-
PDF
- Split View
-
Views
-
Cite
Cite
Pei-Lun Sun, Chao-An Mu, Chi-Chen Fan, Yun-Chen Fan, Jer-Ming Hu, Yu-Ming Ju, Cat favus caused by Microsporum incurvatum comb. nov.: The clinical and histopathological features and molecular phylogeny, Medical Mycology, Volume 52, Issue 3, April 2014, Pages 276–284, https://doi.org/10.1093/mmy/myt023
Close - Share Icon Share
Abstract
Favus is a distinctive form of infection that is caused by exclusively dermatophytes. Its clinical presentation is characterized by scutula, which are concave, thick fungal crusts. The best-known examples of human scalp favus are caused by Trichophyton schoenleinii and those of mouse favus are caused by T. quinckeanum. However, other dermatophytes, such as T. violaceum, T. verrucosum, Microsporum audouinii, M. gallinae, M. gypseum, and M. canis, have been reported sporadically to cause favic lesions. Favus on cats has rarely been mentioned in the literature, and the pathogens with which it has been associated are, for the most part, unknown. Here, we examine four cat favus cases, focusing on clinical presentations and histopathological features. In all cases the etiologic agent was identified as M. incurvatum based on its morphological characteristics and sequences of internal transcribed spacers (ITS) of nuclear ribosomal DNA. Phylogenetic analysis using the neighbor-joining method, which is based on ITS, showed that these four isolates belonged to two strains of M. incurvatum; one strain was a new combination from the basionym Nannizzia incurvata.
Introduction
Favus is a distinctive dermatophyte infection that is characterized by a thick, concave, disc-like fungal crust referred to as scutula. In human scalp favus, the destructive invasion pattern of the etiologic agents may cause permanent hair loss and result in scarring alopecia. Trichophyton schoenleinii is the most frequently reported causative dermatophyte. Once prevalent throughout most of the world, its distribution has decreased due to improvements in living conditions and personal hygiene. Current endemics of T. schoenleinii infection occur mainly in China, Nigeria, and Iran [1]. However, other dermatophytes, including Microsporum audouinii, M. gypseum, M. canis, T. violaceum, and T. verrucosum, have also been reported to cause favic lesions on the scalp as well as glabrous skin in humans [1,2].
Dermatophytes also cause favic lesions in animals. The best known is mouse favus, a disease of field mice caused by T. quinckeanum in many agricultural countries, especially in Eastern Europe. From 1945 to 1960 there was a mouse favus epidemic on the Great Hungarian Plain. A large number of mice were infected by this fungus and transmitted it through their interactions with humans and other small animals [3]. M. gallinae can cause favus in avians. In particular, gallinaceous birds have been reported sporadically in Brazil [4], Costa Rica [5], the United States [6], and recently Japan [7].
Dermatophyte infections are common in cats, but rarely in the form of favus. A small number of cat favus cases have been described [3,8–12], but isolation and identification of causative agents were performed infrequently [11,12]. In this study, we describe four cases of cat favus found at Huai-An Animal Hospital, Taitung, Taiwan (Table 1), including the clinical presentations and histopathological features. Our phylogenetic study, which is based on sequences of nuclear internal transcribed spacers (ITS), revealed that the pathogens belong to two strains of M. incurvatum.
Summary of cat favus cases found in Taiwan.
