-
PDF
- Split View
-
Views
-
Cite
Cite
J. J. James, N. N. Alder, K. H. Mühling, A. E. Läuchli, K. A. Shackel, L. A. Donovan, J. H. Richards, High apoplastic solute concentrations in leaves alter water relations of the halophytic shrub, Sarcobatus vermiculatus, Journal of Experimental Botany, Volume 57, Issue 1, January 2006, Pages 139–147, https://doi.org/10.1093/jxb/erj016
Close - Share Icon Share
Abstract
Predawn plant water potential (Ψw) is used to estimate soil moisture available to plants because plants are expected to equilibrate with the root-zone Ψw. Although this equilibrium assumption provides the basis for interpreting many physiological and ecological parameters, much work suggests predawn plant Ψw is often more negative than root-zone soil Ψw. For many halophytes even when soils are well-watered and night-time shoot and root water loss eliminated, predawn disequilibrium (PDD) between leaf and soil Ψw can exceed 0.5 MPa. A model halophyte, Sarcobatus vermiculatus, was used to test the predictions that low predawn solute potential (Ψs) in the leaf apoplast is a major mechanism driving PDD and that low Ψs is due to high Na+ and K+ concentrations in the leaf apoplast. Measurements of leaf cell turgor (Ψp) and solute potential (Ψs) of plants grown under a range of soil salinities demonstrated that predawn symplast Ψw was 1.7 to 2.1 MPa more negative than predawn xylem Ψw, indicating a significant negative apoplastic Ψs. Measurements on isolated apoplastic fluid indicated that Na+ concentrations in the leaf apoplast ranged from 80 to 230 mM, depending on salinity, while apoplastic K+ remained around 50 mM. The water relations measurements suggest that without a low apoplastic Ψs, predawn Ψp may reach pressures that could cause cell damage. It is proposed that low predawn apoplastic Ψs may be an efficient way to regulate Ψp in plants that accumulate high concentrations of osmotica or when plants are subject to fluctuating patterns of soil water availability.
Introduction
Predawn plant water potential is often considered to reflect soil moisture availability to plants and as such is used to interpret a range of physiological and ecological parameters such as maximum stomatal conductance and transpiration (Reich and Hinckley, 1989; Améglio and Archer, 1996; Mediavilla and Escudero, 2003), growth (Mitchell et al., 1993), and differences in rooting depth, stress tolerance, and habitat partitioning between different species or life stages (Davis and Mooney, 1986; Donovan and Ehleringer, 1994; Peuke et al., 2002; Filella and Peñuelas, 2003). The use and interpretation of Ψw follows from classical water relations models, based on thermodynamics and the Ohm's law analogy, that predict that predawn plant Ψw will equilibrate with soil Ψw in the rooting zone (Slatyer, 1967). However, there is a growing body of evidence suggesting that predawn plant Ψw can be substantially more negative than root-zone soil Ψw in many species. For example, Donovan et al. (2001) documented significant predawn disequilibrium between plant–soil Ψw (hereafter predawn disequilibrium, PDD; sensuDonovan et al., 1999) in 15 out of 15 species surveyed under controlled environmental conditions. For seven of these species PDD ranged from 0.5 to 2.3 MPa. The limited number of field experiments addressing PDD largely support the observations made in controlled environments. In Great Basin, Mojave, and Tunisian desert shrubs PDD ranged from 1.1 to 2.7 MPa (Ourcival et al., 1994; Donovan et al., 2003; James et al., 2005). Comparably large PDD in field populations have also been reported for a range of crop and tree species (see review in Donovan et al., 2001; Bucci et al., 2004). While these observations change thinking about how plants may interact with the soil and atmospheric moisture environment, better understanding of mechanisms driving PDD is needed to evaluate the adaptive significance and physiological and ecological tradeoffs of PDD.
A number of mechanisms that prevent overnight equilibration between plant and soil Ψw have been recognized, including night-time water loss through the canopy or root system, low hydraulic conductivity, high capacitance, and soil moisture heterogeneity (Blake and Ferrell, 1977; Richards and Caldwell, 1987; Ourcival and Berger, 1995; Sellin, 1999). For many halophytes and moderately salt-tolerant desert species, however, even when soils are well-watered and night-time water loss eliminated, PDD can still be significant (0.2–1.6 MPa), suggesting that other mechanisms linked to ion physiology may contribute to PDD in these and other arid-land species (Donovan et al., 2001, 2003).
One putative mechanism that could contribute to large PDD in halophytes is high solute concentration in the leaf apoplast. Although classical water relations assume that Ψs in the leaf apoplast and stem xylem lumen are similar and low (−0.01 MPa) (Ritchie and Hinckley, 1975; Passioura, 1991; Boyer, 1995), experimental observations of predawn leaf Ψw significantly lower than xylem Ψp in halophytes suggests that, in these species, predawn Ψs in the leaf apoplast could be substantial (Donovan et al., 2001, 2003). To understand how this mechanism could contribute to PDD, it is important to recognize that the leaf apoplast is spatially distant from the stem xylem lumen but that these regions are not separated by a membrane. Water movement between the symplast and apoplast involves the plasmalemma, and hence is determined by the sum of pressure and osmotic components, whereas water movement through the apoplast (xylem conduits and cell wall space) is driven solely by pressure differences.
Therefore, in non-transpiring, well-watered plants, equilibration between xylem and apoplast Ψp and between symplast and apoplast Ψw is expected. High solute concentration in the leaf apoplast would lower leaf apoplastic Ψw to a value below that of xylem Ψp. High solute concentrations in the leaf apoplast would not be expected to dissipate overnight into the xylem lumen, and thus reduce stem xylem Ψs, because of relatively low permeability in unstirred layers of intercellular and cell wall spaces and large diffusional distances between the leaf apoplast and stem xylem lumen (Carpita, 1982; Grignon and Sentenac, 1991; Jungk, 1991). If the leaf symplast Ψw is in equilibrium with leaf apoplast Ψw, then leaf Ψw would be significantly lower than stem Ψp, thus contributing to the observed PDD between leaf Ψw and stem Ψp and between leaf and soil Ψw (Donovan et al., 1999). Under these conditions the Ψw difference between the stem xylem and the leaf apoplast would not drive water flow because the pressure difference remains zero. The apoplastic solute accumulation mechanism may be of high magnitude for halophytes growing in saline soils, with Na+ accumulating as the major solute in the leaf apoplast, but a similar mechanism could explain smaller predawn differences that have been observed between stem xylem Ψp and leaf Ψw in glycophytes and halophytes growing in non-saline soils (Donovan et al., 2001). Here K+ might be expected to be the major solute in the leaf apoplast.
