-
PDF
- Split View
-
Views
-
Cite
Cite
Sandrine Mathieu, Nancy Terrier, Jérôme Procureur, Frédéric Bigey, Ziya Günata, A Carotenoid Cleavage Dioxygenase from Vitis vinifera L.: functional characterization and expression during grape berry development in relation to C13-norisoprenoid accumulation, Journal of Experimental Botany, Volume 56, Issue 420, October 2005, Pages 2721–2731, https://doi.org/10.1093/jxb/eri265
Close - Share Icon Share
Abstract
A potential Carotenoid Cleavage Dioxygenase (CCD) gene was identified among a Vitis vinifera L. EST collection and a full-length cDNA (VvCCD1) was isolated. Recombinant expression of VvCCD1 confirmed that the gene encoded a functional CCD. Experimental evidence was obtained that VvCCD1 cleaves zeaxanthin symmetrically yielding 3-hydroxy-β-ionone, a C13-norisoprenoidic compound, and a C14-dialdehyde. Expression of the gene was studied by real-time PCR at different developmental stages of grape berries from Muscat of Alexandria and Shiraz cultivars. A significant induction of the gene expression approaching véraison was observed in both cultivars. In parallel, the C13-norisoprenoid level increased from véraison to maturity in both cultivars.
Introduction
C13-norisoprenoids are terpenoids commonly found in the flowers, fruits, and leaves of many plants (Winterhalter and Rouseff, 2002) and possess interesting flavour aroma properties together with low aroma thresholds. They are present in grape berries (Williams et al., 1992; Razungles et al., 1993; Wirth, 2001) and leaves (Skouroumounis and Winterhalter, 1994; Wirth, 2001) where they occur mainly under glycoconjugated forms (Winterhalter et al., 1990; Williams et al., 1992). They are found among the potent flavour compounds in wines and contribute to floral and fruity attributes (Winterhalter and Schreier, 1994). Therefore, they have been subject to extensive research in recent years with regard to their structure and flavour potential (Williams et al., 1992; Winterhalter and Rouseff, 2002). Carotenoids can be degraded by chemical, photochemical, and oxidase-coupled mechanisms, but the cleavage is not region-specific and leads to the formation of cyclic or linear apocarotenoids with 9, 10, 11, 13, and 15 carbon atoms (Zamora et al., 1988; Wu and Robinson, 1999).
In grape berries, several results supported the hypothesis of the involvement of a region-specific oxygenase in the formation of C13-norisoprenoids: (i) the preponderance of norisoprenoids possessing 13 carbon atoms (Williams et al., 1992), (ii) their common configuration with the parent carotenoids regarding asymmetric centres (Baumes et al., 2002), (iii) the negative correlations observed between the levels of C13-norisoprenoids and carotenoids during grape berry development (Razungles et al., 1993), and (iv) in vivo transfer of 13C markers from carotenoids to C13-norisoprenoids in berries (Baumes et al., 2002).
The first carotenoid dioxygenase, reported in the mid-1950s (Olson and Hayaishi, 1965), was a 15,15′-dioxygenase able to cleave the central carbon 15,15′ double bond of β-carotene giving rise to retinal, the precursor of vitamin A. Recombinant 15,15′-dioxygenases were further purified and characterized (von Lintig and Vogt, 2000; Lindqvist and Andersson, 2002).
Zea mays Vp14, the first carotenoid dioxygenase described in plants, is specific to the 11,12 bond of 9-cis xanthophylls and leads to the formation of xanthoxin, a C15-apocarotenoid, the precursor of abscisic acid (Schwartz et al., 1997). Nine-cis-epoxycarotenoid dioxygenases (NCEDs) have also been described in Arabidopsis thaliana (Tan et al., 2003). Recombinant CCDs cleaving carotenoids symmetrically at the 9,10[9′,10′] bonds, resulting in the formation of C13- and C14-apocarotenoids, were reported in A. thaliana (AtCCD1) (Schwartz et al., 2001) and in Crocus sativus (CsCCD) (Bouvier et al., 2003). In Lycopersicon esculentum, LeCCD1A and LeCCD1B silencing resulted in a significant decrease of the β-ionone content of ripe fruits, implicating a role of these genes in C13-norisoprenoid synthesis in vivo (Simkin et al., 2004a).
The present work describes the isolation of a CCD gene from Vitis vinifera (VvCCD1). To characterize the catalytic activity of VvCCD1, the gene was cloned and expressed in Escherichia coli. The recombinant protein was assayed for cleavage activity towards zeaxanthin. Expression of VvCCD1 in a strain of E. coli accumulating zeaxanthin was also performed for functional analysis. In order to study the putative involvement of VvCCD1 on the synthesis of C13-norisoprenoids in the grape berry, its expression pattern was followed during the grape berry development of two different cultivars: Shiraz, a red-skinned variety and Muscat of Alexandria, a white one. In addition, changes in the C13-norisoprenoid level during ripening in both cultivars were studied.
Materials and methods
Grape berry material
Grape berries from V. vinifera L. cv. Muscat of Alexandria and Shiraz were harvested from 20 June until 10 September 2003, on the INRA-ENSAM open vineyards grown on an artificial substrate composed of sand (Montpellier, France). Fertilization and irrigation were assured by a drip irrigation system. Six different stages of development were chosen. The first stage corresponded to immature green berries, before the critical phase called véraison. Véraison corresponds to the onset of ripening, from which berries soften, become coloured and start to accumulate sugars. The pH increases as a result of rapid malate breakdown (Rüffner, 1982). At véraison, two batches were collected. The first batch was composed of green berries which were still hard, representing the second developmental stage. The second batch was composed of green and soft berries and corresponded to the third developmental stage. After véraison, three more stages were collected according to the alcohol potential content of the berries (Terrier et al., 2001). The fourth stage was composed of berries with a potential alcohol content of 7°, the fifth stage with a potential of 11.6° for Shiraz and 12° for Muscat of Alexandria and, finally, the sixth stage with a potential of 12° for Shiraz and 13.4° for Muscat of Alexandria. Berries were washed, weighed, and counted. Fifty grams were immediately frozen in liquid nitrogen and stored at −80 °C until RNA extraction. The elapsed time between harvest and freezing was less than 30 min. Five hundred grams were stored at −20 °C for norisoprenoid analysis. Before the extraction of RNA and flavour compounds, samples were deseeded and powdered under liquid nitrogen using a Dangoumeau blender.
