-
PDF
- Split View
-
Views
-
Cite
Cite
Ana Sara Monteiro, Beth Okamura, Peter W. H. Holland, Orphan Worm Finds a Home: Buddenbrockia is a Myxozoan, Molecular Biology and Evolution, Volume 19, Issue 6, June 2002, Pages 968–971, https://doi.org/10.1093/oxfordjournals.molbev.a004155
Close - Share Icon Share
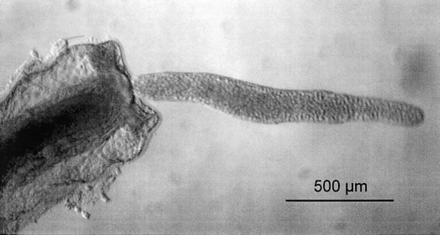
The strange wormlike Buddenbrockia plumatellae and the spore-forming myxozoans are among the most enigmatic animals known to science. Dumortier and van Beneden (1850) first noted worm-shaped parasites inside freshwater bryozoan colonies; these animals were later described by Schröder (1910,1912) and named B. plumatellae (fig. 1 ). This species is almost unique in never having been confidently assigned to an animal phylum, nor has a monotypic phylum been erected for it. Indeed, Nielsen (1995, p. 437) lists Buddenbrockia as one of the last “five enigmatic taxa.” The vermiform shape and the presence of four longitudinal muscle blocks clearly suggest a placement within the Bilateria, perhaps related to nematodes, although it should be noted that Buddenbrockia has neither a gut nor a clear central nervous system.
Myxozoans, in contrast, form plasmodia or hollow sacs in which infective spores are produced. The spores attach to new hosts using penetrative filaments everted from characteristic polar capsules. Because of their anatomical simplicity, myxozoans were long placed within the protists, but rDNA and Hox gene sequences now indicate they are metazoans (Smothers et al. 1994 ; Anderson, Canning, and Okamura 1998 ). The precise phylogenetic placement of Myxozoa within the Metazoa is controversial. If polar capsules are homologues of nematocysts, this character argues for common ancestry with Cnidaria. Such a placement was supported by a combined analysis of rDNA and morphological data (Siddall et al. 1995 ), although several other rDNA analyses have not supported this phylogenetic position (Smothers et al. 1994 ; Schlegel et al. 1996 ; Kim, Kim, and Cunningham 1999 ). In addition, myxozoans have been shown to possess central class Hox genes which is thought to be a uniquely bilaterian character (Anderson, Canning, and Okamura 1998 ; Ferrier and Holland 2001 ).
Here, we report 18S rDNA sequences of B. plumatellae from three widely separate geographical locations and demonstrate near identity with the previously reported 18S rDNA of the myxozoan Tetracapsula bryozoides. We conclude that Buddenbrockia is a myxozoan, a contention also made recently from ultrastructural studies (Okamura et al. 2002 ). We suggest that some species of Myxozoa have two alternative morphological forms, occurring as motile worms and as spore-forming sacs. Furthermore, the anatomy of Buddenbrockia provides strong support for the bilaterian affinities of the Myxozoa.
Buddenbrockia were identified by visual inspection of bryozoan colonies using a dissection microscope. Infections were detected in Plumatella repens (from Barrage de Drennec, Brittany, France), P. fungosa (from the Kennet and Avon Canal, Berkshire, UK; fig. 1 ), and Hyalinella punctata (from Cowan Lake, Ohio). The transparent body walls of bryozoan colonies allowed direct visualization of internal parasites; Buddenbrockia was the only infection observed in all cases. Portions of infected colonies (France and UK) and pure Buddenbrockia specimens released from colonies (Ohio), were placed in 99% ethanol and kept at 4°C before DNA extraction using a standard proteinase K method. Using the PCR primers of Wada (1998) , we amplified the near-complete 18S rDNA gene from P. repens and P. fungosa colonies. A shorter 1.1 kb sequence was amplified from the Ohio Buddenbrockia specimen using the same 5′ primer plus a species-specific 3′ primer. After insertion of PCR products into the T-easy vector (Promega), 20 recombinant clones were sequenced from the France sample, nine from the UK sample, and five from the pure parasite sample (Ohio). The first sample yielded 16 sequences of clear bryozoan origin and four others deduced to be Buddenbrockia. The second sample yielded three bryozoan sequences and six Buddenbrockia (98.6% identity over 1,743 nucleotides between the France and UK samples). The third sample gave one sequence closely matching the above said Buddenbrockia sequences (99.8% identity over 1,077 sites) and no bryozoan sequences; the remaining four were from a freshwater protist, Stentor sp. Commonality between the three samples from different geographical regions (and different bryozoan hosts) gives confidence in the authenticity of the Buddenbrockia sequences. The recovery of Stentor sequences adds further support because it suggests that there was successful PCR amplification from all eukaryotic cells present in the sample.
