-
PDF
- Split View
-
Views
-
Cite
Cite
Matthias Wissuwa, Gloria Gamat, Abdelbagi M. Ismail, Is root growth under phosphorus deficiency affected by source or sink limitations?, Journal of Experimental Botany, Volume 56, Issue 417, July 2005, Pages 1943–1950, https://doi.org/10.1093/jxb/eri189
Close - Share Icon Share
Abstract
Reduced net photosynthesis (Pn) and decreasing shoot and root biomass are typical effects of phosphorus deficiency in plants. Lower biomass accumulation could be the result of reduced Pn (source limitation), but may also be due to direct negative effects of low P availability on growth (sink limitation). Because of the principal importance of root growth for P uptake, this study specifically examined the question whether source or sink limitations were responsible for reduced root growth rates under P deficiency. Rice plants were grown in nutrient solutions with four levels of P supply and at two light treatments and the effect of P×light treatments on growth and carbohydrate distribution was observed. Plants had up to 70% higher Pn when grown with natural (high) light compared with low light. Higher Pn, however, did not lead to additional growth under P deficiency, suggesting that assimilate supply from source leaves to roots was not a limiting factor under P deficiency. This was supported by observations that root starch concentrations increased in P-deficient roots. The comparison of two genotypes with different tolerance to P deficiency showed that the more tolerant one preferentially distributed P to roots where the additional P stimulated root growth and, ultimately, P uptake. The results therefore suggest that source limitation is of little importance under P deficiency. Even at highly sub-optimal tissue P concentrations of below 0.7 mg P g−1 dry weight, plants were able to produce enough assimilates to sustain growth rates that were directly limited by low P availability.
Introduction
Phosphorus (P) deficiency is a major abiotic stress that limits crop productivity on 30–40% of the world's arable land (von Uexküll and Mutert, 1995). In addition to areas of low absolute soil-P content, P deficiency can arise in soils where P is strongly bound to soil particles. This tendency to form insoluble complexes leads to a very low mobility of P in soils. A large root system capable of exploring greater soil volume has therefore been recognized as one important adaptation of plants to ensure a sufficient uptake of P (Sattelmacher et al., 1994; Horst et al., 2001). The root architecture of plants can undergo several changes in response to P deficiency. Increases in lateral root growth and secondary root branching at the expense of primary root elongation was observed in beans (Lynch and Brown, 2001) and arabidopsis (Ticconi et al., 2004). However, Mollier and Pellerin (1999) detected a reduction rather than an increase in lateral root elongation in maize and Wissuwa (2005) showed that P deficiency stimulated root elongation in rice. Benefits of a shift to lateral root growth would be a concentration of roots in the relatively P-rich topsoil (topsoil foraging) and it remains to be seen whether species with a fibrous root system (maize, rice) can achieve the same through changes in root growth angles. One adaptation to P deficiency commonly observed in most species is an increase in root hair length and density, which would improve P uptake through an expansion of root surface area at minimal cost (Gahoonia and Nielsen, 1997; Lynch and Brown, 2001). Another typical response to P deficiency is the increase in root:shoot ratio, possibly due to preferential assimilate distribution to the roots (Fredeen et al., 1989; Mollier and Pellerin, 1999; Vance et al., 2003).
Despite this relative increase in root biomass, P deficiency leads to a reduction in absolute root growth and results from genotypic comparisons in rice (Oryza sativa L.) showed that this reduction was far more pronounced in genotypes with a low tolerance to P deficiency (Wissuwa, 2005). In that study, correlations between P uptake under low-P stress and relative root growth (root dry weight under low-P stress relative to non-stress root growth) were higher (r=0.48) than between P uptake under P deficiency and non-stress root growth potential (r=0.27). This raised the question of how tolerant genotypes were able to maintain relatively high root growth rates despite P deficiency. Two general explanations can be offered. Tolerant plants may have been more efficient in P uptake per root size (higher external P uptake efficiency) and the additional P then drove further root growth, assuming that low P availability affected root biomass accumulation directly. Alternatively, root growth may have been limited by insufficient assimilate supply to roots, possibly due to a negative effect of P deficiency on photosynthetic efficiency.
Concentrations of Pi in the leaf are thought to affect photosynthetic rates via the Pi translocator, an antiporter that facilitates the export of triose phosphate from the stroma to the cytosol in exchange for Pi (Stitt and Quick, 1989). Large amounts of Pi have to be regenerated and retranslocated to the stroma to maintain carbon fixation, as one molecule of Pi is required for every three molecules of CO2 fixed as triose phosphate. Low cytosol Pi concentration may therefore have a negative effect on the Calvin cycle, either by altering levels of phosphorylated intermediates or by affecting the required enzymes through their level of activation (Heldt et al., 1977). It has indeed been shown repeatedly that P deficiency reduces photosynthetic efficiency in crops such as soybeans (Lauer et al., 1989), barley and spinach (Foyer and Spencer, 1986), and wheat (Rodríguez et al., 1998). Others concluded that a reduction in photosynthetic efficiency, though observed, was not the growth-limiting factor under P deficiency. Instead, P deficiency led to an accumulation of assimilates throughout the plant and the fact that they were not used for growth was seen as an indication of sink rather than source limitations under P deficiency (Fredeen et al., 1989). Plénet et al. (2000b) showed that growth of field-grown maize under P deficiency was reduced earlier and to a greater extent than photosynthesis, suggesting that low P availability had a direct effect on growth. These contrasting results may have been caused by different experimental conditions or by interspecific differences in the vulnerability of the photosynthetic complex to low Pi (Foyer and Spencer, 1986).
The question whether growth under P deficiency is reduced as a consequence of source or sink limitations has recently gained practical importance as genetic factors controlling P-deficiency tolerance are detected and their effects investigated. Previous studies have identified and confirmed a major quantitative trait locus (QTL) for P uptake in rice (Wissuwa et al., 2002). A pair of near isogenic lines (NILs) carrying different alleles at this P uptake QTL (Pup1) differed 3-fold in P uptake from a P-deficient soil (Wissuwa and Ae, 2001). The superior NIL maintained higher root growth rates under P deficiency, but only as it started to diverge from intolerant lines in P uptake. It was therefore not possible to determine if differences in root growth preceded and caused differences in P uptake or whether superior root growth was the result of increased P uptake due to higher P uptake per root size (higher external efficiency).
