-
PDF
- Split View
-
Views
-
Cite
Cite
Ichiro Terashima, Yuko T. Hanba, Youshi Tazoe, Poonam Vyas, Satoshi Yano, Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion, Journal of Experimental Botany, Volume 57, Issue 2, January 2006, Pages 343–354, https://doi.org/10.1093/jxb/erj014
Close - Share Icon Share
Abstract
The subject of this paper, sun leaves are thicker and show higher photosynthetic rates than the shade leaves, is approached in two ways. The first seeks to answer the question: why are sun leaves thicker than shade leaves? To do this, CO2 diffusion within a leaf is examined first. Because affinity of Rubisco for CO2 is low, the carboxylation of ribulose 1,5-bisphosphate is competitively inhibited by O2, and the oxygenation of ribulose 1,5-bisphosphate leads to energy-consuming photorespiration, it is essential for C3 plants to maintain the CO2 concentration in the chloroplast as high as possible. Since the internal conductance for CO2 diffusion from the intercellular space to the chloroplast stroma is finite and relatively small, C3 leaves should have sufficient mesophyll surfaces occupied by chloroplasts to secure the area for CO2 dissolution and transport. This explains why sun leaves are thicker. The second approach is mechanistic or ‘how-oriented’. Mechanisms are discussed as to how sun leaves become thicker than shade leaves, in particular, the long-distance signal transduction from mature leaves to leaf primordia inducing the periclinal division of the palisade tissue cells. To increase the mesophyll surface area, the leaf can either be thicker or have smaller cells. Issues of cell size are discussed to understand plasticity in leaf thickness.
Why are sun leaves thicker than shade leaves?
The rate of photosynthesis of C3 leaves strongly depends on the CO2 concentration in the chloroplast, because affinity for CO2 of the primary CO2-fixing enzyme, ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco), is low (von Caemmerer and Quick, 2000). Moreover, the carboxylation of ribulose 1,5-bisphosphate (RuBP) is competitively inhibited by O2, and the oxygenation leads to the energy-consuming photorespiration processes. For C3 plants to perform efficient CO2 fixation in terms of economical use of energy and resources it is essential, therefore, to increase the conductance for CO2 diffusion from the ambient air to the chloroplasts. Since the thickness of C3 leaves is one of the important determinants in conductance for CO2 diffusion and is a key factor to answering the question why sun leaves are thicker than shade leaves, the path of CO2 diffusion is first traced from the ambient air to the chloroplast stroma.
Rubisco
Diffusion of CO2 from the ambient air to the intercellular spaces
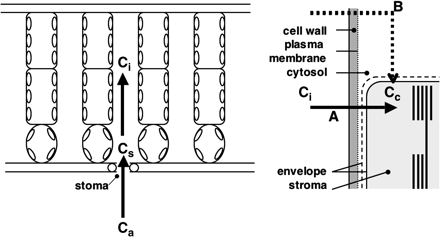
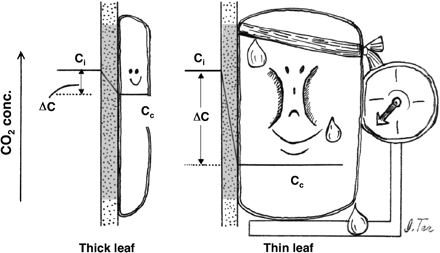
In photosynthesizing C3 leaves, which have no biochemical CO2 concentrating mechanisms, the CO2-concentration at the carboxylation site, Cc, is lower than that in the ambient air, Ca, and CO2 diffuses to the chloroplast stroma along the gradient of CO2 concentration (Fig. 1).
Diffusion of CO2 from the ambient air to the chloroplast stroma. Ca, CO2 concentration in the air; Cs, substomatal CO2 concentration; Ci, intercellular CO2 concentration; Cc, CO2 concentration in the chloroplast stroma. CO2 in the intercellular spaces is dissolved in the water at the cell wall surface and diffuses to the chloroplast stroma through the cell wall, cell membrane, cytosol, and the chloroplast envelope. Because of the large resistance to CO2 diffusion in the liquid phase, CO2 flux via pathway B (dotted line in the right panel) relative to that via pathway A (continuous line) is negligible.
The value of gc,w is relatively constant, irrespective of gs,w values, and thereby the contribution of gc would be significant when gs,w is small. Thus, when some stress factor induces the closure of stomata, calculated Cs using gleaf,w tends to be overestimated (for a review, see Evans et al., 2004). When stomata tend to close, photosynthesis can be non-uniform over the leaf. This also often causes overestimation of Cs (for reviews, see Terashima, 1992; Evans et al., 2004).
When the C3 leaf is vigorously photosynthesizing, the bulk CO2 concentration in the intercellular spaces, Ci, is lower than Cs due to the resistance to CO2 diffusion in the intercellular spaces, rias (Parkhurst, 1994). However, this resistance is usually much smaller than the stomatal resistance, rs. Except for very thick hypostomatous leaves (Parkhurst and Mott, 1990) or succulent leaves with small intercellular spaces (Maxwell et al., 1997), Ci/Cs is >0.9 for hypostomatous leaves (Terashima et al., 2001). The resistance to CO2 diffusion in the intercellular spaces in the amphistomatous leaves is one-third to one-quarter of that in the hypostomatous leaves having the same thickness (Parkhurst et al., 1988; Terashima et al, 2001). Thus, it is unlikely that rias is a major limiting factor of leaf photosynthesis, particularly in the amphistomatous leaves. From here, therefore, Cs will not be distinguished from Ci and only Ci will be used.
CO2 concentration at the carboxylation site in the chloroplast stroma, Cc, in C3 plants is even lower than Ci (Evans and von Caemmerer, 1996; Evans and Loreto, 2000). Two different methods have mainly been used to estimate Cc. One method is based on the comparison of the electron transport rate estimated by the fluorescence method and the gas exchange rate measured simultaneously. This method is simpler but relies on several assumptions. The other one, the concurrent measurement of gas exchange and carbon isotope discrimination, is more complex but gives a more accurate estimation. Cc/Ci, thus obtained for vigorously photosynthesizing C3 leaves, ranges from 0.5 to 0.75.