| Case . | Breed . | Age . | Sex . | Lesion sites . | Treatment . | Outcome . | Pathogen . | Strain No. . |
|---|---|---|---|---|---|---|---|---|
| number . | . | . | . | . | . | . | . | . |
| 1 | Mixed | 2 mo | Male | Right ear | Topical terbinafine | No follow-up | Microsporum | MCCF 108.07 |
| (external aspect) | ointment | incurvatum | ||||||
| 2 | Mixed | 2 mo | Female | Both ears (inner aspect | Griseofulvin 25 mg/kg | Improved | M. incurvatum | MCCF 1687 |
| of right ear and external | twice daily for 2 weeks | |||||||
| aspect of left ear) | ||||||||
| 3 | Mixed | 2 mo | Male | Right ear (external aspect) | Griseofulvin 25 mg/kg | Improved | M. incurvatum | MCCF 1733 |
| twice daily for 4 weeks | ||||||||
| 4 | Mixed | 2 mo | Male | Right hind leg | No | Death, cause | M. incurvatum | MCCF 1752 |
| unknown |
| Case . | Breed . | Age . | Sex . | Lesion sites . | Treatment . | Outcome . | Pathogen . | Strain No. . |
|---|---|---|---|---|---|---|---|---|
| number . | . | . | . | . | . | . | . | . |
| 1 | Mixed | 2 mo | Male | Right ear | Topical terbinafine | No follow-up | Microsporum | MCCF 108.07 |
| (external aspect) | ointment | incurvatum | ||||||
| 2 | Mixed | 2 mo | Female | Both ears (inner aspect | Griseofulvin 25 mg/kg | Improved | M. incurvatum | MCCF 1687 |
| of right ear and external | twice daily for 2 weeks | |||||||
| aspect of left ear) | ||||||||
| 3 | Mixed | 2 mo | Male | Right ear (external aspect) | Griseofulvin 25 mg/kg | Improved | M. incurvatum | MCCF 1733 |
| twice daily for 4 weeks | ||||||||
| 4 | Mixed | 2 mo | Male | Right hind leg | No | Death, cause | M. incurvatum | MCCF 1752 |
| unknown |
Summary of cat favus cases found in Taiwan.
| Case . | Breed . | Age . | Sex . | Lesion sites . | Treatment . | Outcome . | Pathogen . | Strain No. . |
|---|---|---|---|---|---|---|---|---|
| number . | . | . | . | . | . | . | . | . |
| 1 | Mixed | 2 mo | Male | Right ear | Topical terbinafine | No follow-up | Microsporum | MCCF 108.07 |
| (external aspect) | ointment | incurvatum | ||||||
| 2 | Mixed | 2 mo | Female | Both ears (inner aspect | Griseofulvin 25 mg/kg | Improved | M. incurvatum | MCCF 1687 |
| of right ear and external | twice daily for 2 weeks | |||||||
| aspect of left ear) | ||||||||
| 3 | Mixed | 2 mo | Male | Right ear (external aspect) | Griseofulvin 25 mg/kg | Improved | M. incurvatum | MCCF 1733 |
| twice daily for 4 weeks | ||||||||
| 4 | Mixed | 2 mo | Male | Right hind leg | No | Death, cause | M. incurvatum | MCCF 1752 |
| unknown |
| Case . | Breed . | Age . | Sex . | Lesion sites . | Treatment . | Outcome . | Pathogen . | Strain No. . |
|---|---|---|---|---|---|---|---|---|
| number . | . | . | . | . | . | . | . | . |
| 1 | Mixed | 2 mo | Male | Right ear | Topical terbinafine | No follow-up | Microsporum | MCCF 108.07 |
| (external aspect) | ointment | incurvatum | ||||||
| 2 | Mixed | 2 mo | Female | Both ears (inner aspect | Griseofulvin 25 mg/kg | Improved | M. incurvatum | MCCF 1687 |
| of right ear and external | twice daily for 2 weeks | |||||||
| aspect of left ear) | ||||||||
| 3 | Mixed | 2 mo | Male | Right ear (external aspect) | Griseofulvin 25 mg/kg | Improved | M. incurvatum | MCCF 1733 |
| twice daily for 4 weeks | ||||||||
| 4 | Mixed | 2 mo | Male | Right hind leg | No | Death, cause | M. incurvatum | MCCF 1752 |
| unknown |
Case histories and clinical presentations
Case 1
A 2-month-old mixed-breed male stray kitten was brought to the veterinary clinic with a skin lesion of unknown duration. Physical examination revealed a waxy, yellow scutulum surrounded by broken hairs adherent to the right ear. After the scutulum was carefully removed, a figurate ulcerated base was revealed. Microscopic examination of the waxy crust after it was pretreated with 20% potassium hydroxide (KOH) revealed many arthroconidia and hyaline hyphae. Wood's light examination was negative, and the patient was treated with topical terbinafine ointment, but did not return for any follow-up visits.