The objective of this study was to test two alternative hypotheses. (i) Hypothesis A: root growth is limited by insufficient assimilate supply (source limitation); and (ii) hypothesis B: root growth is directly limited by low P supply (sink limitation). A pair of NILs with different alleles at the Pup1 locus was used and the effect of different light and P levels on growth, carbon assimilation, and distribution of P and starch in different plant parts was reported.
Materials and methods
Solution culture experiment
The experiment was carried out during the dry season of 2003 at the International Rice Research Institute (IRRI), Philippines. Genotypes used were NIL-Pup1 (high P uptake; former designation: NIL-C443) and the recurrent parent Nipponbare (low P uptake). A detailed genotypic description is given by Wissuwa et al. (2002). Both lines were germinated on 7 March on moist filter paper and subsequently raised for 10 d in half-strength Yoshida solution (Yoshida et al., 1976) containing P at a concentration of 3 μM. After the pretreatment period, seedlings were transferred to styrofoam sheets floating on full-strength Yoshida solution in 50 l buckets. Nutrient solutions were replaced at 5 d intervals and the pH was adjusted to 5.7 daily. Both genotypes were grown together in the same bucket with two plants of the same genotype per planting hole. Treatments consisted of four different P concentrations in solution (3.2, 6.4, 9.6, and 100 μM P) and two different light intensities (low, natural=high). These levels of P had previously been found to result in growth rates that were similar to ones observed in severely P-deficient (3.2 μM P) or moderately P-deficient soils (9.6 μM P). Plants of the low-light treatment were grown in a growth chamber illuminated with a constant light intensity of 260 μmol m−2 s−1. The growth chamber was set at 70% relative humidity and 30 °C during the 13 h light period and 24 °C during darkness. A greenhouse was used for the natural light treatment. The average natural light intensity during the growth period was 765 μmol m−2 s−1. Average maximum/minimum temperatures in the greenhouse were 30.6/23.4 °C. The experiment was harvested 7 weeks after sowing.
Net photosynthesis was measured on 5 and 7-week-old plants on the youngest fully expanded leaf using a LI-6200 Portable Photosynthesis System (Li-Cor, Lincoln, NB). This system uses ambient light rather than an artificial light source. Measurements were done on a sunny day between 10.00 h and 11.00 h. Following the measurements on week five, one of the two plants planted per hole was removed and brought to the laboratory where leaves, shoots, and roots were separated. Leaf fresh weight was determined and leaf area per plant measured with a Li-Cor-3200 Area Meter (Li-Cor, Lincoln, NB). Leaf, shoot, and root samples were subsequently dried at 65 °C for 3 d and ground to a fine powder. This procedure was repeated with the remaining plant after week 7. The tissue-P concentration of root, shoot, and leaf samples was determined colorimetrically by the phosphovanadate method (Hanson, 1950) after digestion in a mixture of HNO3, HClO4, and H2SO4 (3:1:1; v/v). The carbohydrate contents in leaves and roots were analysed after extraction in 80% ethanol (v/v). Soluble carbohydrates were measured directly on the extract, using anthrone as a colour reagent. The residue after ethanol extraction was washed several times and used for starch analysis following the method of Setter et al. (1994). All data were subjected to analysis of variance using PROC MIXED of SAS (SAS Institute, Cary, NC). The t-test was used for mean comparisons of light treatments within P levels and for mean comparisons of genotypes within light×P levels.
Pot experiment
A highly weathered, P-deficient soil was collected from a farmer's field located in Cavinti, Laguna, Philippines. The soil was roughly ground, passed through a 5 mm sieve and filled into 5.0 l pots. Pre-germinated seeds of Nipponbare and NIL-Pup1 were sown into pots at a rate of five seeds per pot. These were later thinned to maintain two healthy seedlings per pot. Plants were grown under upland conditions for 60 d; during that period they were watered with P-free Yoshida solution once per fortnight and with tap water as needed to maintain water around field capacity. At harvest shoots were removed and roots carefully rinsed in a sieve over running water. Shoot and root samples were dried, weighed, and root sugars and starch determined as described above.
Results
Effect of P supply and light intensity on P uptake and dry matter
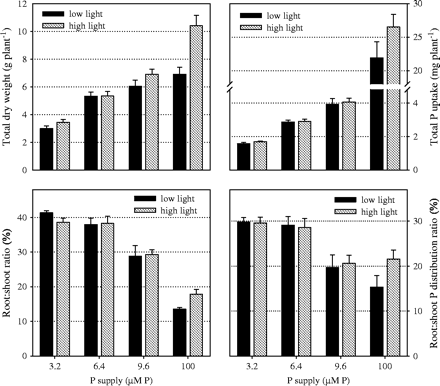
P was supplied at four different levels to study the interaction of light with P supply over a range of deficiencies from severe (3.2 μM P) to moderate (9.6 μM P). Raising the P supply in nutrient solution from 3.2 μM P to 9.6 μM P linearly increased P uptake of plants regardless of light treatments (Fig. 1). Further increases in P uptake in the excess-P treatment (100 μM P), on the other hand, were not linear and the light treatment started to show an effect with less P uptake under low light. These results basically confirm the validity of the experimental setup. When P is deficient, virtually all P added to the nutrient solution is taken up (93–98%), regardless of light treatments. With excess P supply the proportion of P taken up decreases to 40% at low light compared with 50% in high light. The comparison of total dry matter accumulation confirms that P supply was the only limiting factor at 3.2 and 6.4 μM P whereas light was the limiting factor at 100 μM P. The moderate P deficiency treatment represents a transition phase, with P being clearly limiting at high light, but light becoming an additional limitation under low light.