From the intercellular spaces to the chloroplast stroma
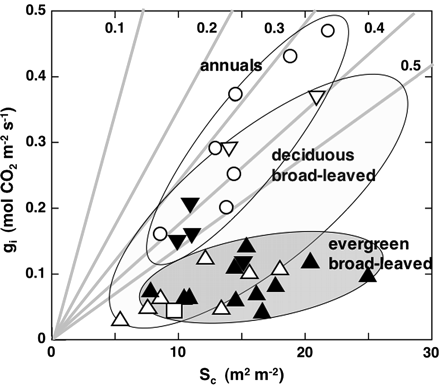
The data of conductance for CO2 diffusion from the substomatal cavity to the chloroplast stroma, gi, are plotted against the cumulated chloroplast surface area that faces the intercellular spaces on a leaf area basis, Sc (Fig. 2). The resistance to CO2 diffusion in the liquid phase for a given distance is 104 of that in the gas phase for the same distance. When pH is greater than 6, the ratio of the cumulated concentration of the inorganic carbon species relative to that of CO2 (ΣC/CO2) is >1 (Nobel, 1999). Then, the diffusion of CO2 will be faster with the increase in ΣC/CO2 if the interconversion among these inorganic carbon species is fast enough. Because cytoplasmic carbonic anhydrase activity would be sufficient (for a review, see Coleman 2000), ΣC/CO2 in the cytosol at pH 7 may be >7. Still, the flux of inorganic carbon species via the pathway like B (dotted line) in the right panel of Fig. 1 should be negligible compared with that of pathway A (continuous line) because of the large liquid phase resistance. Thus, Sc is important as the active area for CO2 diffusion to the chloroplast stroma (Laisk et al., 1970; Raven, 1970, 1977; Nobel, 1977; Evans and Loreto, 2000; Evans et al., 2004).
Internal conductance (gi) plotted against chloroplast surface area, Sc. Open circles, annual herbaceous plants; open triangles, deciduous broad-leaved trees; solid triangles, evergreen broad-leaved trees; an open square, Kalanchoë daigremontiana. Faint lines denote wall conductance per unit leaf area. gw is drawn assuming that p/τ of the cell wall is 0.1 for wall thicknesses of 0.1, 0.2, 0.3, 0.4, and 0.5 μm, respectively. See Table 1 for details of the samples.
The data in Fig. 2 that include plants of various functional types (Table 1) are, however, scattered. Among them, gi values for annual herbs such as crop species are greatest when compared at a given Sc and range from 0.2 to 0.5 mol CO2 m−2 s−1. On the other hand, those of evergreen broad-leaved trees are much lower and range from 0.03 to 0.2 mol CO2 m−2 s−1.
Among the evergreen broad-leaved trees, Citrus spp. showed greater gi than Macadamia integrifolia and the evergreen broad-leaved trees native to Japanese laurel forests (for scientific names and their authorship, see Table 1 and legend to Fig. 3). In mesic deciduous broad-leaved trees, gi values are intermediate between those of annual herbs and evergreen trees. Among them, gi for the tree species that develop leaves successively (Alnus japonica, Populus maximowiczii, and Prunus persica) tends to be greater than those for Acer spp. that flush their leaves in the spring. When the data points are grouped into annuals, including wheat and rice, deciduous broad-leaved trees, and evergreen broad-leaved trees, a tendency that gi increases with the increase in Sc may be apparent.
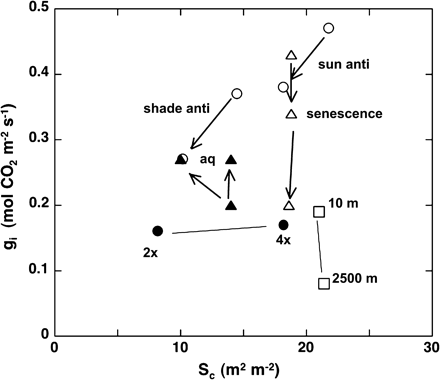
Effects of environmental manipulations, genetic manipulations, and senescence on the Sc versus gi relationship. Open circles, tobacco plants expressing antisense Rubisco small subunits (Evans et al., 1994); solid triangles, rice plants over-expressing barley aquaporin (Hanba et al., 2004); open triangles, senescing wheat leaves (Evans and Vellen, 1996); solid circles, diploid and artificially induced autotetraploid of Phlox drummondii Hook. (P Vyas et al., unpublished observation); open squares, alpine and low land Polygonum cuspidatum Sieb. et Zucc. (Kogami et al., 2001).
Mesophyll surface area, chloroplast surface area and internal conductance of species of various functional types
Species . | Smes (m2 m−2) . | Sc (m2 m−2) . | gi (mol Co2 m−2 s−1) . | Source . |
|---|---|---|---|---|
| Annuals | ||||
| Nicotiana tabacum L. (sun) | 23.7 | 21.8 | 0.470 | Evans et al., 1994 |
| N. tabacum (shade) | 17.0 | 14.5 | 0.370 | Evans et al., 1994 |
| Triticum aestivum L. | 23.5 | 18.8 | 0.430 | Evans and Vallen, 1996 |
| Oryza sativa L. | 19.0 | 14.0 | 0.200 | Hanba et al., 2004 |
| Phaseolus vulgaris L. | 14.3 | 13.0 | 0.290 | Hanba et al., 2003 |
| P. vulgaris | 16.0 | 14.5 | 0.250 | Hanba et al., 2003 |
| Phlox drummondii Hook. | 14.6 | 8.5 | 0.160 | P Vyas et al., unpublished |
| Deciduous broadleaved trees | ||||
| Acer mono Maxim. (17% sun) | 14.0 | 12.0 | 0.125 | Hanba et al., 2001 |
| Ac. mono (full sun) | 12.0 | 8.5 | 0.065 | Hanba et al., 2001 |
| Acer palmatum Thunb.(17% sun) | 8.0 | 5.5 | 0.030 | Hanba et al., 2001 |
| Ac. palmatum (full sun) | 10.0 | 7.5 | 0.052 | Hanba et al., 2001 |
| Acer rufinerve Sieb. et Zucc. (17% sun) | 8.5 | 5 | 0.047 | Hanba et al., 2001 |
| Ac. rufinerve (full sun) | 18.0 | 13.5 | 0.047 | Hanba et al., 2001 |
| Alnus japonica (Thunb.) Steud. | 20.0 | 18.0 | 0.110 | Hanba et al., 2001 |
| Populus maximowiczii A. Henry | 78.0 | 15.5 | 0.100 | Hanba et al., 2001 |
