-
PDF
- Split View
-
Views
-
Cite
Cite
Alexei Solovchenko, Michaela Schmitz‐Eiberger, Significance of skin flavonoids for UV‐B‐protection in apple fruits, Journal of Experimental Botany, Volume 54, Issue 389, 1 August 2003, Pages 1977–1984, https://doi.org/10.1093/jxb/erg199
Close - Share Icon Share
Abstract
An attempt has been made to assess the UV‐B‐protective capacity of phenolic compounds accumulated in superficial structures of plants using apple fruit as a model. Two apple (Malus domestica Borkh.) cultivars (Braeburn and Granny Smith) differing in response to high fluxes of solar radiation were selected and exposed to increasing doses of UV‐B radiation. The extent of UV‐B‐induced damage to photosystem II of apple skin correlated with its quercetin glycoside (but not anthocyanin) content. Granny Smith apples did not demonstrate a pronounced response to high sunlight in terms of the accumulation of phenolic substances in the skin and exhibited similar patterns of Fo, Fm, and Fv/Fm changes in the course of UV‐B irradiation both on sun‐exposed and shaded surfaces of a fruit. Unlike Granny Smith, Braeburn fruits were characterized by a significant accumulation of quercetin glycosides in sun‐exposed skin, however, shaded skin contained these compounds in similar amounts to those in Granny Smith. Accordingly, photosystem II in sun‐exposed skin of Braeburn apples was resistant to high doses of UV‐B radiation (up to 97 kJ m–2), whereas the susceptibility of the photosynthetic apparatus in shaded skin of Braeburn to UV‐B‐induced damage was much higher and similar to that of both sun‐exposed and shaded skin of Granny Smith fruits. Anthocyanins, at least in the amounts found in Braeburn, did not show an additional effect in UV‐B protection.
Received 7 February 2003; Accepted 25 April 2003
Introduction
Higher plants are naturally exposed to high fluxes of solar radiation and therefore they are subjected to relatively high UV‐doses (Caldwell and Flint, 1997). UV‐B is the band of lowest wavelength and highest energy that penetrates the ozone layer of the stratosphere. It comprises about 5% of the whole UV or 0.5% of the total energy of solar radiation (Caldwell and Flint, 1997; Krupa et al., 1998). In spite of the relatively low irradiance, UV‐B radiation could induce severe damage to plants via direct and indirect effects on nucleic acids, proteins and cell membranes (Kulandaivelu and Noorudeen, 1983; Janssen et al., 1998; Schmitz‐Eiberger and Noga, 2001).
On a whole‐plant scale, the effects of UV‐B radiation are apparent as an alteration of plant morphology, a reduction of growth, and a decrease in yield (Tevini and Teramura, 1989; Krupa et al., 1998). For instance, enhanced UV‐B levels, as well as high temperature, play a key role in the development of sunscald in apples (Andrews and Johnson, 1997; Andrews et al., 1998; Schmitz‐Eiberger and Noga, 2001).
The photosynthetic apparatus of higher plants is particularly sensitive to damage by UV‐B radiation (Kulandaivelu and Noorudeen, 1983). The primary targets of UV‐B radiation in photosystem II are the 32 kDa D1 protein of the reaction centre and the water‐oxidizing system (Janssen et al., 1998). Damage to those components results in a decrease in the variable fluorescence level (Janssen et al., 1998; Skórska, 2000) making the measurement of chlorophyll fluorescence parameters such as Fo, Fm, and Fv/Fm suitable for an estimation of UV‐B induced damage to plants (Skórska 2000; Schmitz‐Eiberger and Noga, 2001).
To cope with environmental UV‐irradiation conditions, higher plants have evolved a number of protective mechanisms. Probably, one of the most basic and most studied is attenuation of the radiation by superficial structures of plant organs (Caldwell, 1981; Flint et al., 1985; Kolb et al., 2001). The accumulation of phenolic compounds that selectively absorb UV radiation in the plant cuticle and epidermis (Krauss et al., 1997; Flint et al., 1985; Mazza et al., 2000) probably represents the most cost‐effective strategy for long‐term adaptation in the case of regular and prolonged exposure to elevated doses of solar radiation, including its UV‐B component (Robberecht and Caldwell, 1983; Pietrini et al., 2002; Liakoura et al., 2003).