While solute accumulation in the leaf apoplast during daytime transpiration has been documented in both glycophytes and halophytes (Meinzer and Moore, 1988; Flowers et al., 1991; Canny, 1993), it is not known if high predawn concentrations of leaf apoplastic solutes contribute to the large differences (0.5–2.3 MPa) between predawn leaf and soil Ψw observed in halophytes (Donovan et al., 2001). A better understanding of predawn water and ionic relations in the leaf apoplast is essential for refining predictions of current water relations models. In addition, quantifying the potential magnitude of apoplastic solute concentration and how it may vary with soil salinity could improve understanding of turgor regulation and nutrient relations of halophytes.
The main objective of this study was to determine if high solute accumulation in the leaf apoplast could be a driver of significant PDD documented in well-watered, non-transpiring halophytes. The halophyte Sarcobatus vermiculatus (Hook.) Torrey (Chenopodiaceae) was used as a model because both greenhouse and field experiments have demonstrated large PDD in this species even when other mechanisms known to contribute to PDD have been minimized. In field experiments, Sarcobatus predawn leaf Ψw was 0.9 MPa more negative than stem xylem Ψp. In greenhouse experiments, predawn difference between leaf Ψw and xylem Ψp was 1.22 MPa in plants growing in soils watered with 100 mM NaCl but lower, around 0.5 MPa, for plants growing in non-saline soils (Donovan et al., 1999, 2003). Based on these measurements it was predicted that: (i) predawn solute potential in the leaf apoplast would be substantial, resulting in a significantly lower apoplastic Ψs than stem xylem Ψs; (ii) the major solute contributing to differences in predawn leaf apoplast and stem xylem Ψs would be Na+; and (iii) leaf apoplastic Na+ concentration and corresponding magnitude of leaf apoplastic Ψs, however, would be a function of soil salinity.
The estimation of water relations and the ionic composition of the leaf apoplast is notoriously difficult (Cosgrove and Cleland, 1983; Yu et al., 2000) so two experimental approaches were used to address these objectives. In the first experiment the magnitude of apoplastic Ψs was estimated by calculating the difference between leaf symplast and stem xylem Ψw (Murphy and Smith, 1994). For these calculations, leaf cell Ψp was measured directly with a cell pressure probe and stem xylem Ψp with a pressure chamber. Leaf cell Ψs and stem xylem Ψs were measured with a cryoscopic osmometer. In the second experiment, apoplastic fluid was isolated and concentrations of major apoplastic cations were quantified (Lohaus et al., 2001), allowing estimates of ion contributions to apoplastic Ψs.
Materials and methods
Experiment 1: Leaf symplast and xylem water relations
Plant materials and treatments:
Sarcobatus is a phreatophytic, C3 shrub that readily establishes on saline soils throughout the Great Basin Desert of North America. Naturally established seedlings of Sarcoabtus were collected from the Mono Basin Ecosystem Research Site north of Mono Lake, California, USA, (38° 5′ N, 118° 56′ W, 1958 m elevation) (Toft, 1995; Donovan et al., 1996; Drenovsky and Richards, 2005) and transplanted individually into 4.0 l, 35 cm deep, Tree Pots™ (Stuewe and Sons, Corvallis, OR) with a 3:1 v:v fritted clay:sand medium. Seedlings were grown in a greenhouse at the University of California, Davis campus and received quarter-strength modified Hoagland's solution (Epstein, 1972) four or five times each week. Two months after transplanting, experimental plants were randomly assigned to receive one of three levels of NaCl (0, 100, 300 mM) added to the nutrient solution. The 450 mM NaCl treatment used in Experiment 2 (see below), was not included in the water relations measurements due to logistical constraints associated with the amount of time needed to measure each water relations parameter. Background Na+ levels in the greenhouse watering system were <0.5 mM. Plants were watered with the corresponding salinity treatment for 3 months before measurements were initiated.
Water potential measurements:
The evening before measurements were made, plant canopies were bagged during the night to prevent night-time transpiration completely and soils were watered to field capacity (Donovan et al., 1999, 2003; Snyder et al., 2003). All measurements were made on mature leaves that had emerged and expanded during the salinity treatments. Predawn measurements were made between 04.30 h and 05.00 h. Midday measurements were made between 13.30 h and 14.30 h.
Leaf cell turgor (Ψp) was measured using a cell pressure probe (Husken et al., 1978). Glass microcapillary tips were prepared as described in Shackel et al. (1987). Briefly, borosilicate glass was pulled on a pipette puller (Model 750, Kopf, Tujunga, CA) and bevelled using a modified jet stream microbeveller (Ogden et al., 1978). Tips viewed through a microscope (×100) were aligned in a stream of 0.05 μm alumina grinding compound solution for a total of 1–2 min with pressurized air (∼0.7 MPa) applied to the non-bevelled end to prevent entry of grinding compound into the open tip. Tips were washed in distilled water to remove contaminants adhering to the glass surface. Prior to measurements, pipettes were filled with silicone oil (SF 96/50, Thomas Scientific, Swedesboro, NJ), and attached to a piezo-controlled micromanipulator (Leica Microsystems AG, Wetzlar, Germany) set to a motor speed of 25 μm s−1 and the smallest possible step size (0.5 μm). The micropipette tip and leaf cells were viewed through a microscope (×600) equipped with a vertical illuminator (BHMJ System, Olympus Corp., Melville, NY) and a long working distance (11 mm, ×20 objective, 1-LM546, Olympus Corp.). A video monitor interfaced with the microscope facilitated manipulation of the micropipette and adjustment of the meniscus. Measurements were made on both epidermal and mesophyll cells; results were analysed separately but were pooled for leaf-level water relations comparisons because differences between cell types were small and non-significant under the conditions of these experiments (see below and Results). Cells were penetrated to a distance of 10–15 μm and the oil pressure in the micropipette was adjusted to bring the oil/cell sap meniscus to within ∼5 μm of the cell surface. For mesophyll cell measurement, the micropipette was slowly inserted through the epidermus with the oil under pressure (∼0.1 MPa) until a meniscus was re-established. Ψp was recorded only when the probe pressure had stabilized for 10 min, indicating that cell membranes remained intact (Shackel et al., 1987). Measurements of predawn and midday leaf cell Ψp were made on 3–6 plants for each salinity treatment. Within each replicate, 3–4 cells each from epidermis and mesophyll, were measured to assess and account for cell–cell heterogeneity.