Case 2
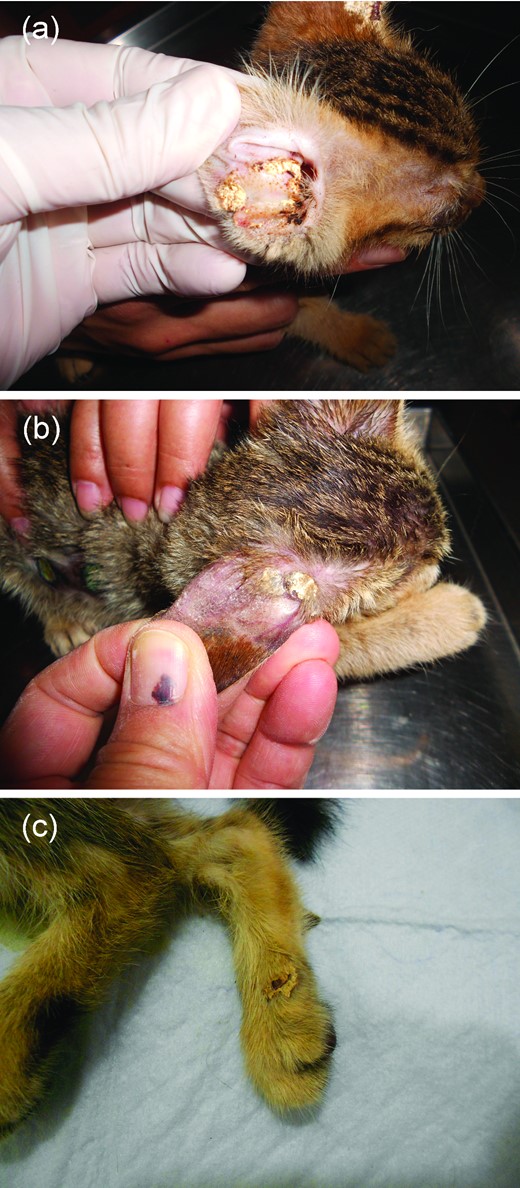
A 2-month-old mixed-breed female stray kitten was brought to the veterinary clinic for a routine health examination before being adopted. She was found to be thin but active, and her appetite was good. After a thorough examination, the kitten was diagnosed to have chemosis and ulceration at the right eye, nasal discharge, and skin lesions on both ears. Tetracycline ophthalmic ointment was prescribed for the corneal ulcer and sulfamethoxazole (5 mg/kg) was prescribed for the bacterial rhinitis. Relative to the ear lesions, yellow scutula arranged in an annular fashion were noted on the external aspect of the left ear, and two large thick scutula with eroded borders and peripheral hair loss were noted on the internal aspect of the right ear (Fig. 1a). Microscopic examination of the scutula after pretreatment with KOH showed massive hyaline hyphae and fragmented arthroconidia. Griseofulvin (25 mg/kg) twice a day was prescribed for the skin lesions; the scutula resolved and only large hair-loss patches were noted on the ears after two weeks of treatment. The owner did not bring the patient back to the clinic for follow-up thereafter.
Clinical pictures of favic scutula found in case 2 (a), case 3 (b) and case 4 (c). This Figure is reproduced in color in the online version of Medical Mycology.