Apple fruit are known to contain large amounts of phenolic substances in the skin. The major flavonoid classes occurring in apple skin are flavonols such as quercetin 3‐glycosides, monomeric and oligomeric flavan‐3‐ols such as catechin, epicatechin and procyanidins and, in red‐coloured cultivars, anthocyanins such as cyanidin 3‐glycosides. Apple fruit also contain considerable amounts of hydroxycinnamic acid derivatives which are mainly represented by chlorogenic acid (Escarpa and González, 1998; Awad and de Jager, 2000; Awad et al., 2000). Flavonoids and chlorogenic acid also contribute to the quality aspects of apple fruits. Their red coloration is primarily due to the flavonoid cyanidin‐3‐galactoside located in the vacuoles of skin cells (Lancaster et al., 1994). However, different groups of phenolic compounds demonstrate non‐uniform behaviour during the adaptation of plants to strong sunlight (Awad et al., 2000; Wang et al., 2000; Winkel‐Shirley, 2001; Ryan et al., 2002). Catechin, as well as the phenolic acid content, does not exhibit a distinct correlation with irradiation conditions in leaves (Bornman et al., 1997; Burchard et al., 2000) and fruits (Awad et al., 2000). By contrast, a dramatic induction of synthesis and accumulation of flavonoids is often observed in response to high (sun)light (Kolb et al., 2001; Reay and Lancaster, 2001; Merzlyak and Solovchenko, 2002). Experiments with transgenic plants demonstrated an up‐regulation of the genes responsible for flavonoid (particularly, kaempferol and quercetin) biosynthesis under elevated UV‐B conditions (Wang et al., 2000; Ryan et al., 2002). Therefore, flavonoids are a particularly interesting group of phenolic substances in connection with the investigation of the high‐sunlight response. However, the UV‐protective efficiency of phenolic ‘sunscreens’ induced in the course of adaptation to high sunlight under natural conditions is still uncertain in many cases, especially in fruits that play a crucial role in plant reproduction.
Apple fruits represent an interesting natural system for the investigation of photo‐adaptation and photo‐damage processes in plants (Andrews and Johnson, 1997; Schmitz‐Eiberger and Noga, 2001). During the development of fruit on the tree, one of its surfaces (sun‐exposed) is predominantly exposed to direct solar radiation whereas the opposite (shaded) surface is illuminated mainly by diffuse sunlight. As a result, the skin of the sun‐exposed surface of apple fruit develop distinct patterns of pigment composition attributable to adaptation to high sunlight (Merzlyak and Solovchenko, 2002). In this study, two apple cultivars (cvs), differing in their visual response to strong sunlight, were used. Fruits of cv. Braeburn possess red pigmentation on sun‐exposed surface due to the accumulation of anthocyanins which is not the case for Granny Smith apples.
The main goal of this study was to estimate the importance of flavonoids accumulated in apple skin in response to strong sunlight for the protection of the apple photosynthetic apparatus from UV‐B‐induced damage. Another aim was to discover whether the anthocyanins that accumulated in sun‐exposed skin of apple fruit have a role in UV‐protection in apple fruit.
Materials and methods
Plant material
Ripe apple (Malus domestica Borkh.) bearing no visual symptoms of damage were used in these experiments. Two cvs, Granny Smith and Braeburn, with different responses to high sunlight were selected for this study. Apple fruits of both cultivars were grown at the Klein‐Altendorf Fruit Research Station (Germany). Fruits of both cultivars were harvested on 12 October 2002 and stored under controlled atmosphere conditions (1.5% O2; 3% CO2) for 3 months. On the date of the investigation, the fruits of both cultivars had attained similar maturity according to the Streif index measurement. The index was calculated as fruit firmness/[soluble solids content×starch index value] (DeLong et al., 1999) and was 0.2 and 0.19 for Braeburn and Granny Smith, respectively.