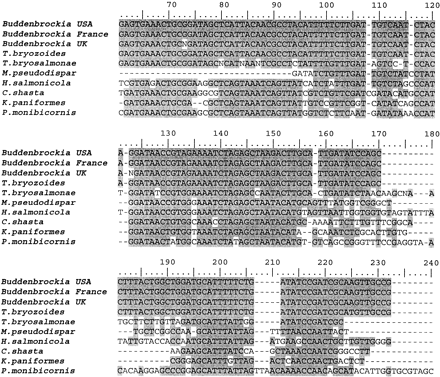
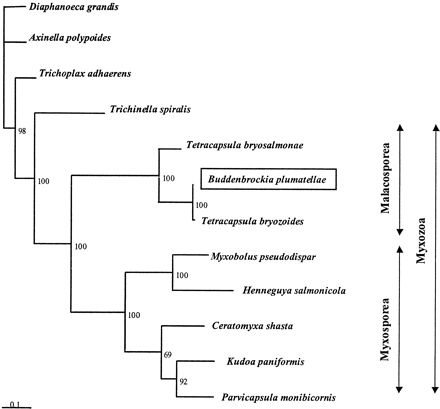
The highest BLAST match obtained for the Buddenbrockia 18S rDNA sequences was with a partial sequence reported for T. bryozoides, a myxozoan of the Class Malacosporea (Anderson, Canning, and Okamura 1999 ; Canning et al. 2000 ). To further examine the extent of this similarity, we amplified and sequenced the near-complete rDNA gene from a sample of T. bryozoides from Dinton Pastures gravel pit, Berkshire, UK. Multiple sequence alignment including T. bryozoides and other representatives of the Phylum Myxozoa revealed that Buddenbrockia has numerous diagnostic rDNA signatures of the malascoporean myxozoans. Indeed, the Buddenbrockia 18S rDNA sequences are almost identical with those of T. bryozoides over the entire gene. Strikingly, the extent of sequence identity between the three Buddenbrockia isolates is comparable with that seen between T. bryozoides and Buddenbrockia and much greater than that between T. bryozoides and the only other described member of this genus, T. bryosalmonae (the infective agent of salmonid proliferative kidney disease; Anderson, Canning, and Okamura 1999 ). Figure 2 illustrates this across a short region of the alignment (full alignment available from the authors). Confirmation of this relationship was obtained by the construction of a phylogenetic tree (fig. 3 ) using maximum-likelihood method implemented using Tree-Puzzle 5.0 (Strimmer and von Haeseler 1996 ).
These analyses indicate that Buddenbrockia and Tetracapsula are congeneric and that Buddenbrockia is a malacosporean myxozoan. It is likely that B. plumatellae and T. bryozoides are actually the same species, perhaps representing facultative stages of a complex life cycle. Our molecular results support a hypothesis made recently from ultrastructural studies of Buddenbrockia (Okamura et al. 2002 ; E. U. Canning et al., personal communication). The latter studies confirmed the presence of four longitudinal muscles blocks, as originally described by Schröder (1912) , and also revealed typical myxozoan polar capsules and spores in Buddenbrockia.