Leaf cell solute potential (Ψs) measurements were made using a direct-reading nanolitre osmometer (Clifton Technical Physics, Hartford, NY). Cell sap from both epidermal and mesophyll cells was collected by aspiration into glass micropipettes using the pressure probe apparatus described above. Samples were discharged into the middle of a reservoir on a silver platform containing immersion oil such that the diameter of the globular sample was typically half that of the reservoir diameter. The platform was pressed onto a cooling stage using thermal grease, and the freezing point was determined by viewing the sample through a microscope. Measurements of predawn and midday leaf cell Ψs were made on 4–7 plants for each salinity treatment. Cell sap samples of mesophyll and epidermal cells for each replicate plant were composited separately from 4–10 cells of each type.
Bulk leaf Ψs was measured on leaves following measurements of cell Ψp and Ψs. Each leaf was excised from the stem, rinsed with distilled water, and blotted dry. The leaf was then ground in a piston press, and expressed sap was drawn into a syringe. The sap was immediately loaded onto paper discs, and the bulk leaf Ψs was measured using a Wescor 5500 vapour pressure osmometer (Wescor Inc., Logan, UT). Measurements of predawn and midday bulk leaf Ψs were made on 3–7 plants for each salinity treatment.
Stem xylem pressure (Ψp) was measured using a pressure chamber (PMS Inc., Corvallis, OR). Measurements of predawn xylem Ψp were made on 4–7 plants for each salinity treatment. Xylem solute potential (Ψs) was measured by expressing xylem sap (approximately 5−10 μl, depending on stem size) from the cut end of a stem in the pressure chamber, collecting the sap on paper discs, and measuring Ψs using the vapour pressure osmometer. Standard precautionary steps to minimize contamination and errors in the xylem Ψp and Ψs measurements were followed (Turner, 1988; Boyer, 1995) which included removal of phloem around the cut surface before measurements were made. Predawn xylem Ψs was measured on 4–7 plants for each salinity treatment. Soil water potential (Ψw) was measured with individually calibrated screen-cage thermocouple psychrometers installed in the centre of the pots and monitored hourly with a data logger (Richards and Caldwell, 1987).
Experiment 2: Leaf apoplastic solutes
Plant materials and treatments:
Seedlings of Sarcobatus were collected from the same location and transplanted using pots and soil media as the plants in Experiment 1. After establishment in an unheated greenhouse at the University of California Davis, plants were randomly grouped into five experimental blocks. Because extraction of apoplastic fluid was only conducted during predawn hours (04.00–06.00 h; see below) it was not possible to harvest all experimental plants at the same time. Blocks were therefore used to account for variation that occurred through time as samples were harvested successively through a 25 week period. Within each block, 40–60 plants were randomly assigned to receive one of four salinity levels (0, 100, 300, 450 mM NaCl) and 6–12 plants per treatment per block were harvested.
The initiation of the salinity treatments for each block was staggered by approximately 3 weeks to allow sufficient time to harvest each block under predawn conditions and to ensure that all plants were exposed to salinity treatments for a similar duration. For the salinity treatments, all plants within a block were moved from the greenhouse to a controlled environment facility. Plants were exposed to an average day/night temperature of 30/8 °C and a photoperiod of 14 h (800 μmol m−2 s−1 PPFD) for 6 weeks. Plants were watered twice a week with the assigned salinity treatment. Essential nutrients were supplied every other week as one-quarter strength modified Hoagland's solution (Epstein, 1972).
Collection and analysis of apoplastic fluid:
The vacuum infiltration technique, slightly modified from the method of Mühling and Sattelmacher (1995) and Lohaus et al. (2001) was used to isolate leaf apoplastic fluid. Extraction of apoplastic fluid began after the replicates within a block had been exposed to salinity treatments for 6 weeks. The apoplastic fluid from all replicates within a block was extracted within a week between 04.00–06.00 h. Approximately 4 g FW of fully expanded leaves were cut at the base of the petiole from the plant with a razor blade, rinsed with deionized water, blotted dry, and were then vacuum infiltrated for 5 min with either 250 mM sorbitol to quantify concentrations of unbound apoplastic cations or 100 mM BaCl2 to quantify concentrations of bound and unbound apoplastic cations. The difference in apoplastic cation concentration between the two infiltration media provides an estimate of bound apoplastic cations. The infiltrated leaves were gently blotted dry, divided into four subsamples, and leaves in each subsample were placed, petiole up, into a 15 ml centrifuge tube. The centrifugation was carried out at 600 g at 4 °C for 6 min. The apoplastic fluid was stored at −25 °C until analysis. Contamination of apoplastic fluid with symplastic fluid was assessed by comparing total soluble protein concentration in samples centrifuged at 600 g and 6000 g (Wimmer et al., 2003). Total soluble protein concentration in samples centrifuged at 600 g was less than 5% of the total protein concentration in samples centrifuged at 6000 g (18±7.1 and 382±30.1 μg ml−1, respectively; mean ±SE, n=30) suggesting that cytoplasmic contamination of apoplastic fluid was minimal with the centrifugation force applied.
Concentrations of Na+, K+, and Ca2+ in the apoplast and leaf sap were analysed with an ion chromatograph (Model DX4500i, Dionex, Sunnyvale, CA) using a cation exchange column (AS12, Dionex) connected to a conductivity detector. Isocratic elution with methanesulphonic acid (20 mmol l−1, 1 ml min−1) was used for the separation of cations. To prevent interference with organic components samples were first filtered through a 0.2 μm membrane filter. Calculation of the ion concentration in the apoplastic water space was determined by multiplying the ion concentration of the fluid extracted with sorbitol with a dilution factor (Lohaus et al., 2001) which was determined using the silicone oil method (Cosgrove and Cleland, 1983) and the indigo carmine method (Husted and Schjoerring, 1995) for apoplastic air and water, respectively.