Sixty fruits of each cultivar were used (a total of 120 fruits). Sun‐exposed and shaded sides of each fruit were estimated visually. Small regions of fruit surface (c. 4 cm2) were selected, which was enough to perform reliable measurement of both chlorophyll fluorescence and pigment content (Merzlyak and Solovchenko, 2002).
UV‐B irradiation conditions
An installation of 10×100 W fluorescent UV‐B (maximum emission energy in 280–310 nm range) lamps (‘Philips’, Germany) was used as the source of UV‐B radiation. A cellulose acetate filter was used for the removal of shortwave UV‐C radiation. Irradiance was measured with a precalibrated spectroradiometer (‘Gröbel’, Karlsruhe, Germany). The linearity of the UV‐B lamps was controlled by the continuous measurement of UV‐B levels in the course of irradiation.
Under these experimental conditions UV‐B irradiance was 11 J m–2 s–1. There were five exposures from 30 min to 150 min at 30 min intervals giving a total incident UV‐B dose of 97.0 kJ m–2 over 150 min, which was sufficient to follow the UV‐B induced damage to photosystem II in real time.
Chlorophyll fluorescence measurements
Measurements were performed on whole fruits after exposing them to UV‐B radiation and then to dark conditions for 30 min (Schmitz‐Eiberger and Noga, 2001). Maximum (Fm) as well as ground (Fo) fluorescence of apple skin were measured using a pulse‐modulation fluorometer (‘PAM–1000’, Walz, Effeltrich, Germany), then the quantum efficiency of open photosystem II centres was calculated as Fv/Fm=(Fm–Fo)/Fo (Maxwell and Johnson, 2000).
Pigment analysis
An extraction procedure allowing the simultaneous assay of chlorophylls, carotenoids, quercetin glycosides, and anthocyanins in an extract was carried out as described by Solovchenko et al. (2001). Apple skin discs (16 mm in diameter and c. 1 mm thickness) were homogenized in chloroform/methanol (2/1, v/v) in the presence of MgO. The homogenates were then filtered through a paper filter and distilled water (1/5 of total extract volume) was added. Then extracts were centrifuged at 3000 g for 10 min until phase separation.
Absorbance of the extracts was measured with a spectrophotometer (‘Lambda 15’, Perkin‐Elmer, USA). Chlorophyll and carotenoid concentrations were quantified in the lower (chloroform) phase using coefficients reported by Wellburn (1994), and the upper (water–methanol) phase of the extract was used for HPLC assay of quercetin glycosides and anthocyanins. Pigment contents were expressed on fruit surface area basis.
A Waters solvent delivery control system with a Sunchrom Marathon sample injector and a Hewlett Packard variable wavelength UV detector were used for the identification and quantification of flavonoids. The column was a 150×2 mm Phenomenex Synergi Polar RP 80 A fitted with a Phenomenex guard column. Chromatograms were recorded using the Waters Millenium program. Solvents used for the elution were (A) 100 ml l–1 acetic acid in water and (B) acetonitrile. Deaeration of the solvent system was achieved by vacuum filtration through a 0.22 µm filter, rapid sparging with helium (100 ml–1 for 10 min) and constant slow bubbling of helium into capped, vented solvent reservoirs (5 ml min–1). Samples (5 µl) were injected onto the column, which was maintained at 30 °C using a Waters column heater. A flow rate of 1 ml min–1 and a linear 20 min solvent gradient from 0–20% acetonitrile, with a 10 min hold at the final concentration was used. The column was; Returned to initial solvent composition over 1 min and re‐equilibrated for 10 min before the next analysis. Eluted components were monitored at 350 nm for flavonols and 530 nm for anthocyanins. The individual compounds were identified and quantified using standard solutions of quercetin and cyanidin‐3‐glycoside (Roth, Karlsruhe, Germany).
Results
Granny Smith fruits possessed green coloration, which was more intensive on the shaded surface. Sun‐exposed surfaces of Braeburn fruits exhibited anthocyanin pigmentation characteristic of this cultivar, whereas shaded surfaces of these fruits were green or pale‐green. The results of pigment analysis (Figs 1, 2) showed that sun‐exposed and shaded apple skin of Granny Smith and Braeburn fruits possessed different patterns of pigment content and composition.
