-
PDF
- Split View
-
Views
-
Cite
Cite
Giulia Arsuffi, Siobhan A Braybrook, Acid growth: an ongoing trip, Journal of Experimental Botany, Volume 69, Issue 2, 5 January 2018, Pages 137–146, https://doi.org/10.1093/jxb/erx390
Close - Share Icon Share
Abstract
Since its first formulation almost 50 years ago, acid growth has had a chequered past complicated by utilization of diverse species and organs for testing alongside necessary but coarse methodology. Within the past 25 years, we have gained new insights into the molecular mechanisms behind the transduction of the signal auxin into the reality of an apoplastic pH shift as well as the effect on cell wall mechanics and the biochemical players within the wall contributing to the resultant growth. In this review, we begin by discussing the historical work and its complications, move on to the modern work and its addition to acid growth, which we finally summarize in an updated model which includes new postulations and questions.
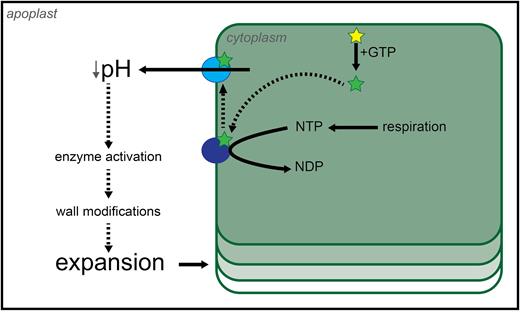
How do cells grow? For plant cells which are bounded by a cell wall, all growth requires modification of the cell wall and its material properties to allow yielding to turgor pressure. Roughly 50 years ago it was hypothesized that decreases in apoplastic pH, stimulated by auxin activation of membrane-bound proton pumps, could be responsible for such modification (Fig. 1; Hager et al., 1971); this is the core of the acid growth theory. Over the subsequent 25 years, various experiments in various systems led to an expanded hypothesis whereby pH manipulation [buffers and fusicoccin (Fc)] could stimulate growth and a drop in pH could increase wall extension and activity of the wall-modifying agent expansin. Over the last 25 years, we have gained further insight into the molecular mechanisms underlying acid growth through the use of new tools. Within this review, we will explore the historical view of acid growth, with a focus on the challenges and contradictions presented in the literature, present the most recent findings in the area, and summarize with an updated model of acid growth.
The first model of acid growth. The mechanistic model proposed by Hager et al. (1971) for auxin-driven growth, adapted here from the original paper, postulated the direct action of auxin onto plasma membrane-localized proton pumps to activate them. In order for this to happen, auxin itself needed to be ‘activated’ by GTP or its precursor ITP. Once active, the proton pumps were hypothesized to hydrolyse available nucleotide triphosphates (NTPs) in order to power proton extrusion. NTP production relies on cellular respiration, which justified the need for aerobic conditions. The increase in apoplastic acidity caused the cell wall to become more extensible by the putative action of modifying agents found in the apoplast, resulting in cell elongation. Inactive auxin, yellow star; active auxin, green star; inactive proton pump, dark blue circle; active proton pump, light blue circle. Hypothetical interactions are shown by dashed lines.
A brief historical review
A signal for pH drop
In the early 1970s, auxin treatment was shown to stimulate proton extrusion into the apoplast as quickly as 20–30 min post-application (Rayle, 1973), in temporal agreement with auxin-induced growth (~20 min; Rayle and Cleland, 1972). Auxin-mediated growth could be reduced if a neutral or basic buffer was coincidentally applied (Hager et al., 1971), indicating that auxin probably acted upstream of acidification. Auxin treatment resulted in a pH drop to ~4.5 (Cleland, 1976), although it has been argued that this may not represent an effective decrease (Kutschera, 1994). Measurements of pH in these studies were done on a bulk level, with segments of organs being floated in liquid and the pH of the liquid being measured. It is plausible that an effective decrease was achieved within specific tissues but this was masked by the bulk pH measurement technique. It is also possible that a drop in pH happens earlier than the 20–30 min recorded, again due to dilution in the bulk technique. A slight modification of this method (Cleland, 1976) involved a small amount of liquid surrounding segments and almost direct contact with the electrode; in this set-up, lower pH drops could be observed upon auxin treatment when compared with the bulk method.