| Prunus persica L. Batsch(sun) | 30.1 | 21.0 | 0.370 | Syvetsen et al., 1995 |
| Pr. persica (shade) | 26.6 | 14.0 | 0.291 | Syvetsen et al., 1995 |
| Evergreen trees | ||||
| Quercus glauca Thunb. ex Murray | 17.0 | 10.8 | 0.066 | Hanba et al., 1999 |
| Q. glauca | 11.2 | 7.8 | 0.076 | Hanba et al., 1999 |
| Q. phillyraeoides A. Gray | 31.4 | 15.4 | 0.143 | Hanba et al., 1999 |
| Cinnamomum camphora (L.) J. Presl | 19.6 | 14.5 | 0.061 | Hanba et al., 1999 |
| Castanopsis sieboldii (Makino) Hatus. | 20.7 | 16.7 | 0.044 | Hanba et al., 1999 |
| Cas. sieboldii | 22.1 | 17.8 | 0.082 | Hanba et al., 1999 |
| Cas. sieboldii | 25.0 | 25.0 | 0.100 | Miyazawa and Terashima, 2001 |
| Ligustrum lucidum Aiton | 20.3 | 10.5 | 0.067 | Hanba et al., 1999 |
| L. lucidum | 28.0 | 14.6 | 0.113 | Hanba et al., 1999 |
| Camellia japonica L. | 41.6 | 20.5 | 0.119 | Hanba et al., 1999 |
| Cam. japonica | 32.9 | 16.2 | 0.069 | Hanba et al., 1999 |
| Citrus paradisi Macfad | 26.2 | 11.0 | 0.208 | Syvetsen et al., 1995 |
| Cit. paradisi | 24.7 | 11.0 | 0.157 | Syvetsen et al., 1995 |
| Citrus limon (L.) Burm.f. | 26.4 | 10.0 | 0.149 | Syvetsen et al., 1995 |
| Macadamia integrifolia Maiden et Betche | 24.4 | 15.0 | 0.114 | Syvetsen et al., 1995 |
| CAM | ||||
| Kalanchoë daigremontiana Hamet et Perr. | 25.4 | 9.8 | 0.050 | Maxwell et al., 1997 |
Species . | Smes (m2 m−2) . | Sc (m2 m−2) . | gi (mol Co2 m−2 s−1) . | Source . |
|---|---|---|---|---|
| Annuals | ||||
| Nicotiana tabacum L. (sun) | 23.7 | 21.8 | 0.470 | Evans et al., 1994 |
| N. tabacum (shade) | 17.0 | 14.5 | 0.370 | Evans et al., 1994 |
| Triticum aestivum L. | 23.5 | 18.8 | 0.430 | Evans and Vallen, 1996 |
| Oryza sativa L. | 19.0 | 14.0 | 0.200 | Hanba et al., 2004 |
| Phaseolus vulgaris L. | 14.3 | 13.0 | 0.290 | Hanba et al., 2003 |
| P. vulgaris | 16.0 | 14.5 | 0.250 | Hanba et al., 2003 |
| Phlox drummondii Hook. | 14.6 | 8.5 | 0.160 | P Vyas et al., unpublished |
| Deciduous broadleaved trees | ||||
| Acer mono Maxim. (17% sun) | 14.0 | 12.0 | 0.125 | Hanba et al., 2001 |
| Ac. mono (full sun) | 12.0 | 8.5 | 0.065 | Hanba et al., 2001 |
| Acer palmatum Thunb.(17% sun) | 8.0 | 5.5 | 0.030 | Hanba et al., 2001 |
| Ac. palmatum (full sun) | 10.0 | 7.5 | 0.052 | Hanba et al., 2001 |
| Acer rufinerve Sieb. et Zucc. (17% sun) | 8.5 | 5 | 0.047 | Hanba et al., 2001 |
| Ac. rufinerve (full sun) | 18.0 | 13.5 | 0.047 | Hanba et al., 2001 |
| Alnus japonica (Thunb.) Steud. | 20.0 | 18.0 | 0.110 | Hanba et al., 2001 |
| Populus maximowiczii A. Henry | 78.0 | 15.5 | 0.100 | Hanba et al., 2001 |
| Prunus persica L. Batsch(sun) | 30.1 | 21.0 | 0.370 | Syvetsen et al., 1995 |
| Pr. persica (shade) | 26.6 | 14.0 | 0.291 | Syvetsen et al., 1995 |
| Evergreen trees | ||||
| Quercus glauca Thunb. ex Murray | 17.0 | 10.8 | 0.066 | Hanba et al., 1999 |
| Q. glauca | 11.2 | 7.8 | 0.076 | Hanba et al., 1999 |
| Q. phillyraeoides A. Gray | 31.4 | 15.4 | 0.143 | Hanba et al., 1999 |
| Cinnamomum camphora (L.) J. Presl | 19.6 | 14.5 | 0.061 | Hanba et al., 1999 |
| Castanopsis sieboldii (Makino) Hatus. | 20.7 | 16.7 | 0.044 | Hanba et al., 1999 |
| Cas. sieboldii | 22.1 | 17.8 | 0.082 | Hanba et al., 1999 |
| Cas. sieboldii | 25.0 | 25.0 | 0.100 | Miyazawa and Terashima, 2001 |
| Ligustrum lucidum Aiton | 20.3 | 10.5 | 0.067 | Hanba et al., 1999 |
| L. lucidum | 28.0 | 14.6 | 0.113 | Hanba et al., 1999 |
| Camellia japonica L. | 41.6 | 20.5 | 0.119 | Hanba et al., 1999 |
| Cam. japonica | 32.9 | 16.2 | 0.069 | Hanba et al., 1999 |
| Citrus paradisi Macfad | 26.2 | 11.0 | 0.208 | Syvetsen et al., 1995 |
| Cit. paradisi | 24.7 | 11.0 | 0.157 | Syvetsen et al., 1995 |
| Citrus limon (L.) Burm.f. | 26.4 | 10.0 | 0.149 | Syvetsen et al., 1995 |
| Macadamia integrifolia Maiden et Betche | 24.4 | 15.0 | 0.114 | Syvetsen et al., 1995 |
| CAM | ||||
| Kalanchoë daigremontiana Hamet et Perr. | 25.4 | 9.8 | 0.050 | Maxwell et al., 1997 |
Mesophyll surface area, chloroplast surface area and internal conductance of species of various functional types
Species . | Smes (m2 m−2) . | Sc (m2 m−2) . | gi (mol Co2 m−2 s−1) . | Source . |
|---|---|---|---|---|
| Annuals | ||||
| Nicotiana tabacum L. (sun) | 23.7 | 21.8 | 0.470 | Evans et al., 1994 |
| N. tabacum (shade) | 17.0 | 14.5 | 0.370 | Evans et al., 1994 |
| Triticum aestivum L. | 23.5 | 18.8 | 0.430 | Evans and Vallen, 1996 |
| Oryza sativa L. | 19.0 | 14.0 | 0.200 | Hanba et al., 2004 |
| Phaseolus vulgaris L. | 14.3 | 13.0 | 0.290 | Hanba et al., 2003 |
| P. vulgaris | 16.0 | 14.5 | 0.250 | Hanba et al., 2003 |
| Phlox drummondii Hook. | 14.6 | 8.5 | 0.160 | P Vyas et al., unpublished |
| Deciduous broadleaved trees | ||||
| Acer mono Maxim. (17% sun) | 14.0 | 12.0 | 0.125 | Hanba et al., 2001 |
| Ac. mono (full sun) | 12.0 | 8.5 | 0.065 | Hanba et al., 2001 |
| Acer palmatum Thunb.(17% sun) | 8.0 | 5.5 | 0.030 | Hanba et al., 2001 |