Pigment content and composition
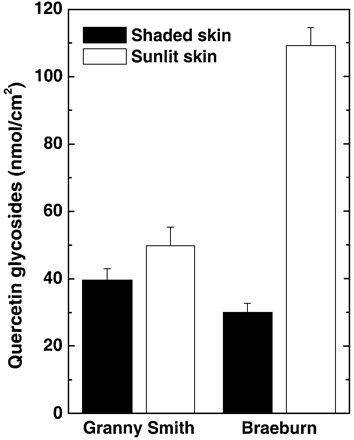
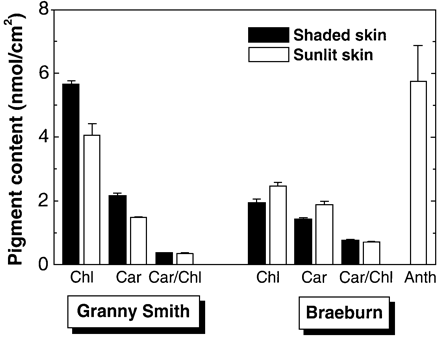
As could be seen from Fig. 1, the quercetin glycoside content of the shaded apple skin of Granny Smith was lower than that of sun‐exposed apple skin and comprised 39.9±3.6 and 49.5±5.4 nmol cm–2, respectively. Shaded apple skin of Braeburn fruits exhibited a lower quercetin glycoside content compared with that of Granny Smith (30.0±2.8 versus 39.9±3.6 nmol cm–2) whereas sun‐exposed apple skin of this cultivar contained about a 3‐fold greater number of the flavonoids (109.2±5.3 nmol cm–2). Anthocyanins were only found in sun‐exposed apple skin of Braeburn apples in amounts up to 6 nmol cm–2 (Fig. 2).
Granny Smith fruits had a higher total chlorophyll content than Braeburn (Fig. 2), and shaded skin of Granny Smith contained more chlorophylls than the sun‐exposed surface of this cv. (5.7±0.1 versus 4.1±0.4 nmol cm–2). The reverse was true for Braeburn where the chlorophyll content of shaded skin was lower than that of sun‐exposed skin (2.5±0.1 versus 1.9±0.1 nmol cm–2). By contrast with pronounced difference in chlorophyll content, skin carotenoid content was close in both cvs. A higher carotenoid content was found in the shaded apple skin of Granny Smith fruits compared with sun‐exposed skin of the same fruits (2.2±0.1 and 1.49±0.1 nmol cm–2, respectively). In the case of Braeburn, sun‐exposed apple skin contained more carotenoids than shaded apple skin (2.2±0.1 and 1.5±0.1 nmol cm–2, respectively).
UV‐B‐screening capacity of apple skin flavonoids
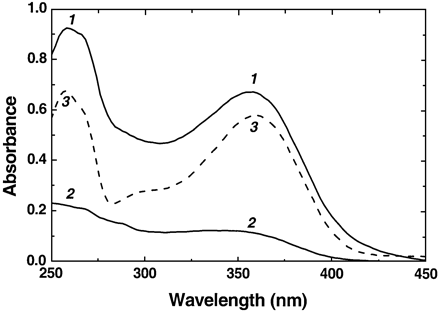
A comparison of the extract absorbance spectra (Fig. 3) showed that, in general, extracts obtained from sun‐exposed apple skin exhibited considerably higher absorbance in the UV region of the spectrum than those from shaded skin. Broad maxima centred at 358 nm accompanied by corresponding short‐wave peaks (near 260 nm) characteristic of quercetin glycoside absorption were present in the spectra of extracts from sun‐exposed skin in both cultivars. These spectral features were much less pronounced in the spectra of shaded skin extracts. A slight increase in the absorbance towards shorter wavelengths was evidence of a contribution by other phenolics (chlorogenic acid and proanthocyanidins) to the extract absorption. According to the data of HPLC and spectral analysis (not shown), flavonoids (represented mainly by quercetin glycosides) were the principal phenolic constituents of apple skin extracts absorbing in the UV‐A and UV‐B regions of the spectrum.