The rDNA, Hox gene, and ultrastructural data, considered together, indicate that myxozoans are part of the bilaterian branch of the animal kingdom. It is still unclear to which group of bilaterians the Myxozoa are most closely related. The presence of four longitudinal muscle blocks in Buddenbrockia suggests possible common ancestry with nematodes; however, our preliminary phylogenetic analysis of 18S rDNA suggests a more basal position within Bilateria (not shown), although long branch lengths urge caution in this conclusion. Whichever position is correct, it now seems clear that Myxozoa evolved from bilaterally symmetrical, wormlike ancestors. We suggest the following evolutionary scenario. The latest common ancestor of all myxozoans will have had both a vermiform phase of its life cycle and probably also a nonmotile stage specialized for production of infective spores. After divergence of the clade into the myxosporeans and the malacosporeans, animals in the former clade lost all vestiges of the bilaterally symmetrical vermiform body plan. Instead, they evolved into plasmodia in which the spores develop. At least some malacosporeans, in contrast, retained the capacity to develop both the worm-shaped form and a saclike spore-producing form. The latter is the usually encountered form, suggesting the motile worm is produced only facultatively. We suggest that B. plumatellae is the vermiform phase of T. bryozoides. Other species of malacosporean myxozoans may have similar motile forms, yet to be discovered.
Edward Holmes, Reviewing Editor
Address for correspondence and reprints: P. W. H. Holland, School of Animal and Microbial Sciences, The University of Reading, Whiteknights, Reading RG6 6AJ, UK. p.w.h.holland@rdg.ac.uk
Fig. 1.—A living Buddenbrockia worm exiting from a zooid of the freshwater bryozoan Plumatella fungosa. Photograph courtesy of Sylvie Tops
Fig. 2.—Partial alignment of 18S rDNA from Buddenbrockia and the myxozoans T. bryozoides, T. bryosalmonae, Myxobolus pseudodispar, Henneguya salmonicola, Kudoa paniformis, Ceratomyxa shasta, Parvicapsula monibicorni, demonstrating very high identity between Buddenbrockia and T. bryozoides. DNA sequences determined here have been deposited with GenBank and given accession numbers AY074913, AY074914, AY074915 (Buddenbrockia), and AY074916 (T. bryozoides). The complete alignment is available from the authors and on www.rubic.ac.uk/amphioxus
Fig. 3.—Molecular phylogenetic analysis of Myxozoa, including Buddenbrockia, based on an 18S rDNA alignment of 1,470 nt length, edited to remove regions of ambiguous alignment. Figures at nodes indicate quartet puzzling support values from 1,000 resamplings of the data. This tree was rooted with a choanozoan (Diaphanoeca), a sponge (Axinella) a placozoan (Trichoplax), and a nematode (Trichinella); other outgroups gave the same root position
We thank Tim Wood for the Ohio sample of Buddenbrockia, Sylvie Tops for the UK Buddenbrockia sample and for photography of the living specimen, and Elizabeth Canning for useful discussions and comments. This research was funded by Fundação para a Ciência e a Tecnologia (A.S.M. PRAXIS XXI/BD/21771/99) and by NERC (Grants NER/A/S/1999/00075 and NER/B/S/2000/00336 to B.O.).
References
———.
Canning E. U., A. Curry, S. W. Feist, M. Longshaw, B. Okamura,
Dumortier B. C., P. J. van Beneden,
Ferrier D. E. K., P. W. H. Holland,
Kim J., W. Kim, W. Cunningham,
Nielsen C.,
Okamura B., A. Curry, T. S. Wood, E. U. Canning,
Schlegel M., J. Lom, A. Stechmann, D. Bernhard, D. Leipe, I. Dykova, M. L. Sogin,
Schröder O.,
———.
Siddall M. E., D. S. Martin, D. Bridge, S. S. Desser, D. K. Cone,
Smothers J. F., C. D. von Dohlen, L. H. Smith Jr., R. D. Spall,
Strimmer K., A. von Haeseler,