| Ac. palmatum (full sun) | 10.0 | 7.5 | 0.052 | Hanba et al., 2001 |
| Acer rufinerve Sieb. et Zucc. (17% sun) | 8.5 | 5 | 0.047 | Hanba et al., 2001 |
| Ac. rufinerve (full sun) | 18.0 | 13.5 | 0.047 | Hanba et al., 2001 |
| Alnus japonica (Thunb.) Steud. | 20.0 | 18.0 | 0.110 | Hanba et al., 2001 |
| Populus maximowiczii A. Henry | 78.0 | 15.5 | 0.100 | Hanba et al., 2001 |
| Prunus persica L. Batsch(sun) | 30.1 | 21.0 | 0.370 | Syvetsen et al., 1995 |
| Pr. persica (shade) | 26.6 | 14.0 | 0.291 | Syvetsen et al., 1995 |
| Evergreen trees | ||||
| Quercus glauca Thunb. ex Murray | 17.0 | 10.8 | 0.066 | Hanba et al., 1999 |
| Q. glauca | 11.2 | 7.8 | 0.076 | Hanba et al., 1999 |
| Q. phillyraeoides A. Gray | 31.4 | 15.4 | 0.143 | Hanba et al., 1999 |
| Cinnamomum camphora (L.) J. Presl | 19.6 | 14.5 | 0.061 | Hanba et al., 1999 |
| Castanopsis sieboldii (Makino) Hatus. | 20.7 | 16.7 | 0.044 | Hanba et al., 1999 |
| Cas. sieboldii | 22.1 | 17.8 | 0.082 | Hanba et al., 1999 |
| Cas. sieboldii | 25.0 | 25.0 | 0.100 | Miyazawa and Terashima, 2001 |
| Ligustrum lucidum Aiton | 20.3 | 10.5 | 0.067 | Hanba et al., 1999 |
| L. lucidum | 28.0 | 14.6 | 0.113 | Hanba et al., 1999 |
| Camellia japonica L. | 41.6 | 20.5 | 0.119 | Hanba et al., 1999 |
| Cam. japonica | 32.9 | 16.2 | 0.069 | Hanba et al., 1999 |
| Citrus paradisi Macfad | 26.2 | 11.0 | 0.208 | Syvetsen et al., 1995 |
| Cit. paradisi | 24.7 | 11.0 | 0.157 | Syvetsen et al., 1995 |
| Citrus limon (L.) Burm.f. | 26.4 | 10.0 | 0.149 | Syvetsen et al., 1995 |
| Macadamia integrifolia Maiden et Betche | 24.4 | 15.0 | 0.114 | Syvetsen et al., 1995 |
| CAM | ||||
| Kalanchoë daigremontiana Hamet et Perr. | 25.4 | 9.8 | 0.050 | Maxwell et al., 1997 |
Species . | Smes (m2 m−2) . | Sc (m2 m−2) . | gi (mol Co2 m−2 s−1) . | Source . |
|---|---|---|---|---|
| Annuals | ||||
| Nicotiana tabacum L. (sun) | 23.7 | 21.8 | 0.470 | Evans et al., 1994 |
| N. tabacum (shade) | 17.0 | 14.5 | 0.370 | Evans et al., 1994 |
| Triticum aestivum L. | 23.5 | 18.8 | 0.430 | Evans and Vallen, 1996 |
| Oryza sativa L. | 19.0 | 14.0 | 0.200 | Hanba et al., 2004 |
| Phaseolus vulgaris L. | 14.3 | 13.0 | 0.290 | Hanba et al., 2003 |
| P. vulgaris | 16.0 | 14.5 | 0.250 | Hanba et al., 2003 |
| Phlox drummondii Hook. | 14.6 | 8.5 | 0.160 | P Vyas et al., unpublished |
| Deciduous broadleaved trees | ||||
| Acer mono Maxim. (17% sun) | 14.0 | 12.0 | 0.125 | Hanba et al., 2001 |
| Ac. mono (full sun) | 12.0 | 8.5 | 0.065 | Hanba et al., 2001 |
| Acer palmatum Thunb.(17% sun) | 8.0 | 5.5 | 0.030 | Hanba et al., 2001 |
| Ac. palmatum (full sun) | 10.0 | 7.5 | 0.052 | Hanba et al., 2001 |
| Acer rufinerve Sieb. et Zucc. (17% sun) | 8.5 | 5 | 0.047 | Hanba et al., 2001 |
| Ac. rufinerve (full sun) | 18.0 | 13.5 | 0.047 | Hanba et al., 2001 |
| Alnus japonica (Thunb.) Steud. | 20.0 | 18.0 | 0.110 | Hanba et al., 2001 |
| Populus maximowiczii A. Henry | 78.0 | 15.5 | 0.100 | Hanba et al., 2001 |
| Prunus persica L. Batsch(sun) | 30.1 | 21.0 | 0.370 | Syvetsen et al., 1995 |
| Pr. persica (shade) | 26.6 | 14.0 | 0.291 | Syvetsen et al., 1995 |
| Evergreen trees | ||||
| Quercus glauca Thunb. ex Murray | 17.0 | 10.8 | 0.066 | Hanba et al., 1999 |
| Q. glauca | 11.2 | 7.8 | 0.076 | Hanba et al., 1999 |
| Q. phillyraeoides A. Gray | 31.4 | 15.4 | 0.143 | Hanba et al., 1999 |
| Cinnamomum camphora (L.) J. Presl | 19.6 | 14.5 | 0.061 | Hanba et al., 1999 |
| Castanopsis sieboldii (Makino) Hatus. | 20.7 | 16.7 | 0.044 | Hanba et al., 1999 |
| Cas. sieboldii | 22.1 | 17.8 | 0.082 | Hanba et al., 1999 |
| Cas. sieboldii | 25.0 | 25.0 | 0.100 | Miyazawa and Terashima, 2001 |
| Ligustrum lucidum Aiton | 20.3 | 10.5 | 0.067 | Hanba et al., 1999 |
| L. lucidum | 28.0 | 14.6 | 0.113 | Hanba et al., 1999 |
| Camellia japonica L. | 41.6 | 20.5 | 0.119 | Hanba et al., 1999 |
| Cam. japonica | 32.9 | 16.2 | 0.069 | Hanba et al., 1999 |
| Citrus paradisi Macfad | 26.2 | 11.0 | 0.208 | Syvetsen et al., 1995 |
| Cit. paradisi | 24.7 | 11.0 | 0.157 | Syvetsen et al., 1995 |
| Citrus limon (L.) Burm.f. | 26.4 | 10.0 | 0.149 | Syvetsen et al., 1995 |
| Macadamia integrifolia Maiden et Betche | 24.4 | 15.0 | 0.114 | Syvetsen et al., 1995 |
| CAM | ||||
| Kalanchoë daigremontiana Hamet et Perr. | 25.4 | 9.8 | 0.050 | Maxwell et al., 1997 |
The main difference among these groups is the thickness of the mesophyll cell wall (δw). The δw in typical annuals, deciduous broad-leaved species, and evergreen broad-leaved species range from 0.1 to 0.2, 0.2 to 0.3, and 0.3 to 0.5 μm, respectively. Within the deciduous tree species, the flush-type species tend to show greater δw than in the successive-type species (Hanba et al., 2001, 2002). Acer rufinerve (100% light) showed a large Sc but a low gi, which is probably explained by its very large δw for a deciduous tree species, 0.25 μm for the palisade tissue cells and 0.46 μm for the spongy tissue cells.