Typical spectra of apple skin extracts shown in Fig. 3 demonstrate that flavonoids occuring in apple skin possess significant absorption, not only in UV‐A (the band of their maximum absorption, Fig. 3, curve 1) but in UV‐B as well. In order to estimate the UV‐B screening capacity of apple skin flavonoids, their contribution to the extract absorption at 300 nm was calculated and expressed on a fruit area basis.
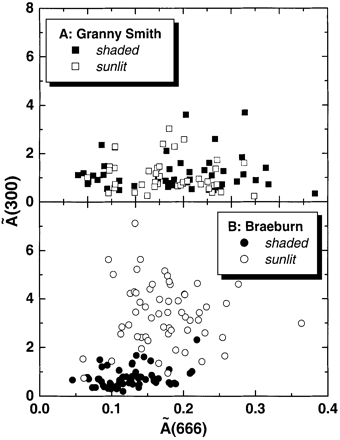
As can be seen from Fig. 4, there was no significant difference in absorption of UV‐B radiation by skin flavonoids between sun‐exposed and shaded surfaces of Granny Smith fruits (Fig. 4A), which was 1.1±0.1 and 1.4±0.2, respectively. By contrast, Braeburn fruits showed a striking difference in the flavonoid contribution to UV‐B absorption of the extracts between sun‐exposed and shaded sides of the fruit (Fig. 4B). The contribution of flavonoids to Ã(300) of the extracts made from shaded skin of Braeburn (0.9±0.1) was close to that of sun‐exposed and shaded skin of Granny Smith, whereas in sun‐exposed skin of Braeburn Ã(300) was approximately four times higher (3.4±0.2).
Changes in chlorophyll fluorescence in the course of UV‐B irradiation
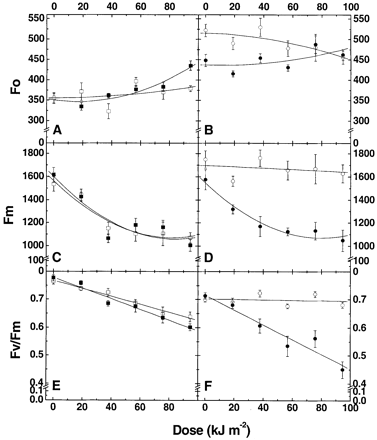
In order to estimate the UV‐B resistance of the photosynthetic apparatus of apple fruits with different pigment content and composition, apple fruits were subjected to UV‐B radiation and dose curves, representing changes in ground (Fo) and maximum (Fm) chlorophyll fluorescence as well as in the Fv/Fm ratio in the course of irradiation, were obtained (Fig. 5).
In intact Granny Smith fruit, nearly the same Fo values were recorded on sun‐exposed and shaded surfaces. An increase in UV‐B dose induced a notable increase in Fo on the shaded (but not on the sun‐exposed) surface (Fig. 5A, closed symbols). In intact Braeburn fruits, the Fo level was higher on the sun‐exposed surface than on the shaded surface, but in the course of irradiation the difference became insignificant (Fig. 5B).
Granny Smith fruits exhibited similar patterns of UV‐induced Fm changes on both surfaces of a fruit (Fig. 5C): UV‐B irradiation up to 40 kJ m–2 induced a sharp decrease in Fm, but a further increase in the dose did not induce such large changes in Fm. The same Fm behaviour in response to UV irradiation was recorded in the shaded surfaces of Braeburn (Fig. 5D, closed symbols). However, on the sun‐exposed side of Braeburn fruits Fm was remarkably stable in whole dose range studied (Fig. 5D, open symbols).
The changes in Fo and Fm described above (Fig. 5A–D) resulted in a steady decline in Fv/Fm in the course of UV‐B irradiation (Fig. 5E, F). The rate of Fv/Fm decrease was similar in both sun‐exposed and shaded surfaces of Granny Smith (Fig. 5E). The shaded surface of Braeburn fruits demonstrated similar dependency between UV‐B dose and Fv/Fm (Fig. 5F, closed symbols), but in sun‐exposed surfaces of these fruits Fv/Fm did not change significantly regardless of UV‐B dose and remained as high as in intact fruits (Fig. 5F, open symbols).
