., 1991; Goldberg et al., 1997; McCusker et al, 2007). Unfortunately, evidence also suggests that mothers and families of children with CHD are themselves at elevated risk for psychological difficulties (Doherty et al., 2009; Vrijmoet-Wiersma, Ottenkamp, van Roozendaal, Grootenhuis, & Koopman, 2009), thus potentiating the risk amplitude of their children. This underlies the rationale for the current study. If maternal and family functioning are compromised, but important in mediating outcomes for these children, then psychological interventions that bolster psychological adjustment and coping in parents, offer a potentially productive focus for study.
Reviews of the evidence for the effectiveness of psychological interventions for children with chronic illness and their families (Barlow & Ellard, 2004; Beale, 2006; Drotar, 2006) have been encouraging. The strongest evidence appears to be for cognitive behavioral interventions for disease management (e.g., to manage pain or promote adherence to treatment), although there have also been positive outcomes reported for interventions (e.g., problem solving and family therapies) designed to promote resilience and adjustment, and reduce distress, in the affected child and family (Kazak, 2005; Sahler et al., 2002; Stein & Jessop, 1991; Stehl et al., 2009). Nevertheless, the evidence base remains emergent. Positive findings are often circumscribed rather than comprehensive across outcome domains studied (Meijssen, Wolf, Koldewijn, van Wassenear, & van Baar, 2010; Stehl et al., 2009), effect sizes vary significantly (Beale, 2006), control groups and follow-up periods beyond 6 months are often lacking (Barlow & Ellard, 2004), and theoretical frameworks and mechanisms of action too infrequently inform intervention design and outcomes studied (Drotar, 2006).
In reviewing the literature specific to CHD, Bellinger and Newburger (2010) highlight the fact that, despite the now extensive literature on determinants of psychosocial and neurodevelopmental outcomes for these children and their families, formal intervention trials have barely begun. De Maso, Gonzalez- Heydrich, Erikson, Grimes, & Strohecker (2000) described high satisfaction ratings, reduced perceptions of social isolation, and increased hope and understanding following a computer-based, narrative therapy, intervention for mothers of children with CHD. However, formal outcome measures and a control group were not included in this study. Our own controlled intervention trial, the Congenital Heart Disease Intervention Programme (CHIP)–Infant study (McCusker, et al., 2009), targeted at parents of infants recently diagnosed with significant CHD and aimed at bolstering mother–infant transactions through psychoeducation, parent skills training, and narrative therapy, did show significant gains for infant mental development, feeding, maternal worry, and anxiety at 6-month follow-up. The present study will present findings at 10-month follow-up for the CHIP–School study—a similar program but adapted to the developmental transition of starting school.
CHIP–School, like the infant study, was underpinned by Thompson’s transactional stress and coping framework (Thompson, Gustafson, Hamlett, & Spock, 1992). This posits that appraisal and coping within the family system, and especially within the mother, mediates the impact of the illness on child outcomes. The CHD literature summarized earlier is consistent with this model. Thus in the CHIP–School study, interventions were essentially about bolstering parenting skills in relation to both general developmental challenges and those specific to parenting a child with CHD, elucidating and challenging unhelpful appraisals and assumptions, and training parents in “problem prevention therapy” to identify and resolve worries and fears. The specific methods used, and adjunctive elements to the program, are described later in the text. The primary purpose of this study was to report on the outcomes following this trial for child and family adjustment.
A secondary aim was to evaluate which factors at baseline assessment (preschool) predicted child adjustment at the end of their first year at school. Although a number of studies have addressed the general question of determinants of outcome, most have been cross-sectional in nature, and few have focused explicitly on periods of key developmental transition.
Methods
Overview of Study Design
This was a randomized controlled trial with three annual recruitment periods (June–August each year, 2001–2003). Following recruitment and baseline assessment (Time 1—T1), participating families were randomly assigned to the CHIP–School Intervention group, or treatment as usual, Control group. Interventions occurred in the first 2 months of the child’s first year at school. Time 2 (T2) data were collected for all participants at the end of the first year at school (on average 10 months after baseline assessment). The main hypotheses were that participation in the Intervention group would result in improved adjustment for the child and mother compared with those in the Control group. Primary outcome measures were child behavioral adjustment and maternal mental health. Secondary outcome measures included days sick, days absent from school, school functioning, maternal worry, health status, and impact of the illness on the family. Baseline factor scores at T1 related to illness, surgery, child and family factors that were regressed against child adjustment at T2 to also examine prospective determinants of outcome.
Participants
Families were recruited for a 3-year period and through a regional center for pediatric cardiology in the UK. Eligibility criteria included having a child who was starting school the following September and who had undergone at least one invasive procedure (open or closed heart surgery) for correction or palliation of a major heart defect (acyanotic and cyanotic). Children with diagnosed neurodevelopmental syndromes were excluded for two reasons. First, we wished to keep the sample as homogenous as possible for CHD, and such syndromes often have behavioral features as part of their phenotypes (Harris, 2008), which could exert independent and interacting effects with CHD on child and maternal adjustment. Second, children with such comorbid presentations present unique challenges and, if included, our intervention protocols would have to have been adjusted or extended for relevance. While this would have been possible, we wished to test the prima facie case for the impact of such a program for children with CHD and their families in the first instance. However, children with other comorbid physical illnesses (e.g., asthma and diabetes) were not excluded, as these were highly prevalent (see later in the text) and were not deemed to require significant modifications to the intervention protocols.
Written invitations came from the consultant pediatric cardiologist known to the family, with responses returned to the research team. Information and consent forms were explicit in outlining the randomized intervention, and follow-up assessment, aspects of the project. Families were informed that if randomized to the control condition, they would continue to receive their usual care in the year between baseline and follow-up assessment. This included regular medical review as dictated by the child’s condition with referral on to any of the other multidisciplinary services if required. The study was approved by the regional ethics committee and complied with the institutional governance framework.
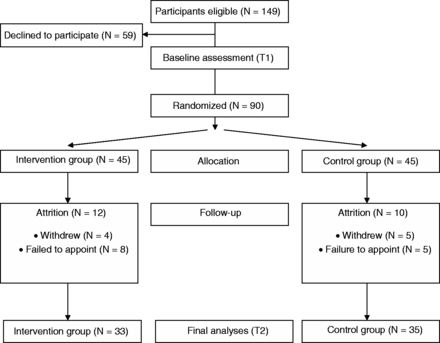
Of the 149 eligible families identified by the pediatric cardiologists from the patient database, 90 agreed to participate. Participants did not differ from non-participants in terms of child age, gender, cyanotic status, surgical procedure, palliative status, or deprivation index scores (all p > .05). Mean age at T1 assessment was 4.6 years (SD = 0.3), and mean time since main operative procedure was 3.8 years (SD = 0.9). Full details of the participants at baseline assessment are outlined and discussed in McCusker et al. (2007).
Following T1 assessment, the 90 families were randomly assigned to either the CHIP–School Intervention or Control group using the Chit method for randomization (Singh, 2006). T2 assessments were conducted at the end of the child’s first year at school (mean time between T1 and T2 = 10.1 months; SD = 0.9; range 9–14 months). Twenty-two families were lost to follow-up (nine withdrew, and for the other 13, it proved impossible to schedule an assessment within the T2 appointment period). Sixty-eight families (76% of baseline sample) were thus retained at T2, 33 in the Intervention, and 35 in the Control group.
Figure 1 summarizes the participation flowchart. Final sample and group characteristics are described later in the text. On the basis of a 0.5 standardized mean difference between groups as indicative of clinical relevance on both primary outcome measures (Child Behavior Checklist [CBCL] and Brief Symptom Inventory (BSI) described later), this final sample size allowed power of 0.9 at p = .05.
Fowchart of enrollment, allocation, attrition, and final group analyses.
Procedures and Intervention
CHIP–School interventions were underpinned by the transactional stress and coping model described earlier (Thompson et al., 1992), by specific knowledge bases as indicated later in the text, and by preliminary discussions with former parent service users from a national children’s heart charity. Formulated by the authored program team, which included academic and clinical practitioners from clinical psychology, pediatric cardiology, pediatrics, and nursing, the intervention included a 1-day workshop for parents, a bicycle exercise stress test, and a follow-up session with families individually. Interventions were delivered at the regional center and always in the same order as described later in the text. An accompanying program manual and personalized fact sheets for parents and community health and education professionals supported the interventions as follows:
1-Day Workshop
A 5-hour group workshop for parent(s) of 9–12 participants occurred for each of the three annual cohorts. Following an overview of the rationale for the intervention and outline of the day, there were three sections to this:
Problem Prevention Therapy: Through small group work and larger group discussion, parents were enabled to define salient, or previously unspoken, worries about their child with CHD, their parenting, and overall family impact. Worries and fears were collated and clustered. The principles and stages of problem-solving therapy (D’Zurrila & Nezu, 1999; Sahler et al., 2002) were introduced, and facilitators modelled this framework using a DO ACT acronym: Define problem and turn into a specific goal(s); Option brainstorm; Assess pros and cons of various option; Choose a strategy; Take action and evaluate. Parents then had a chance to work together in small groups applying the procedure to their own worries and fears, with some facilitation and guidance from the therapists. Typical worries related to promoting independence, managing challenging behaviors in a child with a “weak” heart, safe activity levels for the child, knowing when the child is “really sick,” ensuring siblings did not feel neglected, and what to tell the child about their condition. This approach aimed to promote problem-solving skills in a way that would be generalizable beyond the worries of the current developmental period.