Effect of P supply and light intensity on dry weight, P uptake and on the relative distribution of dry weight and P between root and shoot (harvested 7 weeks after sowing). Values shown are combined averages for genotypes Nipponbare and NIL-Pup1 (bars represent standard errors). Plants were grown in nutrient solution at four levels of P supply and two levels of light intensity (low=260 μmol m−2 s−1; natural=765 μmol m−2 s−1).
How P and dry matter were distributed between the root and shoot was mostly affected by P supply (Fig. 1). At the lowest P level the highest proportion of P (30%) and of dry matter (40%) was found in roots. This decreased to 20% for P and 30% for dry matter under moderate P deficiency. When P was not limiting, less than 20% of P and dry matter were distributed to roots. Root growth and P supply to roots decreased even further in the low-light treatment in favour of leaf development, presumably to maximize light interception.
A detailed description of dry matter and P distribution between the root and shoot is shown in Table 1, together with respective tissue-P concentrations. A clear distinction can be made between excess P and the three P-deficient treatments. In the excess P-treatment, plants had very high root and shoot tissue P concentrations and showed significant effects of the light treatment for all measured traits. Higher tissue-P concentrations in the low-light treatment at lower root and shoot dry matter confirmed that light was the growth-limiting factor. Plants grown under P deficiency showed highly suboptimal P concentrations in root (<0.5 mg P g−1 RDM) and shoot tissue (<0.75 mg P g−1 SDM). P treatments had an effect on RDM but light intensity did not, which possibly indicated that P supply had a direct effect on root growth. Shoot dry matter, however, did show a small response to light, particularly under moderate P deficiency (9.6 μM P), where plants grown under high light had slightly higher SDM (+15%) at lower shoot P concentrations (−11%) compared with plants grown under low light. Light, therefore, started to have an effect at the moderate P-deficiency level, but at more severe P deficiency this effect was either absent (6.4 μM P) or small (3.2 μM P).
Dry matter and P distribution between root and shoot as influenced by P supply and light intensity
P supply (μM) . | Light . | Root DMa (g) . | Root Pa (mg) . | Root P concentrationa (mg g−1) . | Shoot DMa (g) . | Shoot Pa (mg) . | Shoot P concentrationa (mg g−1) . |
|---|---|---|---|---|---|---|---|
| 3.2 | Low | 0.88 ns | 0.36 ns | 0.41 * | 2.12 * | 1.22 ns | 0.58 ** |
| High | 0.98 ns | 0.36 ns | 0.36 * | 2.54 * | 1.33 ns | 0.52 ** | |
| 6.4 | Low | 1.47 ns | 0.61 ns | 0.41 ns | 3.86 ns | 2.26 ns | 0.59 ** |
| High | 1.48 ns | 0.62 ns | 0.43 ns | 3.69 ns | 2.33 ns | 0.64 ** | |
| 9.6 | Low | 1.38 ns | 0.63 ns | 0.47 ns | 4.61 ** | 3.31 ns | 0.73 ** |
| High | 1.54 ns | 0.68 ns | 0.44 ns | 5.29 ** | 3.37 ns | 0.66 ** | |
| 100 | Low | 0.86 ** | 2.92 ** | 3.40 ** | 6.31 ** | 19.01 ** | 3.02 ** |
| High | 1.55 ** | 4.80 ** | 3.10 ** | 8.62 ** | 21.71 ** | 2.52 ** | |
| HSDb | 0.30 | 0.21 | 0.14 | 1.05 | 0.74 | 0.14 |
P supply (μM) . | Light . | Root DMa (g) . | Root Pa (mg) . | Root P concentrationa (mg g−1) . | Shoot DMa (g) . | Shoot Pa (mg) . | Shoot P concentrationa (mg g−1) . |
|---|---|---|---|---|---|---|---|
| 3.2 | Low | 0.88 ns | 0.36 ns | 0.41 * | 2.12 * | 1.22 ns | 0.58 ** |
| High | 0.98 ns | 0.36 ns | 0.36 * | 2.54 * | 1.33 ns | 0.52 ** | |
| 6.4 | Low | 1.47 ns | 0.61 ns | 0.41 ns | 3.86 ns | 2.26 ns | 0.59 ** |
| High | 1.48 ns | 0.62 ns | 0.43 ns | 3.69 ns | 2.33 ns | 0.64 ** | |
| 9.6 | Low | 1.38 ns | 0.63 ns | 0.47 ns | 4.61 ** | 3.31 ns | 0.73 ** |
| High | 1.54 ns | 0.68 ns | 0.44 ns | 5.29 ** | 3.37 ns | 0.66 ** | |
| 100 | Low | 0.86 ** | 2.92 ** | 3.40 ** | 6.31 ** | 19.01 ** | 3.02 ** |
| High | 1.55 ** | 4.80 ** | 3.10 ** | 8.62 ** | 21.71 ** | 2.52 ** | |
| HSDb | 0.30 | 0.21 | 0.14 | 1.05 | 0.74 | 0.14 |
Plants were grown in nutrient solution at four levels of P supply and two levels of light intensity. A growth chamber at a constant light intensity of 260 μmol m−2 s−1 was used for the low-light treatment and a greenhouse was used for the high-light treatment (average light intensity during the growth period was 765 μmol m−2 s−1). Seven-week-old plants were harvested and values shown are combined averages for genotypes Nipponbare and NIL-Pup1.
*, **Difference between low and high light within P levels significant (t-test at P <0.05 and P <0.01, respectively); ns, not significant.
Tukey's HSD (P <0.05) for the comparison of P treatment effects.