Role of quercetin glycosides accumulated under high‐sunlight conditions in UV‐B protection
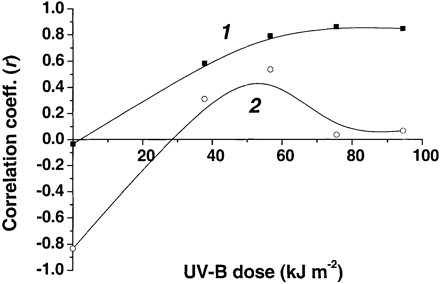
The experiments demonstrated that the photosynthetic apparatus in the skin of sun‐exposed surfaces of Braeburn fruits was most resistant to UV‐B induced damage (Fig. 5D, F). The highest content of quercetin glycosides (Fig. 1) was also recorded here. However, the sun‐exposed skin of Braeburn simultaneously contained significant amounts of anthocyanins (Fig. 2) whereas shaded apple skin of these fruits and both surfaces of Granny Smith were anthocyanin‐free. In order to find out whether anthocyanins contribute to UV‐B protection of the photosynthetic apparatus in Braeburn, the correlation coefficient (r) for dependency between quercetin glycoside or anthocyanin content and Fv/Fm magnitude was calculated and plotted versus UV‐B dose (Fig. 6). No correlation was found between Fv/Fm and skin content of quercetin glycosides in case of intact fruits (r ∼ 0, Fig. 6). In the course of UV‐B irradiation, the correlation between Fv/Fm and content of the flavonoids became stronger (Fig. 6, curve 1) reaching its maximum at c. 70 kJ m–2 (r >0.8). By contrast, a low correlation between anthocyanins and Fv/Fm was recorded during UV‐B irradiation of the same fruits (Fig. 6, curve 2).
Discussion
Plants are adapted to and respond to natural solar UV‐B radiation that varies constantly during the day (Caldwell, 1981; Ryan et al., 2002). Therefore, the estimations of UV‐B‐protective mechanism efficiency using plants grown in growth chambers under unnatural artificial UV and visible irradiation conditions are often misleading (Ryan et al., 2002). This work represents an attempt to estimate the significance of phenolic compounds, accumulated in response to strong sunlight under natural conditions, in the protection of the plant photosynthetic apparatus from UV‐B‐induced damage.
The results of pigment analysis revealed that fruits of Granny Smith and Braeburn exhibited different patterns of pigment content and composition as a result of adaptation to strong sunlight (Fig. 2). Anthocyanin‐free fruits (Granny Smith) showed a decrease in chlorophyll contents in sun‐exposed apple skin accompanied by a proportional decrease in carotenoids. This was not the case in anthocyanin‐containing Braeburn fruits (Fig. 2) which possessed higher chlorophyll and carotenoid contents in sun‐exposed apple skin. This is consistent with the results of previous studies which showed that the adaptation of anthocyanin‐free fruits to elevated sunlight conditions is carried out by means of lowering the chlorophyll content, whereas anthocyanin‐containing apple fruits contained equivalent or even higher amounts of chlorophyll in sun‐exposed skin as compared with shaded skin (Merzlyak and Solovchenko, 2002). Higher skin chlorophyll content in anthocyanin‐containing fruits was probably due to a photoprotective function of the anthocyanins, which reduce the danger of photo‐oxidative damage by means of limiting the amount of light quanta absorbed by the chlorophyll (Gould et al., 2000; Merzlyak and Chivkunova, 2000; Pietrini et al., 2002).
However, as well as the presence of anthocyanins, sun‐exposed skin of Braeburn fruits was characterized by a massive accumulation of flavonoids, which was not observed in the shaded skin of this cultivar (Fig. 1). As could be seen from Fig. 1, both surfaces of Granny Smith fruits possessed moderate skin flavonoid content close to that in shaded surface of Braeburn.