Psychoeducation: this section provided a medical review of diagnoses, treatments, prognoses, and outcomes. The emphasis was on normalization and promoting activity and independence in the child. Future scenarios regarding negotiating issues of health insurance, employment, fertility, and pregnancy were discussed. In addition, every family received a personalized factsheet (rather than the generic condition specific factsheets available in the unit) prepared by the pediatric cardiologists. Such individualized information sheets were used to render information more personally relevant, credible, and memorable (Kreuter & Holt, 2001).
Parenting skills: The afternoon sessions of the workshop focused on parenting skills with links made with the worry list generated earlier in the day. Generic parent training skills (e.g., child-centerd play and effective communication, praise, and motivation and basic reinforcement principles) together with skills specific to parenting a child with CHD (preparation for medical procedures, information giving, liaison with school, looking after the rest of the family, and so forth) were discussed and applied to specific scenarios raised by participants. Across scenarios therapists highlighted unhelpful beliefs (e.g., “my child has been through too much to worry about discipline,” “lying about what is happening will protect my child’s feelings,” and so forth.) and gently challenged as roadblocks to effective parenting.
In addition to the personalized factsheets, families were furnished with an illustrated program manual, summarizing all aspects of the workshop. Additional resources and information related to the workshop content were included.
A Bicycle Exercise Stress Test
One of the most pervasive worries apparent in the workshop related to what was a “safe” level of physical activity for the child. This was consistent with the current literature (Moola, Fusco, & Kirch, 2011). Sessions were scheduled 1–4 weeks after the parent workshop, with all children in the Intervention group and their parents, where they underwent a specially designed bicycle exercise stress test. This was considered important to objectively review the child’s exercise capability but, more importantly, to demonstrate to parents observing the test that their child could perform vigorous exercise safely, thus challenging fears about the risks of physical activity. The bicycle was designed to include a lighting panel that became more illuminated as a “reward” for increasing effort and duration of exercise performed by the child. Continuous ECG monitoring was performed during the exercise and recovery period, and cardiologists drew attention to the continuing normality of the ECG rhythms, despite increased heart rates and prolonged exertion.
An Individual Parental Session
These occurred 1–4 weeks after the bicycle test and were facilitated by one of the project psychologists (ND). This session had three foci: (a) reviewing parental experiences and gains from the workshop and bicycle test, (b) reviewing how they had been applying problem prevention therapy to date and helping them with future worry applications, and (c) psychoeducation to promote the psychological health of siblings and parents of children with a chronic illness.
Outreach to Community Services
Finally, the personalized factsheets described earlier were circulated to general medical practitioners, community pediatricians, and school teachers. Although there is little literature on this, the parent service users consulted during project development had suggested that a significant source of confusion and unease for them had been the relative ignorance about complex CHD in these non-specialist, but “first-line” educational and health professionals. This had sometimes led to overly cautious advice, anxiety-provoking referral back to the regional center, and over-protection and lowered expectations at school. The personalized fact sheets to these professionals were deemed to be helpful in affirming a normalization ethos in management.
The program was delivered consistently by two pediatric clinical psychologists, three pediatric cardiologists, and a pediatric cardiology nurse specialist. Although no ratings of treatment fidelity were undertaken, having the same therapists and health professionals deliver the same sessions across the 3 years maximized standardization of delivery. Where therapist–family interactions varied appropriately in the follow-up individual sessions, supervision and case note review of the psychologist who facilitated these sessions (ND) by the senior consultant psychologist (CMC) in the project, helped ensure interventions remained within the aims and parameters of these sessions as described.
Measures
Preschool Predictor Variables
Most of the studies examining determinants of behavioral outcomes for children with CHD, as outlined earlier, have been cross-sectional in nature. From these, however, a number of factors of predictive significance have been identified and were thus of theoretical interest for the prospective regression analyses conducted here. These included illness and surgical factors, child factors, and family factors. Specific variables assessed at T1 were operationalized as follows:
Illness/Surgery Factors
Cyanotic status: Children’s diagnoses as derived from the medical chart suggested their lesion(s) were either acyanotic (blood oxygenation not compromised) or cyanotic (blood oxygenation compromised). This was a dichotomous variable.
Surgery: This was another dichotomous variable that indicated whether the surgery or surgeries undertaken involved cardiopulmonary bypass (open) surgery or not (closed surgery). Information pertaining to this was taken from surgical records.
Palliative status: Again a dichotomous variable, extracted from surgical and medical records, this indicated whether the lesion(s) had been fully corrected or not (palliation only).
Total number of surgical procedures and total number of days in hospital (attained from medical records) were continuous variables and were used as indicators of disease severity.
Child Factors
Gender
Comorbid illness: Although children with neurodevelopmental syndromes were excluded as discussed earlier, there was a 38% prevalence (26/68) of other comorbid illnesses in our sample, such as asthma and diabetes. For the purposes of this analysis, participants were dichotomously categorized as having, or not having, another chronic illness.
Cognitive functioning: Two cognitive factor scores (one related to verbal skills and one related to perceptual-motor skills) were extracted by a principal component analysis of performance on 10 specific cognitive scales taken from the Wechsler Preschool and Primary Scale of Intelligence (Wechsler, 1989) and A Developmental Neuropsychological Assessment (NEPSY;Korkman, Kirk, & Kemp, 1998). These had been assessed at T1 and before study randomization. Further details of these cognitive scales, cohort profile scores, and the principal component analysis may be found in our article related to the baseline attributes of these children (McCusker et al., 2007).
Family Factors
Maternal mental health: This was assessed using the BSI (Derogatis, 1993). The BSI is a 53-item self-report symptom inventory (likert scored) designed to measure psychological symptoms in psychiatric, medical, and community samples. There are nine subscale dimensions, but in the current study, only the Global Severity Index (GSI) T score was used. The author reports the stability coefficient for the GSI score as approximately 0.9 across a range of studies with medical and psychiatric populations, with it also demonstrating satisfactory internal consistency (0.71–0.85 across symptom dimensions) and validity (when assessed against other clinical and psychometric rating scales of psychopathology).
Maternal worry: This was assessed using the Maternal Worry Scale (De Vet & Ireys, 1998), which is an 11-item (likert scored) self-report scale, designed to measure levels of worry (rather than psychopathology per se) in mothers of children with chronic illness. The authors describe good internal consistency (0.94), test–retest reliability (0.84), and satisfactory construct validity in relation to measures of maternal mental health and child adjustment. The measure was completed at T1 and also T2.
Family composition: A dichotomous variable, this represented whether one or two parents/guardians were living at home with the child at T1.
Maternal and paternal employment: These were dichotomous variables coded as employed (full or part time) or not at T1.
Deprivation index score: This was a continuous variable that reflects the degree of socio-economic deprivation in the neighbourhood area indexed by the family post code (zip code) at T1 (Townsend, Phillimore, & Beattie, 1988).
Outcome Measures—Child Adjustment
The CBCL (Achenbach, 1991) has been the most extensively used instrument by authors investigating behavioral outcomes following CHD. Although there are three global and seven specific subscales in this 113-item behavior problem inventory, only the Total Problem Behavior T-score was used in analyses here. T scores > 63 were deemed to be in the clinically significant range as per author guidance. It was completed by mothers at both T1 and T2, and as well as examining T-score changes across time, changes in the proportion of children in the clinically significant range were also examined. Although some items of the Internalizing scale have been criticized for confounding somatic features when applied to pediatric illness populations (Perrin, Stein, & Drotar, 1991), the CBCL has been subject to extensive psychometric investigations with generally satisfactory internal and test–retest reliability and validity data reported.
The Comprehensive Behavior Rating Scale for Children (CBRSC; Neeper, Lahey, & Frick, 1990) is a 70-item (likert scored) teacher rating scale of school functioning. This measure was chosen in preference to the teacher version of the CBCL, as it has 9-scaled T-scores that not only relate to behavioral adjustment (Oppositional-Conduct Disorders, Motor Hyperactivity, Anxiety, Sluggish Tempo, Daydreaming, and Social Competence) but also to academic and cognitive competencies (Inattention-Disorganization, Reading Problems, and Cognitive Deficits). Test–retest reliability indices for subscales are reported as high (0.84–0.97) with satisfactory validity assessments against criterion groups and other commonly used measures. This was completed by teachers at T2 (i.e., at the end of the first year at school).