Statistical analysis
Effects of soil salinity on leaf and xylem water relations and leaf apoplastic Na+, K+, and Ca2+ concentrations were analysed using ANOVA. Assumptions of ANOVA were evaluated using Shapiro–Wilk test for normality and Levene's test for homogeneity of variance. When these assumptions were violated, ANOVA models were weighted by the inverse of the variance (Neter et al., 1990). Following ANOVA, differences between treatments were determined using the Ryan–Einot–Gabriel–Welsch multiple range test (SAS, 2001).
Results and discussion
Experiment 1: Leaf symplast and xylem water relations
In all treatments Sarcobatus exhibited significant predawn disequilibria of large magnitude. Under well-watered conditions with canopies bagged during the night to eliminate night-time canopy water loss completely, Sarcobatus predawn leaf symplast Ψw was 1.70–2.37 MPa more negative than predawn soil Ψw with PDD between leaf and soil water potential increasing with higher substrate salinity (Table 1). These results are largely consistent with field and greenhouse studies that have demonstrated large PDD based on discrepancies between psychrometric measurements of predawn soil and bulk leaf Ψw. For example, under controlled environmental conditions Sarcobatus bulk leaf predawn Ψw was around 1.4 MPa more negative than soil Ψw, but in the field was up to 2.7 MPa more negative than soil Ψw (Donovan et al., 1999, 2003). Similarly, under controlled environmental conditions bulk leaf predawn water potentials were 2 and 1 MPa more negative than soil Ψw for the Tunisian desert shrubs Artemisia herba-alba and Anthyllis henoniana, respectively (Ourcival and Berger, 1995). This work extends these findings by demonstrating that large PDD exists between individual leaf cell symplastic Ψw and stem xylem Ψw under well-watered, uniform soil conditions and when shoot water loss is eliminated.
Predawn leaf symplast (cell measurements) and stem xylem water (Ψw), solute (Ψs) and pressure (Ψp) potential (MPa) for Sarcobatus growing in three salinity treatments (mean ±SE, see methods for sample size)
Treatment (mM NaCl) . | . | Leaf symplast (MPa) . | Leaf apoplast (MPa) . | Stem xylem (MPa) . | Soil (MPa) . |
|---|---|---|---|---|---|
| 0 | Ψw | −1.71±0.07 a = = = = = | −1.71 | −1.07±0.08 b | −0.01±0.01 c |
| Ψs | −1.95±0.07 | (−0.75) | −0.16±0.02 | ||
| Ψp | 0.32±0.03 | −0.96 = = = = = = = | −0.96±0.08 | ||
| 100 | Ψw | −2.61±0.11 a = = = = = | −2.61 | −1.23±0.06 b | −0.45±0.01 c |
| Ψs | −3.03±0.09 | (−1.57) | −0.20±0.05 | ||
| Ψp | 0.39±0.01 | −1.03 = = = = = = = | −1.03±0.06 | ||
| 300 | Ψw | −3.65±0.11 a = = = = = | −3.65 | −1.74±0.69 b | −1.28±0.16 c |
| Ψs | −3.93±0.08 | (−2.12) | −0.24±0.05 | ||
| Ψp | 0.34±0.01 | −1.53 = = = = = = = | −1.53±0.07 |
Treatment (mM NaCl) . | . | Leaf symplast (MPa) . | Leaf apoplast (MPa) . | Stem xylem (MPa) . | Soil (MPa) . |
|---|---|---|---|---|---|
| 0 | Ψw | −1.71±0.07 a = = = = = | −1.71 | −1.07±0.08 b | −0.01±0.01 c |
| Ψs | −1.95±0.07 | (−0.75) | −0.16±0.02 | ||
| Ψp | 0.32±0.03 | −0.96 = = = = = = = | −0.96±0.08 | ||
| 100 | Ψw | −2.61±0.11 a = = = = = | −2.61 | −1.23±0.06 b | −0.45±0.01 c |
| Ψs | −3.03±0.09 | (−1.57) | −0.20±0.05 | ||
| Ψp | 0.39±0.01 | −1.03 = = = = = = = | −1.03±0.06 | ||
| 300 | Ψw | −3.65±0.11 a = = = = = | −3.65 | −1.74±0.69 b | −1.28±0.16 c |
| Ψs | −3.93±0.08 | (−2.12) | −0.24±0.05 | ||
| Ψp | 0.34±0.01 | −1.53 = = = = = = = | −1.53±0.07 |
Symplast Ψs and Ψp include measurements of both epidermal and mesophyll cells (see Materials and methods for experimental approach and measurement techniques; see also Table 2). All plant canopies were bagged during the night period before predawn measurements to minimize night-time shoot water loss. Different letters indicate significantly different predawn Ψw between leaf symplast, stem xylem, and soil within a salinity treatment (P <0.05). Based on water relations theory, leaf apoplastic Ψw was assumed to be in equilibrium with leaf symplast Ψw (indicated by = = =). Likewise, leaf apoplastic Ψp also was assumed to be in equilibrium with stem xylem Ψp (indicated by = = =). Based on these equilibrium assumptions which match experimental conditions, the contribution of apoplastic solute potential Ψs to apoplastic water potential was estimated (shown in parentheses).