Dry matter and P distribution between root and shoot as influenced by P supply and light intensity
P supply (μM) . | Light . | Root DMa (g) . | Root Pa (mg) . | Root P concentrationa (mg g−1) . | Shoot DMa (g) . | Shoot Pa (mg) . | Shoot P concentrationa (mg g−1) . |
|---|---|---|---|---|---|---|---|
| 3.2 | Low | 0.88 ns | 0.36 ns | 0.41 * | 2.12 * | 1.22 ns | 0.58 ** |
| High | 0.98 ns | 0.36 ns | 0.36 * | 2.54 * | 1.33 ns | 0.52 ** | |
| 6.4 | Low | 1.47 ns | 0.61 ns | 0.41 ns | 3.86 ns | 2.26 ns | 0.59 ** |
| High | 1.48 ns | 0.62 ns | 0.43 ns | 3.69 ns | 2.33 ns | 0.64 ** | |
| 9.6 | Low | 1.38 ns | 0.63 ns | 0.47 ns | 4.61 ** | 3.31 ns | 0.73 ** |
| High | 1.54 ns | 0.68 ns | 0.44 ns | 5.29 ** | 3.37 ns | 0.66 ** | |
| 100 | Low | 0.86 ** | 2.92 ** | 3.40 ** | 6.31 ** | 19.01 ** | 3.02 ** |
| High | 1.55 ** | 4.80 ** | 3.10 ** | 8.62 ** | 21.71 ** | 2.52 ** | |
| HSDb | 0.30 | 0.21 | 0.14 | 1.05 | 0.74 | 0.14 |
P supply (μM) . | Light . | Root DMa (g) . | Root Pa (mg) . | Root P concentrationa (mg g−1) . | Shoot DMa (g) . | Shoot Pa (mg) . | Shoot P concentrationa (mg g−1) . |
|---|---|---|---|---|---|---|---|
| 3.2 | Low | 0.88 ns | 0.36 ns | 0.41 * | 2.12 * | 1.22 ns | 0.58 ** |
| High | 0.98 ns | 0.36 ns | 0.36 * | 2.54 * | 1.33 ns | 0.52 ** | |
| 6.4 | Low | 1.47 ns | 0.61 ns | 0.41 ns | 3.86 ns | 2.26 ns | 0.59 ** |
| High | 1.48 ns | 0.62 ns | 0.43 ns | 3.69 ns | 2.33 ns | 0.64 ** | |
| 9.6 | Low | 1.38 ns | 0.63 ns | 0.47 ns | 4.61 ** | 3.31 ns | 0.73 ** |
| High | 1.54 ns | 0.68 ns | 0.44 ns | 5.29 ** | 3.37 ns | 0.66 ** | |
| 100 | Low | 0.86 ** | 2.92 ** | 3.40 ** | 6.31 ** | 19.01 ** | 3.02 ** |
| High | 1.55 ** | 4.80 ** | 3.10 ** | 8.62 ** | 21.71 ** | 2.52 ** | |
| HSDb | 0.30 | 0.21 | 0.14 | 1.05 | 0.74 | 0.14 |
Plants were grown in nutrient solution at four levels of P supply and two levels of light intensity. A growth chamber at a constant light intensity of 260 μmol m−2 s−1 was used for the low-light treatment and a greenhouse was used for the high-light treatment (average light intensity during the growth period was 765 μmol m−2 s−1). Seven-week-old plants were harvested and values shown are combined averages for genotypes Nipponbare and NIL-Pup1.
*, **Difference between low and high light within P levels significant (t-test at P <0.05 and P <0.01, respectively); ns, not significant.
Tukey's HSD (P <0.05) for the comparison of P treatment effects.
Effect of P supply and light intensity on photosynthetic efficiency and carbohydrate accumulation
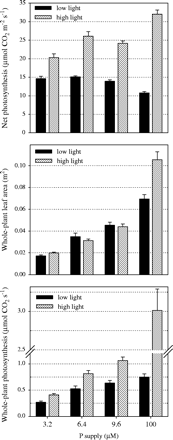
Net photosynthesis (Pn) was always higher under natural light (Fig. 2) and this difference increased with increasing P supply. Under natural light, the addition of P increased Pn from 20 μmol CO2 m−2 s−1 at 3.2 μM P to 32 μmol CO2 m−2 s−1 at 100 μM P. Such an increase was not observed under low light intensity. Net photosynthesis remained rather constant at around 15 μmol CO2 m−2 s−1 throughout P-deficient conditions and decreased to 11 μmol CO2 m−2 s−1 with excess P. Estimates of total CO2 fixation were obtained by multiplication of net photosynthetic rates by total leaf area (Fig. 2). Differences in Pn and whole plant leaf area both affected whole plant photosynthesis. Differences between light treatments within P treatments were mainly due to low Pn levels at low light. However, at a given light level, differences between P treatments were mainly due to increases in leaf area with increasing P supply.
Effect of P supply and light intensity on net photosynthesis, plant leaf area and on estimated whole-plant photosynthesis. This estimate was obtained by multiplying net photosynthesis by whole-plant leaf area. Values shown are combined averages for genotypes Nipponbare and NIL-Pup1, both grown in nutrient solution containing four levels of P (bars represent standard errors; measurements taken 7 weeks after sowing).
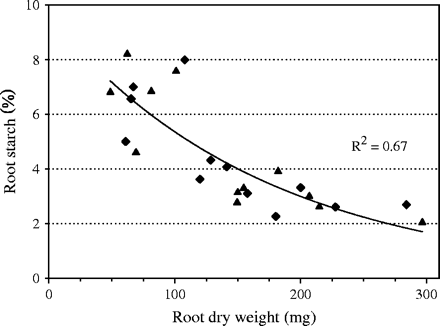
Sugar and starch concentrations in leaves of plants grown under low light were higher (Table 2), despite lower levels of CO2 assimilation in the low light treatment. Leaf starch concentrations were lowest in the excess-P treatment which would be expected if insufficient P supply in the chloroplasts negatively affected photosynthesis through reduced assimilate export. However, root starch concentrations increased with decreasing P supply and this observation indicated that assimilate supply to roots was not a limiting factor for root growth under P deficiency. Even more pronounced differences in root starch concentrations were found in plants grown in highly P-deficient soil. In that experiment, root size was inversely related to root starch concentrations (Fig. 3). It appears, therefore, that root growth in soil (and solution) was not inhibited by a lack of assimilate supply to roots.
Relationship between root dry weight and root starch content of 60-d-old rice plants grown in 5.0 l pots filled with highly P-deficient soil (Nipponbare (filled triangles), NIL-Pup1 (filled diamonds)).