Isolation of a potential CCD gene
A V. vinifera L. cv. Shiraz grape berry EST database (Terrier et al., 2001) was screened using standard BLAST with the amino acid sequence of the previously described AtCCD1 as query (Schwartz et al., 2001). Among the sequences presenting significant homologies, the clone called TT264D02 was the longest. This clone was entirely sequenced and corresponded to a full-length cDNA. The sequence has been deposited in the GenBank database under the accession number AY856353.
Total RNA extraction, reverse transcription, and real-time PCR
Total RNA was extracted from berries as previously described by Sarni-Manchado et al. (1997) and accurately quantified by fluorometry with Ribogreen reagent (Molecular probes, Leiden, The Netherlands). First-strand cDNA synthesis was performed in triplicate in a 20 μl reaction volume, with 500 ng of total RNA as the template, 500 ng of oligo(dT)12–18, and 200 units of SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA), in accordance with the manufacturer's instructions.
For real-time PCR experiments, oligonucleotide primers were designed and their specificity was checked using the TIGR Grape Gene Index containing the latest V. vinifera ESTs (Release 4.0, September 2004). The primer pair was as follows: forward primer 5′-CGTCCATGACGAGAAAACTG-3′ and reverse primer 5′-AGGCATGAAAACCGTATGGA-3′. The amplicon length was 119 bp.
Real-time PCR was performed in triplicate on 1 μl cDNA from each cultivar at each developmental stage using a model 7700 Sequence Detection System (Applied Biosystems, Warrington, UK) and the SYBR-Green PCR Master kit (Applied Biosystems). Data were calculated from the calibration curve and normalized using the constitutively expressed EF1α gene (Terrier et al., 2005).
Analysis of C13-norisoprenoids from grape berries
Extraction of free and glycosidically bound norisoprenoidic compounds:
Extraction and analysis were performed in triplicate for each cultivar at each developmental stage. Seventy grams of powder were taken into 100 ml of H2O milliQ containing 40 mmol l−1 of gluconolactone to inhibit glycosidase activities. The mixture was stirred, centrifuged (15 min, 6000 g, 4 °C) and 37.7 μg of 4-nonanol (solubilized in ethanol) was added to the supernatant as an internal standard. The supernatant was filtered through a cellulose nitrate membrane filter (5 μm) and loaded on a SPE RP18 column (LiChrolut, Merck). Free norisoprenoids were eluted with 60 ml of pentane/dichloromethane (2:1, v/v). The organic phase was concentrated to 500 μl by distillation through a Vigreux and Dufton column and stored at −20 °C until analysed by GC-FID and GC-MS.
Glycosidically bound norisoprenoids were eluted with 25 ml of methanol. After elimination of the solvent under vacuum, the residue was taken into 300 μl of phosphate-citrate buffer (0.1 mol 1−1, pH 5.0) and washed with pentane/dichloromethane (2:1, v/v) (5×1 ml). 100 μl of a glycosidase preparation (70 mg ml−1, AR-2000, Gist-Brocades, Seclin, France) were added and the mixture was incubated at 40 °C for 16 h (Günata et al., 1985). Released volatiles from glycosides were extracted with pentane/dichloromethane (2:1, v/v) (5×1 ml). The organic extract was spiked with 37.7 μg of 4-nonanol, concentrated to 500 μl as above and stored at −20 °C until analysis with GC-FID and GC-MS.
GC-FID and GC-MS analysis conditions:
Free and enzymatically liberated norisoprenoids were analysed using a Varian 3800 gas chromatograph equipped with a DB-Wax (J&W Scientific, Folson, CA) capillary column (30 m ×0.25 mm; 0.25 μm film thickness). The flow of hydrogen carrier gas was 1 ml min−1. The oven was kept at 60 °C for 3 min, then programmed to 245 °C at 3 °C min−1, and kept at 245 °C for 20 min. The FID and injector temperatures were maintained at 250 °C. One microlitre of sample was injected using the splitless mode. The levels of the volatile compounds were expressed as 4-nonanol equivalents.
A Varian 3800 gas chromatograph coupled to a Saturn ion-trap mass spectrometer was used for the identification of norisoprenoids. The capillary column and the oven temperature were as above. Mass spectra were recorded in electron impact (EI) ionization mode. The ion trap, the manifold, and the transfer line temperatures were set, respectively, at 150 °C, 45 °C and 250 °C. Mass spectra were scanned in the range of m/z 29–350 amu at 1 s intervals. Identification was carried out by comparing the linear retention index and EI mass spectra with data from authentic compounds (Wirth et al., 2001).
Cloning of VvCCD1 into a zeaxanthin accumulating E. coli strain
VvCCD1 was amplified by PCR using the forward and reverse primers 5′-ATGGCGGAGAAGGAGGAGCAA-3′ and 5′-CCCCGAATTCTCAAAGTTTTGCTTGTTCTTT-3′. The resulting fragment was cloned into the SmaI site of pGEX-2T (Amersham Pharmacia Biotech) allowing the expression of a functional glutathione-S-transferase (GST)-VvCCD1 fusion protein upon induction with IPTG (isopropyl-β-D-thiogalactopyranoside) inducible promoter. Plasmid pGEX-2T (negative control) and the plasmid pGEX-VvCCD1 were cloned into E. coli JM101 (pACCAR25ΔcrtX) (Misawa et al., 1995).
This strain expresses carotenoid biosynthetic genes from Erwinia uredovora, allowing the synthesis of zeaxanthin. Colonies were selected on solid LB plates containing ampicillin (100 μg ml−1) and chloramphenicol (34 μg ml−1).