Study Questionnaire Items—Parents and teachers were also asked to complete the following:
Days “sick”—at both T1 and T2, parents were asked to record the number of days in the previous 3 months that they perceived their child to have been “sick.” This was defined as “in need of medical attention” at T1 and “in need of medical attention (e.g., sufficient to warrant time off school)” at T2.
Days off school—at T2 only, teachers were asked to record the number of days the child had missed school during the previous year.
Remedial help—at T2, teachers were asked to record whether the child was in receipt of any remedial help at school for literacy, numeracy, or behavior needs. This was a dichotomous variable.
Physical Education (PE) participation—again at T2, teachers were asked to record whether the child’s PE participation was comparable with, or below, other children in the same class. This was a dichotomous variable.
Outcome Measures—Family Functioning
As well as being used as a predictor variable at T1 for child adjustment at T2, the BSI (Derogatis, 1993) was the primary outcome measure of maternal mental health at T2. Psychometric properties are described as aforementioned. GSI T-scores were used to examine differential changes from T1 to T2 in mothers in both groups, together with the proportion of each group in the clinically significant range (T > 63). Responses were received from too few fathers across both time periods to analyse.
Similarly, the Maternal Worry Scale (De Vet & Ireys, 1998) was used at both T1 and T2, and psychometric properties are described earlier in the text. This secondary outcome measure was deemed particularly relevant to the CHIP–School interventions related to problem solving, as described earlier, and had been shown to be reduced in mothers following the CHIP–Infant study (McCusker et al., 2009). Differential changes in maternal scores between groups from T1 to T2 were assessed.
The Impact on Family Scale (Stein & Reissman, 1980) is a 24-item (likert scored) self-report scale designed specifically to measure the perceived impact of having a child with a chronic illness on four domains of family functioning—financial (e.g., loss of income through care requirements), family strain (e.g., restrictions imposed by illness on family activities), personal strain (e.g., perceived burden of care on the self), and mastery (e.g., illness experience bringing the family closer and promoting enhanced competencies). The authors describe satisfactory reliability and validity with a median internal reliability of 0.81 reported for mothers of children with chronic illness. This secondary outcome scale was completed by the mother in the current study at both T1 and T2.
Study Questionnaire Items
Parents were asked to record the following additional secondary outcomes at T2:
Whether they had experienced any significant physical illnesses since T1, and this was defined as “in need of medical attention.”
Whether they had experienced any significant emotional or psychological difficulties since T1, and this was defined as “in need of medical, psychological, or psychiatric attention.”
Acceptability of Program
Around 2–3 weeks after completing the CHIP–School intervention, a program evaluation questionnaire was forwarded to mothers where they rated the degree to which each program element (i.e., workshop, manual, bicycle exercise test, factsheet, and one-to-one session) was helpful on a 5-point likert scale. In addition, mothers were asked to endorse five forced choice alternative statements related to the program objectives of increasing knowledge about CHD, reducing worry about the child’s future, reducing worry about the child’s physical health, improving confidence about safe activity levels, and improving confidence in parenting skills (e.g., “I feel just as worried about my child’s future as before the CHIP sessions” OR “I feel less worried about my child’s future than before the CHIP sessions”). Finally, open-ended feedback was requested that was content analysed into themes.
Overview of Statistical Analyses
Unfortunately, responses were not received from fathers in sufficient quantities at T2 to analyse, and thus T1 to T2 changes are computed for data returned from mothers only. Data met assumptions for parametric analyses, and the statistical and clinical significance of any differential change on the outcome measures detailed earlier from T1 to T2 was computed using a 2 × 2 mixed analysis of variance, where the statistical significance of the interaction term was of interest together with the associated measure of clinical significance, partial eta squared. Eta squared is also reported as a check on the reliability on the partial eta-squared statistic (Levine & Hullet, 2002). In addition, the clinical significance of outcomes was further assessed by comparing the proportion of children, in both groups, in the clinically significant range of the CBCL at T1 and T2. Similar analyses were computed for the proportion of mothers in the clinically significant range of the BSI at T1 and T2. These were the primary outcome measures, and hypotheses were that child and maternal adjustment factor scores would improve across time in the Intervention compared with the Control group. Additional secondary outcome measures related to these domains were included as outlined earlier. Where such outcome measures were only recorded at T2 (e.g., school functioning measures), t-tests were used to compare differences between groups. Bonferonni corrections were applied where there were several statistical analyses within a given construct domain (e.g., maternal adjustment and impact on family scales).
Three multiple linear regressions were conducted with each group of factors assessed at T1 for their predictive significance for child adjustment at T2 (i.e., the illness/surgery, child and family T1 factors outlined earlier). Factors were included as informed by previous cross-sectional research as noted earlier. Those factor scores that emerged as having unique statistical significance from each domain were included in a higher order regression analysis to determine the proportion of variance they predicted collectively.
Results
Final Group Characteristics
Those lost to follow-up from T1 to T2 (N = 22) did not differ from those retained (N = 68) in terms of cyanotic status, surgery, co-morbid illness, gender, deprivation index scores, family composition, or parental employment (all p > .3). There were differences between the two groups on some variables at T1. Families who were lost to follow-up tended to have children with higher levels of behavioral problems (CBCL) and mothers with higher maternal worry and GSI scores (all p < .05). The potential influence of these differences is considered later in the text.
Of the final retained sample, 33 were in the Intervention and 35 in the Control group. Although attrition inevitably compromises randomization, the final groups did not differ from each other in terms of cyanotic status, surgical procedure, palliative status, total number of procedures, days in hospital, comorbid illness, gender, deprivation index scores, family composition, or parental employment (Table I). Nor did they differ on any of the T1 predictor variables of interest noted earlier (all p > .05).
Demographic and Medical Characteristics of Intervention and Control Groups
| . | Intervention (N = 33) . | Control (N = 35) . | p . |
|---|---|---|---|
| Child age (N) | 33 | 35 | |
| Mean years at T2 (SD) | 5.5 (0.3) | 5.4 (0.3) | .71 |
| Child gender (N) | 33 | 35 | |
| Male | 24 (73%) | 20 (59%) | .23 |
| Cyanotic Status (N) | 33 | 35 | .49 |
| Acyanotic | 20 (61%) | 24 (69%) | |
| Cyanotic | 13 (39%) | 11 (31%) | |
| Surgical procedure (N) | 33 | 34 | .92 |
| Open | 20 (61%) | 21 (62%) | |
| Closed | 13 (39%) | 13 (38%) | |
| Palliative status (N) | 33 | 35 | .4 |
| Corrected | 28 (84%) | 32 (91%) | |
| Palliative | 5 (15%) | 3 (9%) | |
| Total days in hospital (N) | 33 | 34 | |
| Mean days (SD) | 18.3 (23.1) | 25.4 (21.6) | .19 |
| Total number of procedures (N) | 33 | 34 | |
| Mean days (SD) | 1.94 (1.2) | 1.85 (1.2) | .77 |
| Other childhood illness (N) | 33 | 35 | .19 |
| None | 23 (70%) | 19 (54%) | |
| Any other | 10 (30%) | 16 (46%) | |
| Family composition (N) | 33 | 35 | .58 |
| Lone parent | 6 (18%) | 5 (14%) | |
| Two parents at home | 27 (82%) | 30 (86%) | |
| Maternal employment (N) | 33 | 35 | |
| Not employed | 15 (46%) | 18 (51%) | |
| Employed | 18 (54%) | 17 (49%) | |
| Paternal employment (N) | 32 | 34 | .64 |
| Not employed | 4 (13%) | 2 (6%) | |
| Employed | 27 (87%) | 31 (94%) | |
| Deprivation index scorea (N) | 33 | 33 | .41 |
| Mean Townsend score (SD) | 0.081 (2.62) | 0.716 (3.53) |
| . | Intervention (N = 33) . | Control (N = 35) . | p . |
|---|---|---|---|
| Child age (N) | 33 | 35 | |
| Mean years at T2 (SD) | 5.5 (0.3) | 5.4 (0.3) | .71 |
| Child gender (N) | 33 | 35 | |
| Male | 24 (73%) | 20 (59%) | .23 |
| Cyanotic Status (N) | 33 | 35 | .49 |
| Acyanotic | 20 (61%) | 24 (69%) | |
| Cyanotic | 13 (39%) | 11 (31%) | |
| Surgical procedure (N) | 33 | 34 | .92 |
| Open | 20 (61%) | 21 (62%) | |
| Closed | 13 (39%) | 13 (38%) | |
| Palliative status (N) | 33 | 35 | .4 |
| Corrected | 28 (84%) | 32 (91%) | |
| Palliative | 5 (15%) | 3 (9%) | |
| Total days in hospital (N) | 33 | 34 | |
| Mean days (SD) | 18.3 (23.1) | 25.4 (21.6) | .19 |
| Total number of procedures (N) | 33 | 34 | |
| Mean days (SD) | 1.94 (1.2) | 1.85 (1.2) | .77 |
| Other childhood illness (N) | 33 | 35 | .19 |
| None | 23 (70%) | 19 (54%) | |
| Any other | 10 (30%) | 16 (46%) | |
| Family composition (N) | 33 | 35 | .58 |
| Lone parent | 6 (18%) | 5 (14%) | |
| Two parents at home | 27 (82%) | 30 (86%) | |
| Maternal employment (N) | 33 | 35 | |
| Not employed | 15 (46%) | 18 (51%) | |
| Employed | 18 (54%) | 17 (49%) | |
| Paternal employment (N) | 32 | 34 | .64 |
| Not employed | 4 (13%) | 2 (6%) | |
| Employed | 27 (87%) | 31 (94%) | |
| Deprivation index scorea (N) | 33 | 33 | .41 |
| Mean Townsend score (SD) | 0.081 (2.62) | 0.716 (3.53) |
aTownsend scores—deprivation index scores from zip codes (Townsend, Phillimore, & Beattie, 1988).