Case 3
A 2-month-old mixed-breed male stray kitten with diarrhea lasting a few days was seen at the veterinary clinic. Coccidiosis was diagnosed, and sulfamethoxazole (5 mg/kg) was prescribed. The general appearance of his skin was normal at the time of initial presentation. However, 1 week later, when the kitten was brought back for follow-up related to the diarrhea, the caretaker indicated that the kitten had abnormalities on one ear. Clinically, there were two adjacent round (approximately 0.5 cm in diameter), yellow scutula on the external aspect of the right ear. Surrounding the scutula was a large hair-loss patch that involved approximately three-quarters of the ear surface (Fig. 1b). Fungal elements were microscopically observed in the scutulum specimens. One scutulum, which was completely removed with a pair of forceps, was subjected to histopathological examination and fungal culturing. The kitten was negative for feline leukemia virus and feline immunodeficiency virus. Oral griseofulvin, 25 mg/kg twice a day for 4 weeks, was prescribed for the fungal infection, which gradually resolved.
Case 4
A 2-month-old mixed-breed male stray kitten was found dying on the street and brought to the veterinary clinic for treatment. Upon arrival, the kitten was suffering from hypothermia (36.9°C) and dehydration and died 1 h later despite emergent management. No autopsy was performed, and the cause of death remains unknown. A skin lesion on his right hind leg was noted incidentally during the physical examination. It was a thick, yellow favic scutulum with a waxy surface (Fig. 1c). The lesion was composed of massive numbers of hyaline arthroconidia and hyaline hyphae, as seen by microscopy. The diseased skin was excised and subjected to histopathological examination and fungal culturing.
Materials and methods
Histopathological studies
Specimens taken from cases 3 and 4 were subjected to histopathological evaluation. In case 3, the favus lesion on the right ear was removed by forceps; in case 4, the whole favus lesion was excised from the right hind leg with a scalpel. The specimens were fixed with formaldehyde and further processed in the Department of Pathology, Macaky Memorial Hospital, Taipei, Taiwan.
Mycological studies
Specimens taken from favus lesions for all cases were inoculated onto Sabouraud glucose agar and Mycosel agar slants (BD, Maryland, USA) and incubated at room temperature. A portion from each developing colony was subcultured onto a 9-cm plastic plate that contained potato dextrose agar. Pure cultures were subjected to morphological examinations and subsequent molecular studies.
DNA extraction, amplification, and sequencing
Mycelia were prepared from pure cultures of all isolates; the procedures for this and for DNA extraction have been described previously [13]. The internal transcribed spacer regions and 5.8S ribosomal DNA (ITS1-5.8S-ITS2) were amplified with the primer pair ITS1/ITS4. The polymerase chain reaction (PCR) condition followed that of Hsieh et al. [14], with minor modifications. The PCR products were purified and then sequenced with the ABI Prism model 3730 ×1 DNA analyzer (Applied Biosystems, Foster City, CA, USA).
Phylogenetic study
Included in the phylogenetic analysis were sequences of Microsporum species from the four isolates obtained from cat favus reported here and mating strains of the following Microsporum species: M. incurvatum (CBS 172.64, CBS 173.64, M. gypseum (CBS 170.64, CBS 171.64), and M. fulvum (CBS 167.64, CBS 168). In addition, 13 sequences of M. incurvatum and 1 of M. gypseum obtained from GenBank were included in the analyses. Details of the fungal isolates/strains and the fungal sequences obtained from GenBank are listed in Table 2. MEGA software, version 5.10 [15], was used for alignment and phylogenetic analysis. CBS 511.73 Trichophyton erinacei was used as an outgroup. Neighbor-joining trees were generated for ITS1 only and ITS1-5.8S-ITS2. Maximum composite likelihood was used as the substitution model.