Most experiments were conducted on coleoptiles (maize and oat) and epi- or hypocotyls (pea, soybean, and sunflower), and involved abrasion or removal of the cuticle to allow chemical access; commonly several organ segments would be stacked to facilitate measurements of growth (Kutschera and Schopfer, 1985b). These experimental necessities imposed several constraints on the interpretation of results: first, it was impossible to gain any tissue-level resolution; secondly, mechanical perturbation of the samples by fragment excision, peeling, and abrasion raised concerns about whether growth was due to a normal response to the substance or exaggerated by the removal of the mechanically constraining epidermis (Kutschera, 1994). It is still unclear which tissue within the organ segment was responding to auxin and whether all tissues responded in the same way. There are several studies which add to the debate over whether the epidermis acts as the main target of auxin action (Diehl et al., 1940; Kutschera et al., 1987; Cleland, 1991; Rayle et al., 1991; Kutschera, 1992); a key role for the epidermis might prove problematic when it is often perturbed to allow solutions to enter the organ. The first experiments to address this question involved fine dissection of sunflower hypocotyls into tissues such as pith and cortex, and examination of their differential growth responses to auxin (Diehl et al., 1940). A review on the role of the epidermis in growth, so-called ‘tissue tension’, can be found in Peters and Tomos (1996) and in relation to auxin-induced growth in Kutschera and Niklas (2007). The literature on tissue-related responses and growth merits careful consideration when approaching this subject; it is likely that the epidermis responds to auxin, allowing growth, whereas the inner tissues are primed for growth already, although this may be highly organ dependent
Exploring pH
While auxin was known to stimulate growth, it was not until the 1970s that the acid growth theory proposed that some of this effect might be through apoplast acidification; acid buffer treatments were able to stimulate growth in organ segments (Hager et al., 1971; Rayle, 1973). The growth response was almost immediate (Rayle and Cleland, 1970, 1980), confirming the placement of the pH drop just before growth but after auxin. Growth increased proportionally with a decrease in pH from 6 to 2 (Tepfer and Cleland, 1979; Kutschera and Schopfer, 1985b). Acid buffers led to a transient rapid growth response which levelled off (Rayle and Cleland, 1980; McQueen-Mason et al., 1992). This is in contrast to auxin-induced growth which is more sustained (Rayle and Cleland, 1970).
The application of Fc has always been one of the most effective ways to stimulate growth in vitro. Its application caused rapid acidification of the surrounding buffers (Cleland, 1976; Rayle and Cleland, 1980), proton excretion (Kutschera and Schopfer, 1985a), and coincident rapid growth with an extremely fast rate (~5 min; Rayle and Cleland, 1980). The magnitude of response changed depending on the concentration of Fc applied (Lado et al., 1973). Fc causes the irreversible activation of proton pumps, essentially turning the acid growth system on and leaving it on (Marre, 1979). It is possible that this irreversibility is responsible for the fast and large magnitude of response. This also implies that if pH is globally and permanently kept low, the growth magnitude increases. Combinatorial use of Fc and buffers has helped clarify some points. The application of neutral buffers abolished Fc-induced growth (Kutschera and Schopfer, 1985a). Low pH buffers can mimic, and even surpass, the growth stimulated by Fc (Kutschera and Schopfer, 1985a, b). At pH 4, the value at which pH stabilizes after Fc treatment, the addition of Fc to the acidic buffer stimulates no further growth (Kutschera and Schopfer, 1985a, b). This means that the growth-promoting action of Fc can be replaced by a concentration of protons in the apoplast corresponding to that measured when Fc is added (Kutschera and Schopfer, 1985a), an equivalence that does not hold true for auxin (Kutschera and Schopfer, 1985b).
A mechanical response in the cell wall
The mechanical effect of acid growth on the cell wall has been investigated for as long as acid growth has. The first experiments, and most of those which followed, were conducted on thawed frozen epicotyls, hypocotyl, or coleoptiles (Rayle et al., 1970; Tepfer and Cleland, 1979) or plasmolysed hypocotyls (Hager et al., 1971). When effectively dead organs were used for experiments, growth was simulated by applying an external weight (Hager et al., 1971) or utilization of Instron-type extensometers (Rayle et al., 1970). In these instances, it became key to measure growth in intact living samples, alongside organ extension by mechanical weight (e.g. Rayle, 1973; Rayle and Cleland, 1980; Cleland, 1984; McQueen-Mason et al., 1992). Addition of low pH buffers caused sample extension under load as rapidly as 1–15 min post-treatment (Rayle et al., 1970), and extension was seen to increase proportionally with pH decrease (Hager et al., 1971). Upon auxin treatment, wall extensibility increased rapidly as well (Cleland, 1967; Rayle and Cleland, 1970; Rayle, 1973; Kutschera and Schopfer, 1986). In these studies, extensibility was often split into two parts, elastic and plastic extensibility (Cleland, 1967; Rayle and Cleland, 1970). The plastic extensibility has been theorized to be that which is most relatable to growth (Cosgrove, 1993); however, there still exists some debate on whether plastic extensibility measured was really plastic or simply a viscoelastic deformation which was not given sufficient recovery time (Hohl and Schopfer, 1992). In the end, our understanding of wall mechanical measurements as they relate to growth is still in its infancy.