A clonal herbaceous perennial, Polygonum cuspidatum (synonymous to Reynoutria japonica Houttuyn), is a well-known pioneer plant as the first colonizer of volcanic deserts (Adachi et al., 1996). The plants grown at 2500 m a.s.l. in a volcanic desert on Mt Fuji-san, Japan, had a thick δw of 0.35–0.42 μm, while the same species at 10 m a.s.l. had a δw of 0.22–0.29 μm. In agreement with the difference in wall thickness, gi for the plants from 2500 m was 0.076 mol m−2 s−1 while that for the plants from 10 m was 0.2 mol m−2 s−1 (Kogami et al., 2001; see Fig. 3). When autotetraploidy was artificially induced in Phlox drummondii, the leaves became thicker and Sc increased. However, at the same time, cell wall thickness increased considerably (from 0.12 to 0.24 μm), which would partly explain the absence of the increase in gi with the increase in Sc (P Vyas, unpublished observation; see Fig. 3).
Sc represents the area for CO2 dissolution and δw represents the path length for CO2 diffusion in the liquid phase. Thus, it is understandable, that gi is proportional to Sc and inversely related to δw.
The role of aquaporins
In addition to the two physical factors dealt with above, the role of aquaporins in CO2 diffusion will be highlighted. Changes in gi without marked changes in Sc and/or cell δw have been reported. For example, Evans and Vellen (1996) followed senescing wheat leaves and observed the drastic decrease in gi without marked changes in Sc (Fig. 3). Perhaps δw did not increase greatly either. Decreases in gi have been reported for plants under water stress (Flexas et al., 2002) and salt stress (Delfine et al., 1998, 1999). These studies indicate that CO2 permeability of the plasma membrane or chloroplast envelope might have changed.
Aquaporins, the most abundant proteins in plant plasma membranes, transport water molecules according to the gradient of the water potential (Maurel, 1997; Kjellbom et al., 1999). However, there are reports indicating that some animal aquaporins transport CO2 (Cooper and Boron, 1998; Nakhoul et al., 1998; Yang et al., 2000).
Terashima and Ono (2002) estimated gi using the concurrent measurements of gas exchange and fluorescence in the leaves of Vicia faba L. and Phaseolus vulgaris L. before and after the application of HgCl2, an inhibitor of most of the aquaporins, to the petiole. Because gi and hydraulic conductivity of the mesophyll cells decreased at the same concentration range of HgCl2, it is proposed that aquaporins are involved in diffusion of CO2 across the plasma membrane. Application of chloromercuribenzene sulphonate that would not permeate membranes easily gave similar results (I Terashima, Y Tazoe, V Oja, A Laisk, unpublished results). Bernacchi et al. (2002) observed a clear peak at 35–37 °C in the temperature dependence of gi measured by the fluorescence method in tobacco, which suggests the involvement of protein(s) in CO2 diffusion across the plasma membrane.
Recently, it was shown that the aquaporin 1 of Nicotiana tabacum L., expressed in Xenopus oocytes, transfers CO2 (Uehlein et al., 2003). In this study, tobacco aquaporin 1 (NtAQP1) was expressed in Xenopus oocytes and the CO2 permeability was monitored as the decrease in pH with a pH micro-electrode. Hanba et al. (2004) attempted to over-express an aquaporin of Hordeum vulgare L. (HvPIP2; 1) in rice (Oryza sativa L.). By contrast to their original expectation, they obtained plants with varying aquaporin contents. The concurrent measurement of the gas exchange and carbon isotope discrimination of these leaves revealed that gi clearly increases with the increase in aquaporin abundance.
Besides the abundance of aquaporins, conductance of CO2 through aquaporins could also be regulated, for example, by pH and/or protein phosphorylation, because water permeability has been reported to be regulated by pH (Tournaire-Roux et al., 2003), and by protein phosphorylation (Maurel, 1997; Kjellbom et al., 1999). These possibilities are to be studied.
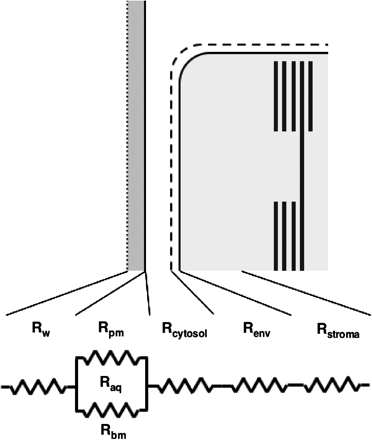
Components of the internal conductance
For the moment, let us neglect Rcytosol, because the distance between the plasma membrane and chloroplast envelope is very small in many wild plants, and the abundance of inorganic carbon species, mostly
Wall
Nobel (1999) assumed that the p/τ of the mesophyll cell wall is around 0.3. Because DC at 20 °C is not very different from the diffusion coefficient of CO2 (see below), 1.7×10−9 m2 s−1, he used pDC/τ of 5×10−10 m2 s−1.
It is known that the permeability obtained by the pressurizing technique is greater than that by the diffusion method if the material has bulky holes or pipes (Gutknecht, 1967). The p/τ value of 3, therefore, indicates that there are holes or pipes in the cell wall.
Accurate measurements of wall resistance to diffusion of water and CO2 with higher plants are needed. It should be noted that one cannot use the pressure probe method in the pressurizing mode to determine wall resistance.
Assuming p/τ =0.1, gw (see the grey lines in Fig. 2) was calculated. If p/τ is less than about 0.1, then wall conductance is an important factor determining internal conductance. Of course, p/τ values would vary considerably, because of the variation in cell wall constituents across species. However, it is proposed that the p/τ value is around 0.1 or lower, because the difference in gi among leaves having a given Sc, but from different functional groups, is roughly explained by δw.
The resistance to CO2 diffusion in the liquid phase is 104 of that in the gas phase. If p/τ is 0.1, then the wall thickness of 0.3 μm will correspond to an air layer 30 mm thick. Thus, wall resistance would be an important limiting factor of photosynthesis.
Plasma membrane
When HgCl2 was applied to the leaflets of Phaseolus vulgaris L., gi decreased by up to 60% depending on the concentration of HgCl2 (Terashima and Ono, 2002). Some aquaporins are known to be insensitive to HgCl2 (Kjellbom et al., 1999) and, therefore, a part of the residual conductance could be attributed to such insensitive aquaporins. As already mentioned above, the tobacco aquaporin (NtAQP1) has been shown to transport CO2 across the plasma membrane of Xenopus oocytes (Uehlein et al., 2003)
Hanba et al. (2004) have reported that rice leaves expressing various amounts of barley aquaporin (HvPIP2,1) showed differences in gi, the range of which corresponds to 50% of the gi in the control plants. gi in tobacco plants expressing antisense aquaporin 1 was lower than that of the control plants by 40%, while the plants over-expressing aquaporin 1 showed gi greater than that in the control plants by 30% (Flexas et al., 2004; International Photosynthesis Congress, 2004, Montreal; J Flexas, personal communication).
There are >10 different aquaporin species that are expressed in the plasma membrane of plants (Kjellbom et al., 1999). It is not known how many of these are responsible for the transport of CO2. Thus, CO2 transport activity in these aquaporins should be examined on a one by one basis. Then, the contribution of aquaporins to the overall conductance of the plasma membrane should be studied.