Concentration of starch and reducing sugars in root and leaf tissue as influenced by P supply and light intensity
P supply (μM) . | Light . | Root starcha (%) . | Leaf starcha (%) . | Root sugarsa (%) . | Leaf sugarsa (%) . |
|---|---|---|---|---|---|
| 3.2 | Low | 4.30 * | 2.80 ** | 0.98 ns | 3.29 ** |
| High | 3.76 * | 2.34 ** | 0.99 ns | 2.57 ** | |
| 6.4 | Low | 3.97 ** | 2.92 ** | 0.94 ns | 3.75 ** |
| High | 3.43 ** | 2.34 ** | 0.91 ns | 2.62 ** | |
| 9.6 | Low | 3.49 ns | 2.86 * | 0.90 ns | 4.23 ** |
| High | 3.59 ns | 2.54 * | 0.86 ns | 2.86 ** | |
| 100 | Low | 2.94 * | 2.12 ** | 0.83 ns | 3.25 ns |
| High | 3.26 * | 1.69 ** | 0.76 ns | 2.52 ns | |
| HSDb | 0.35 | 0.31 | 0.14 | 0.61 |
P supply (μM) . | Light . | Root starcha (%) . | Leaf starcha (%) . | Root sugarsa (%) . | Leaf sugarsa (%) . |
|---|---|---|---|---|---|
| 3.2 | Low | 4.30 * | 2.80 ** | 0.98 ns | 3.29 ** |
| High | 3.76 * | 2.34 ** | 0.99 ns | 2.57 ** | |
| 6.4 | Low | 3.97 ** | 2.92 ** | 0.94 ns | 3.75 ** |
| High | 3.43 ** | 2.34 ** | 0.91 ns | 2.62 ** | |
| 9.6 | Low | 3.49 ns | 2.86 * | 0.90 ns | 4.23 ** |
| High | 3.59 ns | 2.54 * | 0.86 ns | 2.86 ** | |
| 100 | Low | 2.94 * | 2.12 ** | 0.83 ns | 3.25 ns |
| High | 3.26 * | 1.69 ** | 0.76 ns | 2.52 ns | |
| HSDb | 0.35 | 0.31 | 0.14 | 0.61 |
Plants were grown in nutrient solution at four levels of P supply and two levels of light intensity (low=260 μmol m−2 s−1; high=765 μmol m−2 s−1). Seven-week-old plants were harvested and values shown are the combined averages for genotypes Nipponbare and NIL-Pup1.
*, **Difference between low and high light within P levels significant (t-test at P <0.05 and P <0.01, respectively); ns, not significant.
Tukey's HSD (P <0.05) for the comparison of P treatment effects.
Concentration of starch and reducing sugars in root and leaf tissue as influenced by P supply and light intensity
P supply (μM) . | Light . | Root starcha (%) . | Leaf starcha (%) . | Root sugarsa (%) . | Leaf sugarsa (%) . |
|---|---|---|---|---|---|
| 3.2 | Low | 4.30 * | 2.80 ** | 0.98 ns | 3.29 ** |
| High | 3.76 * | 2.34 ** | 0.99 ns | 2.57 ** | |
| 6.4 | Low | 3.97 ** | 2.92 ** | 0.94 ns | 3.75 ** |
| High | 3.43 ** | 2.34 ** | 0.91 ns | 2.62 ** | |
| 9.6 | Low | 3.49 ns | 2.86 * | 0.90 ns | 4.23 ** |
| High | 3.59 ns | 2.54 * | 0.86 ns | 2.86 ** | |
| 100 | Low | 2.94 * | 2.12 ** | 0.83 ns | 3.25 ns |
| High | 3.26 * | 1.69 ** | 0.76 ns | 2.52 ns | |
| HSDb | 0.35 | 0.31 | 0.14 | 0.61 |
P supply (μM) . | Light . | Root starcha (%) . | Leaf starcha (%) . | Root sugarsa (%) . | Leaf sugarsa (%) . |
|---|---|---|---|---|---|
| 3.2 | Low | 4.30 * | 2.80 ** | 0.98 ns | 3.29 ** |
| High | 3.76 * | 2.34 ** | 0.99 ns | 2.57 ** | |
| 6.4 | Low | 3.97 ** | 2.92 ** | 0.94 ns | 3.75 ** |
| High | 3.43 ** | 2.34 ** | 0.91 ns | 2.62 ** | |
| 9.6 | Low | 3.49 ns | 2.86 * | 0.90 ns | 4.23 ** |
| High | 3.59 ns | 2.54 * | 0.86 ns | 2.86 ** | |
| 100 | Low | 2.94 * | 2.12 ** | 0.83 ns | 3.25 ns |
| High | 3.26 * | 1.69 ** | 0.76 ns | 2.52 ns | |
| HSDb | 0.35 | 0.31 | 0.14 | 0.61 |
Plants were grown in nutrient solution at four levels of P supply and two levels of light intensity (low=260 μmol m−2 s−1; high=765 μmol m−2 s−1). Seven-week-old plants were harvested and values shown are the combined averages for genotypes Nipponbare and NIL-Pup1.
*, **Difference between low and high light within P levels significant (t-test at P <0.05 and P <0.01, respectively); ns, not significant.
Tukey's HSD (P <0.05) for the comparison of P treatment effects.
Genotypic differences in root growth as affected by P supply and light intensity
Differences between a pair of NILs that differ for the Pup1 allele are shown in Table 3. Genotype Nipponbare (N) lacks the positive Pup1 allele while NIL-Pup1 carries the allelic variant that confers tolerance to P deficiency due to higher relative root growth under P deficiency. Genotypic differences were not significant at the 3.2 and the 100 μM P levels, so only data from the intermediate P-deficiency levels are shown. Genotypic differences for all traits were more pronounced in the low-light treatment where NIL-Pup1 had significantly more total and root dry matter. This was due to differences in P and DM allocation between roots and shoots. The NIL had consistently higher root:shoot ratios with regard to P and DM and this difference increased in the low-light treatment. Since both genotypes were grown together in the same container, some degree of competition for P existed between genotypes. With more root biomass the NIL was able to take up more P which, in turn, allowed for additional biomass accumulation. That P uptake depended mainly on root biomass and not on P uptake efficiency of roots (mg P taken up per unit root DM) was suggested by the observation that Nipponbare appeared to be more efficient, yet was inferior in total P uptake.