Strains/isolates and sequences used for analysis in this study.
| Name used in this study . | Original name . | Strain number . | Source . | Location . | GenBank accession number . |
|---|---|---|---|---|---|
| Microsporum incurvatum | MCCF 108.07 | Cat, favus | Taitung, Taiwan | KC784359 | |
| M. incurvatum | MCCF 1687 | Cat, favus | Taitung, Taiwan | KC784360 | |
| M. incurvatum | MCCF 1733 | Cat, favus | Taitung, Taiwan | KC784361 | |
| M. incurvatum | MCCF 1752 | Cat, favus | Taitung, Taiwan | KC784362 | |
| M. incurvatum | Arthroderma incurvatum | CBS 172.64 | Human hair | United Kingdom | KC784356 |
| M. incurvatum | A. incurvatum | CBS 173.64 | Human skin | United Kingdom | KC784357 |
| M. incurvatum | A. incurvatum | KMU 2981 | Human skin | Nara, Japan | AB613251 |
| M. incurvatum | A. incurvatum | KMU 2982 | Human skin | Wakayama, Japan | AB613252 |
| M. incurvatum | A. incurvatum | KMU 2986 | Human skin | Oita, Japan | AB613253 |
| M. incurvatum | A. incurvatum | KMU 2987 | Human skin | Oita, Japan | AB613254 |
| M. incurvatum | A. incurvatum | IFM 41290 | Human | Nagasaki, Japan | AB193689 |
| M. incurvatum | A. incurvatum | IFM 41067 | Human | Japan | AB193685 |
| M. incurvatum | A. incurvatum | IFM 46926 | Human | Amami, Japan | AB193700 |
| M. incurvatum | A. incurvatum | IFM 5296 | Human | Japan | AB193672 |
| M. incurvatum | A. incurvatum | IFM 46793 | Human | Peking, China | AB193697 |
| M. incurvatum | A. incurvatum | IFM 59508 | Human skin | Sri Lanka | AB613257 |
| M. incurvatum | A. incurvatum | IFM 41133 | Human | Brazil | AB193687 |
| M. incurvatum | A. incurvatum | IFM 46456 | Unknown | Costa Rica | AB613256 |
| M. incurvatum | A. incurvatum | IFM 46457 | Unknown | Costa Rica | AB613258 |
| M. gypseum | A. gypseum | SM 8377 | Human | Japan | AB593398 |
| M. gypseum | A. gypseum | CBS 170.64 | Human skin | United Kingdom | KC784354 |
| M. gypseum | A. gypseum | CBS 171.64 | Soil | Australia | KC784355 |
| M. fulvum | A. fulvum | CBS 167.64 | Soil | Hungary | KC771283 |
| M. fulvum | A. fulvum | CBS 168.64 | Soil | Hungary | KC784353 |
| Trichophyton erinacei | Trichophyton erinacei | CBS 511.73 | Hedgehog | New Zealand | KC784358 |
| Name used in this study . | Original name . | Strain number . | Source . | Location . | GenBank accession number . |
|---|---|---|---|---|---|
| Microsporum incurvatum | MCCF 108.07 | Cat, favus | Taitung, Taiwan | KC784359 | |
| M. incurvatum | MCCF 1687 | Cat, favus | Taitung, Taiwan | KC784360 | |
| M. incurvatum | MCCF 1733 | Cat, favus | Taitung, Taiwan | KC784361 | |
| M. incurvatum | MCCF 1752 | Cat, favus | Taitung, Taiwan | KC784362 | |
| M. incurvatum | Arthroderma incurvatum | CBS 172.64 | Human hair | United Kingdom | KC784356 |
| M. incurvatum | A. incurvatum | CBS 173.64 | Human skin | United Kingdom | KC784357 |