Demographic and Medical Characteristics of Intervention and Control Groups
| . | Intervention (N = 33) . | Control (N = 35) . | p . |
|---|---|---|---|
| Child age (N) | 33 | 35 | |
| Mean years at T2 (SD) | 5.5 (0.3) | 5.4 (0.3) | .71 |
| Child gender (N) | 33 | 35 | |
| Male | 24 (73%) | 20 (59%) | .23 |
| Cyanotic Status (N) | 33 | 35 | .49 |
| Acyanotic | 20 (61%) | 24 (69%) | |
| Cyanotic | 13 (39%) | 11 (31%) | |
| Surgical procedure (N) | 33 | 34 | .92 |
| Open | 20 (61%) | 21 (62%) | |
| Closed | 13 (39%) | 13 (38%) | |
| Palliative status (N) | 33 | 35 | .4 |
| Corrected | 28 (84%) | 32 (91%) | |
| Palliative | 5 (15%) | 3 (9%) | |
| Total days in hospital (N) | 33 | 34 | |
| Mean days (SD) | 18.3 (23.1) | 25.4 (21.6) | .19 |
| Total number of procedures (N) | 33 | 34 | |
| Mean days (SD) | 1.94 (1.2) | 1.85 (1.2) | .77 |
| Other childhood illness (N) | 33 | 35 | .19 |
| None | 23 (70%) | 19 (54%) | |
| Any other | 10 (30%) | 16 (46%) | |
| Family composition (N) | 33 | 35 | .58 |
| Lone parent | 6 (18%) | 5 (14%) | |
| Two parents at home | 27 (82%) | 30 (86%) | |
| Maternal employment (N) | 33 | 35 | |
| Not employed | 15 (46%) | 18 (51%) | |
| Employed | 18 (54%) | 17 (49%) | |
| Paternal employment (N) | 32 | 34 | .64 |
| Not employed | 4 (13%) | 2 (6%) | |
| Employed | 27 (87%) | 31 (94%) | |
| Deprivation index scorea (N) | 33 | 33 | .41 |
| Mean Townsend score (SD) | 0.081 (2.62) | 0.716 (3.53) |
| . | Intervention (N = 33) . | Control (N = 35) . | p . |
|---|---|---|---|
| Child age (N) | 33 | 35 | |
| Mean years at T2 (SD) | 5.5 (0.3) | 5.4 (0.3) | .71 |
| Child gender (N) | 33 | 35 | |
| Male | 24 (73%) | 20 (59%) | .23 |
| Cyanotic Status (N) | 33 | 35 | .49 |
| Acyanotic | 20 (61%) | 24 (69%) | |
| Cyanotic | 13 (39%) | 11 (31%) | |
| Surgical procedure (N) | 33 | 34 | .92 |
| Open | 20 (61%) | 21 (62%) | |
| Closed | 13 (39%) | 13 (38%) | |
| Palliative status (N) | 33 | 35 | .4 |
| Corrected | 28 (84%) | 32 (91%) | |
| Palliative | 5 (15%) | 3 (9%) | |
| Total days in hospital (N) | 33 | 34 | |
| Mean days (SD) | 18.3 (23.1) | 25.4 (21.6) | .19 |
| Total number of procedures (N) | 33 | 34 | |
| Mean days (SD) | 1.94 (1.2) | 1.85 (1.2) | .77 |
| Other childhood illness (N) | 33 | 35 | .19 |
| None | 23 (70%) | 19 (54%) | |
| Any other | 10 (30%) | 16 (46%) | |
| Family composition (N) | 33 | 35 | .58 |
| Lone parent | 6 (18%) | 5 (14%) | |
| Two parents at home | 27 (82%) | 30 (86%) | |
| Maternal employment (N) | 33 | 35 | |
| Not employed | 15 (46%) | 18 (51%) | |
| Employed | 18 (54%) | 17 (49%) | |
| Paternal employment (N) | 32 | 34 | .64 |
| Not employed | 4 (13%) | 2 (6%) | |
| Employed | 27 (87%) | 31 (94%) | |
| Deprivation index scorea (N) | 33 | 33 | .41 |
| Mean Townsend score (SD) | 0.081 (2.62) | 0.716 (3.53) |
aTownsend scores—deprivation index scores from zip codes (Townsend, Phillimore, & Beattie, 1988).
Child Outcomes
Outcomes for the child are summarized in Table II. Although there was a greater drop in the mean CBCL Total Problem Behavior Score in the Intervention compared with the Control group, and although numbers in the clinically significant range decreased from 21.2–12.1% in the Intervention group and increased in the Control group from 11.4–14.3%, these differences did not reach levels of statistical significance (all p > .1). However, statistically and clinically significant findings summarized in Table II did suggest that children in the Control group were perceived as “sick” on a greater number of days in the preceding 3 months and missed more days during their first year at school through illness than those in the Intervention group. This statistical significance (both p = .02) across outcomes was matched by effect sizes (partial eta- and eta-squared) of moderate–large magnitudes (Cohen, 1988). These differences are not accounted for by objective indicators of CHD severity, palliative status, surgical history, or the presence of other chronic illnesses that, as summarized in Table I, did not differ between groups.
Child Outcomes at T1 – T2 in the Intervention and Control Groups
| . | Intervention group (N = 33) . | Control group (N = 35) . | . | . | ||
|---|---|---|---|---|---|---|
| Outcome measure . | T1 . | T2 . | T1 . | T2 . | F/t/chi square (df) . | p (partial eta squared; eta squared) . |
| CBCL (N) | 33 | 35 | ||||
| Total problem score (SD) | 50.2 (11.2) | 47.7 (12.6) | 48.9 (10.3) | 48.0 (11.7) | 0.52a(1,63) | .48 (0.008; 0.008) |
| (95% confidence intervals) | (46.5–53.9) | (43.4–52.0) | (45.4–52.6) | (43.7–52.3) | ||
| N (%) clinically significant range – T1 | 7 (21.2%) | — | 4 (11.4%) | — | 1.19b(1) | .27 |
| N (%) clinically significant range – T2 | — | 4 (12.1%) | — | 5 (14.3%) | 0.07b(1) | 0.79 |
| Days sick (N) | 33 | 34 | ||||
| Mean days sick past 3 months (SD) | 4.4 (6.4) | 3.3 (3.6) | 3.7 (7.3) | 7.7 (10.6) | 5.61a (1,65) | .02 (0.08; 0.08) |
| (95% confidence intervals) | (2.0–6.8) | (0.6–6.1) | (1.3–6.0) | (5.0–10.5) | ||
| School functioning (N) | 33 | 34 | ||||
| Remedial input – N (%) | — | 8 (24.2%) | — | 8 (23.5%) | 0.02b (1) | .98 |
| Mean days off school since start (SD) | — | 4.9 (4.6) | — | 9.7 (11.1) | 2.30c (65) | .02 (0.06; 0.05) |
| (95% confidence intervals) | (0.64–8.93) | |||||
| Below peers PE participation – N (%) | — | 3 (9.1%) | — | 5 (14.7%) | 0.64b | .42 |
| . | Intervention group (N = 33) . | Control group (N = 35) . | . | . | ||
|---|---|---|---|---|---|---|
| Outcome measure . | T1 . | T2 . | T1 . | T2 . | F/t/chi square (df) . | p (partial eta squared; eta squared) . |
| CBCL (N) | 33 | 35 | ||||
| Total problem score (SD) | 50.2 (11.2) | 47.7 (12.6) | 48.9 (10.3) | 48.0 (11.7) | 0.52a(1,63) | .48 (0.008; 0.008) |
| (95% confidence intervals) | (46.5–53.9) | (43.4–52.0) | (45.4–52.6) | (43.7–52.3) | ||
| N (%) clinically significant range – T1 | 7 (21.2%) | — | 4 (11.4%) | — | 1.19b(1) | .27 |
| N (%) clinically significant range – T2 | — | 4 (12.1%) | — | 5 (14.3%) | 0.07b(1) | 0.79 |
| Days sick (N) | 33 | 34 | ||||
| Mean days sick past 3 months (SD) | 4.4 (6.4) | 3.3 (3.6) | 3.7 (7.3) | 7.7 (10.6) | 5.61a (1,65) | .02 (0.08; 0.08) |
| (95% confidence intervals) | (2.0–6.8) | (0.6–6.1) | (1.3–6.0) | (5.0–10.5) | ||
| School functioning (N) | 33 | 34 | ||||
| Remedial input – N (%) | — | 8 (24.2%) | — | 8 (23.5%) | 0.02b (1) | .98 |
| Mean days off school since start (SD) | — | 4.9 (4.6) | — | 9.7 (11.1) | 2.30c (65) | .02 (0.06; 0.05) |
| (95% confidence intervals) | (0.64–8.93) | |||||
| Below peers PE participation – N (%) | — | 3 (9.1%) | — | 5 (14.7%) | 0.64b | .42 |
aF statistic for the interaction effect.