The agent of change
While changes in pH and applications of auxin were known to stimulate organ extension, experiments involving heat-killing of organs or enzyme inhibitors such as copper led experimenters to hypothesize that enzymes were involved (Hager et al., 1971; Tepfer and Cleland, 1979). However, it was not until the discovery of expansin in cucumber that the strongest pH-responsive mechanistic player in the apoplast was revealed (McQueen-Mason et al., 1992; for a review of other proteins, see McQueen-Mason, 1997). The protein expansin was able to induce elongation in living and dead organ segments, in a pH-dependent manner (McQueen-Mason et al., 1992). The seminal expansin work also showed that expansin activity was correlated positionally along the cucumber hypocotyl with growth (McQueen-Mason et al., 1992). Cucumber wall extract was able to stimulate elongation in other species of eudicots and monocots, but was slightly less effective in grasses (McQueen-Mason et al., 1992). Expansin application to apical meristems was able to induce outgrowth (Fleming et al., 1997), and more recently has been used to manipulate leaf shape (Pien et al., 2001). As far as mechanism of action, expansin has not been shown to have enzymatic activity but does appear to facilitate the loosening of the cell wall via xyloglucan slipping (Cosgrove, 2000). In the simplest case, expansins exist within the apoplastic space and wait for shifts in pH to regulate their activity; given the time scales of Fc and auxin action on pH and wall extension, this is a plausible mechanism regulating short-term (without requirement for new material synthesis) cell growth. While expansin has been shown to be a prolific stimulator of growth, activated by acidification of the apoplast, it is likely that other wall components and modifiers are involved as well; the cell wall is a complex material.
The need for RNA and protein synthesis
The protein synthesis inhibitor cycloheximide (CHX) has been shown to block auxin-induced growth (Cleland, 1970; Kutschera and Schopfer, 1985a), proton extrusion upon auxin treatment (Rayle et al., 1970; Rayle, 1973; Cleland, 1976; Rayle and Cleland, 1980; Kutschera and Schopfer, 1985a; Edelmann and Schopfer, 1989), and auxin-induced wall extensibility (Cleland, 1970). CHX treatment could not block proton secretion upon Fc treatment (Cleland, 1970; Kutschera and Schopfer, 1985a). The RNA synthesis inhibitor cordycepin was shown to have a similar effect on blocking auxin-induced growth (Edelmann and Schopfer, 1989). These experiments strongly indicated that auxin-induced acid growth requires active transcription and translation, although the molecular mechanisms behind this, and their links to proton pump activation, were undiscovered at the time. Recent findings on the role of protein synthesis are described in depth by Kutschera and Wang (2016, and references therein). Among these findings, one of the most notable is the observation of highly electron-dense particles in the outer epidermal wall of intact growing maize coleoptiles; the particles disappear upon fragment excision, but auxin application is able to restore their formation as well as promote fragment elongation, in contrast to Fc and acidic buffer which only affect elongation. However, auxin is not able to induce the formation of these particles if CHX is applied. Based on these data, the particles have been hypothesized to be auxin-dependent cell wall-loosening complexes likely to be proteinaceous.
The historical literature, of which a snapshot has been presented here, is plentiful, but also rife with contradiction. The reasons behind these contradictions are equally opaque but may be due to: the manipulation and abrasion of organs, diverse species being utilized, variable concentrations of applied chemicals, the lack of molecular biological investigation, and the global nature of growth, pH, and mechanical measurements—all of which were necessary concessions given the tools of the time. Next, we present recent findings which have helped to refine the acid growth theory, provided new tools of exploration, and yielded new experimental questions.
New techniques and new data
As is the case for several plant processes, most of the new insights into the acid growth theory come from the study of the angiosperm model species Arabidopsis thaliana. Despite resulting in a narrower perspective, the use of a single, well-characterized model brought several advantages: new imaging and molecular biology techniques were developed and applied to this model more readily than to any other plant. These developments ultimately led to the elucidation of the signalling pathway going from auxin perception to the activation of the proton pumps which acidify the apoplast and promote growth, as well as a better understanding of pH dynamics and cell wall mechanics.