| M. incurvatum | A. incurvatum | KMU 2981 | Human skin | Nara, Japan | AB613251 |
| M. incurvatum | A. incurvatum | KMU 2982 | Human skin | Wakayama, Japan | AB613252 |
| M. incurvatum | A. incurvatum | KMU 2986 | Human skin | Oita, Japan | AB613253 |
| M. incurvatum | A. incurvatum | KMU 2987 | Human skin | Oita, Japan | AB613254 |
| M. incurvatum | A. incurvatum | IFM 41290 | Human | Nagasaki, Japan | AB193689 |
| M. incurvatum | A. incurvatum | IFM 41067 | Human | Japan | AB193685 |
| M. incurvatum | A. incurvatum | IFM 46926 | Human | Amami, Japan | AB193700 |
| M. incurvatum | A. incurvatum | IFM 5296 | Human | Japan | AB193672 |
| M. incurvatum | A. incurvatum | IFM 46793 | Human | Peking, China | AB193697 |
| M. incurvatum | A. incurvatum | IFM 59508 | Human skin | Sri Lanka | AB613257 |
| M. incurvatum | A. incurvatum | IFM 41133 | Human | Brazil | AB193687 |
| M. incurvatum | A. incurvatum | IFM 46456 | Unknown | Costa Rica | AB613256 |
| M. incurvatum | A. incurvatum | IFM 46457 | Unknown | Costa Rica | AB613258 |
| M. gypseum | A. gypseum | SM 8377 | Human | Japan | AB593398 |
| M. gypseum | A. gypseum | CBS 170.64 | Human skin | United Kingdom | KC784354 |
| M. gypseum | A. gypseum | CBS 171.64 | Soil | Australia | KC784355 |
| M. fulvum | A. fulvum | CBS 167.64 | Soil | Hungary | KC771283 |
| M. fulvum | A. fulvum | CBS 168.64 | Soil | Hungary | KC784353 |
| Trichophyton erinacei | Trichophyton erinacei | CBS 511.73 | Hedgehog | New Zealand | KC784358 |
Strains/isolates and sequences used for analysis in this study.
| Name used in this study . | Original name . | Strain number . | Source . | Location . | GenBank accession number . |
|---|---|---|---|---|---|
| Microsporum incurvatum | MCCF 108.07 | Cat, favus | Taitung, Taiwan | KC784359 | |
| M. incurvatum | MCCF 1687 | Cat, favus | Taitung, Taiwan | KC784360 | |
| M. incurvatum | MCCF 1733 | Cat, favus | Taitung, Taiwan | KC784361 | |
| M. incurvatum | MCCF 1752 | Cat, favus | Taitung, Taiwan | KC784362 | |
| M. incurvatum | Arthroderma incurvatum | CBS 172.64 | Human hair | United Kingdom | KC784356 |
| M. incurvatum | A. incurvatum | CBS 173.64 | Human skin | United Kingdom | KC784357 |
| M. incurvatum | A. incurvatum | KMU 2981 | Human skin | Nara, Japan | AB613251 |
| M. incurvatum | A. incurvatum | KMU 2982 | Human skin | Wakayama, Japan | AB613252 |
| M. incurvatum | A. incurvatum | KMU 2986 | Human skin | Oita, Japan | AB613253 |
| M. incurvatum | A. incurvatum | KMU 2987 | Human skin | Oita, Japan | AB613254 |
| M. incurvatum | A. incurvatum | IFM 41290 | Human | Nagasaki, Japan | AB193689 |
| M. incurvatum | A. incurvatum | IFM 41067 | Human | Japan | AB193685 |
| M. incurvatum | A. incurvatum | IFM 46926 | Human | Amami, Japan | AB193700 |
| M. incurvatum | A. incurvatum | IFM 5296 | Human | Japan | AB193672 |
| M. incurvatum | A. incurvatum | IFM 46793 | Human | Peking, China | AB193697 |
