-
PDF
- Split View
-
Views
-
Cite
Cite
Shukla Das, Bineeta Kashyap, Madhumita Barua, Neelima Gupta, Rumpa Saha, Lakshmi Vaid, Alok Banka, Nasal rhinosporidiosis in humans: new interpretations and a review of the literature of this enigmatic disease, Medical Mycology, Volume 49, Issue 3, April 2011, Pages 311–315, https://doi.org/10.3109/13693786.2010.526640
Close - Share Icon Share
Abstract
Rhinosporidiosis is a disease caused by Rhinosporidium seeberi which primarily affects the mucosa of the nose, conjunctiva and urethra. While it is endemic in some Asian regions, isolated cases are reported in other parts of the world as a result of the socio-cultural phenomenon of the migration. Its manifestation is a polypoid mass growing inside the affected cavity and the only treatment is surgical excision. Rhinosporidiosis is a condition which both clinicians and microbiologists should keep in mind when managing patients with nasal masses even those from non endemic areas. It is critical in such cases to follow the clinical course to ensure against recurrence of the disease. This study describes the clinical features, diagnosis, and treatment of rhinosporidiosis of the nose and nasopharynx in a series of three cases in East Delhi, India.
Introduction
Rhinosporidiosis has been known for over 100 years since its first description in Argentina. It is a rare chronic granulomatous disease endemic in some areas of Asia, such as south India and Sri Lanka, but infections have been reported to have occurred in the Americas, Europe and Africa. It is more commonly found in the tropics due to increased migration of those who have acquired rhinosporidiosis in their native Asian countries to the west [1]. Rhinosporidiosis is a chronic and localized infection of the mucus membranes and the lesions present clinically as polypoid, soft masses (sometimes pedunculated) of the nose, throat, ear, and even the genitalia in both sexes. The presumed mode of infection from the natural aquatic habitat of Rhinosporidium seeberi is through the traumatized epithelium (‘transepithelial infection’) most commonly in nasal sites [2].
The etiological agent is Rhinosporidium seeberi, whose taxonomy has been debated in the last decades since the microorganism is intractable to isolation and microbiological culture [3]. In the 1890s an apparent sporozoan parasite was described in nasal polyps and was named Coccidium seeberia after the protozoal subdivision Coccidia. Later in the early 1900s the life cycle of the organism was described and it was argued to be a fungus with a proposed name Rhinosporidium seeberi. Since then, the microbe has been considered a fungus by most microbiologists, although its taxonomy has been debated. Through phylogenetic analysis of Rhinosporidium seeberi 18S rRNA gene, this group of pathogens was originally identified by Ragan et al. as in the DRIP clade (acronym derived from Dermocystidum, rosette agent, Ichthyophonus and Psorospermium) [4]. Herr et al. replaced it with the term Mesomycetozoa (between fungi and animals) [5]. The phylogenetic distribution of this novel group of parasites suggests that the features they possibly share with early diverging animals and fungi may offer clues on the appearance of this ancestor. The phylogenetic hunt reassured that Rhinosporidium seeberi produces endosporulating cells in their infected host and the presence of chitin synthase genes reduced the divergence of its existence.
The infectious agent forms round and thick-walled sporangia in the submucosa of the affected site, varying from 10–200 mm in size, which are visible as white dots in the mucosa containing smaller ‘daughter cells’ (called ‘sporangiospores’). It can be visualized with fungal stains such as Gomori methenamine silver (GMS) and periodic acid-Schiff (PAS), as well as with standard haematoxylin and eosin (H&E) staining. The only curative approach is the surgical excision combined with electrocoagulation. The failure to propagate Rhinosporidium seeberi in vitro has prevented the determination of its in vitro sensitivity to drugs that might have clinical application. Recurrence, dissemination in anatomically close sites and local secondary bacterial infections are the most frequent complications [1].
We present here a series of three cases of rhinosporidiosis that presented in our tertiary care hospital in East Delhi, emphasizing the clinical presentation, diagnosis and management for the prevention of recurrence. To the best of our knowledge this is the first report of rhinosporidiosis from this part of Delhi.