Predawn leaf symplast (cell measurements) and stem xylem water (Ψw), solute (Ψs) and pressure (Ψp) potential (MPa) for Sarcobatus growing in three salinity treatments (mean ±SE, see methods for sample size)
Treatment (mM NaCl) . | . | Leaf symplast (MPa) . | Leaf apoplast (MPa) . | Stem xylem (MPa) . | Soil (MPa) . |
|---|---|---|---|---|---|
| 0 | Ψw | −1.71±0.07 a = = = = = | −1.71 | −1.07±0.08 b | −0.01±0.01 c |
| Ψs | −1.95±0.07 | (−0.75) | −0.16±0.02 | ||
| Ψp | 0.32±0.03 | −0.96 = = = = = = = | −0.96±0.08 | ||
| 100 | Ψw | −2.61±0.11 a = = = = = | −2.61 | −1.23±0.06 b | −0.45±0.01 c |
| Ψs | −3.03±0.09 | (−1.57) | −0.20±0.05 | ||
| Ψp | 0.39±0.01 | −1.03 = = = = = = = | −1.03±0.06 | ||
| 300 | Ψw | −3.65±0.11 a = = = = = | −3.65 | −1.74±0.69 b | −1.28±0.16 c |
| Ψs | −3.93±0.08 | (−2.12) | −0.24±0.05 | ||
| Ψp | 0.34±0.01 | −1.53 = = = = = = = | −1.53±0.07 |
Treatment (mM NaCl) . | . | Leaf symplast (MPa) . | Leaf apoplast (MPa) . | Stem xylem (MPa) . | Soil (MPa) . |
|---|---|---|---|---|---|
| 0 | Ψw | −1.71±0.07 a = = = = = | −1.71 | −1.07±0.08 b | −0.01±0.01 c |
| Ψs | −1.95±0.07 | (−0.75) | −0.16±0.02 | ||
| Ψp | 0.32±0.03 | −0.96 = = = = = = = | −0.96±0.08 | ||
| 100 | Ψw | −2.61±0.11 a = = = = = | −2.61 | −1.23±0.06 b | −0.45±0.01 c |
| Ψs | −3.03±0.09 | (−1.57) | −0.20±0.05 | ||
| Ψp | 0.39±0.01 | −1.03 = = = = = = = | −1.03±0.06 | ||
| 300 | Ψw | −3.65±0.11 a = = = = = | −3.65 | −1.74±0.69 b | −1.28±0.16 c |
| Ψs | −3.93±0.08 | (−2.12) | −0.24±0.05 | ||
| Ψp | 0.34±0.01 | −1.53 = = = = = = = | −1.53±0.07 |
Symplast Ψs and Ψp include measurements of both epidermal and mesophyll cells (see Materials and methods for experimental approach and measurement techniques; see also Table 2). All plant canopies were bagged during the night period before predawn measurements to minimize night-time shoot water loss. Different letters indicate significantly different predawn Ψw between leaf symplast, stem xylem, and soil within a salinity treatment (P <0.05). Based on water relations theory, leaf apoplastic Ψw was assumed to be in equilibrium with leaf symplast Ψw (indicated by = = =). Likewise, leaf apoplastic Ψp also was assumed to be in equilibrium with stem xylem Ψp (indicated by = = =). Based on these equilibrium assumptions which match experimental conditions, the contribution of apoplastic solute potential Ψs to apoplastic water potential was estimated (shown in parentheses).
Predawn leaf symplast Ψw also was significantly more negative than predawn xylem Ψw in all treatments (Table 1). Under experimental conditions that eliminated night-time shoot and root water loss, Ψw of the leaf symplast and adjacent leaf apoplast should equilibrate overnight. Similarly, equilibration between the leaf apoplast Ψp and xylem Ψp is assumed because these areas are not separated by a membrane. Under these experimental conditions and equilibrium assumptions, the large discrepancy between predawn xylem Ψp and leaf symplast Ψw suggests a predawn leaf apoplastic Ψs ranging from −0.75 to −2.12 MPa (Table 1). These calculated predawn leaf apoplastic Ψs values are more negative with increasing soil salinity. Further, these predawn leaf apoplastic Ψs values are substantially more negative than the xylem Ψs of Sarcobatus observed in this experiment (Table 1) and measured in the field (Donovan et al., 1996). Equilibration between leaf apoplast and xylem Ψs is not necessarily expected because of the relatively large diffusional distance between these areas and the low diffusion rates of ions in the leaf apoplast (Carpita, 1982; Jungk, 1991; Canny, 1995). Previous studies on actively growing tissues of crop plants have demonstrated that apoplastic Ψs can be as much as 0.3 MPa lower than xylem Ψs during the daytime when transpiration rates are high (Cosgrove and Cleland, 1983; Meinzer and Moore, 1988). These results suggest that even for mature tissue under minimal transpiration conditions, predawn leaf apoplast Ψs can be very low and that predawn leaf apoplast Ψs does not equilibrate with predawn xylem Ψs. As a consequence, low leaf apoplast Ψs reduces predawn leaf apoplastic Ψw to a value significantly lower than predawn xylem Ψw.
As expected, predawn leaf symplast Ψw decreased with increasing salinity (Table 1). The effect of salinity on cell water relations, however, was similar between mesophyll and epidermal cells under the minimal transpiration conditions of these experiments (Table 2). Although cell Ψp declined during the day with transpirational water loss, cell Ψp remained remarkably consistent between cell types and substrate salinities. As a result, for both cell types the decline in cell Ψw with increasing salinity was largely due to a decline in Ψs but not cell Ψp. Fricke et al. (1994) and Fricke (1997) demonstrated that Ψp in barley leaves was largely unaffected by substrate salinity and that, while cell Ψs declined with increasing salinity, changes in Ψs were similar between epidermal and mesophyll cells. Also consistent with these observations, Clipson et al. (1985) demonstrated that cell Ψp in the halophyte Suaeda maritima was not affected by soil salinity ranging from 0 to 400 mM NaCl despite large changes in cell Ψs. Although spatial gradients in Ψp and Ψs in leaf tissue are commonly reported during daytime measurements, the uniformity in epidermal and mesophyll Ψp, as well as the close agreement between bulk leaf Ψs and the Ψs of individual cells during predawn measurement conditions (Table 2), indicates that leaf cell Ψw is relatively homogenous within these mature leaves and that this estimate of leaf cell Ψw is a good measure of bulk leaf Ψw.