Investigating the transcriptional control behind acid growth
Growth is a long-term process requiring transcriptional changes within growing cells. Auxin-mediated transcriptional changes have been demonstrated to occur within minutes of auxin treatment, and auxin-induced growth requires transcription and translation (McClure and Guilfoyle, 1987; Fendrych et al., 2016; see previous section). The major mechanism of auxin perception in plants is the TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX–AUXIN/INDOLE-3-ACETIC ACID (TIR1/AFB–AUX/IAA) nuclear co-receptor (Dharmasiri et al., 2005; Kepinski and Leyser, 2005). When the concentration of auxin is sufficiently high, the hormone bridges the interaction between the F-box TIR1/AFB proteins and the Aux/IAA transcriptional repressors, leading to the degradation of Aux/IAA proteins (for a review, see Strader and Zhao, 2016). Functional TIR1/AFB–Aux/IAA signalling is required for auxin-driven apoplast acidification and growth, as shown by Aux/IAA-inducible overexpressors (Leyser et al., 1996) which fail to respond to auxin and show agravitropism upon induction (Fendrych et al., 2016). The TIR1/AFB–Aux/IAA pathway induces apoplast acidification in Arabidopsis, in part via the SMALL AUXIN UP-RNA (SAUR) protein family (Spartz et al., 2014). Auxin stimulus promotes expression of this group of short-lived proteins, which in turn cause activation of the plasma membrane (PM) H+-ATPase, leading to a decrease in apoplastic pH (Spartz et al., 2014). More specifically, H+-ATPase activity is regulated by the phosphorylation of threonine residues in the proton pump C-terminal domain (Takahashi et al., 2012). SAURs promote threonine phosphorylation and simultaneously inhibit the activity of type 2C protein phosphatases (PP2Cs), thus maintaining the PM H+-ATPase in its phosphorylated—and active—state (Spartz et al., 2014). Perturbing this pathway has profound effects on growth. Stabilization of SAUR19 by fusion to green fluorescent protein (GFP) results in auxin-independent elongation and apoplast acidification (Fendrych et al., 2016). A highly expressing inducible inhibitor of auxin signalling (dominant negative axr3-1) was able to block auxin-mediated growth and pH drops in the hypocotyl (Fendrych et al., 2016); however, the native axr3-1 mutant could only partially supress auxin-induced growth and H+-ATPase phosphorylation (Takahashi et al., 2012). These data may result from having more or less suppression of the signalling pathway.
The transcriptional response to auxin also mediates the expression of cell wall-remodelling agents. Laskowski et al. (2006) found that Arabidopsis roots treated with exogenous auxin showed induced expression of genes encoding expansins, pectin methylesterases (PMEs), and pectate lyases. Given the different effects which auxin exerts on aerial organs compared with roots (Dunser and Kleine-Vehn, 2015), it remains to be tested if the regulatory effect of auxin on cell wall-remodelling agent gene expression changes in a tissue-dependent manner. In addition, spatial and temporal analysis of cell wall-modifying agent gene transcription will provide a wealth of data for comparison with auxin signalling and growth dynamics; an excellent example of temporal analysis during lateral root emergence provides strong evidence for auxin-mediated wall modification (Lewis et al., 2013).
Changes in pH in muro
The activation of PM H+-ATPases should lead to rapid apoplast acidification. One of the historical points of contention for acid growth centred on the absolute value of the apoplastic pH and where (on cellular and tissue levels) pH changes might act. Values obtained from whole organs, via contact solution pH, or from microelectrodes inside plant tissues have been treated as equivalent measures, resulting in seemingly contradictory data. The advent of fluorescent pH probes such as pHusion (Gjetting et al., 2012), pHluorin (Gao et al., 2004), Pt-GFP (Geilfus et al., 2014), and HPTS (8-hydroxypyrene-1,3,6-trisulphonic acid; Barbez et al., 2017) means that we now have the ability to observe changes in apoplastic pH without having to disturb the organ mechanically by peeling or abrasion. In addition, these sensors have changed the resolution at which pH is measured, by allowing single-cell pH profiling. According to Yu et al. (2000), the great variation in pH values across different tissues, species, and experiments, extending from 3.5 to 8.3, can ultimately be pinned down to the common misconception that the apoplast can be treated as a homogeneous space instead of an ensemble of compartments. For the same reason, values reproducibly cluster in different parts of the pH scale depending on the method of choice (Yu et al., 2000). Combined with high resolution microscopy, fluorescent probes are able partially to overcome this issue by mapping apoplastic pH to the single cell. Enhanced photostability of these sensors, alongside their fast and response to pH changes, also allow fine temporal resolution, ranging from a few minutes to several hours.
In a recent study, elongation in the Arabidopsis hypocotyl was examined at the cellular level alongside changes in an apoplastically targeted pHusion sensor (apo-pHusion; Gjetting et al., 2012). Fendrych et al. (2016) found that apoplastic acidification, elongation, and auxin transcriptional response all happen ~20 min after auxin application in the etiolated Arabidopsis hypocotyl. Apolastic acidification upon auxin treatment, but not Fc treatment, was dependent on the auxin signal perception machinery (see section above). This was the first time that auxin perception, apoplast acidification, and elongation were examined at this spatial scale. The power of the apo-pHusion approach was especially evident when auxin-induced pH changes in the apoplast were examined in vivo on gravitropic responses (Fendrych et al., 2016).