Case presentations
Case 1
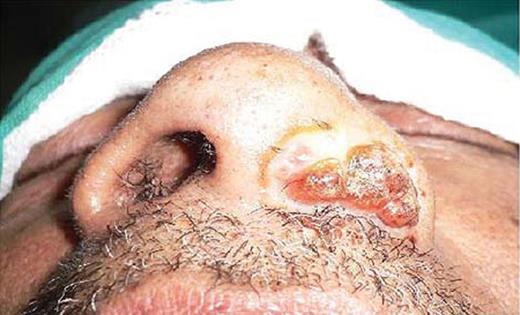
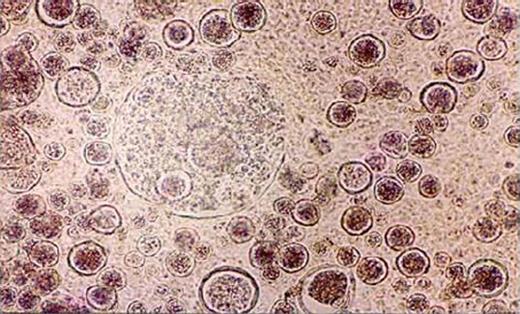
A 65-year-old male patient, native of Bihar and farmer by profession, presented to the Otorhinolaryngology Outpatients' Department (OPD) with complaints of nasal obstruction, occasional epistaxis and a painless mass in the left nostril which had been gradually protruding outside the nostril over the last 3–4 years. He gave a history of two conventional nasal surgeries for excision of rhinosporidiosis nasal mass in various peripheral hospitals in Bihar in the past, but no further details were available. He could not recall any other suggestive history of trauma or contact with contaminated water in the past. On anterior rhinoscopy examination there was an erythematous, papillomatous mass, protruding from the left nostril and almost completely occupying the cavity (Fig. 1). It was strawberry-like in appearance with occasional grayish areas. The mass was friable and had a few whitish dots on the under surface. The rest of the clinical examination was within normal limits. On the basis of history and examination, rhinosporidiosis was made as the first differential diagnosis. The mass was excised in toto and the base was cauterized and sent for microbiological and histological assessment. Examination of KOH mounts of tissue from the protruding nasal mass revealed the presence of multiple sporangia filled with endospores in various sizes and stages of development which morphologically resembled sporangia of Rhinosporidium seeberi (Fig. 2). Studies of the haematoxylin and eosin stain sections demonstrated the presence of globular cysts of varying sizes representing sporangia in different stages of development surrounded by a mixed inflammatory infiltrate comprising lymphocytes, eosinophils and neutrophils. The patient was put on Dapsone 100 mg twice daily after surgery for 3 months. The patient was discharged and was later lost to follow up.
Clinical photograph showing polypoidal mass protruding from the left nasal cavity.
KOH mount preparation of the protruding nasal mass showing multiple sporangia filled with endospores.
Case 2
A 25-year-old man, a native of Bihar, presented to the OPD of Otorhinolaryngology, of our tertiary care hospital with a history of foreign body sensation and a small painless mass in the left nostril, associated with occasional epistaxis and nasal discharge at intermittent intervals of 7–8 months duration. Although there was no history of trauma, there was a history of animal handling and contact with contaminated water (pond). He was a milkman by profession, residing in a suburban village near to the hospital. Physical examination showed a peduncular polyp, pinkish red in colour, nearly 10 mm diameter, attached by a narrow pedicle to the septum. An apparent diagnosis of rhinosporidiosis was made. The mass was resected and the base of the implant was electrocuted. The whole mass was sent for histopathological and microbiological study and the patient was placed on Dapsone, 100 mg once daily, for 6 months. The diagnosis was confirmed on histological examination. Macroscopically, the mass was pink and of fleshy consistency, studded with scattered gray-white spots on the surface. Examination of the 10% KOH preparations demonstrated the presence of multiple sporangia, approximately 200–300 mm in diameter, with a hyaline wall filled with endospores. The mass section was stained with a hematoxylin and eosin stain and studies showed multiple giant cells and lymphocytes around the mature sporangium. Microscopically, the lesion had the characteristic features of rhinosporidiosis, i.e., hyper plastic squamous epithelium, edematous fibro-connective tissue containing many thick-walled globular cysts (sporangia), which in turn contained numerous endospores. He made an uneventful recovery and on a 1-year follow up after surgery he did not have any recurrence.
Case 3
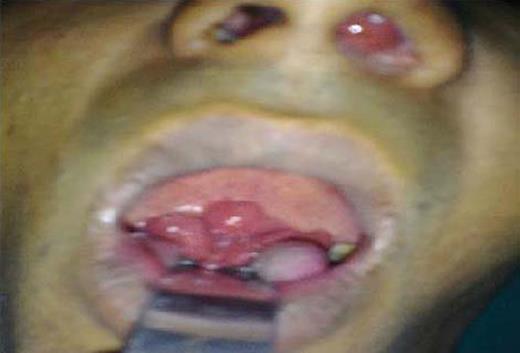
A 30-year-old man, native of Uttar Pradesh and a laborer by profession, presented with complaints of nasal obstruction, epistaxis and a change in voice of 4–5 month duration. On examination he had a polypoidal reddish congested mass in the left nostril occupying the whole nasal cavity causing expansion of the frontonasal process (Fig. 3). He also had fullness in the left lower eyelid. CT scan of the paranasal sinuses revealed an infiltrative soft tissue mass filling the nasal cavity and maxillary antrum, with extensions into the nasopharynx and palatal region (Fig. 4). Per operatively the lesion was found to involve the posterior pharyngeal wall and had attachments to the palate along with a presence in the nasopharynx and nasal cavity. Wide surgical excision with cautery to the base of these polypoidal extensions of the lesion was done. The nasal mass was sent to the Microbiology and Pathology department for mycological investigations. A diagnosis of nasal rhinosporidiosis was made on the basis of the examination of a KOH mount, haematoxylin and eosin and PAS-stained tissue sections. The patient was then put on Dapsone 100 mg daily and asked to return for follow up after 3 months.