Predawn and midday leaf epidermal and mesophyll cell pressure (Ψp) and solute (Ψs) potentials (mean ±SE) for Sarcobatus growing under three salinity treatments
Treatment (mM NaCl) . | Cell type or bulk . | Predawn . | . | Midday . | . | ||
|---|---|---|---|---|---|---|---|
. | . | Ψp . | Ψs . | Ψp . | Ψs . | ||
| 0 | Epidermal | 0.31±0.04 | −2.05±0.15 | 0.25±0.05 | −2.48±0.18 | ||
| Mesophyll | 0.33±0.05 | −1.91±0.08 | 0.34±0.08 | −2.16±0.14 | |||
| Bulk | NM | −1.88±0.14 | NM | −2.32±0.21 | |||
| 100 | Epidermal | 0.38±0.03 | −2.94±0.24 | 0.25±0.08 | −3.02±0.29 | ||
| Mesophyll | 0.40±0.01 | −3.05±0.03 | 0.21±0.07 | −2.82±0.23 | |||
| Bulk | NM | −3.11±0.16 | NM | −3.14±0.23 | |||
| 300 | Epidermal | 0.35±0.02 | −4.09±0.06 | 0.20±0.02 | −3.95±0.17 | ||
| Mesophyll | 0.33±0.03 | −3.88±0.23 | 0.22±0.03 | −3.88±0.24 | |||
| Bulk | NM | −3.27±0.10 | NM | −3.68±0.11 | |||
Treatment (mM NaCl) . | Cell type or bulk . | Predawn . | . | Midday . | . | ||
|---|---|---|---|---|---|---|---|
. | . | Ψp . | Ψs . | Ψp . | Ψs . | ||
| 0 | Epidermal | 0.31±0.04 | −2.05±0.15 | 0.25±0.05 | −2.48±0.18 | ||
| Mesophyll | 0.33±0.05 | −1.91±0.08 | 0.34±0.08 | −2.16±0.14 | |||
| Bulk | NM | −1.88±0.14 | NM | −2.32±0.21 | |||
| 100 | Epidermal | 0.38±0.03 | −2.94±0.24 | 0.25±0.08 | −3.02±0.29 | ||
| Mesophyll | 0.40±0.01 | −3.05±0.03 | 0.21±0.07 | −2.82±0.23 | |||
| Bulk | NM | −3.11±0.16 | NM | −3.14±0.23 | |||
| 300 | Epidermal | 0.35±0.02 | −4.09±0.06 | 0.20±0.02 | −3.95±0.17 | ||
| Mesophyll | 0.33±0.03 | −3.88±0.23 | 0.22±0.03 | −3.88±0.24 | |||
| Bulk | NM | −3.27±0.10 | NM | −3.68±0.11 | |||
Measurements for cell Ψp and Ψs were made using a cell pressure probe and nanoliter osmometer, respectively. Bulk leaf tissue was sampled for Ψs to compare with individual cell measurements but bulk leaf tissue Ψp was not measured (NM).
Predawn and midday leaf epidermal and mesophyll cell pressure (Ψp) and solute (Ψs) potentials (mean ±SE) for Sarcobatus growing under three salinity treatments
Treatment (mM NaCl) . | Cell type or bulk . | Predawn . | . | Midday . | . | ||
|---|---|---|---|---|---|---|---|
. | . | Ψp . | Ψs . | Ψp . | Ψs . | ||
| 0 | Epidermal | 0.31±0.04 | −2.05±0.15 | 0.25±0.05 | −2.48±0.18 | ||
| Mesophyll | 0.33±0.05 | −1.91±0.08 | 0.34±0.08 | −2.16±0.14 | |||
| Bulk | NM | −1.88±0.14 | NM | −2.32±0.21 | |||
| 100 | Epidermal | 0.38±0.03 | −2.94±0.24 | 0.25±0.08 | −3.02±0.29 | ||
| Mesophyll | 0.40±0.01 | −3.05±0.03 | 0.21±0.07 | −2.82±0.23 | |||
| Bulk | NM | −3.11±0.16 | NM | −3.14±0.23 | |||
| 300 | Epidermal | 0.35±0.02 | −4.09±0.06 | 0.20±0.02 | −3.95±0.17 | ||
| Mesophyll | 0.33±0.03 | −3.88±0.23 | 0.22±0.03 | −3.88±0.24 | |||
| Bulk | NM | −3.27±0.10 | NM | −3.68±0.11 | |||
Treatment (mM NaCl) . | Cell type or bulk . | Predawn . | . | Midday . | . | ||
|---|---|---|---|---|---|---|---|
. | . | Ψp . | Ψs . | Ψp . | Ψs . | ||
| 0 | Epidermal | 0.31±0.04 | −2.05±0.15 | 0.25±0.05 | −2.48±0.18 | ||
| Mesophyll | 0.33±0.05 | −1.91±0.08 | 0.34±0.08 | −2.16±0.14 | |||
| Bulk | NM | −1.88±0.14 | NM | −2.32±0.21 | |||
| 100 | Epidermal | 0.38±0.03 | −2.94±0.24 | 0.25±0.08 | −3.02±0.29 | ||
| Mesophyll | 0.40±0.01 | −3.05±0.03 | 0.21±0.07 | −2.82±0.23 | |||
| Bulk | NM | −3.11±0.16 | NM | −3.14±0.23 | |||
| 300 | Epidermal | 0.35±0.02 | −4.09±0.06 | 0.20±0.02 | −3.95±0.17 | ||
| Mesophyll | 0.33±0.03 | −3.88±0.23 | 0.22±0.03 | −3.88±0.24 | |||
| Bulk | NM | −3.27±0.10 | NM | −3.68±0.11 | |||
Measurements for cell Ψp and Ψs were made using a cell pressure probe and nanoliter osmometer, respectively. Bulk leaf tissue was sampled for Ψs to compare with individual cell measurements but bulk leaf tissue Ψp was not measured (NM).
Accounting for the entire difference between predawn soil and leaf Ψw still requires the large predawn discrepancy between soil and xylem Ψw to be explained. In this experiment, predawn xylem Ψw was about 1 MPa more negative than soil Ψw (Table 1). This study's experimental conditions of bagging and homogeneous soil moisture around the entire root system eliminated night-time transpiration and hydraulic redistribution. Yet pressure chamber readings indicated tension remaining in the stem xylem. While similar observations of large PDD between soil and xylem Ψw have been observed in a range of species (Donovan et al., 2001) the mechanisms driving this disequilibrium remain unknown. One possibility is that this disequilibrium is caused by high concentrations of apoplastic solutes in the root intercellular spaces (Stirzaker and Passioura, 1996; Donovan et al., 1999). Data on root water potential components were not quantified in this study. Such data are needed, however, to account for the soil–xylem discrepancy that was observed and to evaluate mechanisms contributing to this difference.