Even more recently, the pH indicator HPTS was used to elucidate the dynamics of growth, apoplastic acidification, and auxin signalling in Arabidopsis root epidermal cells (Barbez et al., 2017). For the first time, all three factors were observed at a cellular resolution in an organ where the validity of the acid growth theory has historically been controversial. Apoplast acidification per se was shown to stimulate epidermal root cell elongation, but high auxin levels (both endogenous and exogenous) did not trigger apoplast acidification in the same way as was seen in the hypocotyl (Fendrych et al., 2016). Instead, auxin induced a biphasic response starting with a rapid alkalinization of the apoplast followed a few hours later by acidification. Given that no cell expansion ensued, the authors concluded that the initial rise in apoplastic pH must have an inhibitory effect on cell growth in roots. Following that, they went on to show that the receptor-like kinase FERONIA mediates alkalinization and that this phenomenon, together with growth inhibition, is not observed in fer-4 mutants. Adding to the results of Fendrych et al. (2016) described above, functional auxin signal transduction was sho wn to be required for a normal root gravitropic response. Crucially, apoplast alkalinization is a necessary intermediate step.
FERONIA also links to acid growth at the level of cell wall modifications. Its ligand RALF4 is co-regulated with pectin-modifying agents (Wolf and Höfte, 2014) among which are PMEs, most of which are known to have an alkaline pH optimum (Sénéchal et al., 2014). Low pH is also known to activate the expansin family of cell wall-remodelling agents (Cosgrove, 2016; see section above)). The mechanism and kinetics of expansin action, however, remain unclear. Based on a recent model of cell wall architecture (Park and Cosgrove, 2012), their primary site of action has been hypothesized to be xyloglucan-rich biomechanical hotspots where cellulose microfibrils are in close contact (Wang et al., 2013). Further characterization of their action, including of their potential enzymatic activity, is required to strengthen our knowledge of the link between apoplast pH decrease and cell wall remodelling leading to mechanical changes.
We believe that further use of fluorescent pH sensors will shed even more light on the temporal and spatial dynamics of the acid growth theory, especially if a reliable calibration method can be achieved so that absolute values of pH are obtainable (Gjetting et al., 2012; Barbez et al., 2017). In addition, sub muro imaging of pH dynamics may also prove informative, but would probably require super-resolution microscopy.
Measuring mechanical properties at a cellular level
The result of wall acidification (e.g. through expansin activity) is wall remodelling leading to cell expansion. Organ-level mechanical studies have demonstrated that expansin activity leads to increased extensibility in living and dead organs (see section above). The introduction of micro-indentation methods such as atomic force microscopy (AFM) has served as a means of testing the changes in cellular and subcellular mechanical properties brought about by auxin (Braybrook and Peaucelle, 2013; Milani et al., 2013; Braybrook, 2017). These techniques are applicable to living samples and allow the combination of genetic, biochemical, and biomechanical observations. Recently AFM-based indentation has added new information to the relationship between auxin and wall mechanics and revealed a role for pectin: auxin was shown to trigger a decrease in cell wall rigidity dependent on pectin de-esterification prior to organ emergence in the Arabidopsis shoot meristem (Braybrook and Peaucelle, 2013). When the de-esterification of pectin was prevented, auxin was no longer able to drive primordium formation (Braybrook and Peaucelle, 2013), indicating that pectin biochemical changes are a necessary part of the cell wall-remodelling events caused by auxin. De-methylated pectin can follow two paths: be degraded by polygalacturonases or cross-link and rigidify with calcium. While the former may be favoured in the shoot meristem, the latter seems most likely in the elongating coleoptile or hypocotyl given that acid-induced growth and wall extensibility were suppressed by calcium addition (Tepfer and Cleland, 1979; Prat et al., 1984).
Early mechanical measurements on the whole-organ level focused on plastic extensibility, or viscoelastic extensibility (see section above), and it is still unclear how modern elasticity measurements relate to these historical measurements (Cosgrove, 2016). Current indentation-based methods measure elasticity at the cell and tissue level, but this property does not necessarily equal cell wall extensibility resulting in growth. There are several reasons for this, some of which are of a technical nature while others are largely dependent on our knowledge gaps about how cell wall architecture is achieved and changes over time to modulate growth (for a review, see Cosgrove, 2016). As those gaps are filled, we anticipate that the role of other mechanical properties of the wall, such as viscoelasticity, will become clearer and require new methods for further investigation.