bChi-square statistic between groups.
ct statistic between groups.
Child Outcomes at T1 – T2 in the Intervention and Control Groups
| . | Intervention group (N = 33) . | Control group (N = 35) . | . | . | ||
|---|---|---|---|---|---|---|
| Outcome measure . | T1 . | T2 . | T1 . | T2 . | F/t/chi square (df) . | p (partial eta squared; eta squared) . |
| CBCL (N) | 33 | 35 | ||||
| Total problem score (SD) | 50.2 (11.2) | 47.7 (12.6) | 48.9 (10.3) | 48.0 (11.7) | 0.52a(1,63) | .48 (0.008; 0.008) |
| (95% confidence intervals) | (46.5–53.9) | (43.4–52.0) | (45.4–52.6) | (43.7–52.3) | ||
| N (%) clinically significant range – T1 | 7 (21.2%) | — | 4 (11.4%) | — | 1.19b(1) | .27 |
| N (%) clinically significant range – T2 | — | 4 (12.1%) | — | 5 (14.3%) | 0.07b(1) | 0.79 |
| Days sick (N) | 33 | 34 | ||||
| Mean days sick past 3 months (SD) | 4.4 (6.4) | 3.3 (3.6) | 3.7 (7.3) | 7.7 (10.6) | 5.61a (1,65) | .02 (0.08; 0.08) |
| (95% confidence intervals) | (2.0–6.8) | (0.6–6.1) | (1.3–6.0) | (5.0–10.5) | ||
| School functioning (N) | 33 | 34 | ||||
| Remedial input – N (%) | — | 8 (24.2%) | — | 8 (23.5%) | 0.02b (1) | .98 |
| Mean days off school since start (SD) | — | 4.9 (4.6) | — | 9.7 (11.1) | 2.30c (65) | .02 (0.06; 0.05) |
| (95% confidence intervals) | (0.64–8.93) | |||||
| Below peers PE participation – N (%) | — | 3 (9.1%) | — | 5 (14.7%) | 0.64b | .42 |
| . | Intervention group (N = 33) . | Control group (N = 35) . | . | . | ||
|---|---|---|---|---|---|---|
| Outcome measure . | T1 . | T2 . | T1 . | T2 . | F/t/chi square (df) . | p (partial eta squared; eta squared) . |
| CBCL (N) | 33 | 35 | ||||
| Total problem score (SD) | 50.2 (11.2) | 47.7 (12.6) | 48.9 (10.3) | 48.0 (11.7) | 0.52a(1,63) | .48 (0.008; 0.008) |
| (95% confidence intervals) | (46.5–53.9) | (43.4–52.0) | (45.4–52.6) | (43.7–52.3) | ||
| N (%) clinically significant range – T1 | 7 (21.2%) | — | 4 (11.4%) | — | 1.19b(1) | .27 |
| N (%) clinically significant range – T2 | — | 4 (12.1%) | — | 5 (14.3%) | 0.07b(1) | 0.79 |
| Days sick (N) | 33 | 34 | ||||
| Mean days sick past 3 months (SD) | 4.4 (6.4) | 3.3 (3.6) | 3.7 (7.3) | 7.7 (10.6) | 5.61a (1,65) | .02 (0.08; 0.08) |
| (95% confidence intervals) | (2.0–6.8) | (0.6–6.1) | (1.3–6.0) | (5.0–10.5) | ||
| School functioning (N) | 33 | 34 | ||||
| Remedial input – N (%) | — | 8 (24.2%) | — | 8 (23.5%) | 0.02b (1) | .98 |
| Mean days off school since start (SD) | — | 4.9 (4.6) | — | 9.7 (11.1) | 2.30c (65) | .02 (0.06; 0.05) |
| (95% confidence intervals) | (0.64–8.93) | |||||
| Below peers PE participation – N (%) | — | 3 (9.1%) | — | 5 (14.7%) | 0.64b | .42 |
aF statistic for the interaction effect.
bChi-square statistic between groups.
ct statistic between groups.
In terms of school functioning, there were no differences in proportions between groups in receipt of any type of remedial education or those who were perceived by their teachers to participate less in PE compared with classroom peers (Table II). Moreover, CBRSC factor score differences were uniformly non-significant (multivariate F = 0.85; df = 1, 61; p = .59). Mean scores, summarized in Table III, generally varied around the average T score across both the Intervention and Control groups and proportions in the clinically significant range (recommended by the authors as T > 65) did not deviate from those in the standardization sample.
Mean (SD) CBRSC Teacher Ratings at T2 and N in Clinically Significant Range (T > 65)
| . | Intervention group (N = 33) . | Control group (N = 30) . | Univariate t-test statistic . | p . |
|---|---|---|---|---|
| Inattention | 50.2 (10.6) | 47.5 (8.1) | 1.29 | .26 |
| 4 (12%) | 0 (0%) | |||
| Reading | 52.1 (11.6) | 49.3 (8.5) | 1.13 | .29 |
| 5 (15%) | 2 (7%) | |||
| Cognitive deficits | 48.2 (7.8) | 47.2 (5.6) | 0.32 | .57 |
| 1 (3%) | 0 (0%) | |||
| Oppositional behavior | 48.5 (10.7) | 46.4 (8.3) | 0.69 | .41 |
| 2 (6%) | 2 (7%) | |||
| Hyperactivity | 49.6 (8.9) | 47.1 (7.7) | 1.43 | .24 |
| 3 (9%) | 2 (7%) | |||
| Anxiety | 48.5 (10.9) | 48.9 (8.7) | 0.02 | .89 |
| 3 (9%) | 2 (7%) | |||
| Sluggish tempo | 51.5 (10.3) | 48.5 (7.5) | 1.66 | .20 |
| 5 (15%) | 1 (3%) | |||
| Daydreaming | 49.2 (9.8) | 46.4 (6.4) | 1.88 | .18 |
| 1 (3%) | 0 (0%) | |||
| Social competence | 48.3 (11.6) | 45.1 (12.2) | 1.13 | .29 |
| 5 (15%) | 1 (3%) |
| . | Intervention group (N = 33) . | Control group (N = 30) . | Univariate t-test statistic . | p . |
|---|---|---|---|---|
| Inattention | 50.2 (10.6) | 47.5 (8.1) | 1.29 | .26 |
| 4 (12%) | 0 (0%) | |||
| Reading | 52.1 (11.6) | 49.3 (8.5) | 1.13 | .29 |
| 5 (15%) | 2 (7%) | |||
| Cognitive deficits | 48.2 (7.8) | 47.2 (5.6) | 0.32 | .57 |
| 1 (3%) | 0 (0%) | |||
| Oppositional behavior | 48.5 (10.7) | 46.4 (8.3) | 0.69 | .41 |
| 2 (6%) | 2 (7%) | |||
| Hyperactivity | 49.6 (8.9) | 47.1 (7.7) | 1.43 | .24 |
| 3 (9%) | 2 (7%) | |||
| Anxiety | 48.5 (10.9) | 48.9 (8.7) | 0.02 | .89 |
| 3 (9%) | 2 (7%) | |||
| Sluggish tempo | 51.5 (10.3) | 48.5 (7.5) | 1.66 | .20 |
| 5 (15%) | 1 (3%) | |||
| Daydreaming | 49.2 (9.8) | 46.4 (6.4) | 1.88 | .18 |
| 1 (3%) | 0 (0%) | |||
| Social competence | 48.3 (11.6) | 45.1 (12.2) | 1.13 | .29 |
| 5 (15%) | 1 (3%) |
Mean (SD) CBRSC Teacher Ratings at T2 and N in Clinically Significant Range (T > 65)
| . | Intervention group (N = 33) . | Control group (N = 30) . | Univariate t-test statistic . | p . |
|---|---|---|---|---|
| Inattention | 50.2 (10.6) | 47.5 (8.1) | 1.29 | .26 |
| 4 (12%) | 0 (0%) | |||
| Reading | 52.1 (11.6) | 49.3 (8.5) | 1.13 | .29 |
| 5 (15%) | 2 (7%) | |||
| Cognitive deficits | 48.2 (7.8) | 47.2 (5.6) | 0.32 | .57 |
| 1 (3%) | 0 (0%) | |||
| Oppositional behavior | 48.5 (10.7) | 46.4 (8.3) | 0.69 | .41 |
| 2 (6%) | 2 (7%) | |||
| Hyperactivity | 49.6 (8.9) | 47.1 (7.7) | 1.43 | .24 |
| 3 (9%) | 2 (7%) | |||
| Anxiety | 48.5 (10.9) | 48.9 (8.7) | 0.02 | .89 |
| 3 (9%) | 2 (7%) | |||
| Sluggish tempo | 51.5 (10.3) | 48.5 (7.5) | 1.66 | .20 |
| 5 (15%) | 1 (3%) | |||
| Daydreaming | 49.2 (9.8) | 46.4 (6.4) | 1.88 | .18 |
| 1 (3%) | 0 (0%) | |||
| Social competence | 48.3 (11.6) | 45.1 (12.2) | 1.13 | .29 |
| 5 (15%) | 1 (3%) |
| . | Intervention group (N = 33) . | Control group (N = 30) . | Univariate t-test statistic . | p . |
|---|---|---|---|---|