Experiment 2: Leaf apoplastic solutes
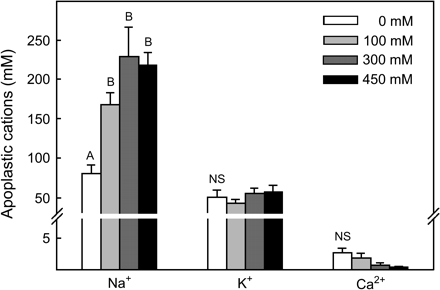
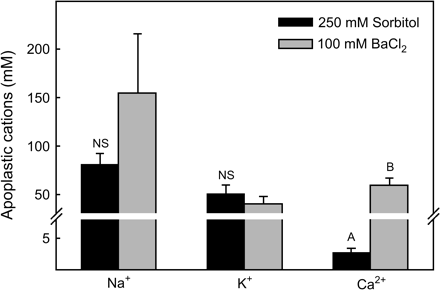
Predawn leaf apoplastic Na+ and K+ concentrations were substantial in all treatments (Fig. 1). These leaf apoplastic Na+ and K+ concentrations were 25- and 5-fold greater, respectively, than Sarcobatus xylem ion concentrations reported previously (Donovan et al., 1996). Leaf apoplastic Na+ significantly increased in Sarcobatus plants receiving additional NaCl in the watering solution (P=0.007), but this increase did not significantly differ among the 100, 300, and 450 mM salinity treatments (Fig. 1). Leaf apoplastic K+ was not significantly affected by soil salinity (P >0.05). Apoplastic Na+ and K+ did not increase significantly when leaves were infiltrated with BaCl2 compared with when leaves were infiltrated with sorbitol (Fig. 2; P >0.05) whereas Ca2+ concentrations did increase (P <0.05), suggesting that the majority of Na+ and K+ in the apoplast is soluble while the majority of Ca2+ is tightly sorbed to fixed anions on the cell walls (Mühling and Sattelmacher, 1995; Mühling and Läuchli, 2002).
Concentration of soluble Na+, K+, and Ca2+ in the leaf apoplast of Sarcobatus growing in four salinity treatments (mean ±SE, n=30–55). Each replicate measurement was made on approximately 4 g FW of leaves infiltrated with 250 mM sorbitol and centrifuged at 600 g for 6 min. Letters indicate significant differences in cation concentration (P <0.05) between salinity treatments. Note scale change across y-axis break.
Concentration of soluble (250 mM sorbitol infiltration solution) and exchangeable plus soluble (100 mM BaCl2 infiltration solution) Na+, K+, and Ca2+ in the leaf apoplast of Sarcobatus (mean ±SE, n=44–86). Values for the two different infiltration solutions were averaged across the four salinity treatments. Letters indicate significant differences in cation concentration (P <0.05) between infiltration solutions. Note scale change across y-axis break.
The relatively high leaf apoplastic K+ and Na+ under low salinity and the substantial increase in apoplastic Na+ at high salinity strongly suggests that these ions are major drivers of the low apoplastic Ψs of Sarcobatus observed in the first experiment and contribute to the large PDD observed in this species in previous greenhouse and field studies. Using the van't Hoff relation, and assuming that Na+ and K+ completely dissociate with Cl− in the apoplast, the estimated combined Ψs of these ions is about −0.6 MPa in the 0 mM NaCl treatment and as low as −1.4 MPa with 300 mM NaCl. While other apoplastic solutes such as sugars and amino acids probably further reduce Ψs of the leaf apoplast in Sarcobatus (Lohaus et al., 2001), the contribution of Na+ and K+ alone corresponds to 80% and 65% of the predawn apoplastic Ψs calculated in Experiment 1 for plants grown in the 0 and 300 mM salinity treatments, respectively. While a similar pattern of apoplastic ion concentrations may be expected for other halophytes, low predawn apoplastic Ψs observed in glycophytes, which tend to exclude Na+ at the root surface and sequester what Na+ is absorbed in roots and stems (Läuchli and Epstein, 1990), are probably due to high K+ or other solute (e.g. sugars) levels.
While the concentration of leaf apoplastic Na+ and K+ observed in Sarcobatus under predawn conditions were much higher than values typically found in crop plants during daytime conditions (Lohaus et al., 2001; Mühling and Läuchli, 2002; Wimmer et al., 2003), high apoplastic solute concentrations have been reported during daytime measurements in some systems. For example, when rice (Orzya sativa) and Suaeda maritima are grown under salinity stress, apoplastic Na+ can reach concentrations exceeding 500 mM as a result of the evaporative separation of water and Na+ in the leaf apoplast during daytime transpiration (Flowers and Yeo, 1986; Flowers et al., 1991). Similarly, Canny (1995) documented leaf apoplastic K+ levels ranging from 20 to 200 mM in Helianthus annuus during the daytime. In Helianthus, however, K+ did not appear to accumulate in the transpirational stream. Instead, high apoplastic K+ levels appeared to be a result of rapid symplastic influx and efflux of K+ and the recirculation of K+ between the leaf symplast and phloem.
These results suggest that high apoplastic ion concentrations in Sarcobatus could result from either residual build-up from daytime transpiration or ion transport between the symplast and apoplast. In the field, however, the difference between predawn leaf and xylem Ψw was similar between Sarcobatus plants that had canopies bagged overnight to eliminate night-time transpiration and plants where canopies were left unbagged, indicating similar predawn leaf apoplastic Ψs (Donovan et al., 2003). This suggests that the high apoplastic Na+ and K+ levels in Sarcobatus are not a simple function of transpiration rate but may be highly regulated by ion transport between the symplast and apoplast. If ion concentrations in this compartment are regulated, then the logical question remains whether there is a functional role for high apoplastic solute concentrations or if this trait is a simple by-product related to some other aspect of ion physiology or metabolism.