At this stage, only a rough estimation can be made. Let us assume that the CO2 diffusion through aquaporins in a P. vulgaris leaf is completely suppressed by HgCl2 and gi decreased from 0.3 mol m−2 s−1 to 0.12 mol m−2 s−1. If p/τ, cell wall thickness, and Sc are 0.1, 0.1 μm, and 13 m2 m−2, respectively, then gw will be 0.9 mol m−2 s−1. If it is further assumed that internal resistance is the sum of wall resistance and membrane resistance only, the membrane conductance values before and after the HgCl2 treatment are 0.45 and 0.138 mol m−2 s−1. The corresponding conductance for the unit chloroplast surface area, Gaq and Gbm is calculated to be 0.024 and 0.011 mol m−2 s−1, or 5.86×10−4 and 2.69×10−4 m s−1, respectively. These calculations may indicate that more than two-thirds of CO2 molecules transported across the plasma membrane are via aquaporins.
It should also be pointed out that the effects of changes in abundance or conductivity of aquaporins on total internal conductance is largest in annual plants with thin cell walls but smallest in the evergreen broad-leaved tree species.
In summary, gi is determined by Sc, wall thickness, and by abundance and/or conductivity of aquaporins. These aquaporins that transport CO2 may be called ‘cooporins’ to highlight CO2-porins that are co-operating with other photosynthetic components such as carbonic anhydrase.
Why are sun leaves thicker than shade leaves?
The light-saturated rate of leaf photosynthesis per unit area (Pmax) in C3 plants strongly depends on leaf nitrogen content and photosynthetic components such as Rubisco, cytochrome f, H+-ATPase, and reaction centres. Pmax is also strongly correlated with structural parameters such as leaf thickness, leaf mass per area, mesophyll surface area (Smes), and chloroplast surface area (Sc). Importance of Sc as an area for CO2 dissolution and for the CO2 diffusion pathway has been already discussed.
Let us consider the drawdown of CO2 concentration from the intercellular spaces to the stroma (ΔC=Ci–Cc) for a unit chloroplast surface area (Fig. 5). The drawdown is proportional to the flux of CO2 across the cell wall, plasma membrane, cytosol, and chloroplast envelope per unit chloroplast surface area and to the internal resistance, Ri. With the increase in the amount of Rubisco per unit chloroplast surface area, the CO2 flux increases. However, the photosynthetic rate per Rubisco decreases because Cc decreases. From the viewpoint of efficiency of Rubisco use (or nitrogen use), thicker leaves with greater Sc would be advantageous because the amount of Rubisco per Sc becomes smaller and thereby Cc would increase. On the other hand, the construction and maintenance costs of thick leaves are expensive. Also, the drawdown of CO2 concentration during the diffusion in the intercellular spaces, Cs–Ci, becomes greater with the increase in leaf thickness, which thus causes a decrease in the bulk Ci. In the latter two aspects, thick leaves are not at all advantageous.
Consequences of the difference in Rubisco content per unit chloroplast surface area. Given that the Rubisco content per leaf area is identical, thinner leaves having a smaller Sc should have more Rubisco per Sc. Then Rubisco in the thinner leaves would operate at a lower CO2 concentration.
Effects of various aspects of mesophyll structure, in particular mesophyll thickness on photosynthesis, were evaluated using a one-dimensional model of CO2 diffusion in the C3 leaf (Terashima et al., 2001). In this model, mesophyll is composed of columnar cells, the lateral surfaces of which are fully occupied by chloroplasts. When mesophyll thickness was increased, keeping the Rubisco content per leaf area constant, Sc increases and Rubisco content per Sc decreases. The model leaves are either hypostomatous or amphistomatous. The main results can be summarized as follows:Obviously, neither (i) nor (ii) explains the strong relationship between Pmax and Sc. Thus (iii) or constraints of this kind explain the strong relationships between Pmax and leaf morphological parameters such as mesophyll thickness and Sc. In other words, leaf thickness is determined as a compromise between the increase in chloroplast surface area for CO2 dissolution and the decrease in the construction and maintenance costs of the leaf. If such the economical optimum is strongly favoured, Sc/Smes can be expected to be very high. Moreover, thickness of chloroplasts or Rubisco/Sc would not vary much. Both are roughly true in nature (see below). Thus, this would be the answer to the question: why are sun leaves thicker than shade leaves?
(i) When mesophyll thickness was increased keeping the Rubisco content per leaf area constant, the rate of photosynthesis per leaf area increased at first due to the increase in Cc, attained the peak value, and then gradually decreased. The gradual decrease was due to the gradual decrease in Ci due to the increased rias. The thickness that gives the peak value was identical for the leaves with various Rubisco contents per leaf area.
(ii) The thickness that gives the maximum photosynthetic rate for the amphistomatous leaves was greater than that for the hypostomatous leaves because rias in the former were one-quarter to one-third of that in the latter leaves.
(iii) The mesophyll thickness that realizes a given photosynthetic rate per unit mesophyll thickness increased with the increase in the Rubisco content per leaf area.
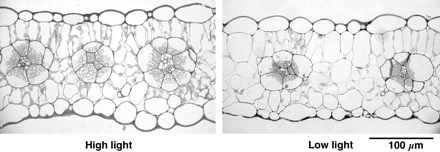
Because C4 plants have CO2 concentration mechanisms, and the CO2 fixation enzyme is located in the cytosol of mesophyll cells, the arguments above are exclusively for C3 plants. The changes in leaf thickness or mesophyll cell surface area have not been intensively studied for C4 plants. It was found recently that the thickness of the leaves of Amaranthus cruentus L., an NAD-ME-type C4 dicotyledonous plant, did not respond to growth in the light (Fig. 6; Tazoe et al., 2005). For responses of C4 photosynthesis to growth photosynthetic photon flux densities (PPFD), see the review by Sage (2006).
Sun and shade leaves of Amaranthus cruentus L. The plants were grown in a glasshouse. The midday PPFD for high-light plants was 1600 μmol m−2 s−1, while that for low-light plants was around 500 μmol m−2 s−1. High-light and low-light leaves showed a Pmax of about 30 and 15 μmol CO2 m−2 s−1, respectively (Y Tazoe, unpublished micrographs).
How do C3 sun leaves have greater Sc than shade leaves?
In many deciduous broad-leaved tree species, sun leaves have a greater Sc than shade leaves. In sun leaves, the height of the palisade tissue is greater than in shade leaves. In some cases, thickening of the palisade tissue is accompanied by periclinal cell division as well as cell elongation of the palisade tissue cells.