| Inattention | 50.2 (10.6) | 47.5 (8.1) | 1.29 | .26 |
| 4 (12%) | 0 (0%) | |||
| Reading | 52.1 (11.6) | 49.3 (8.5) | 1.13 | .29 |
| 5 (15%) | 2 (7%) | |||
| Cognitive deficits | 48.2 (7.8) | 47.2 (5.6) | 0.32 | .57 |
| 1 (3%) | 0 (0%) | |||
| Oppositional behavior | 48.5 (10.7) | 46.4 (8.3) | 0.69 | .41 |
| 2 (6%) | 2 (7%) | |||
| Hyperactivity | 49.6 (8.9) | 47.1 (7.7) | 1.43 | .24 |
| 3 (9%) | 2 (7%) | |||
| Anxiety | 48.5 (10.9) | 48.9 (8.7) | 0.02 | .89 |
| 3 (9%) | 2 (7%) | |||
| Sluggish tempo | 51.5 (10.3) | 48.5 (7.5) | 1.66 | .20 |
| 5 (15%) | 1 (3%) | |||
| Daydreaming | 49.2 (9.8) | 46.4 (6.4) | 1.88 | .18 |
| 1 (3%) | 0 (0%) | |||
| Social competence | 48.3 (11.6) | 45.1 (12.2) | 1.13 | .29 |
| 5 (15%) | 1 (3%) |
Maternal and Family Outcomes
Bonferonni corrections were applied here to standard levels of statistical significance (p < .05) where domain construct scores were related. Thus for maternal mental health and maternal worry, the threshold for statistical significance became p = .025, and for the four impact on family factor scores the level became p = .0125.
A number of statistically significant differences were evident between groups at T2 compared with T1 (Table IV). Although scores on the Maternal Worry Scale did not vary differentially between groups across time, and remained fairly constant in both groups, T scores on the GSI of the BSI dropped in the Intervention group between T1 and T2, whereas a rise was evident in the Control group. This interaction was statistically significant (p = .005) and the partial eta-squared (0.12) and eta-squared score (0.11) suggested a large effect size. Moreover, although mothers in both groups did not differ in terms of the proportions who reported having experienced significant physical illness between T1 and T2 (12.1% and 11.8% in the Intervention and Control group, respectively), there was a statistically significant difference (p = .03) between groups in the proportions who suggested they had experienced significant emotional difficulties across the same time period (3% in the Intervention group and 21% in the Control group), a finding that was consistent with current GSI profile scores at T2.
Maternal and Family Outcomes at T1 – T2 in the Intervention and Control Groups
| . | Intervention group (N = 33) . | Control group (N = 35) . | . | . | ||
|---|---|---|---|---|---|---|
| Outcome measure . | T1 . | T2 . | T1 . | T2 . | F/chisquare (df) . | p (partial eta squared; eta squared) . |
| Maternal BSI (N) | 33 | 31 | ||||
| GSI Mean T- Scores (SD) | 50.6 (10.1) | 43.4 (9.8) | 48.7 (10.0) | 49.8 (10.2) | 8.30a (1,62) | .005 (0.12; 0.11) |
| (95% confidence intervals) | (47.1–54.1) | (40.0–46.9) | (45.1–52.3) | (46.2–53.4) | ||
| N (%) clinically significant range – T1 | 4 (12.1%) | — | 3 (9.6%) | — | 0.66b (1) | .71 |
| N (%) clinically significant range – T2 | — | 1 (3%) | — | 4 (12.9%) | 2.16b (1) | .14 |
| Maternal worry scale (N) | 32 | 32 | ||||
| Mean worry scale score (SD) | 16.3 (4.0) | 16.8 (3.7) | 15.9 (4.7) | 16.0 (4.7) | 0.25a (1,62) | .62 (0.004; 0.004) |
| (95% confidence intervals) | (14.8–17.9) | (15.3–18.2) | (14.4–17.5) | (14.5–17.5) | ||
| Maternal health since baseline (N) | 33 | 34 | ||||
| N (%) – any physical illness | — | 4 (12.1%) | — | 4 (11.8%) | 0.002b (1) | .96 |
| N (%) – any emotional difficulties | — | 1 (3%) | — | 7 (21%) | 4.91b (1) | .03 |
| Impact on family scale (N) | 32 | 31 | ||||
| Family strain – mean (SD) | 17.2 (4.1) | 15.8 (4.4) | 16.1 (4.3) | 17.0 (3.7) | 5.44a (1,61) | .02 (0.08; 0.08) |
| (95% confidence intervals) | (15.8–18.7) | (14.3–17.2) | (14.6–17.6) | (15.5–18.4) | ||
| Financial impact – mean (SD) | 7.8 (2.5) | 8.0 (3.5) | 7.3 (2.5) | 8.0 (2.5) | 0.59a (1,61) | .44 (0.01; 0.01) |
| (95% confidence intervals) | (6.9–8.7) | (6.9–9.1) | (6.4–8.2) | (6.9–9.1) | ||
| Personal strain – mean (SD) | 12.0 (3.1) | 11.3 (3.3) | 10.9 (3.7) | 12.1 (3.5) | 7.18a (1,61) | .01 (0.11; 0.10) |
| (95% confidence intervals) | (10.8–13.2) | (10.1–12.5) | (9.7–12.1) | (10.9–13.3) | ||
| Mastery – mean (SD) | 15.9 (1.9) | 16.2 (1.9) | 16.1 (2.4) | 16.3 (2.0) | 0.13a (1,61) | .72 (0.002; 0.002) |
| (95% confidence intervals) | (15.1–16.4) | (15.5–16.9) | (15.4–16.9) | (15.6–17.0) | ||
| . | Intervention group (N = 33) . | Control group (N = 35) . | . | . | ||
|---|---|---|---|---|---|---|
| Outcome measure . | T1 . | T2 . | T1 . | T2 . | F/chisquare (df) . | p (partial eta squared; eta squared) . |
| Maternal BSI (N) | 33 | 31 | ||||
| GSI Mean T- Scores (SD) | 50.6 (10.1) | 43.4 (9.8) | 48.7 (10.0) | 49.8 (10.2) | 8.30a (1,62) | .005 (0.12; 0.11) |
| (95% confidence intervals) | (47.1–54.1) | (40.0–46.9) | (45.1–52.3) | (46.2–53.4) | ||
| N (%) clinically significant range – T1 | 4 (12.1%) | — | 3 (9.6%) | — | 0.66b (1) | .71 |
| N (%) clinically significant range – T2 | — | 1 (3%) | — | 4 (12.9%) | 2.16b (1) | .14 |
| Maternal worry scale (N) | 32 | 32 | ||||
| Mean worry scale score (SD) | 16.3 (4.0) | 16.8 (3.7) | 15.9 (4.7) | 16.0 (4.7) | 0.25a (1,62) | .62 (0.004; 0.004) |
| (95% confidence intervals) | (14.8–17.9) | (15.3–18.2) | (14.4–17.5) | (14.5–17.5) | ||
| Maternal health since baseline (N) | 33 | 34 | ||||
| N (%) – any physical illness | — | 4 (12.1%) | — | 4 (11.8%) | 0.002b (1) | .96 |
| N (%) – any emotional difficulties | — | 1 (3%) | — | 7 (21%) | 4.91b (1) | .03 |
| Impact on family scale (N) | 32 | 31 | ||||
| Family strain – mean (SD) | 17.2 (4.1) | 15.8 (4.4) | 16.1 (4.3) | 17.0 (3.7) | 5.44a (1,61) | .02 (0.08; 0.08) |
| (95% confidence intervals) | (15.8–18.7) | (14.3–17.2) | (14.6–17.6) | (15.5–18.4) | ||
| Financial impact – mean (SD) | 7.8 (2.5) | 8.0 (3.5) | 7.3 (2.5) | 8.0 (2.5) | 0.59a (1,61) | .44 (0.01; 0.01) |
| (95% confidence intervals) | (6.9–8.7) | (6.9–9.1) | (6.4–8.2) | (6.9–9.1) | ||
| Personal strain – mean (SD) | 12.0 (3.1) | 11.3 (3.3) | 10.9 (3.7) | 12.1 (3.5) | 7.18a (1,61) | .01 (0.11; 0.10) |
| (95% confidence intervals) | (10.8–13.2) | (10.1–12.5) | (9.7–12.1) | (10.9–13.3) | ||
| Mastery – mean (SD) | 15.9 (1.9) | 16.2 (1.9) | 16.1 (2.4) | 16.3 (2.0) | 0.13a (1,61) | .72 (0.002; 0.002) |
| (95% confidence intervals) | (15.1–16.4) | (15.5–16.9) | (15.4–16.9) | (15.6–17.0) | ||
aF statistic for the interaction effect.