Hocq et al. (2017) recently proposed an interesting model whereby pectin de-esterification not only reduces cell wall rigidity, but also contributes to localized apoplast acidification and its downstream events. Indeed, it would make little sense to assume that the biochemical changes of pectin chains had no effect on the molecular environment of the apoplast. Interestingly, the major pectin in Arabidopsis, homogalacturonan, can spontaneously de-esterify in alkaline conditions, driving pectin either to cross-link with calcium (if available) or towards degradation, while probably lowering apoplastic pH. This provides more possible mechanisms by which the pectin–pH loop might affect and be affected by acid growth.
Measuring growth at a cellular level
The ultimate process of interest here is growth, and growth kinematics has a long history, beginning at the organ level (Silk and Erickson, 1979). Organ-level kinematics still has uses today and can be very useful in the study of non-model species and their growth (Stahlberg et al., 2015; Solly et al., 2016). High-resolution light imaging has been employed to track smaller and smaller surface landmarks, resulting in an almost cell-level resolution of growth (Bastien et al., 2016; Fendrych et al., 2016). Confocal-based imaging methods combined with stains or transgenic markers have allowed for the tracking of cell-level growth. Computational tools to analyse cell dimension changes such as length, width, volume, and surface area have allowed for a detailed quantitative analysis of cell-level growth to be achieved: ImageJ, CellSet, MorphoGraphx, and PointTracker (Kuchen et al., 2012; Pound et al., 2012; Schneider et al., 2012; Barbier de Reuille et al., 2015); however, these methods are still limited by imaging depth, often being restricted to the epidermis. The application of these cell-level growth-tracking methods alongside measuring transcriptional responses, pH dynamics, and cell wall mechanics will provide a more detailed picture of the mechanisms linking auxin and acid growth.
The need for alternative species
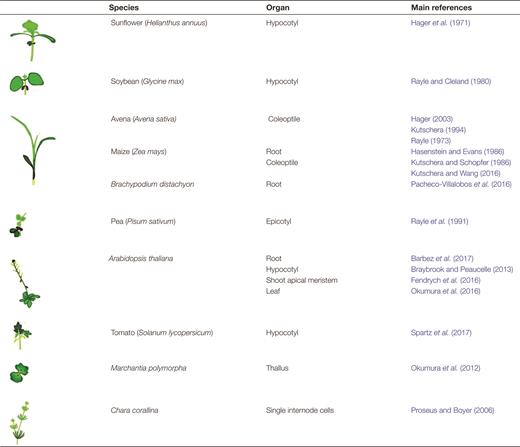
The data behind the acid growth theory initially came from hypocotyls, epicotyls, and coleoptiles of a variety of monocot and dicot species, most commonly oat, pea, and maize, and beginning with sunflower (see section above). Utilizing plant molecular biological resources in the model dicot A. thaliana has provided a depth of knowledge which would have been unattainable otherwise. However, new flowering plant models have provided crucial insight into the effects of auxin on growth, and continuing to explore the diversity of acid growth mechanisms, in both species and organs, will be crucial to our understanding of auxin-mediated growth (Table 1).
The study of the acid growth theory requires the inclusion of a broad range of species.The seminal paper by Hager et al. (1971) referring to auxin-induced elongation as ‘acid growth’ for the first time was based on experiments carried out on sunflower (Helianthus annuus). During the following 25 years, the set of species used to probe the growth-promoting effects of auxin were most commonly soybean, pea, oat, and maize. The rapid rise of Arabidopsis as a model plant, bringing with it molecular knowledge and tools, narrowed the focus of several areas of plant research including that of auxin-driven growth. In the last couple of decades, however, the use of new and existing models has gathered momentum and now no longer only includes angiosperms but also representatives of more basal plant lineages (e.g. Marchantia polymorpha) as well as algae (e.g. Chara corallina). This table indicates some starting example papers, and the species and organs they utilized, for the reader. The references include classic and modern examples
Tomato has been used to confirm the role of SAUR19 in auxin-mediated hypocotyl elongation (Spartz et al., 2017), while studies in Brachypodium revealed that increased elongation in response to constitutively high levels of endogenous auxin is not coupled to increased proton excretion in roots (Pacheco-Villalobos et al., 2016). The Brachypodium experimental results differ from those in Arabidopsis (Barbez et al., 2017), which may indicate species-specific differences or, alternatively, differences in spatial and temporal resolution; either of these possibilities supports the need for further experimentation. Growth, in isolation, of the epidermis and mesophyll tissues from the Argentum pea leaf increases with incubation in a low pH buffer (Stahlberg et al., 2015). Cultured tobacco cells displayed an increase in cell wall elasticity after 1 h of auxin exposure, although the detailed dynamics were not studied with respect to growth and pH (Braybrook, 2017).