Crude dioxygenase preparation
Escherichia coli BL21(DE3) (Stratagene, La Jolla, California, USA) was transformed with pGEX-VvCCD1 and cultivated at 37 °C in 100 ml of 2× YT broth containing ampicillin (100 μg ml−1). When the culture optical density (600 nm) reached 1.6, 125 μmol l−1 of IPTG was added to induce the production of the GST-VvCCD1 fusion. The temperature was decreased to 18 °C to overcome the formation of inclusion bodies and the culture was incubated for a further 20 h. Cells were pelleted by centrifugation at 4 °C (5000 g) and resuspended in 5 ml of PBS (phosphate buffer saline) containing a protease inhibitor cocktail (Complete, Roche Applied Science), 4 mmol l−1 DTT and 1% (v/v) Triton X-100 (Sigma). Cells were disrupted by sonication and the lysate was centrifuged at 30 000 g for 30 min (4 °C). The supernatant was filtered through a 0.22 μm membrane and the resulting dioxygenase preparation was stored at −20 °C.
In vitro assay with recombinant GST-VvCCD1
500 μl of a zeaxanthin (Extrasynthèse, France) solution (0.5 g l−1 in acetone) were mixed with 0.025 g of Tween 40. After evaporation of acetone under nitrogen, the mixture was resuspended in 5 ml of 0.1 mol l−1bis-tris buffer (pH 7.0). The reaction mixture was composed of 600 μl of the substrate solution, 500 μl of the supernatant, 150 μl of 50 μmol l−1 FeSO4 and 150 μl of 10 mmol l−1 DTT buffered with 0.1 mol l−1bis-tris buffer (pH 7.0) to a final volume of 1.5 ml. The assay medium was incubated at 30 °C for 3 h.
Extraction of apocarotenoids, GC-MS and HPLC analysis
2.8 μg of 3-oxo-α-ionol (in ethanol) as the internal standard were added to the assay medium and apocarotenoids were extracted with 3 vols of pentane/dichloromethane (2:1, v/v). The organic phase was concentrated under a stream of nitrogen to 100 μl and analysed by GC-MS and HPLC.
For GC-MS analysis, the equipment and the conditions were those previously described. The analysis was performed in SIS (Selected Ion Storage) mode. The selected ions were, respectively, 108 and 152 for 3-oxo-α-ionol, 175 and 193 for 3-hydroxy-β-ionone.
HPLC equipment with an online diode array detector (Waters) mounted with a reverse phase column (LiChroCART 250-4 Purospher® RP18; 250×4 mm; 5 μm; Merck) was used. The flow rate was 1 ml min−1. The gradient was as follows: 0–4 min, 50% acetonitrile; 4–20 min, 50–100% acetonitrile; 100% acetonitrile from 20–60 min. 4,9-dimethyldodeca-2,4,6,8,10-pentaene-1,12-dialdehyde (C14-dialdehyde, BASF, Germany) and zeaxanthin were identified through the injection of reference compounds and their absorption spectrum.
Results
Obtaining a full-length cDNA from the V. vinifera EST library and sequence analysis
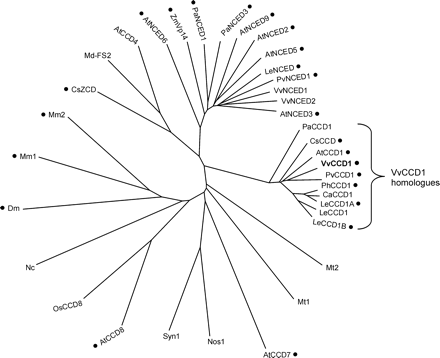
VvCCD1 was obtained by in silico screening of an EST collection from the grape berry cv. Shiraz (Terrier et al., 2001). Among the different clones exhibiting homologies with AtCCD1 and belonging to the same contig, one clone corresponded to a full-length cDNA. VvCCD1 contained an open reading frame of 1629 nucleotides. The predicted polypeptide contained 542 amino acids with a calculated mass of 61.1 kDa and a pI of 6.8. Moreover, a transmembrane helix of 20 amino acids was predicted (TM Pred) (data not shown), as for the two homologues AtCCD1 and CsCCD (Schwartz et al., 2001; Bouvier et al., 2003). VvCCD1 belongs to the Carotenoid Cleavage Dioxygenases class (Fig. 1). This class includes AtCCD1 and CsCCD which share 81% and 78% homology with VvCCD1, respectively, and the recently described PhCCD1 (Simkin et al., 2004b), LeCCD1A and LeCCD1B (Simkin et al., 2004a) with 82%, 82%, and 76% homologies respectively. Thus, VvCCD1 is clearly distinct from the NCEDs class, with 35% homology on average (Fig. 1). Besides these two classes, other dioxygenases have also been reported. Among them, VvCCD1 shows 39% homology with CsZCD (Bouvier et al., 2003) but only 16% with AtCCD7 and AtCCD8 (Schwartz et al., 2004). Dm, Mm1, and Mm2 corresponding to mammalian 15,15′-β-carotene dioxygenases, are more related to AtCCD7 and AtCCD8 than to VvCCD1.
Radial phylogram representation from Clustal W (EMBL-EBI) of the deduced amino acid sequences of V. vinifera Carotenoid Cleavage Dioxygenase and homologues. Functionally characterized dioxygenases are marked by a closed circle. The abbreviations refer to: At, Arabidopsis thaliana (AtCCD1, NP_191911.1; AtCCD4, NP_193652.1; AtCCD7, NP_182026.1; AtCCD8, NP_195007.2; AtNCED2, NP_193569.1; AtNCED3, NP_188062.1; AtNCED5, NP_174302.1; AtNCED6, NP_189064.1; AtNCED9, NP_177960.1); Ca, Capsicum annuum (CaCCD1, Y14164); Cs, Crocus sativus (CsCCD, AJ132927; CsZCD, AJ489276); Dm, Drosophila melanogaster (Dm, CAB93141.1); Le, Lycopersicon esculentum (LeCCD1A, AY576001; LeCCD1B, AY576002; LeCCD1, AJ489278; LeNCED, CAB10168.1); Md, Malus×domestica (Md-FS2, CAB07784.1); Mm, Mus musculus (Mm1, AAG33982.1; Mm2, CAC28026.1); Mt, Mycobacterium tuberculosis (Mt1, CAB09380; Mt2, CAB08511.1); Nc, Neurospora crassa (Nc, XP_331998.1); Nos, Nostoc sp. PCC 7120 (Nos1, BAB75983); Os, Oryza sativa (OsCCD8, BAB63485.1); Pa, Persea americana (PaCCD1, AAK00622.1; PaNCED1, AAK00632.1, PaNCED3, AAK00623.1); Ph, Petunia hybrida (PhCCD1, AY576003); Pv, Phaseolus vulgaris (PvCCD1, AAK38744.1; PvNCED1, AAF26356.1); Syn, Synechocystis sp. PCC 6803 (Syn1, BAA18428.1); Vv, Vitis vinifera (VvCCD1, AY856353; VvNCED1, AY337613; VvNCED2, AY337614); Zm, Zea mays (ZmVp14, AAB62181.1).