bChi-square statistic between groups.
Maternal and Family Outcomes at T1 – T2 in the Intervention and Control Groups
| . | Intervention group (N = 33) . | Control group (N = 35) . | . | . | ||
|---|---|---|---|---|---|---|
| Outcome measure . | T1 . | T2 . | T1 . | T2 . | F/chisquare (df) . | p (partial eta squared; eta squared) . |
| Maternal BSI (N) | 33 | 31 | ||||
| GSI Mean T- Scores (SD) | 50.6 (10.1) | 43.4 (9.8) | 48.7 (10.0) | 49.8 (10.2) | 8.30a (1,62) | .005 (0.12; 0.11) |
| (95% confidence intervals) | (47.1–54.1) | (40.0–46.9) | (45.1–52.3) | (46.2–53.4) | ||
| N (%) clinically significant range – T1 | 4 (12.1%) | — | 3 (9.6%) | — | 0.66b (1) | .71 |
| N (%) clinically significant range – T2 | — | 1 (3%) | — | 4 (12.9%) | 2.16b (1) | .14 |
| Maternal worry scale (N) | 32 | 32 | ||||
| Mean worry scale score (SD) | 16.3 (4.0) | 16.8 (3.7) | 15.9 (4.7) | 16.0 (4.7) | 0.25a (1,62) | .62 (0.004; 0.004) |
| (95% confidence intervals) | (14.8–17.9) | (15.3–18.2) | (14.4–17.5) | (14.5–17.5) | ||
| Maternal health since baseline (N) | 33 | 34 | ||||
| N (%) – any physical illness | — | 4 (12.1%) | — | 4 (11.8%) | 0.002b (1) | .96 |
| N (%) – any emotional difficulties | — | 1 (3%) | — | 7 (21%) | 4.91b (1) | .03 |
| Impact on family scale (N) | 32 | 31 | ||||
| Family strain – mean (SD) | 17.2 (4.1) | 15.8 (4.4) | 16.1 (4.3) | 17.0 (3.7) | 5.44a (1,61) | .02 (0.08; 0.08) |
| (95% confidence intervals) | (15.8–18.7) | (14.3–17.2) | (14.6–17.6) | (15.5–18.4) | ||
| Financial impact – mean (SD) | 7.8 (2.5) | 8.0 (3.5) | 7.3 (2.5) | 8.0 (2.5) | 0.59a (1,61) | .44 (0.01; 0.01) |
| (95% confidence intervals) | (6.9–8.7) | (6.9–9.1) | (6.4–8.2) | (6.9–9.1) | ||
| Personal strain – mean (SD) | 12.0 (3.1) | 11.3 (3.3) | 10.9 (3.7) | 12.1 (3.5) | 7.18a (1,61) | .01 (0.11; 0.10) |
| (95% confidence intervals) | (10.8–13.2) | (10.1–12.5) | (9.7–12.1) | (10.9–13.3) | ||
| Mastery – mean (SD) | 15.9 (1.9) | 16.2 (1.9) | 16.1 (2.4) | 16.3 (2.0) | 0.13a (1,61) | .72 (0.002; 0.002) |
| (95% confidence intervals) | (15.1–16.4) | (15.5–16.9) | (15.4–16.9) | (15.6–17.0) | ||
| . | Intervention group (N = 33) . | Control group (N = 35) . | . | . | ||
|---|---|---|---|---|---|---|
| Outcome measure . | T1 . | T2 . | T1 . | T2 . | F/chisquare (df) . | p (partial eta squared; eta squared) . |
| Maternal BSI (N) | 33 | 31 | ||||
| GSI Mean T- Scores (SD) | 50.6 (10.1) | 43.4 (9.8) | 48.7 (10.0) | 49.8 (10.2) | 8.30a (1,62) | .005 (0.12; 0.11) |
| (95% confidence intervals) | (47.1–54.1) | (40.0–46.9) | (45.1–52.3) | (46.2–53.4) | ||
| N (%) clinically significant range – T1 | 4 (12.1%) | — | 3 (9.6%) | — | 0.66b (1) | .71 |
| N (%) clinically significant range – T2 | — | 1 (3%) | — | 4 (12.9%) | 2.16b (1) | .14 |
| Maternal worry scale (N) | 32 | 32 | ||||
| Mean worry scale score (SD) | 16.3 (4.0) | 16.8 (3.7) | 15.9 (4.7) | 16.0 (4.7) | 0.25a (1,62) | .62 (0.004; 0.004) |
| (95% confidence intervals) | (14.8–17.9) | (15.3–18.2) | (14.4–17.5) | (14.5–17.5) | ||
| Maternal health since baseline (N) | 33 | 34 | ||||
| N (%) – any physical illness | — | 4 (12.1%) | — | 4 (11.8%) | 0.002b (1) | .96 |
| N (%) – any emotional difficulties | — | 1 (3%) | — | 7 (21%) | 4.91b (1) | .03 |
| Impact on family scale (N) | 32 | 31 | ||||
| Family strain – mean (SD) | 17.2 (4.1) | 15.8 (4.4) | 16.1 (4.3) | 17.0 (3.7) | 5.44a (1,61) | .02 (0.08; 0.08) |
| (95% confidence intervals) | (15.8–18.7) | (14.3–17.2) | (14.6–17.6) | (15.5–18.4) | ||
| Financial impact – mean (SD) | 7.8 (2.5) | 8.0 (3.5) | 7.3 (2.5) | 8.0 (2.5) | 0.59a (1,61) | .44 (0.01; 0.01) |
| (95% confidence intervals) | (6.9–8.7) | (6.9–9.1) | (6.4–8.2) | (6.9–9.1) | ||
| Personal strain – mean (SD) | 12.0 (3.1) | 11.3 (3.3) | 10.9 (3.7) | 12.1 (3.5) | 7.18a (1,61) | .01 (0.11; 0.10) |
| (95% confidence intervals) | (10.8–13.2) | (10.1–12.5) | (9.7–12.1) | (10.9–13.3) | ||
| Mastery – mean (SD) | 15.9 (1.9) | 16.2 (1.9) | 16.1 (2.4) | 16.3 (2.0) | 0.13a (1,61) | .72 (0.002; 0.002) |
| (95% confidence intervals) | (15.1–16.4) | (15.5–16.9) | (15.4–16.9) | (15.6–17.0) | ||
aF statistic for the interaction effect.
bChi-square statistic between groups.
Finally, with bonferonni corrections, Table IV highlights a statistically significant difference between groups on the personal strain subscale of the Impact on Family Scale. Specifically, mothers’ responses indicated reduced levels of personal strain in the Intervention group between T1 and T2, whereas levels increased across time in the Control group. The interaction effect was statistically significant (p = .01) with partial eta-squared (0.11) and eta-squared (0.10) suggesting a large effect size. The statistical significance level (p = .02) on the family strain subscale failed to reach the bonferonni corrected level although the eta- and partial eta-squared statistics (both = 0.08) suggested a moderate effect size difference here.