Effect of P supply and light intensity on dry matter and P distribution between root and shoot in a pair of 7-week-old near isogenic lines that differ for the Pup1 locus, which confers tolerance to P deficiency in the field
P supply (μM) . | Light . | Line . | Total DMa (g) . | Root DMa (g) . | R:Sh ratioa (%) . | Total Pa (mg) . | Root Pa (mg) . | R:Sh P ratioa (%) . | Root P concentration (mg g−1) . | Shoot P concentrationa (mg g−1) . | P uptake/ root DMa (mg g−1) . | Pn (μmol m−2 s−1) . | Leaf areaa (cm2) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6.4 | Low | N | 4.67 * | 1.18 ** | 34.0 ** | 2.76 | 0.50 | 22.1 ** | 0.43 | 0.65 ** | 2.18 ** | 15.1 | 300 |
| Low | Pup1 | 5.96 * | 1.76 ** | 41.9 ** | 2.96 | 0.71 | 31.6 ** | 0.40 | 0.54 ** | 1.59 ** | 15.9 | 395 | |
| High | N | 4.99 | 1.33 | 36.4 | 2.96 | 0.59 | 24.9 * | 0.45 | 0.70 ** | 2.21 | 25.7 | 301 | |
| High | Pup1 | 5.67 | 1.62 | 40.1 | 2.85 | 0.65 | 29.5 * | 0.42 | 0.60 ** | 1.79 | 28.5 | 322 | |
| 9.6 | Low | N | 4.89 ** | 1.02 ** | 27.1 | 3.30 ** | 0.47 ** | 16.6 * | 0.46 | 0.77 * | 3.04 * | 13.6 | 394 * |
| Low | Pup1 | 7.19 ** | 1.66 ** | 30.2 | 4.54 ** | 0.77 ** | 20.5 * | 0.46 | 0.70 * | 2.57 * | 14.7 | 512 * | |
| High | N | 6.31 | 1.42 | 29.0 | 3.72 | 0.58 * | 18.1 | 0.42 | 0.66 | 2.42 | 24.2 | 410 | |
| High | Pup1 | 7.48 | 1.69 | 29.7 | 4.33 | 0.80 * | 22.4 | 0.47 | 0.67 | 2.47 | 24.5 | 467 |
P supply (μM) . | Light . | Line . | Total DMa (g) . | Root DMa (g) . | R:Sh ratioa (%) . | Total Pa (mg) . | Root Pa (mg) . | R:Sh P ratioa (%) . | Root P concentration (mg g−1) . | Shoot P concentrationa (mg g−1) . | P uptake/ root DMa (mg g−1) . | Pn (μmol m−2 s−1) . | Leaf areaa (cm2) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6.4 | Low | N | 4.67 * | 1.18 ** | 34.0 ** | 2.76 | 0.50 | 22.1 ** | 0.43 | 0.65 ** | 2.18 ** | 15.1 | 300 |
| Low | Pup1 | 5.96 * | 1.76 ** | 41.9 ** | 2.96 | 0.71 | 31.6 ** | 0.40 | 0.54 ** | 1.59 ** | 15.9 | 395 | |
| High | N | 4.99 | 1.33 | 36.4 | 2.96 | 0.59 | 24.9 * | 0.45 | 0.70 ** | 2.21 | 25.7 | 301 | |
| High | Pup1 | 5.67 | 1.62 | 40.1 | 2.85 | 0.65 | 29.5 * | 0.42 | 0.60 ** | 1.79 | 28.5 | 322 | |
| 9.6 | Low | N | 4.89 ** | 1.02 ** | 27.1 | 3.30 ** | 0.47 ** | 16.6 * | 0.46 | 0.77 * | 3.04 * | 13.6 | 394 * |
| Low | Pup1 | 7.19 ** | 1.66 ** | 30.2 | 4.54 ** | 0.77 ** | 20.5 * | 0.46 | 0.70 * | 2.57 * | 14.7 | 512 * | |
| High | N | 6.31 | 1.42 | 29.0 | 3.72 | 0.58 * | 18.1 | 0.42 | 0.66 | 2.42 | 24.2 | 410 | |
| High | Pup1 | 7.48 | 1.69 | 29.7 | 4.33 | 0.80 * | 22.4 | 0.47 | 0.67 | 2.47 | 24.5 | 467 |
Plants were grown in nutrient solution at four levels of P supply and two levels of light intensity (low=260 μmol m−2 s−1; high=765 μmol m−2 s−1).
*, **Difference between lines within P and light levels significant (t-test at P <0.05 and P <0.01), respectively.