Clinical photograph showing mass in the left nasal cavity and polypoidal masses coming from the nasopharynx region and protruding into the oropharynx.
CT Scan of paranasal sinuses (axial cut) showing soft tissue density mass filling the left nasal cavity, maxillary antrum and extending into the nasopharynx.
Discussion
This study documents and reports for the first time the incidence of nasal rhinosporidiosis in the region of East Delhi. The disease has been reported from about 70 countries with diverse geographical features [2,6]. Infrequently, isolated cases are reported in other parts of the world, mainly due to migration [1,7]. In contrast with more recent fungal infections, some aspects of the taxonomy, morphology, ontogenesis and epidemiology of those caused by Rhinosporidium seeberi remain controversial and have not been resolved. Though now related to a group of fish parasites referred to as the DRIP clade, most microbiologists initially considered it a fungus on the basis of its property to be stained by fungal stains such as GMS and PAS [1]. A study done by Silva et al. using phylogenetic analysis of the complete internal transcribed spacer sequences raises the possibility that the genus Rhinosporidium may possess multiple host-specific strains and indicates that Rhinosporidium seeberi recovered from humans could have diverged according to its geographical location [8]. These authors indicated that a combination of host specificities and resistance of Rhinosporidium to grow in culture may account for the failure to produce experimental rhinosporidiosis. Ajello and Mendoza effectively proposed its class Mesomycetozoa [9]. Recent studies done using fluorescent in-situ-hybridization techniques provide evidence that its natural habitat are water reservoirs and perhaps soil contaminated by waste [10]. In addition, other aquatic micro-organisms might be relevant to a possible synergistic action in the establishment of natural rhinosporidiosis. There are examples of such synergism between bacteria and parasites, e.g., lactobacilli with Trichomonas and Wolbachia with filarial nematodes. The class Mesomycetozoa has two orders, that is, the Dermocystida and the Ichthyophonida. In the order Dermocystida is the family Rhinosporideaceae which includes Rhinosporidium seeberi, Dermocystidium spp. and the rosette agent. In the order Ichthyophonida, the class Ichthyophonae has members with phylogenetic features in common with the genus Ichthyophonus and Psorospermium [9].
The route of transmission of Rhinosporidium remains unclear even though the presumed mode of infection from the natural aquatic habitat of Rhinosporidium seeberi is through the traumatized epithelium (‘transepithelial infection’), most commonly in nasal sites. Various modes of spread have been documented by several workers including; (i) auto-inoculation through spillage of endospores from polyps after trauma or surgery, (ii) haematogenous dissemination to distant sites, (iii) lymphatic routes, and (iv) sexual [11]. The disease is prevalent in rural settings, particularly among individuals working or in contact with contaminated soil, stagnant water (ponds, or lakes) or sand. In our case series, one patient did give a history of contact with contaminated pond water and all three cases involved patients from rural areas. Contact with feces of infected livestock or waterfowl and even working in contaminated agricultural fields has also been reported as risk factors. A curious feature in the incidence of the disease is that while several hundred people bathe in the stagnant waters, only a few develop progressive disease. This might indicate the existence of predisposing, though obscure, factors in the host. The possibility that nonspecific immune reactivity in the host, blood group and HLA types has been suggested by various investigations as possibly important in the pathogenesis of Rhinosporidium seeberi to establish an initial focus of infection [2].
As the disease has a slow course, lesions may be present for many years before the patients become symptomatic which was true in our cases. Rhinosporidiosis manifests as tumor-like masses, usually of the nasal mucosa or ocular, conjunctivae of humans and animals. Patients with nasal involvement often have masses leading to nasal obstruction or bleeding due to polyp formation and it can spread to the nasopharynx, oropharynx, and the maxillary antrum, as was evident in one of our cases. The diagnosis is established by observing the characteristic appearance of the organism in tissue biopsies and CT scans. The lesion is friable, a vascular pedunculated or sessile polyp, with a surface studded with tiny white dots due to spores beneath the epithelium, giving a ‘strawberry-like’ appearance which was evident in all of our three cases. This made the clinical diagnosis relatively easy to establish. Apart from this appearance, lesions have been associated with other areas in the head and neck region and urethral, vaginal and rectum [12]. Systemic disease is rare but can include multiple mucocutaneous, hepatic, renal, pulmonary, splenic or bone lesions, associated with fever, wasting, and even death [13].