Functional characterization of VvCCD1
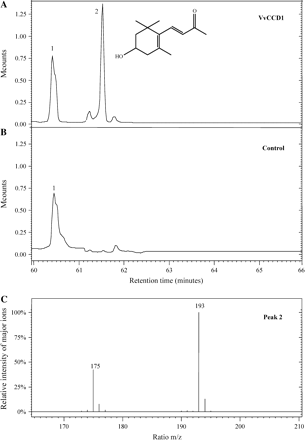
To investigate whether VvCCD1 encoded a functional CCD, co-expression of VvCCD1 in a strain of E. coli accumulating zeaxanthin was performed. Colonies clearly failed to develop a yellow colour contrary to the negative control (Fig. 2). Further studies were conducted on the production of recombinant VvCCD1 by E. coli growing in liquid medium. CCD activity was tested on supernatants from sonicated E. coli cells using zeaxanthin as the substrate. GC-MS analysis revealed the presence of 3-hydroxy-β-ionone (peak 2) (m/z 175, 193) (Fig. 3A, C). This compound was not detected in the control (Fig. 3B).
Escherichia coli carrying zeaxanthin biosynthetic genes and transformed with pGEX-VvCCD1 (VvCCD1) or pGEX-2T (control).
GC-MS analysis of the reaction assay containing zeaxanthin and supernatant from E. coli carrying (A) pGEX-VvCCD1 (VvCCD1) or (B) empty pGEX-2T (control). (C) Mass spectrum of peak 2 corresponding to 3-hydroxy-β-ionone. Note that peak 1 corresponds to the internal standard, 3-oxo-α-ionol.
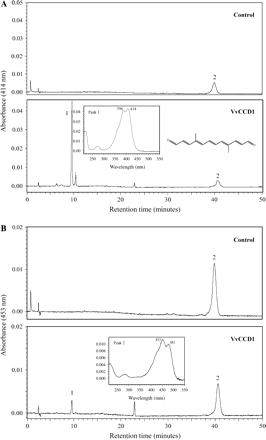
Furthermore, HPLC analysis at 414 nm showed the presence of a compound (peak 1) eluted at 9.6 min with absorbance maxima at 396 nm and 414 nm (Fig. 4A). This compound was absent in the control. When the reference compound (4,9-dimethyldodeca-2,4,6,8,10-pentaene-1,12-dialdehyde) was injected in the same conditions, identical results were obtained (data not shown). Peak 2 corresponded to zeaxanthin (Fig. 4B) with absorbance maxima at 453 nm and 481 nm. This compound was more abundant in the control than in VvCCD1.
HPLC analysis and online diode-array spectra. (A) Absorbance measured at 414 nm. Peak 1, with maxima absorption at 396 and 414 nm, corresponded to 4,9-dimethyldodeca-2,4,6,8,10-pentaene-1,12-dialdehyde (C14-dialdehyde) detected or not in the assay using enzymatic supernatant from E. coli carrying pGEX-VvCCD1 (VvCCD1) or empty pGEX-2T (control). (B) Absorbance measured at 453 nm. Peak 2 corresponded to zeaxanthin, with maxima absorption at 453 and 481 nm.
Expression profile of VvCCD1 during grape berry development
VvCCD1 expression was measured by real-time PCR at different grape berry developmental stages in two cultivars, Shiraz and Muscat of Alexandria. It was verified that the primer pair used for the PCR reaction had the same yield in amplification for both cultivars.
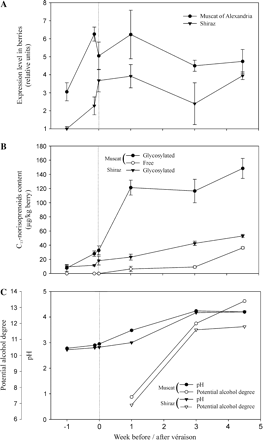
Results were normalized by the expression of EF1α, a gene which expression is constant during grape berry development, and expressed as the variation in the expression level of five stages of ripening compared with the first stage which corresponds to immature berries of Shiraz (Fig. 5A). Results indicated a significant induction of VvCCD1 expression in the grape berries of the two cultivars studied during the week preceding véraison, with a 2-fold induction for Muscat of Alexandria and a nearly 4-fold one for Shiraz. After véraison, the expression of the gene remained almost stable throughout the ripening stages. Furthermore, results demonstrated that the VvCCD1 expression level was higher in Muscat of Alexandria berries than in Shiraz ones during grape berry development.
Analysis of grape berry development in two different cultivars, Muscat of Alexandria and Shiraz. (A) Expression profile of VvCCD1 in berries obtained by real-time PCR. Levels are expressed in units relative to the level of the constitutive EF1α gene and to the first stage of Shiraz berries. (B) Changes in the levels of free and glycosylated C13-norisoprenoids in the corresponding cultivars. (C) pH and potential degree of alcohol. Week 0 corresponded to véraison.