The importance of stretching beyond angiosperm species to incorporate more ancient members of the plant kingdom cannot be stressed enough. Not only do the latter often show reduced genetic redundancy, but they also help identify conserved genes and pathways underlying auxin-driven growth. The liverwort Marchantia polymorpha, for example, is being used as a model alongside Arabidopsis to elucidate the regulation of proton pumps by photosynthetic products (Okumura et al., 2012, 2016). The green alga Chara corallina has been exploited to expand on the previous finding that the inhibitory effect of auxin on maize roots is quenched by the application of the calcium chelator EGTA (Hasenstein and Evans, 1986; Proseus and Boyer, 2006), once again suggesting a role for pectin cross-linking in auxin-driven growth.
Current view and outstanding questions
So far, we have described the historical development of the acid growth theory and how recent tools could help settle some of its most controversial points. However, several aspects of the theory remain contentious, starting from its validity being potentially restricted to certain organs only (Luthen and Bottger, 1993; Kutschera, 2006). In contrast to the growth-promoting effects that it has on aerial organs, the application of auxin inhibits root elongation, potentially because of the tighter interplay of cell division and elongation that takes place in roots (Pacheco-Villalobos et al., 2016).
Part of the present controversy revolves around the long-standing problem of measuring apoplastic pH. Despite the advantages of fluorescent pH sensors, we still lack a reliable way to quantify absolute apoplastic pH. Methods to measure it are continuously being refined, but their resolution is still too coarse (for instance, whole-organ resolution in Villiers and Kwak, 2013) to be fully informative, while methods going down to the cellular level are not reliable at the quantitative level because of problematic calibration and possible bleeding of signal from the endomembrane system into signal from the apoplast (Gjetting et al., 2012). Subapoplast imaging resolution has the potential not only to solve signal overlap but also to provide useful information on local changes in pH, such as near sites of pectin delivery. Obtaining absolute pH values will be crucial to make a connection between auxin-driven apoplast acidification and the optimal conditions for cell wall-modifying agents to operate.
Auxin-induced apoplastic acidification is dependent on the activity of PM H+-ATPases (Takahashi et al., 2012), but these are by far not the only players involved. Mutants in the CAX gene family of tonoplast-localized Ca2+/H+ antiporters show a 3-fold increase in apoplastic Ca2+ concentration together with altered cell wall mechanical properties (Conn et al., 2011), reduced expression of cell wall-modifying agent transcripts (Conn et al., 2011), higher apoplastic pH (Cho et al., 2012), and perturbed auxin transport (Cho et al., 2012). The vacuolar H+-pyrophosphatase gene AVP1 has also been associated with alterations in auxin transport and changes in apoplastic pH (Li et al., 2005). The apoplastic pH was found to be lower in AVP1OX mutants and higher in avp1-1 mutants, and was hypothesized to be associated with the recycling of the auxin efflux carrier PIN1 (Li et al., 2005). However, given the lack of auxin phenotypes of the AVP1 loss-of-function mutant fugu5 and the secondary T-DNA insertion present in avp1-1 in a different gene involved in auxin transport, the involvement of AVP1 in acid growth has been questioned (Schilling et al., 2016). These are only two examples of processes which have not been historically associated with acid growth until recent times. We anticipate that, as other areas of plant molecular physiology advance alongside the field of acid growth and new and existing tools are refined, it will become easier to draw parallels between auxin perception and signalling, changes in apoplastic pH, and growth responses.
A revised model of acid growth
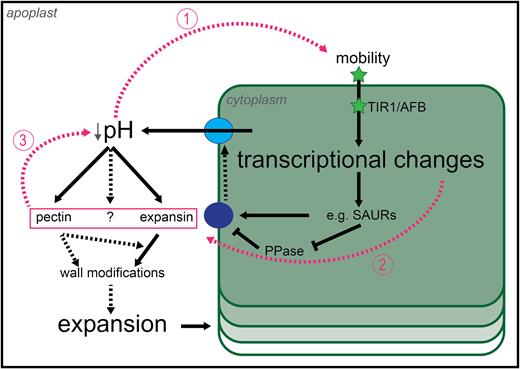
The historical and current data on acid growth leaves us with a model which is at once parsimonious yet provides new questions. At the core of the model is a decrease in apoplastic pH, which leads to changes in the cell wall, resulting in growth. We propose that this change in pH may be mediated by auxin-induced changes in gene transcription which may affect the wall directly (increased wall-modifying agent gene transcription) or indirectly (changing H+-ATPase activity). As outlined in Fig. 2, once auxin is perceived by a cell, through the TIR1/AFB–AUX/IAA co-receptor, transcriptional changes occur very rapidly, leading to the activation of PM H+-ATPases. Co-incidentally, the transcriptional response probably involves induction of wall-modifying agent gene transcription (kinetics unknown at this time). These two mechanisms would lead to a decrease in apoplastic pH, increasing the activity of wall-modifying agents as well as an increase in the quantity of agents able to modify the cell wall architecture.