Effect of P supply and light intensity on dry matter and P distribution between root and shoot in a pair of 7-week-old near isogenic lines that differ for the Pup1 locus, which confers tolerance to P deficiency in the field
P supply (μM) . | Light . | Line . | Total DMa (g) . | Root DMa (g) . | R:Sh ratioa (%) . | Total Pa (mg) . | Root Pa (mg) . | R:Sh P ratioa (%) . | Root P concentration (mg g−1) . | Shoot P concentrationa (mg g−1) . | P uptake/ root DMa (mg g−1) . | Pn (μmol m−2 s−1) . | Leaf areaa (cm2) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6.4 | Low | N | 4.67 * | 1.18 ** | 34.0 ** | 2.76 | 0.50 | 22.1 ** | 0.43 | 0.65 ** | 2.18 ** | 15.1 | 300 |
| Low | Pup1 | 5.96 * | 1.76 ** | 41.9 ** | 2.96 | 0.71 | 31.6 ** | 0.40 | 0.54 ** | 1.59 ** | 15.9 | 395 | |
| High | N | 4.99 | 1.33 | 36.4 | 2.96 | 0.59 | 24.9 * | 0.45 | 0.70 ** | 2.21 | 25.7 | 301 | |
| High | Pup1 | 5.67 | 1.62 | 40.1 | 2.85 | 0.65 | 29.5 * | 0.42 | 0.60 ** | 1.79 | 28.5 | 322 | |
| 9.6 | Low | N | 4.89 ** | 1.02 ** | 27.1 | 3.30 ** | 0.47 ** | 16.6 * | 0.46 | 0.77 * | 3.04 * | 13.6 | 394 * |
| Low | Pup1 | 7.19 ** | 1.66 ** | 30.2 | 4.54 ** | 0.77 ** | 20.5 * | 0.46 | 0.70 * | 2.57 * | 14.7 | 512 * | |
| High | N | 6.31 | 1.42 | 29.0 | 3.72 | 0.58 * | 18.1 | 0.42 | 0.66 | 2.42 | 24.2 | 410 | |
| High | Pup1 | 7.48 | 1.69 | 29.7 | 4.33 | 0.80 * | 22.4 | 0.47 | 0.67 | 2.47 | 24.5 | 467 |
P supply (μM) . | Light . | Line . | Total DMa (g) . | Root DMa (g) . | R:Sh ratioa (%) . | Total Pa (mg) . | Root Pa (mg) . | R:Sh P ratioa (%) . | Root P concentration (mg g−1) . | Shoot P concentrationa (mg g−1) . | P uptake/ root DMa (mg g−1) . | Pn (μmol m−2 s−1) . | Leaf areaa (cm2) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6.4 | Low | N | 4.67 * | 1.18 ** | 34.0 ** | 2.76 | 0.50 | 22.1 ** | 0.43 | 0.65 ** | 2.18 ** | 15.1 | 300 |
| Low | Pup1 | 5.96 * | 1.76 ** | 41.9 ** | 2.96 | 0.71 | 31.6 ** | 0.40 | 0.54 ** | 1.59 ** | 15.9 | 395 | |
| High | N | 4.99 | 1.33 | 36.4 | 2.96 | 0.59 | 24.9 * | 0.45 | 0.70 ** | 2.21 | 25.7 | 301 | |
| High | Pup1 | 5.67 | 1.62 | 40.1 | 2.85 | 0.65 | 29.5 * | 0.42 | 0.60 ** | 1.79 | 28.5 | 322 | |
| 9.6 | Low | N | 4.89 ** | 1.02 ** | 27.1 | 3.30 ** | 0.47 ** | 16.6 * | 0.46 | 0.77 * | 3.04 * | 13.6 | 394 * |
| Low | Pup1 | 7.19 ** | 1.66 ** | 30.2 | 4.54 ** | 0.77 ** | 20.5 * | 0.46 | 0.70 * | 2.57 * | 14.7 | 512 * | |
| High | N | 6.31 | 1.42 | 29.0 | 3.72 | 0.58 * | 18.1 | 0.42 | 0.66 | 2.42 | 24.2 | 410 | |
| High | Pup1 | 7.48 | 1.69 | 29.7 | 4.33 | 0.80 * | 22.4 | 0.47 | 0.67 | 2.47 | 24.5 | 467 |
Plants were grown in nutrient solution at four levels of P supply and two levels of light intensity (low=260 μmol m−2 s−1; high=765 μmol m−2 s−1).
*, **Difference between lines within P and light levels significant (t-test at P <0.05 and P <0.01), respectively.
Despite allocating relatively more P to shoots and maintaining higher P concentrations in leaves, Nipponbare did not show an increase in Pn. The light intensity factor had a much stronger influence on Pn than P treatments. Internal shoot P concentrations also had no effect on Pn, at least over the range in P supply of 6.4 to 9.6 μM P.
Discussion
This study was conducted to determine whether low P availability limited root growth due to a reduction in net photosynthesis, which would, in turn, have supplied insufficient assimilates to sustain growth (source limitation, hypothesis A), or whether insufficient P supply limited root growth directly (sink limitation, hypothesis B). That P deficiency has a negative effect on net photosynthesis has repeatedly been shown (Foyer and Spencer, 1986; Usuda and Shimogawara, 1991; Rodriguez et al., 1998) and results obtained here under normal light (Fig. 2) further confirm this effect as net photosynthesis was reduced by up to 37%. This, however, does not automatically suggest that reduced growth was due to source limitations. To answer this question it may be helpful to predict where and how carbohydrate reserves would change if source or sink was limiting.
Low levels of available Pi can reduce photosynthetic efficiency via the Pi translocator, an antiporter that facilitates the export of triose phosphate from the stroma to the cytosol in exchange for Pi (Stitt and Quick, 1989). Low cytosol Pi concentrations should, therefore, have a negative effect on the Calvin cycle, either by altering levels of phosphorylated intermediates or by affecting the required enzymes through their level of activation (Heldt et al., 1977) or regeneration (Rao and Terry, 1989). As a result of these changes, triose phosphate export from chloroplast would be reduced and starch would accumulate in leaf tissue while the export of carbohydrates to roots should decrease (Rao and Terry, 1989; Stitt and Quick, 1989). If P deficiency reduced growth rates directly before Pn was affected, excess carbohydrates would be available in the system and Fredeen et al. (1989) showed that carbon transport from source leaves was not impaired under P deficiency in young plants. Roots are generally regarded as stronger sinks than leaves under P deficiency since root:shoot ratios typically increase (Mollier and Pellerin, 1999; Vance et al., 2003). Consequently, one could expect the continuous export of excess carbohydrates to roots and the subsequent accumulation in roots once growth ceases. Low sink demand would later lead to the accumulation of carbohydrates in source leaves and, ultimately, to a reduction of Pn due to end-product synthesis limitation (Pieters et al., 2001).
Under both scenarios, starch would accumulate in the source leaves, but only if P was directly limiting growth (sink limitation) would starch also accumulate in roots. Our data showed that this was the case in the solution experiment (Table 2) as well as in plants grown in P-deficient soil (Fig. 3). In that experiment, roots of the plants most affected by P deficiency reached very high concentrations of starch (8%) that were much higher than levels (2–3%) typically found in unstressed plants (Yamamoto, 1989). This clearly suggests that reductions in root growth under P deficiency were not caused by source limitations, but were due to a more direct negative effect of low P availability on growth.