Program Acceptability
In addition to the formal outcome measures described earlier, the acceptability of the CHIP–School program to the Intervention group mothers was assessed by an anonymous evaluation questionnaire completed 2–3 weeks after program end. Returns were received from 30 mothers (90%). Benefits in terms of understanding the child’s heart condition, confidence in parenting skills, confidence in safe activity levels for the child, and reduced worry about the child’s health and future were perceived by the vast majority of mothers. This ranged from 70% endorsement of reduced worry about the child’s future to 97% suggesting they were now more confident about what were safe activity levels for the child. Related to this, the mean likert score for the helpfulness of program elements was >4 on a 5-point scale for all elements (workshop, bicycle exercise test, individual session, manual factsheets, and child factsheets) with mean rating = 4.8.
It is beyond the scope of this paper to discuss the themes derived from a content analysis of the open-ended feedback provided. However, the most common themes related to: (1) improved knowledge and skills, (2) appreciation of the shared empathy with other families attained through the workshop, (3) attitude change regarding the child’s exercise capacity and (4) appreciation of CHIP manual, factsheet resources and information to schools. Interestingly, especially given the absence of any significant differences between groups on the Maternal Worry Scale, a final theme related to the continuation of worry despite feeling better equipped to manage this.
Prospective Regression Analyses
Three prospective regression analyses were conducted where factor or variable scores at T1 (illness/surgical, child factors, and family factors) were regressed against child behavioral adjustment (CBCL scores) at T2. The regression model for illness/surgical factors (cyanosis, surgery, palliative status, total number of procedures, and total days in hospital) was not significant (adjusted R2 = 0.043; F = 0.49, p = .78).
The regression model for child factors (gender, comorbid illness, verbal skills, and perceptual-motor skills) was statistically significant (adjusted R2 = 0.141; F = 3.17, p = .02), although only the perceptual-motor skills cognitive factor score made a unique statistically significant contribution (standardized beta = –0.343, p = .01). Similarly, the regression model for family factors (deprivation index score, maternal and paternal employment, family composition, maternal mental health, and maternal worry) was statistically significant (adjusted R2 = 0.192; F = 3.34, p = .007). Maternal mental health (standardized beta = 0.289, p = .035) and maternal worry (standardized beta = 0.315, p = .014) each made unique contributions of statistical significance to the model.
A final regression model was computed, which included only these three statistically significant variables across the child and family domains. Together, cognitive functioning as indexed by perceptual-motor skills, maternal mental health, and maternal worry at T1, just before the child started school, accounted for 27.9% of the variance on child behavioral adjustment at T2, the end of the first year at school (adjusted R2 = 0.279; F = 7.83, p < .001).
Discussion
The importance of attending to psychological factors in the clinical management of children with chronic illness has been sufficiently recognised over recent years that national guidelines increasingly call for such interventions to be integrated within care pathways (e.g., Institute of Medicine, 2007; National Institute for Clinical Excellence, 2005). However, the evidence base for the efficacy of such interventions is in its infancy and especially in relation to promoting the emotional adjustment and resilience of these children and their families. Previous intervention studies have been compromised in terms of theoretical drivers, control conditions, power, specificity of the intervention, and limited follow-up periods; positive outcomes as a proportion of outcomes assessed have been modest (Barlow & Ellard, 2004; Beale, 2006; Kibby, Tyc, & Mulhern, 1998).
The CHIP program, as far as the authors are aware, represents the first major trial of a psychological intervention, founded on psychological theory and original in its composition, to be delivered to children with CHD and their families and at a key developmental transition in their lives. CHIP–School shares many of the same principles as the previously reported CHIP–Infant study (McCusker et al., 2009). These included a focus on maternal and family functioning, parent–child transactions, individualized psychoeducation, and outreach to community health and, in the current study, education providers. However, the specific elements of the CHIP–School intervention presented here were tailored to the developmental transition of starting school. Thus, specific elements related to promoting independence and activity levels, managing developmentally relevant behaviors, and promoting resilience in collaborating with medical care procedures.
Positive gains were found at 10-month follow-up in terms of maternal mental health and perceived personal strain in the family. These are important findings, given the significance of maternal adjustment for child outcomes. Moreover, the children in the Intervention group missed fewer days from school and were perceived to have been sick less often than those in the Control group. Together, these are encouraging findings that support weaving psychological interventions into the program of care delivered to children with CHD, and arguably chronic illness in general.
Positive gains were not, however, demonstrated on all outcome measures. Although the proportion of children whose CBCL scores were in the clinically significant range nearly halved in the Intervention group, and increased slightly in the Control group, statistical comparisons were not significant. Greater attrition in children with higher CBCL scores at T1, as noted earlier, will have compromised statistical variability and the expected effect size difference. More fundamentally, although the CBCL has been extensively used in pediatric outcome research, it may measure more trait, than state like, behaviors, and be less sensitive to the more preventive aspirations, which typically underpin psychological interventions with pediatric populations (Kazak, 2005).
No gains were evident on the teacher completed CBRSC scales. Impact on school functioning may have been a too distal effect from the specific foci of the program that related to parent–child transactions. Moreover, fewer children overall were in the clinically significant range of these teacher-completed scales than the parent CBCL scales. This divergence between parent and teacher ratings of child adjustment is noteworthy in itself and has been discerned in other studies (Bellinger et al., 2009).
Supplementing the positive gains found on formal outcome measures, program acceptability data suggested high levels of parental appreciation of program elements. The vast majority of mothers’ subjective reports were of gains accrued in terms of knowledge, understanding, and parenting competencies. All elements of the CHIP–School program were appreciated.
In addition to analyses of differential outcomes across groups, the present study examined the predictive significance of medical, child factors, and family factors at baseline assessment (preschool) for behavioral adjustment at the end of the first year at school. Previous cross-sectional analyses with this population at T1 have highlighted the importance of maternal mental health, worry, parenting style, marital status, and acyanotic disease status (McCusker et al., 2007). Fewer factors were significant in our prospective regression equations and the model involving illness and surgical variables explained very little of the variance on behavioral outcomes and was not statistically significant. Three factors emerged as key. These included child cognitive functioning, as also noted in the Bellinger studies (Bellinger et al., 2009), and two maternal factors—maternal mental health and maternal worry. Such longitudinal findings support previous cross-sectional studies in highlighting the importance of maternal adjustment over illness or surgical factors in understanding long-term outcomes for these children. They also further emphasize the importance of psychological interventions, such as outlined in the present article, for improving outcomes for these children.
There are a number of limitations in this study that must be considered. We could not control for the non-specific impact of having the additional contact time involved in delivering the specifics of the intervention. However, the additional resources required to achieve this, and include independent ratings of treatment fidelity, not included in this study, is certainly indicated in future research trials, given these encouraging findings. Participants were not blind to their group status, and perhaps more importantly, those who delivered the program were from the research team. This had the potential to create bias such as participants wishing to please those with whom they had worked, especially in the program acceptability feedback. This cannot be discounted. However, on the formal outcome measures, profiles were differential (rather than uniformly positive from the Intervention group), which suggested discriminating responses. Furthermore, there were no subjective judgements required in scoring the questionnaires, which were returned against a participant code, rather than by name or group status.
It was also regrettable that response rate was insufficient from fathers for analysis. However, in line with the Thompson model, the focus was on optimizing maternal participation and a requirement that both parents attend all sessions and complete all questionnaires, would have compromised the overall number of cases we had and study viability. Paternal, and other independent, raters of appropriate outcomes should be an important focus in future research of such trials.
The potential limitations of the CBCL with pediatric populations have been noted earlier. Future research may consider using measures related to competencies and resilience, more suited to prevention than treatment initiatives. Alternatively, those subsets of children at greatest psychosocial risk might form the target population for intervention trials (Drotar, 2006; Kazak, 2005), but this will require multi-center trials or appreciation of research designs other than the RCT (e.g., case series designs).
The CHIP studies are innovative in targeting interventions at key developmental transitions. This principle might inform future intervention research, as effects are likely to be enhanced at these critical times of challenge, adjustment and change. This CHIP research program has, to date, focused on parents of newly diagnosed infants and, in this current study, when children were entering school. Although we included children with other comorbid physical illnesses, we did not include children with neurodevelopmental syndromes in this arm of the study. These were included, however, in our CHIP–Infant study, and findings therein (McCusker et al., 2009) support generalizability potential. Moreover, the principles of this intervention program would appear to have generalizable potential to other pediatric populations and transitions, such as adolescence and from child to adult services.
In sum, longitudinal data have been presented in this paper that confirms the central importance of maternal factors for later adjustment in children with significant CHD. Perhaps, more importantly, we have presented a theoretically driven, but relatively brief psychological intervention program, targeted at a key developmental transition for these children and families, which we have shown to be of benefit when tested in an RCT. Benefits accrued for maternal mental health, family functioning, perceived sickness in the child, and school attendance are significant and have already shaped subsequent practices at our center. This should inform practice elsewhere, and the generalizability of such a program to other pediatric populations merits consideration.
Funding
This work was supported by the National Lottery Charities Board/Community Fund of Great Britain (URN: MRB 217787).
Conflicts of interest: None declared.