Changes in the C13-norisoprenoid level during ripening
To study a potential correlation between the induction of VvCCD1 and the C13-norisoprenoid synthesis in grape berry, the latter were analysed for both cultivars at the developmental stages previously described (Fig. 5B). Only norisoprenoids possessing 13 carbon atoms were detected as volatile or potentially volatile apocarotenoids. As already observed (Winterhalter et al., 1990; Wirth et al., 2001), most of them were detected mainly under glycoconjugated forms. In both cultivars, glycosylated bound compounds were already present at a low level before véraison and increased significantly after véraison (Fig. 5B). Shiraz berries exhibited a progressive increase throughout ripening while a dramatic increase was observed for the Muscat of Alexandria cultivar during the first week following véraison. Moreover, the total C13-norisoprenoid level was nearly three times higher in the mature berries of Muscat of Alexandria than in those of Shiraz. Free norisoprenoids, 4-oxo-β-ionol and 3-hydroxy-β-ionone, were only detected in ripening berries of Muscat of Alexandria berries from one week after véraison and thereafter (Table 1). Among the 11 norisoprenoids detected, the major ones were 3-oxo-α-ionol and 3-hydroxy-7,8-dihydro-β-ionone for both cultivars. In addition, 3-hydroxy-β-ionone and 4-oxo-β-ionol were abundant in the Muscat of Alexandria cultivar. Qualitative differences were observed. Some glycosylated compounds were present in one cultivar only. Indeed, 3-hydroxy-5,6-epoxy-β-ionone was not detected at all in Muscat of Alexandria berries whereas 3,4-dihydro-α-ionone and 4-oxo-β-ionol were absent in Shiraz berries.
Levels (μg kg−1 berry) of free and glycosylated C13-norisoprenoids from Muscat of Alexandria and Shiraz berries at different developmental stages (nd: not detected; HB: hard berries; SB: soft berries)
. | Week before/after véraison . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | −1 . | 0 . | . | 1 . | 3 . | 4.5 . | |||||
. | . | HB . | SB . | . | . | . | |||||
| Muscat of Alexandria | |||||||||||
| Major bound compounds | |||||||||||
| 4-oxo-β-ionol | nd | 6.23 | 6.48 | 16.11 | 33.89 | 49.61 | |||||
| 3-hydroxy-7,8-dihydro-β-ionone | 0.27 | 0.57 | 1.06 | 15 | 27.73 | 33.57 | |||||
| 3-oxo-α-ionol | 2.08 | 13.36 | 15.52 | 64.65 | 26.61 | 33.91 | |||||
| Minor bound compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | nd | nd | 2.99 | 2.5 | 0.53 | |||||
| 3-hydroxy-5,6-epoxy-β-ionone | nd | nd | nd | nd | nd | nd | |||||
| 3-hydroxy-β-damascone | 1.78 | 2.63 | 3.33 | 5.11 | 4.64 | 7.9 | |||||
| 3-hydroxy-7,8-dihydro-β-ionol | 1.42 | 1.91 | 1.99 | 6.4 | 9.84 | 8.27 | |||||
| 3-hydroxy-7,8-dehydro-β-ionol | 0.69 | 0.72 | 1.05 | 1.69 | 2.03 | 3.11 | |||||
| 3,4-dihydro-α-ionone | nd | nd | nd | 1.44 | 1.81 | 2.32 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer I) | 0.76 | 1.37 | 1.58 | 3.73 | 3.04 | 2.96 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer II) | 0.87 | 1.3 | 1.67 | 4.17 | 4.44 | 6.24 | |||||
| Free compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | nd | nd | 5.88 | 6 | 30.2 | |||||
| 4-oxo-β-ionol | nd | nd | nd | 0.53 | 3.22 | 6.03 | |||||
| Shiraz | |||||||||||
| Major bound compounds | |||||||||||
| 3-oxo-α-ionol | 4.05 | 4.07 | 8 | 11.47 | 16.79 | 17.95 | |||||
| 3-hydroxy-7,8-dihydro-β-ionone | 1.45 | 1.04 | 1.02 | 2.28 | 6.99 | 12.4 | |||||
| Minor bound compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | 0.59 | 1.5 | 0.81 | 2.44 | 3.63 | |||||
| 3-hydroxy-5,6-epoxy-β-ionone | nd | 0.49 | 0.51 | 1.17 | 5.22 | 5.38 | |||||
| 3-hydroxy-β-damascone | 0.05 | 1.2 | 1.57 | 2.44 | 5.58 | 3.4 | |||||
| 3-hydroxy-7,8-dihydro-β-ionol | 1.74 | 1.37 | 1.09 | 1.33 | 1.52 | 2.39 | |||||
| 3-hydroxy-7,8-dehydro-β-ionol | 0.86 | 0.64 | 2.59 | 0.84 | 0.93 | 2.52 | |||||
| 3,4-dihydro-α-ionone | nd | nd | nd | nd | nd | nd | |||||
| 3,4-dihydro-3-oxoactinidol (isomer I) | 0.48 | 1.39 | 0.92 | 1.54 | 0.57 | 4.1 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer II) | 0.76 | 0.86 | 0.95 | 1.14 | 2.55 | 1.26 | |||||
| 4-oxo-β-ionol | nd | nd | nd | nd | nd | nd | |||||
. | Week before/after véraison . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | −1 . | 0 . | . | 1 . | 3 . | 4.5 . | |||||
. | . | HB . | SB . | . | . | . | |||||
| Muscat of Alexandria | |||||||||||
| Major bound compounds | |||||||||||
| 4-oxo-β-ionol | nd | 6.23 | 6.48 | 16.11 | 33.89 | 49.61 | |||||
| 3-hydroxy-7,8-dihydro-β-ionone | 0.27 | 0.57 | 1.06 | 15 | 27.73 | 33.57 | |||||
| 3-oxo-α-ionol | 2.08 | 13.36 | 15.52 | 64.65 | 26.61 | 33.91 | |||||
| Minor bound compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | nd | nd | 2.99 | 2.5 | 0.53 | |||||
| 3-hydroxy-5,6-epoxy-β-ionone | nd | nd | nd | nd | nd | nd | |||||
| 3-hydroxy-β-damascone | 1.78 | 2.63 | 3.33 | 5.11 | 4.64 | 7.9 | |||||
| 3-hydroxy-7,8-dihydro-β-ionol | 1.42 | 1.91 | 1.99 | 6.4 | 9.84 | 8.27 | |||||