Two contrasting light treatments were employed to test hypothesis A further. If Pi would limit Pn, increasing the light intensity should not lead to substantial gains in Pn. That higher light intensity increased Pn by up to 70% (6.4 μM P), even under highly suboptimal tissue P concentrations of around 0.6 mg P g−1 DM, suggested that Pn can substantially tolerate P deficiency if light is not limiting (1.0 mg P g−1 DM is considered the deficiency threshold; Dobermann and Fairhurst, 2000). Furthermore, if root growth was restricted due to source limitations, any increase in Pn should also lead to additional root growth. However, higher Pn rates under natural light were not transformed into biomass when P was highly limiting (3.2 and 6.4 μM P). Low photosynthetic rates under low light were, therefore, sufficient to supply growing organs with the assimilates required to sustain growth rates that were limited by another factor such as low P availability. These results provided additional evidence to reject hypothesis A in favour of hypothesis B.
The general model put forward in support of source limitation asserts that P deficiency first reduces leaf area (Khamis et al., 1990; Mollier and Pellerin, 1999) and that assimilates no longer needed for leaf area expansion are partitioned to roots initially to maintain high root growth rates. Once these excess assimilates are used up, the smaller leaf area no longer supplies enough carbohydrates and root growth rates decrease due to source limitations. The initial step in this chain of events (reduction in leaf expansion/initiation), however, was not attributed to source limitations (Plénet et al., 2000a), but rather to some direct but not fully understood effect of low Pi on leaf expansion. This raises the question why only leaf growth would be directly affected by Pi but not root growth. A more parsimonious model could stipulate that both root and shoot growth are directly affected by Pi availability and that the increase in root:shoot ratio frequently observed under P deficiency is causally due to greater P rather than carbohydrate partitioning to roots. Data from this study would support such an alternative model as P deficiency increased relative P allocation to roots, particularly in the tolerant genotype. What the exact direct effect of low Pi availability is remains unresolved. It has been suggested that the accumulation of putrescine in response to P deficiency mediates growth inhibition in rice (Shih and Kao, 1996) while recent evidence points to an even more complex system of Pi sensing and signalling pathways that affect meristem activity and ultimately root growth (Hammond et al., 2004; Ticconi et al., 2004).
The comparison of Nipponbare with NIL-Pup1 revealed how balancing the two stresses, P deficiency and low light, could affect overall plant growth. P deficiency favours assimilate distribution to roots (Mollier and Pellerin, 1999; Vance et al., 2003) whereas low irradiance has the opposite effect (Wilson, 1988; Cruz, 1997). Carbohydrate partitioning in stressed plants, therefore, appears to be directed by the need to overcome limiting factors, in this case by increasing root growth under P deficiency or leaf area under low light (Wilson, 1988). Two limiting factors were combined in this experiment and the data showed that P deficiency had a stronger effect than low light as root:shoot ratios increased under P deficiency, regardless of light level (Fig. 1). Once P was no longer limiting (100 μM P), low light had the reverse effect. However, Nipponbare and NIL-Pup1 differed slightly in how they responded to low light under P deficiency. Assimilate and P distribution to roots in NIL-Pup1 was only affected by decreasing P supply (from 9.6 to 3.2 μM P) and the contrary effect of low light was not detectable. Nipponbare, however, showed a slight additional response to low light, having lower assimilate and P distribution to roots relative to NIL-Pup1 (Table 3). With less P going to roots, Nipponbare produced significantly less root dry matter and that led to lower P uptake. The strategy of NIL-Pup1, maintaining root growth and P uptake rather than allocating more P to shoots in favour of maintaining total CO2 assimilation, therefore appears to be beneficial, particularly if competition for the factor P occurs.
Model simulations suggested that preferential distribution of P to roots is a highly effective way to improve tolerance to P deficiency (Wissuwa, 2003). To attribute the effect of the Pup1 locus to preferential P distribution to roots may, however, be premature, considering that results of the present study were obtained in low-P nutrient solutions. In soil, other factors such as the solubilization of soil-bound P forms could be of greater importance and recent evidence showed that NIL-Pup1 was more efficient in accessing these soil-bound P forms (Wissuwa, 2005). That significant differences between Nipponbare and NIL-Pup1 were detected for two potential tolerance mechanisms could indicate that the Pup1 locus represents a regulatory gene that affects several tolerance mechanisms. Results of the present study clearly showed, however, that these are related to overcoming sink rather than source limitations.
This work was, in part, funded by the German ‘Gesellschaft fuer Technische Zusammenarbeit’ (GTZ).
References
Cruz P.
Dobermann A, Fairhurst TH.
Foyer C, Spencer C.
Fredeen AL, Rao IM, Terry N.
Gahoonia TS, Nielsen NE.
Hammond JP, Broadley MR, White PJ.
Hanson WC.
Heldt HW, Chon CJ, Maronde D, Herold A, Stankovic ZS, Walker DA, Kraminer A, Kirk MR, Heber U.
Horst WJ, Kamh M, Jibrin JM, Chude VO.
Khamis S, Chaillou S, Lamaze T.
Lauer MJ, Pallardy SG, Blevins DG, Randall DD.
Lynch JP, Brown KM.
Mollier A, Pellerin S.
Pieters AJ, Paul MJ, Lawlor DW.
Plénet D, Etchebest S, Mollier A, Pellerin S.
Plénet D, Mollier A, Pellerin S.
Rao IM, Terry N.
Rodríguez D, Keltjens WG, Goudriaan J.
Sattelmacher B, Horst WJ, Becker HC.
Setter TL, Ella ES, Valdez AP.
Shih C-Y, Kao CH.
Stitt M, Quick WP.
Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S.
Usuda H, Shimogawara K.
Uexküll HR von, Mutert E.
Vance CP, Uhde-Stone C, Allan DL.
Wilson JB.
Wissuwa M.
Wissuwa M.
Wissuwa M, Ae N.
Wissuwa M, Wegner J, Ae N, Yano M.
Yamamoto Y.




Comments