| M. incurvatum | A. incurvatum | IFM 59508 | Human skin | Sri Lanka | AB613257 |
| M. incurvatum | A. incurvatum | IFM 41133 | Human | Brazil | AB193687 |
| M. incurvatum | A. incurvatum | IFM 46456 | Unknown | Costa Rica | AB613256 |
| M. incurvatum | A. incurvatum | IFM 46457 | Unknown | Costa Rica | AB613258 |
| M. gypseum | A. gypseum | SM 8377 | Human | Japan | AB593398 |
| M. gypseum | A. gypseum | CBS 170.64 | Human skin | United Kingdom | KC784354 |
| M. gypseum | A. gypseum | CBS 171.64 | Soil | Australia | KC784355 |
| M. fulvum | A. fulvum | CBS 167.64 | Soil | Hungary | KC771283 |
| M. fulvum | A. fulvum | CBS 168.64 | Soil | Hungary | KC784353 |
| Trichophyton erinacei | Trichophyton erinacei | CBS 511.73 | Hedgehog | New Zealand | KC784358 |
| Name used in this study . | Original name . | Strain number . | Source . | Location . | GenBank accession number . |
|---|---|---|---|---|---|
| Microsporum incurvatum | MCCF 108.07 | Cat, favus | Taitung, Taiwan | KC784359 | |
| M. incurvatum | MCCF 1687 | Cat, favus | Taitung, Taiwan | KC784360 | |
| M. incurvatum | MCCF 1733 | Cat, favus | Taitung, Taiwan | KC784361 | |
| M. incurvatum | MCCF 1752 | Cat, favus | Taitung, Taiwan | KC784362 | |
| M. incurvatum | Arthroderma incurvatum | CBS 172.64 | Human hair | United Kingdom | KC784356 |
| M. incurvatum | A. incurvatum | CBS 173.64 | Human skin | United Kingdom | KC784357 |
| M. incurvatum | A. incurvatum | KMU 2981 | Human skin | Nara, Japan | AB613251 |
| M. incurvatum | A. incurvatum | KMU 2982 | Human skin | Wakayama, Japan | AB613252 |
| M. incurvatum | A. incurvatum | KMU 2986 | Human skin | Oita, Japan | AB613253 |
| M. incurvatum | A. incurvatum | KMU 2987 | Human skin | Oita, Japan | AB613254 |
| M. incurvatum | A. incurvatum | IFM 41290 | Human | Nagasaki, Japan | AB193689 |
| M. incurvatum | A. incurvatum | IFM 41067 | Human | Japan | AB193685 |
| M. incurvatum | A. incurvatum | IFM 46926 | Human | Amami, Japan | AB193700 |
| M. incurvatum | A. incurvatum | IFM 5296 | Human | Japan | AB193672 |
| M. incurvatum | A. incurvatum | IFM 46793 | Human | Peking, China | AB193697 |
| M. incurvatum | A. incurvatum | IFM 59508 | Human skin | Sri Lanka | AB613257 |
| M. incurvatum | A. incurvatum | IFM 41133 | Human | Brazil | AB193687 |
| M. incurvatum | A. incurvatum | IFM 46456 | Unknown | Costa Rica | AB613256 |
| M. incurvatum | A. incurvatum | IFM 46457 | Unknown | Costa Rica | AB613258 |
| M. gypseum | A. gypseum | SM 8377 | Human | Japan | AB593398 |
| M. gypseum | A. gypseum | CBS 170.64 | Human skin | United Kingdom | KC784354 |
| M. gypseum | A. gypseum | CBS 171.64 | Soil | Australia | KC784355 |
| M. fulvum | A. fulvum | CBS 167.64 | Soil | Hungary | KC771283 |
| M. fulvum | A. fulvum | CBS 168.64 | Soil | Hungary | KC784353 |