Revised model of auxin-driven cell growth. The revised model confirms parts of the old model and refutes others (see text). Solid black arrows indicate interactions which have been shown to happen, while dashed black arrows indicate interactions for which we only have partial evidence and which require further investigation. Pink dashed arrows connect the components of the positive feedback loop which are hypothesized to sustain auxin-driven growth over time. High levels of auxin, achieved either by diffusion or by polar transport (or both), trigger downstream transcriptional events leading to the activation of plasma membrane proton pumps and consequent acidification of the apoplast. Auxin may also affect cell wall modifications by regulating the transcription of cell wall-modifying agents. The change in apoplastic pH leads to cell wall modifications by enzyme activation, and such modifications potentially feed-back onto pH itself by changing the local biochemical environment. A more acidic pH is also going to change the protonation status of auxin and, consequently, its ability to cross the plasma membrane by diffusion, closing the loop. Auxin, green star; inactive proton pump, dark blue circle; active proton pump light blue circle. Hypothetical interactions are shown by dashed lines.
We have proposed several positive feed-back points within the acid growth model that could allow for continuation of growth and even its increase over time (Fig. 2, pink lines). First, changes in apoplastic pH would probably increase the diffusive mobility of apoplastic auxin into the cell where it would feed into transcriptional responses. Secondly, depending on the kinetics of induction, the activation of wall-modifying agent genes induced by auxin may provide an additional boost to the system since, thirdly, changes in wall structure and biochemistry may lead to decreases in pH themselves (e.g. pectin de-methylation; Hocq et al., 2017). These three potential interactions in the model could contribute to its robustness and dynamics in time. It should be mentioned that auxin enters and exits cells by active transport as well as diffusion, and it is possible that these mechanisms are so efficient that changes in diffusion cannot impact the absolute amount of auxin within a cell, limiting the effect of this feed-back loop.
The model proposed here lends new questions to investigation. We still have only a basic understanding of the kinetics of auxin transcriptional response as it relates to acid growth and wall modification, which needs more depth. Add to this kinetics a tissue and cell type context and the task becomes challenging and exciting. With respect to wall-modifying agent activities and their relationship to pH, again we have very basic knowledge about which pH values are optimal for which agents, how much each architectural change contributes to growth mechanics, and if changes in architecture might feed-back onto mobility of other agents. It is highly likely that there are other wall-modifying agents to be considered (beyond expansins and PME/PMEIs), including but not limited to xyloglucan endotransglucosylase/hydrolases and polygalacturonases. It is also not yet understood how activities of these agents might alter pH, and these dynamics are worth further study as well. In addition, the contributions of diffusive auxin uptake versus active auxin uptake in such a dynamic system require investigation. Lastly, something which has not been touched upon here at all, one might wonder how acid growth ceases especially when positive feed-back loops might be involved?
Additions to the current model
Modern reformulations are extending the original acid growth theory by the inclusion of other factors. Dunser and Kleine-Vehn (2015) proposed a mechanism, which they baptized ‘the acid growth balloon theory’, whereby auxin-driven changes in vacuolar volume are the key player behind cell elongation, underlining the importance of ion transport in acid growth. As described in a previous section, Hocq et al. (2017) postulated that the de-esterification status of pectin itself changes apoplastic pH, closing the loop between the biochemistry of the cell wall and auxin.. Finally, Okumura et al. (2016) showed that sugar activates PM H+-ATPases and hypothesize that this observation might be the result of sugar-induced activation of SAUR transcription. Further investigation is required to understand how processes associated with auxin-induced elongation are internally regulated. As correctly underlined by Niklas and Kutschera (2012), however, it is important to bear in mind that these systems do not work in isolation and that the insights derived from their study will become truly informative only when an integrative approach is adopted. In other words, as is often the case in plant development, the whole is more than just the sum of its parts, and this holds true for cells and tissues as well as for molecular pathways.
Abbreviations:
Acknowledgements
GA is supported by a BBSRC Doctoral Training Partnership through the University of Cambridge. SAB and work within the group is funded by The Gatsby Charitable Foundation (GAT3396/PR4).
References
Author notes
Present address: Department of Molecular, Cell and Developmental Biology, UCLA, 610 Charles E Young Dr East, Los Angeles, CA 90095, USA.




Comments