Regulation of Na+ and K+ concentrations in the leaf apoplast might be a mechanism allowing desert shrubs and halophytes to regulate Ψp in the leaf symplast (Tomos, 1988; Tomos et al., 1992). While much work has focused on understanding how plant cells increase or maintain Ψp during drought or high soil salinity by accumulation of compatible solutes and/or ions in the symplast, there has been little recognition of how high apoplastic solute concentrations might prevent the occurrence of excessive Ψp if leaves accumulate substantial quantities of osmotica. This may be the case for Sarcobatus. For example, even under low-to-moderate salinity, Na+ concentrations in leaves of Sarcobatus can exceed 8–10% in field- and greenhouse-grown plants (Richards, 1994; Donovan et al., 1997). In this experiment, this high Na+ accumulation resulted in predawn leaf Ψs ranging from −1.95 to −3.93 MPa (Table 1). If the leaf mesophyll and epidermal cells equilibrated with the Ψw of the xylem without the contribution of leaf apoplastic solutes, cell Ψp would be expected to increase from 0.32 MPa to 0.88 MPa under 0 mM NaCl while cell Ψp would increase from 0.34 MPa to 2.2 MPa under 300 mM NaCl. Turgor pressure of well-watered plant cells generally ranges from 0.3 to 1.0 MPa (Tomos, 1988). In this experiment, Ψp in mesophyll and epidermal cells averaged about 0.35 MPa and remained remarkably constant over a range of external salinities despite large decreases in cell Ψs. This suggests that, even under moderate soil salinity, the predawn Ψp that Sarcobatus would have to achieve to equilibrate with xylem Ψw without the contribution of apoplastic solutes would probably be too high for mature leaf cell function and could cause cell damage or inhibit cell–cell transport processes (Oparka and Dam, 1992; Moreshet et al., 1999). Because of the small volume of the apoplast relative to the symplast, regulation of solute concentration in this compartment may be a very efficient means to regulate Ψp. Similarly, Matthews and Shackel (2005) proposed that apoplastic solutes are important in preventing the occurrence of excessive Ψp in fleshy fruits, which accumulate substantial concentrations of sugars (–3.5 MPa for grapes) as part of normal development, even under irrigated conditions.
Conclusion
Taken together, these experiments demonstrate that ion concentrations in the leaf apoplast can be substantial in a model halophyte, greatly reducing Ψs in the leaf apoplast and creating a large disequilibrium between predawn leaf and xylem Ψw, which contrasts with classical expectations of predawn plant–soil water relations. Regulating ion concentrations in the leaf apoplast appears to be an important turgor regulation mechanism in halophytes and arid-land plants that accumulate high concentrations of osmotica in the symplast to maintain Ψp and high stomatal conductance during the daytime (Flowers et al., 1977; Romo and Haferkamp, 1989) but then need to prevent excess Ψp from occurring during the night-time when transpiration decreases and xylem Ψw increases. In addition to diurnal regulation of Ψp, rapid regulation of symplastic Ψp through apoplastic osmotic adjustments may be particularly important in ecological situations when plants that have developed high levels of compatible solutes in leaves experience rapid increases in soil Ψw. This would happen, for example, when roots of salt-accumulating arid-land riparian (e.g. Tamarisk spp.) and phreatophytic plants (e.g. Sarcobatus) access relatively fresh groundwater or experience large pulses of summer rain. This mechanism of Ψp regulation is also predicted to be important in salt-marsh species exposed to variable fresh water and salt water inputs. Further work is need, however, to understand the extent that apoplastic solutes prevent excess Ψp when water status recovers overnight and how this can vary between species and environmental conditions.
Present address: Medical Biochemistry and Genetics, Texas A&M University Health Science Center, College Station, TX 77840, USA.
Present address: Institute of Plant Nutrition, Justus-Liebig-University, Ludwigstraße 23, D-35390 Giessen, Germany.
We thank R Drenovsky, R O'Dell, and B Richards for help with plant collection and laboratory work. This research was supported by NSF grants IBN-9816670 to LA Donovan and IBN-9903004 and IBN-0416581 to JH Richards. Additional support was provided by the California Agricultural Experiment Station and an NSF Graduate Fellowship to NN Alder. JH Richards also thanks the UC Valentine Eastern Sierra Reserve for support.
References
Améglio T, Archer P.
Blake J, Ferrell WK.
Bucci SJ, Scholz FG, Guillermo G, Meinzer FG, Hinojosa JA, Hoffmann WA, Agusto CF.
Canny MJ.
Canny MJ.
Carpita NC.
Clipson NJW, Tomos AD, Flowers TJ, Jones RGW.
Cosgrove DJ, Cleland RE.
Davis SD, Mooney HA.
Donovan LA, Ehleringer JR.
Donovan LA, Grise DJ, West JB, Pappert RA, Alder NN, Richards JH.
Donovan LA, Linton MJ, Richards JH.
Donovan LA, Richards JH, Muller MW.
Donovan LA, Richards JH, Linton MJ.
Donovan LA, Richards JH, Schaber EJ.
Drenovsky RE, Richards JH.
Filella I, Peñuelas J.
Flowers TJ, Hajibagheri MA, Yeo AR.
Flowers TJ, Troke PF, Yeo AR.
Flowers TJ, Yeo AR.
Fricke W.
Fricke W, Leigh RA, Tomos AD.
Grignon C, Sentenac H.
Husken D, Steudle E, Zimmermann U.
Husted S, Schjoerring JK.
James JJ, Tiller RL, Richards JH.
Jungk AO.
Läuchli A, Epstein E.
Lohaus G, Pennewiss K, Sattelmacher B, Hussmann M, Mühling KH.
Matthews MA, Shackel KA.
Mediavilla S, Escudero A.
Meinzer FC, Moore PH.
Mitchell RJ, Zutter BR, Green TH, Perry MA, Gjerstad DH, Glover GR.
Moreshet S, Yao C, Aloni B, Karni L, Fuchs M, Stanghellini C.
Mühling KH, Läuchli A.
Mühling KH, Sattelmacher B.
Murphy R, Smith JAC.
Neter J, Wasserman W, Kutner MH.
Ogden TE, Citron MC, Pierantoni R.
Oparka KJ, Dam P.
Ourcival JM, Berger A.
Ourcival JM, Floret C, Lefloch E, Pontanier R.
Peuke AD, Schraml C, Hartung W, Rennenberg H.
Reich PB, Hinckley TM.
Richards JH.
Richards JH, Caldwell MM.
Ritchie GA, Hinckley TM.
Romo JT, Haferkamp MR.
Sellin A.
Shackel KA, Matthews MA, Morrison JC.
Snyder KA, Richards JH, Donovan LA.
Stirzaker RJ, Passioura JB.
Toft CA.
Tomos AD.
Tomos AD, Leigh RA, Palta JA, Williams JHH.
Turner NC.
Wimmer MA, Mühling KH, Läuchli A, Brown PH, Goldbach HE.




Comments