| 3-hydroxy-7,8-dehydro-β-ionol | 0.69 | 0.72 | 1.05 | 1.69 | 2.03 | 3.11 | |||||
| 3,4-dihydro-α-ionone | nd | nd | nd | 1.44 | 1.81 | 2.32 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer I) | 0.76 | 1.37 | 1.58 | 3.73 | 3.04 | 2.96 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer II) | 0.87 | 1.3 | 1.67 | 4.17 | 4.44 | 6.24 | |||||
| Free compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | nd | nd | 5.88 | 6 | 30.2 | |||||
| 4-oxo-β-ionol | nd | nd | nd | 0.53 | 3.22 | 6.03 | |||||
| Shiraz | |||||||||||
| Major bound compounds | |||||||||||
| 3-oxo-α-ionol | 4.05 | 4.07 | 8 | 11.47 | 16.79 | 17.95 | |||||
| 3-hydroxy-7,8-dihydro-β-ionone | 1.45 | 1.04 | 1.02 | 2.28 | 6.99 | 12.4 | |||||
| Minor bound compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | 0.59 | 1.5 | 0.81 | 2.44 | 3.63 | |||||
| 3-hydroxy-5,6-epoxy-β-ionone | nd | 0.49 | 0.51 | 1.17 | 5.22 | 5.38 | |||||
| 3-hydroxy-β-damascone | 0.05 | 1.2 | 1.57 | 2.44 | 5.58 | 3.4 | |||||
| 3-hydroxy-7,8-dihydro-β-ionol | 1.74 | 1.37 | 1.09 | 1.33 | 1.52 | 2.39 | |||||
| 3-hydroxy-7,8-dehydro-β-ionol | 0.86 | 0.64 | 2.59 | 0.84 | 0.93 | 2.52 | |||||
| 3,4-dihydro-α-ionone | nd | nd | nd | nd | nd | nd | |||||
| 3,4-dihydro-3-oxoactinidol (isomer I) | 0.48 | 1.39 | 0.92 | 1.54 | 0.57 | 4.1 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer II) | 0.76 | 0.86 | 0.95 | 1.14 | 2.55 | 1.26 | |||||
| 4-oxo-β-ionol | nd | nd | nd | nd | nd | nd | |||||
Levels (μg kg−1 berry) of free and glycosylated C13-norisoprenoids from Muscat of Alexandria and Shiraz berries at different developmental stages (nd: not detected; HB: hard berries; SB: soft berries)
. | Week before/after véraison . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | −1 . | 0 . | . | 1 . | 3 . | 4.5 . | |||||
. | . | HB . | SB . | . | . | . | |||||
| Muscat of Alexandria | |||||||||||
| Major bound compounds | |||||||||||
| 4-oxo-β-ionol | nd | 6.23 | 6.48 | 16.11 | 33.89 | 49.61 | |||||
| 3-hydroxy-7,8-dihydro-β-ionone | 0.27 | 0.57 | 1.06 | 15 | 27.73 | 33.57 | |||||
| 3-oxo-α-ionol | 2.08 | 13.36 | 15.52 | 64.65 | 26.61 | 33.91 | |||||
| Minor bound compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | nd | nd | 2.99 | 2.5 | 0.53 | |||||
| 3-hydroxy-5,6-epoxy-β-ionone | nd | nd | nd | nd | nd | nd | |||||
| 3-hydroxy-β-damascone | 1.78 | 2.63 | 3.33 | 5.11 | 4.64 | 7.9 | |||||
| 3-hydroxy-7,8-dihydro-β-ionol | 1.42 | 1.91 | 1.99 | 6.4 | 9.84 | 8.27 | |||||
| 3-hydroxy-7,8-dehydro-β-ionol | 0.69 | 0.72 | 1.05 | 1.69 | 2.03 | 3.11 | |||||
| 3,4-dihydro-α-ionone | nd | nd | nd | 1.44 | 1.81 | 2.32 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer I) | 0.76 | 1.37 | 1.58 | 3.73 | 3.04 | 2.96 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer II) | 0.87 | 1.3 | 1.67 | 4.17 | 4.44 | 6.24 | |||||
| Free compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | nd | nd | 5.88 | 6 | 30.2 | |||||
| 4-oxo-β-ionol | nd | nd | nd | 0.53 | 3.22 | 6.03 | |||||
| Shiraz | |||||||||||
| Major bound compounds | |||||||||||
| 3-oxo-α-ionol | 4.05 | 4.07 | 8 | 11.47 | 16.79 | 17.95 | |||||
| 3-hydroxy-7,8-dihydro-β-ionone | 1.45 | 1.04 | 1.02 | 2.28 | 6.99 | 12.4 | |||||
| Minor bound compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | 0.59 | 1.5 | 0.81 | 2.44 | 3.63 | |||||
| 3-hydroxy-5,6-epoxy-β-ionone | nd | 0.49 | 0.51 | 1.17 | 5.22 | 5.38 | |||||
| 3-hydroxy-β-damascone | 0.05 | 1.2 | 1.57 | 2.44 | 5.58 | 3.4 | |||||
| 3-hydroxy-7,8-dihydro-β-ionol | 1.74 | 1.37 | 1.09 | 1.33 | 1.52 | 2.39 | |||||
| 3-hydroxy-7,8-dehydro-β-ionol | 0.86 | 0.64 | 2.59 | 0.84 | 0.93 | 2.52 | |||||
| 3,4-dihydro-α-ionone | nd | nd | nd | nd | nd | nd | |||||
| 3,4-dihydro-3-oxoactinidol (isomer I) | 0.48 | 1.39 | 0.92 | 1.54 | 0.57 | 4.1 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer II) | 0.76 | 0.86 | 0.95 | 1.14 | 2.55 | 1.26 | |||||
| 4-oxo-β-ionol | nd | nd | nd | nd | nd | nd | |||||
. | Week before/after véraison . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | −1 . | 0 . | . | 1 . | 3 . | 4.5 . | |||||
. | . | HB . | SB . | . | . | . | |||||
| Muscat of Alexandria | |||||||||||
| Major bound compounds | |||||||||||
| 4-oxo-β-ionol | nd | 6.23 | 6.48 | 16.11 | 33.89 | 49.61 | |||||
| 3-hydroxy-7,8-dihydro-β-ionone | 0.27 | 0.57 | 1.06 | 15 | 27.73 | 33.57 | |||||
| 3-oxo-α-ionol | 2.08 | 13.36 | 15.52 | 64.65 | 26.61 | 33.91 | |||||
| Minor bound compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | nd | nd | 2.99 | 2.5 | 0.53 | |||||
| 3-hydroxy-5,6-epoxy-β-ionone | nd | nd | nd | nd | nd | nd | |||||
| 3-hydroxy-β-damascone | 1.78 | 2.63 | 3.33 | 5.11 | 4.64 | 7.9 | |||||
| 3-hydroxy-7,8-dihydro-β-ionol | 1.42 | 1.91 | 1.99 | 6.4 | 9.84 | 8.27 | |||||
| 3-hydroxy-7,8-dehydro-β-ionol | 0.69 | 0.72 | 1.05 | 1.69 | 2.03 | 3.11 | |||||
| 3,4-dihydro-α-ionone | nd | nd | nd | 1.44 | 1.81 | 2.32 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer I) | 0.76 | 1.37 | 1.58 | 3.73 | 3.04 | 2.96 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer II) | 0.87 | 1.3 | 1.67 | 4.17 | 4.44 | 6.24 | |||||