The results of skin extract analysis (Fig. 3) as well as HPLC data (not shown) revealed that quercetin glycosides are the principal group of flavonoids accumulated in apple skin in response to high sunlight. Data shown in Fig. 4 represent the contribution of quercetin glycosides to the absorption of apple skin extracts per unit fruit surface. Granny Smith did not demonstrate a distinct response in terms of quercetin glycoside accumulation since the contribution of these substances to UV‐B absorption of skin extracts was similar on both sun‐exposed and shaded surfaces (Fig. 4A). By contrast, Braeburn responded to an enhanced level of sun radiation by a significant accumulation of quercetin glycoside, resulting in a c. 2‐fold increase in their contribution to absorption of skin extract at 300 nm (Fig. 4B). Taking into account that pigments incorporated in turbid and high‐scattering plant tissue can absorb significantly more light than equivalent amounts of pigment in an extract due to increased optical path length (Butler, 1964), quercetin glycosides in Braeburn skin could absorb nearly all the incident UV‐B radiation. Therefore, apple skin flavonoids are able to serve as efficient sunscreens which filter out most of the solar UV‐B radiation. It should be noted that, flavonoids accumulated in fruits during on‐tree ripening are not subjected to metabolic turnover and are retained even after prolonged storage in darkness (Awad and de Jager, 2000; Merzlyak and Solovchenko, 2002).
Further experiments were designed to estimate the significance of other phenolic substances accumulated in apple skin in response to high fluxes of sun radiation (and hence to elevated UV‐B levels) and their contribution to enhanced resistance of apple photosynthetic apparatus. Apple fruits with different skin phenolic content were subjected to UV‐B irradiation and UV‐B‐induced damage to photosystem II was monitored by means of chlorophyll fluorescence measurements.
The analysis of UV‐B‐induced Fo, Fm and Fv/Fm changes revealed that the resistance of the photosynthetic apparatus of apple fruit to UV‐B irradiation correlated with skin phenolic content (Figs 5, 6). In the case of moderate flavonoid content (both surfaces of Granny Smith or in the shaded surface of Braeburn) UV‐B irradiation induced severe damage to the photosynthetic machinery of apple fruit, which was apparent as a decline in Fm and Fv/Fm values (Fig. 5). An increase in Fo level on the shaded surfaces of both cultivars indicates that UV‐B radiation mainly damaged the reaction centres of photosystem II in these experiments (Maxwell and Johnson, 2000). The extent of the UV‐B induced decrease in the Fm and Fv/Fm parameters exhibited high correlation with apple skin quercetin glycoside content (r2 >0.8 in both cultivars, not shown).
Granny Smith, as well as shaded surfaces of Braeburn, characterized by similar flavonoid contents, also exhibited similar patterns of Fm and Fv/Fm decrease in response to UV‐B irradiation (cf. Fig. 5C, D, E, F). According to data of pigment analysis, sun‐exposed skin of Braeburn possessed the highest flavonoid content among other variants (Fig. 1) and demonstrated remarkable UV‐B‐resistance of photosystem II, which did not show signs of damage at doses up to 97 kJ m–2 (Fig. 5D, F).
The correlation between Fv/Fm and skin quercetin glycosides content, negligible in intact fruits, significantly increases in the course of irradiation (Fig. 6, curve 1). It is probable that, under such conditions, the integrity of photosystem II became dependent on the screening exerted by quercetin glycosides contained in the apple skin. This could be a reason for an increase in the correlation between Fv/Fm and quercetin glycoside content observed at high UV‐B doses.
Only a weak correlation was found between apple skin anthocyanin content and Fv/Fm during UV‐B irradiation (Fig. 6, curve 2). This is in agreement with the results of pigment analysis (Figs 1, 2). Taking into account those data and data on the spectral properties of anthocyanins, which characterized by low extinction coefficients in the UV‐B (Strack and Wray, 1989), the contribution of quercetin glycosides to the UV absorbance of apple skin extracts must be much higher than that of anthocyanins. These considerations support the hypothesis of a limited significance of anthocyanins in the UV‐protection of plants (Woodall and Stewart, 1998), at least when they occur in low or moderate amounts. It should also be noted that massive accumulation of anthocyanins in apple skin generally takes place after the degradation of the bulk of the chlorophyll in the course of ripening, therefore, the Braeburn fruits used here represent the anthocyanin content adequate for the most of the on‐tree ripening period (Saure, 1990; Merzlyak and Solovchenko 2002).