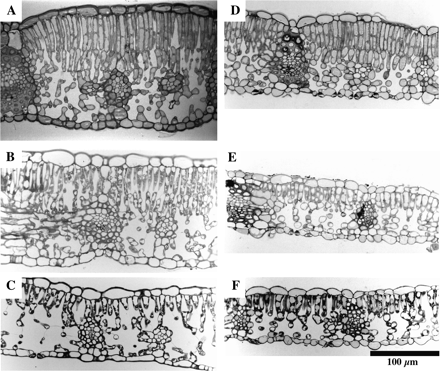
Some ecotypes of Japanese beech (Fagus crenata Blume), including those on the Pacific side, differentiate sun leaves with thick palisade tissue comprising two cell layers (T Koike, personal communication; Fig. 7). In such ecotypes, not only the number of leaves (Kozlowski and Clausen, 1966) but also the number of cell layers in the palisade tissue of these leaves is determined in the winter buds, by early winter of the year prior to leaf unfolding (Eschrich et al., 1989). When the sun-exposed branches with young expanding leaves of F. crenata were shaded by shade cloths, the resultant leaves showed intermediate characteristics: they had the palisade tissue that comprised two cell layers but the height of the palisade tissue and Pmax were lower than those in the fully exposed sun leaves (Uemura et al., 2000; A Uemura and A Ishida, personal communication). These strongly indicate that several different signals are used for the determination of characteristics of sun leaves, including multi-layered palisade tissue, greater cell height, larger Sc, higher contents of photosynthetic enzymes, and higher stomatal frequency and conductance. At least, the current-year PPFD and previous-year PPFD play different roles (Kimura et al., 1998; Uemura et al., 2000).
Sun and shade leaves of Fagus crenata Blume (A–C) and Fagus japonica Maxim. (D–F). (A) and (D) were from fully exposed shoots; (B) and (E) were from the shoots that had been exposed but were shaded from early spring until sampling in mid-summer; (C) and (F) were from the lower shaded shoots. Note that the palisade tissues of F. japonica had only one cell layer irrespective the light conditions (Uemura et al., 2000). Original micrographs of I Terashima.
Using Chenopodium album L., an annual herb, Yano and Terashima (2001) established a more sophisticated experimental system and examined the differentiation processes of sun and shade leaves. The plants were shaded in various ways and the effects of these shade treatments on the properties of the developing leaves were examined. When mature leaves were exposed to high light, the developing leaves, irrespective of their light environments, formed palisade tissue with two cell layers. On the other hand, when mature leaves were shaded, palisade tissue with one cell layer was formed. These results clearly showed that the light environment of mature leaves determined the number of cell layers in the palisade tissue of new leaves. There must be a signal transduction system that conveys the signal(s) from the mature leaves to the developing leaves (Yano and Terashima, 2001).
Yano and Terashima (2004) also conducted a detailed developmental study of sun and shade leaves of C. album. Whether the plants were grown under typical sun or shade conditions, the number of cells in the palisade tissue per leaf was almost identical. Moreover, in sun leaves, anticlinal and periclinal divisions occur almost synchronously. Thus, the signal from the mature leaves regulates the direction of cell division. In the future sun leaves, the signal probably induces periclinal division in addition to anticlinal division, while the signal from the shaded mature leaves only allows the cells to divide anticlinally (Yano and Terashima, 2004).
Yano and Terashima (2001, 2004) hypothesized that the signal is the abundance of photosynthates; when the photosynthates from mature leaves are abundant, leaves would develop into sun leaves. Currently, the effects of the sucrose content of the agar on leaf development in Arabidopsis thaliana (L.) Heynh. are being examined. There is a clear indication that the number of cell layers in the palisade tissue increases with increasing sucrose content (S Yano, H Tsukaya, personal communication).
Stomatal frequency and morphology of epidermal cells in young leaves are also regulated by the environment of the mature leaves (Lake et al., 2001; Thomas et al., 2003; Coupe et al., 2006), although the signal transduction mechanisms involved may be different from those that regulate the direction of cell division. For the palisade tissue cells to elongate, the local light environment of the developing leaves appears to be essential, although detailed studies have not been conducted.
On the other hand, chloroplast properties are mostly determined by the local light environment of the developing leaves (Terashima and Hikosaka, 1995; Yano and Terashima, 2001). It is known that there is a gradient in light environment within a leaf (Terashima and Saeki, 1983, 1985) and that chloroplasts within the leaf acclimate to their respective light environments (Terashima and Inoue, 1985a, b). When the leaves were inverted or irradiated from the bottom after their unfolding, the properties of chloroplasts changed and acclimated to their new light environments (Terashima and Takenaka, 1986; Terashima et al., 1986). Thus, acclimation of chloroplast properties to their local light environments is very plastic.
After maturation, leaves of some species respond to changes in the light environment. When grown with sufficient nutrients, most of the mesophyll cell surfaces facing the intercellular spaces are occupied by chloroplasts. However, it was shown that, for the shade leaves of Chenopodium album, unoccupied spaces are indispensable for re-acclimating to brighter light environments (Oguchi et al., 2003). When the shade leaves of C. album were exposed to a higher light for growth, an increase in Sc was observed. This increase in Sc was not accompanied by an increase in mesophyll surface area. Oguchi et al. (2005) examined shade leaves of three deciduous tree species, Betula ermanii Cam., Fagus crenata Blume, and Acer rufinerve Sieb. et Zucc. In F. crenata mesophyll surfaces were fully occupied by chloroplasts and did not increase Pmax when the plants were exposed to a higher growth PPFD. On the other hand, shade leaves of B. ermanii that had unoccupied mesophyll surfaces increased Pmax in response to the exposure. Shade leaves of A. rufinerve elongated palisade tissue cells and increased Pmax in response to the increase in growth PPFD.
In Hedera helix L., the mature palisade tissue cells divided periclinally in response to the increase in growth PPFD (Bauer and Thoni, 1988). Such division of the palisade tissue cells has not been described for other species. However, for the evergreen leaves of extended longevity, such plastic adjustment may be important. Recently, mechanisms of the sun and shade leaf differentiation were reviewed elsewhere (Terashima et al., 2005).
Plasticity of leaf structure: importance of cell size
In this review, attention was first drawn to the importance of having sufficient Sc for efficient photosynthesis. Mechanisms responsible for the regulation of Sc are also discussed. These arguments are based on an assumption that cell diameter would not change. However, in nature, the size of mesophyll cells varies greatly and the leaf can increase Sc and decrease resistance to CO2 diffusion in the intercellular spaces by decreasing cell size (Terashima et al., 2001, 2005; Miyazawa et al., 2003). The leaf with smaller cells is also mechanically tougher (Terashima et al., 2001). Actually, mesophyll cell size differs considerably across species (Terashima et al., 2001). However, leaves do not have very small cells. This could be because leaves exhibiting considerable rates of leaf area expansion, adequate heat capacitance, high efficiency of resource use, etc. have been favoured by natural selection (Terashima et al., 2001). The merits or demerits of having large and small mesophyll cells are summarized in Table 2. If the leaves have large Sc with large cells, as is found in annual herbs, the leaves should be thick. Such leaves would expand quicker, keep their shape by turgor, and have stomata on both leaf surfaces. The leaves with extended longevity tend to have small cells (Terashima et al., 2001). We mentioned previously that alpine plants tend to have smaller mesophyll cells (Terashima et al., 2005). However, their cells are not necessarily smaller than those of related lowland species (Körner, 1999). It is also worth mentioning that the mesophyll cell sizes of bonsai plants are comparable with those of plants grown normally (Körner et al., 1989). Although such leaves with small cells are mechanically tougher, area expansion would be slower. Moreover, the volume ratio of chloroplasts/nucleus in small cells might be smaller. Because N and P are important constituents of nucleic acids or proteins in nuclei as well, the cost, which may be called ‘information cost,’ would be much greater in leaves of small cells. If this informational cost is large, the nitrogen use efficiency of photosynthetic production would decrease. If this is very important, cell size would be greater in leaves under low availability of P and/or N,. There are other features such as water relationships with respect to cell size. Comprehensive studies approaching the diversity in leaf thickness based on causes and consequences of cell size should be made.