| Free compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | nd | nd | 5.88 | 6 | 30.2 | |||||
| 4-oxo-β-ionol | nd | nd | nd | 0.53 | 3.22 | 6.03 | |||||
| Shiraz | |||||||||||
| Major bound compounds | |||||||||||
| 3-oxo-α-ionol | 4.05 | 4.07 | 8 | 11.47 | 16.79 | 17.95 | |||||
| 3-hydroxy-7,8-dihydro-β-ionone | 1.45 | 1.04 | 1.02 | 2.28 | 6.99 | 12.4 | |||||
| Minor bound compounds | |||||||||||
| 3-hydroxy-β-ionone | nd | 0.59 | 1.5 | 0.81 | 2.44 | 3.63 | |||||
| 3-hydroxy-5,6-epoxy-β-ionone | nd | 0.49 | 0.51 | 1.17 | 5.22 | 5.38 | |||||
| 3-hydroxy-β-damascone | 0.05 | 1.2 | 1.57 | 2.44 | 5.58 | 3.4 | |||||
| 3-hydroxy-7,8-dihydro-β-ionol | 1.74 | 1.37 | 1.09 | 1.33 | 1.52 | 2.39 | |||||
| 3-hydroxy-7,8-dehydro-β-ionol | 0.86 | 0.64 | 2.59 | 0.84 | 0.93 | 2.52 | |||||
| 3,4-dihydro-α-ionone | nd | nd | nd | nd | nd | nd | |||||
| 3,4-dihydro-3-oxoactinidol (isomer I) | 0.48 | 1.39 | 0.92 | 1.54 | 0.57 | 4.1 | |||||
| 3,4-dihydro-3-oxoactinidol (isomer II) | 0.76 | 0.86 | 0.95 | 1.14 | 2.55 | 1.26 | |||||
| 4-oxo-β-ionol | nd | nd | nd | nd | nd | nd | |||||
Discussion
In the past, correlations between the levels of carotenoids and C13-norisoprenoids during grape berry ripening (Razungles et al., 1993), common chemical configuration of norisoprenoids and relevant parent carotenoids, and isotopic labelling studies (Baumes et al., 2002) have suggested the existence of carotene dioxygenases able to yield norisoprenoids from carotenoids. Actually, the present work demonstrates the existence of a transcript encoding a CCD in grape berries highly homologous to other plant CCDs.
VvCCD1 corresponded to TIGR contig TC44975 from the latest version (Release 4.0, September 2004). This contig is composed of 48 ESTs, mainly found in libraries from grape berries at véraison (0.24% of total ESTs from this library) or from leaves in late season (0.16% of total ESTs from this library). In tomato for example, LeCCD1B which is implicated in the C13-norisoprenoid synthesis during fruit development, represents 0.11% of total transcripts in the intermediate red fruit (Simkin et al., 2004a). LeCCD1B expression is lower in the leaf, with 0.04% of total transcripts.
These results showed that recombinant VvCCD1 catalyses the symmetrical cleavage of zeaxanthin to generate 3-hydroxy-β-ionone and 4,9-dimethyldodeca-2,4,6,8,10-pentaene-1,12-dialdehyde. Assays with lutein, one of the major carotenoids in grape berries, generated the same products as with zeaxanthin (data not shown). No cleavage product could be detected in these experimental conditions when β-carotene was used as substrate. It now appears that VvCCD1 is a 9,10[9′,10′]-carotenoid cleavage dioxygenase. Most of the VvCCD1 homologues (Fig. 1) reported so far have been functionally characterized and demonstrated to catalyse the symmetrical cleavage of carotenoids at the 9,10[9′,10′] bonds. Consequently, it was hypothesized that VvCCD1 and homologues might act as dimers. Different NCEDs have also been functionally characterized. The specificity of this class towards 9-cis xanthophylls and their asymmetric cleavage at the 11,12 bond clearly distinguish them from CCDs. In grapevine, VvNCED1 and VvNCED2 have recently been described to possess the same characteristics as others NCEDs (Soar et al., 2004) (Fig. 1). A plastidic localization was also predicted and VvNCED1 expression has been correlated with an increase in abscisic acid concentration in water-stressed leaves.
Contrary to NCEDs, and since no chloroplastic transit peptide was predicted (ChloroP 1.1) (Emanuelsson et al., 1999), a cytoplasmic location of VvCCD1 is hypothesized. Previous immunohistochemical experiments have confirmed a cytoplasmic location of CsCCD (Bouvier et al., 2003). In vitro localization assays also showed that LeCCD1A and B were not imported into chloroplasts (Simkin et al., 2004a). However, a putative transmembrane domain was predicted for VvCCD1 as for the homologous dioxygenases AtCCD1 and CsCCD. Because carotenoids are located in plastidic membranes, VvCCD1 could be attached to the outer membrane of plastids. Moreover, better recovery of VvCCD1 in presence of Triton X-100 in the sonicated extracts (data not shown) suggests that VvCCD1 could be associated with membranes.
The VvCCD1 expression has been studied during the grape berry development of two cultivars, together with the accumulation of C13-norisoprenoids. The C13-norisoprenoid total level was found to be higher in Muscat of Alexandria berries than in Shiraz ones throughout maturation (Fig. 5B). This could be a consequence of the higher VvCCD1 expression in Muscat compared with Shiraz berries (Fig. 5A). Since there is a one week delay between VvCCD1 induction and the accumulation of volatile compounds, a direct relationship can not be established.