Collectively, the results obtained in this work suggest that flavonoids (represented mainly by quercetin glycosides) accumulated in apple skin during acclimation to strong sunlight can serve as an efficient UV‐B screen. As a consequence, they play an important role in the resistance of the photosynthetic apparatus to the UV‐B component of sun radiation. Anthocyanins did not exhibit a detectable synergistic effect in UV‐B protection, at least in the amounts recorded in this study, and seemingly served for protection from damage only by radiation in the blue‐green part of the visible spectrum (Merzlyak and Chivkunova, 2000).
Acknowledgements
The authors are grateful to Professor MN Merzlyak, Dr SI Pogosyan and Professor Noga for helpful discussions. We also thank the ‘Ministerium für Umwelt, Naturschutz, Landwirtschaft und Verbraucher schutz’, NRW, Germany, for financial support.
Fig. 1. Quercetin glycoside content of skin from sun‐exposed and shaded surfaces of Granny Smith and Braeburn apples (n=60); mean ±SE.
Fig. 2. Pigment content of sun‐exposed skin compared with that of shaded skin of Granny Smith and Braeburn apples (n=60); mean ±SE.
Fig. 3. Typical absorption spectra of the water–methanol fraction of extracts (diluted 8‐fold) obtained from (1) sun‐exposed and (2) shaded skin of Braeburn apples as well as (3) spectrum of pure rutin.
Fig. 4. Contribution of quercetin glycosides (at 300 nm) versus that of chlorophylls (at 666 nm) to the absorption of extracts obtained from shaded and sun‐exposed skin of Granny Smith and Braeburn apples. Absorbance, Ã, was calculated as Ã=A×V×l–1×S–1, where A, V, S, and l are absorbance, volume of extract (ml), fruit skin area (cm2) taken for pigment extraction, and cell pathlength (cm), respectively.
Fig. 5. Changes in chlorophyll fluorescence in shaded (closed symbols) and sun‐exposed (open symbols) skin in the course of UV‐B irradiation of Granny Smith (A, C, E) and Braeburn (B, D, F) apples; mean ±SE, n=10.
Fig. 6. Changes in correlation between Fv/Fm and (1) quercetin glycoside or (2) anthocyanin content in the course of UV‐B irradiation of Braeburn apples (n=10).In each case, error of correlation coefficient was less than 0.01.
References
AndrewsPK, Johnson JR.
AndrewsPK, Johnson JR, Fahy D.
AwadMA, de Jager A.
AwadMA, de Jager A, van Westing LM.
BornmanJF, Reuber S, Cen Y‐P, Weissenböck G.
BurchardP, Bilger W, Weissenböck G.
ButlerW.
CaldwellMM.
CaldwellMM, Flint SD.
DeLongJM, Prange RK, Harrison PA.
EscarpaA, González MC.
FlintSD, Jordan PW, Caldwell MM.
GouldKG, Markham KR, Smith RH, Goris JJ.
JanssenMAK, Gaba V, Greenberg BM.
KolbCA, Käser MA, Copecký J, Zotz G, Riederer M, Pfündel EE.
KraussP, Markstädter C, Riederer M.
KrupaSV, Kickert RN, Jäger H‐J.
KulandaiveluG, Noorudeen AM.
LancasterJE, Grant JE, Lister CE, Taylor MC.
LiakouraV, Bornman JF, Karabourniotis G.
MaxwellK, Johnson GN.
MazzaCA, Boccalandro HE, Giordano CV, Battista D, Scopel AL, Ballaré CL.
MerzlyakMN, Chivkunova OB.
MerzlyakMN, Solovchenko AE.
PietriniF, Iannelli MA, Massacci A.
ReayPF, Lancaster JE.
RobberechtR, Caldwell MM.
RyanKG, Swinny EE, Markham KR, Winefeld C.
Schmitz‐EibergerM, Noga G.
SkórskaE.
SolovchenkoAE, Chivkunova OB, Merzlyak MN, Reshetnikova IV.
StrackD, Wray V.
TeviniM, Teramura AH.
WangH, Arakawa O, Motomura Y.
WellburnAR.
Winkel‐ShirleyB.




Comments