Possible effects of cell size on leaf characteristics
. | Small cells . | Large cells . |
|---|---|---|
| Leaf thickness for the same Sc | Thin | Thick |
| Mechanical strength | Strong | Weak |
| Maintenance of leaf morphology | Cell walls (armour) | Turgor (balloon) |
| Longevity | Long | Short |
| Heat capacity | Small | Large |
| Intercellular resistance to CO2 diffusion | Small | Large |
| Stomatal occurrence | Hypostomatous | Amphistomatous |
| Area expansion | Slow | Fast |
| Informational cost (nuclear N and P/chloroplast N and P) | High | Low |
. | Small cells . | Large cells . |
|---|---|---|
| Leaf thickness for the same Sc | Thin | Thick |
| Mechanical strength | Strong | Weak |
| Maintenance of leaf morphology | Cell walls (armour) | Turgor (balloon) |
| Longevity | Long | Short |
| Heat capacity | Small | Large |
| Intercellular resistance to CO2 diffusion | Small | Large |
| Stomatal occurrence | Hypostomatous | Amphistomatous |
| Area expansion | Slow | Fast |
| Informational cost (nuclear N and P/chloroplast N and P) | High | Low |
Possible effects of cell size on leaf characteristics
. | Small cells . | Large cells . |
|---|---|---|
| Leaf thickness for the same Sc | Thin | Thick |
| Mechanical strength | Strong | Weak |
| Maintenance of leaf morphology | Cell walls (armour) | Turgor (balloon) |
| Longevity | Long | Short |
| Heat capacity | Small | Large |
| Intercellular resistance to CO2 diffusion | Small | Large |
| Stomatal occurrence | Hypostomatous | Amphistomatous |
| Area expansion | Slow | Fast |
| Informational cost (nuclear N and P/chloroplast N and P) | High | Low |
. | Small cells . | Large cells . |
|---|---|---|
| Leaf thickness for the same Sc | Thin | Thick |
| Mechanical strength | Strong | Weak |
| Maintenance of leaf morphology | Cell walls (armour) | Turgor (balloon) |
| Longevity | Long | Short |
| Heat capacity | Small | Large |
| Intercellular resistance to CO2 diffusion | Small | Large |
| Stomatal occurrence | Hypostomatous | Amphistomatous |
| Area expansion | Slow | Fast |
| Informational cost (nuclear N and P/chloroplast N and P) | High | Low |
Present address: Department of Botany, University of Delhi, Delhi 110 007, India
Present address: National Institute for Basic Biology, Myodaiji-cho, Okazaki 444-8585, Japan
We thank the Society for Experimental Biology for the symposium on plant plasticity. We also thank the organizer of the symposium, Dr Owen Atkin, for his kind invitation to the symposium. Drs K Noguchi and A Antonio Suzuki, and Mr K Yoshida read an early draft and made insightful comments. IT would like to dedicate this review paper to late Dr Hiroshi Terashima, his father and a gynecologist, who passed away on 22 February 2005, to thank him for his continual encouragement.
References
Adachi N, Terashima I, Takahashi M.
Bauer H, Thoni W.
Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP.
Coleman JR.
Cooper GJ, Boron WF.
Coupe SA, Palmer BJ, LAke JA, Overy SA, Oxborough K, Woodward FI, Gray JE, Quick WP.
Cowan IR.
Delfine S, Alvino A, Villani MC, Loreto F.
Delfine S, Alvino A, Zacchini M, Loreto F.
Eschrich WR, Burchardt R, Essamah S.
Evans JR, Loreto F.
Evans JR, Terashima I, Hanba YT, Loreto F.
Evans JR, Vellen L.
Evans JR, von Caemmerer S.
Evans JR, von Caemmerer S, Setchell BA, Hudson GS.
Farquhar GD, Sharkey TD.
Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD.
Flexas J, Bota J, Escalona JM, Sampol B, Medrano H.
Gutknecht J.
Hanba YT, Kogami H, Terashima I.
Hanba YT, Kogami H, Terashima I.
Hanba YT, Miyazawa S-I, Kogami H, Terashima I.
Hanba YT, Miyazawa S-I, Terashima I.
Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M.
Kamiya N, Tazawa M, Takata T.
Kimura K, Ishida A, Uemura A, Matsumoto Y, Terashima I.
Kjellbom P, Karlsson C, Johansson I, Karlsson M, Johanson U.
Körner C, Pelaez Menendez-Riedl S, John PC.
Kogami H, Hanba YT, Kibe T, Terashima I, Masuzawa T.
Kozlowski TT, Chausen JJ.
Laisk A, Oja V, Rahi M.
Leegood RC.
Maurel C.
Maxwell C, von Caemmerer S, Evans JR.
Miyazawa S-I, Makino A, Terashima I.
Miyazawa S-I, Terashima I.
Nakhoul NL, Davis BA, Romero MF, Boron WF.
Nobel PS.
Oguchi R, Hikosaka K, Hirose T.
Oguchi R, Hikosaka K, Hirose T.
Okuda K, Ueno S, Mine I.
Parkhurst DF, Mott KA.
Parkhurst DF, Wong S-C, Farquhar GD, Cowan IR.
Raven JA.
Raven JA.
Raven JA, Farquhar GD.
Raven JA, Glidewell SM.
Roy H, Andrews TJ.
Sage RF, McKown AD.
Stahlberg R, Van Volkenburgh E.
Syvertsen JP, Lloyd J, McConchie C, Kriedemann PE, Farquhar GD.
Tazoe Y, Noguchi K, Terashima I.
Terashima I.
Terashima I, Araya T, Miyazawa S-I, Sone K, Yano S.
Terashima I, Hikosaka K.
Terashima I, Inoue Y.
Terashima I, Inoue Y.
Terashima I, Miyazawa S-I, Hanba YT.
Terashima I, Ono K.
Terashima I, Saeki T.
Terashima I, Saeki T.
Terashima I, Sakaguchi S, Hara N.
Terashima I, Takenaka A.
Thomas PW, Woodward FI, Quick WP.
Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, Bligny R, Maurel C.
Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R.
Uemura A, Ishida A, Nakano T, Terashima I, Tanabe H, Matsumoto Y.
von Caemmerer S.
von Caemmerer S, Quick WP.
Yang B, Fukuda N, Van Hoek A, Matthay MA, Ma T, Verkman AS.
Yano S, Terashima I.




Comments