Except for 3-hydroxy-β-ionone and 3-hydroxy-5,6-epoxy-β-ionone, cleavage products of zeaxanthin (or lutein) and violaxanthin, respectively, the majority of the C13-norisoprenoids detected in the study are not the carotenoid primary cleavage products but probably their metabolites (Table 1). When their chemical structures are examined, the modifications could be attributed to the actions of oxidases and reductases.
Furthermore, glycosyltransferases clearly play a role since most of the metabolites are detected under their glycosylated form. It is highly likely that all the enzymes that carry out further metabolization of the primary cleavage products are probably present in grape berries, as already observed in the biosynthesis of terpenes (Suga and Hirata, 1990). Indeed, when β-ionone was administrated to a grape cell suspension culture, several oxygenated, reduced, and glycosylated norisoprenoidic compounds were observed (Wirth et al., 2003). These further enzymatic modifications could, in part, explain the delay observed between VvCCD1 induction and the C13-norisoprenoid accumulation. A delay was also observed between the induction of the S-linalool synthase gene (LIS) and linalool synthesis during grape berry development of the Muscat of Frontignan cultivar (Ebang-Oke et al., 2003).
It is interesting to note that, contrary to other genes that play a role in secondary metabolism pathways, VvCCD1 is induced at the early stages of the grape berry development. Moreover, the plausible norisoprenoidic metabolites of VvCCD1 are already present at these stages. Hence, VvCCD1 could serve as a marker in the determination of the grape berry maturity induction phase. On the contrary, for example, anthocyanin biosynthetic genes are induced after véraison when anthocyanins begin to accumulate in grape berries (Boss et al., 1996). Terpene biosynthetic genes also present some differences in expression profile during ripening. Sesquiterpene synthase and monoterpene synthase transcripts are not detected during the early stages of grape berry development and valencene synthase transcripts accumulate 4 weeks after véraison (Lücker et al., 2004). In the same way, LIS is expressed throughout ripening, but is predominantly induced in the third week post-véraison (Ebang-Oke et al., 2003). In addition, linalool synthesis reaches a peak 4 weeks after véraison.
C13-norisoprenoids accumulated earlier during grape berry development than others secondary metabolites. This could suggest their involvement in the regulation of grapevine development, as already suggested in other plants. For example, 3-hydroxy-β-ionone, a norisoprenoid present in grape berry, has been shown to accumulate in etiolated bean seedlings on exposure to light, playing a potential role in the light-induced inhibition of hypocotyl elongation (Kato-Noguchi, 1992; Kato-Noguchi et al., 1993). However, the functions of norisoprenoids still remain obscure. In grapevine, they could play a role of attractants, favouring seed scattering by animals. The function of the C14-dialdehyde generated by the symmetrical cleavage of zeaxanthin also remains unclear. This compound is supposed to be the precursor of rosafluene, a natural product of roses (Eugster and Märki-Fischer, 1991). Its role in plant development and regulation can also not be excluded. A C18-apocarotenoid resulting from the sequential cleavage action of AtCCD7 and AtCCD8 has been shown to play a role in the regulation of lateral branching (Sorefan et al., 2003). The availability of transgenic grapevine plants altered in CCD1 expression could help to define the function of apocarotenoids more precisely.
Other V. vinifera genes could also encode different CCDs. AtCCD7 possesses a different substrate specificity when compared with AtCCD1 (Schwartz et al., 2004). No homologue of AtCCD7 has been found in the latest database unigene set of V. vinifera ESTs (TIGR, Release 4.0, September 2004). The complete grape genome sequencing will help to determine whether other CCD enzymes are involved in the biosynthesis of C13-norisoprenoids.
We gratefully thank SH Schwartz and B Camara for their helpful suggestions, B Camara for sending the strain of E. coli accumulating zeaxanthin and H Ernst from BASF Company for providing 4,9-dimethyldodeca-2,4,6,8,10-pentaene-1,12-dialdehyde. Thanks to C Romieu and A Ageorges for access to ESTs grape bank, Y Bayssac for HPLC analysis, R Ratomahenina, and S Vialet for technical assistance. We also thank the Languedoc-Rousillon region for the financial contribution of the GC-MS equipment. S Mathieu has been supported by a grant from the French ‘Ministère de l'Education Nationale, de l'Enseignement Supérieur et de la Recherche’.
References
Baumes RL, Wirth J, Bureau S, Günata Z, Razungles A.
Boss PK, Davies C, Robinson SP.
Bouvier F, Suire C, Mutterer J, Camara B.
Ebang-Oke J, de Billerbeck G, Ambid C.
Emanuelsson O, Nielsen H, von Heijne G.
Eugster CH, Märki-Fischer E.
Günata Z, Bayonove C, Baumes RL, Cordonnier RE.
Kato-Noguchi H.
Kato-Noguchi H, Kosemura S, Yamasura S, Hasegawa K.
Lindqvist A, Andersson S.
Lücker J, Bowen P, Bohlmann J.
Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W.
Olson JA, Hayaishi O.
Razungles A, Günata Z, Pinatel S, Baumes RL, Bayonove CL.
Rüffner HP.
Sarni-Manchado P, Verries C, Tesnière C.
Schwartz SH, Qin X, Loewen MC.
Schwartz SH, Qin X, Zeevart JA.
Schwartz SH, Tan BC, Gage DA, McCarty DR, Zeevart JA.
Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ.
Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark DG, Klee HJ.
Skouroumounis GK, Winterhalter P.
Soar C, Speirs J, Maffei S, Loveys B.
Sorefan K, Booker J, Haurogne K, et al.
Suga T, Hirata T.
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR.
Terrier N, Ageorges A, Aball P, Romieu C.
Terrier N, Glissant D, Grimplet J, et al.
von Lintig J, Vogt K.
Williams PJ, Sefton MA, Francis IL.
Winterhalter P, Rouseff R.
Winterhalter P, Schreier P.
Winterhalter P, Sefton MA, Williams P.
Wirth J.
Wirth J, Guo W, Baumes RL, Günata Z.
Wirth J, Sauvage FX, Baumes RL, Günata Z.
Wu Z, Robinson S.





Comments