The disease is more common in younger age groups as has been observed by various authors. An uncommon pathogen, typically restricted to tropical areas and seems to occur more in the younger age group, more so in men, as this group is frequently occupationally active (agriculturists, sand workers, divers etc). Less outdoor activity and less chance of contact with animals could explain fewer incidences among women [14]. This finding was evident in our series of cases as two of the three cases were in young adults and all three were males. In addition, Indian social, cultural habits and the custom of bathing in open ponds expose individuals to several innocuous water-borne organisms. The epidemiology of rhinosporidiosis still remains unclear and the phylogenetic relationship of its life cycle creates difficulties in understanding the actual incidence of infection and the populations of patients at risk. Many investigations are therefore needed to understand whether rhinosporidiosis is acquired in particular communities or if unrecognized factors exist that may explain the emerging epidemiology of this infection.
With no significant travel history to the known endemic states of India for rhinosporidiosis, nor having any contact with infected patients as per the history given by them, we presumed that the patients in our cases acquired the infection locally. This is truer for the first two cases due to either occupational predisposition to the disease or the patients' history of contact with a contaminated pond. To clearly say whether these are imported cases from endemic areas or are cases detected in newer geographical areas due to the expansion of the ecological niche of Rhinosporidium seeberi, is difficult at this stage due to various reasons. Firstly, elaborate and extensive research is needed to identify the niches of Rhinosporidium seeberi in different geographical areas. Secondly, in Delhi, due to constant migration of labor from neighboring Indian states where the disease is endemic, the possibility of such infections can never be ruled out. In addition, inadequate diagnostic infrastructure in many states of our country could contribute to misdiagnosis or under diagnosis of this disease. Moreover, given the modern day propensity for travel and the ability of these agents to remain dormant for years, infections acquired by patients in restricted geographic regions can manifest symptoms outside of that area. Whether a genetic predisposition or merely increased exposure in the environment leads to infection is unclear. Further, natural animal hosts, soil habitats or water reservoirs could be analyzed to identify the ecological density of Rhinosporidium seeberi.
Spontaneous regression of rhinosporidial growths has been noted in animals and in humans but is rare. Therefore, medical and/or surgical intervention is necessary. Wide local surgical excision with electro-coagulation of the base of the lesions is the treatment of choice to reduce the risk of recurrence, though this may be associated with significant morbidity due to hemorrhage and nasal septal perforation. So, limited surgical excision and adjuvant medical therapies, including antifungals such as griseofluvin and amphotericin B, trimethoprim-sulphadiazine, and sodium stibogluconate have been tried with varied success [6,15–17]. All drugs were endospore-static rather than endosporicidal. Data on antimicrobial drug resistance in Rhinosporidium seeberi is lacking. The strains obtained from human and animal rhinosporidiosis have shown genetic variations which might explain the variation of responses to some drugs [17]. The only drug appearing to have clinical promise is Dapsone [18]. It arrests the maturation of sporangia and promotes fibrosis in the stroma, when used as an adjunct to surgery [2]. It could therefore be expected that presurgical Dapsone would minimize both the hemorrhage by its promotion of fibrosis, as well as preventing the colonization and infection of new sites after the release of endospores from the surgically traumatized polyps [17]. Laser and endoscopic excision promises to be the mainstream treatment of nasal/nasopharyngeal rhinosporidiosis in the future [6]. All our patients had complete excision with wide surgical margins and cautery of the base of the lesion and all of them were treated subsequently with Dapsone. As far as recurrence of the disease is concerned we presume that only our first case was a recurrent case as he gave a history which included two previous surgeries in a peripheral hospital in Bihar for the same condition.
In conclusion, in a non-endemic areas like Delhi, India, rhinosporidiosis is uncommon, may pose a diagnostic challenge. However, with a significant proportion of the migrant population from the endemic states, it is likely that it will be observed more frequently in future. It is thus prudent for both clinicians and microbiologists to keep this condition in mind when managing patients with nasal masses even from non-endemic areas. Moreover, it will be very crucial to follow in the next few years the clinical course of these patients to exclude the possibility of recurrence of the lesion, which usually occurs after an extended time period, to evaluate the best treatment for this infection. Nevertheless, rhinosporidiosis continues to be an enigma and a large number of further studies from endemic and non-endemic areas are needed. To the best of our knowledge, our case series is one of the few nasal and sino-nasopharyngeal rhinosporidiosis reported from Delhi and the first from East Delhi.
Declaration of interest: The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper.
Source of funding: None.
References
This paper was first published online on Early Online on 19 October 2010.