| Trichophyton erinacei | Trichophyton erinacei | CBS 511.73 | Hedgehog | New Zealand | KC784358 |
Results
Histopathological findings
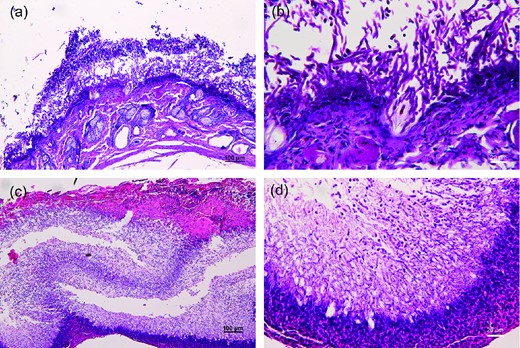
In case 4, remnants of the stratum corneum could be seen at the edge of the lesion. Infundibula of hair follicles were dilated and filled with fungal elements, and the hair shaft within was surrounded by hyphae (Fig. 2a and 2b). Beneath the stratum corneum was a thick layer of vertically oriented and interwoven fungal hyphae. The hyphae had regular septations, and some were fragmented into rectangular arthroconidia. Although examination was with a low-power microscope, the fungal elements formed horizontal alternating dense and loose zones, which can be seen clearly with both hematoxylin and eosin and periodic acid-Schiff stains (Fig. 2a). The epidermis beneath the fungal layer was severely disrupted by the fungal hyphae (Fig. 2b). Many inflammatory cells had infiltrated the dermis. The sebaceous glands and eccrine ducts were intact, and no dermal invasion by fungi was noted. The density of fungal elements in the stratum corneum decreased and the volume of normal epidermis increased away from the central zone. The lesion features of case 3 were similar to those of case 4, and striking zonations of scutula were also clearly demonstrated in this specimen (Fig. 2c). The dermal portion was not contained in the sections because the lesion was separated from the destructed epidermal base during sampling, which was performed using forceps. Destruction of epidermis and vertical growth of fungal hyphae were seen (Fig. 2d).
Histopatholgy of specimens of case 4 (a,b) and case 3 (c,d). (a) The scutulum was composed of a thin layer of stratum corneum, main fungal portion, and a destructed, necrotic lower epidermis. Hair follicles and hairs were invaded by fungal hyphae (100X, PAS stain) (b) Higher magnification of Fig. 2(a). (c) Horizontal zonations of fungal portion of scutulum (100X, H&E stain). (d) Destruction of lower epidermis by fungal hyphae (400X, H&E stain). This Figure is reproduced in color in the online version of Medical Mycology.
Mycological studies
The colony and microscopic features were similar in all four isolates. The colonies were fast growing and had a powdery cinnamon-colored surface. Many fusiform thin-walled macroconidia with transverse septa and a fine granular surface were observed by microscopy, all of which contributed to identification of the isolates as Microsporum incurvatum.
Phylogenetic study
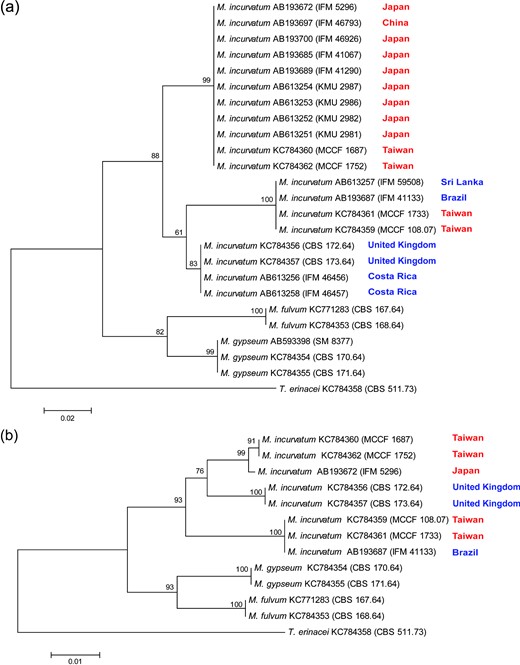
The sequences of MCCF 108.07 and MCCF 1733 (617 nucleotides) were identical, as were those of MCCF 1687 and MCCF 1752 (619 nucleotides). There were 27 nucleotide differences between these two sets of sequences. The phylogenetic trees showed that M. incurvatum, M. gypseum, and M. fulvum formed separate clades; each clade was supported by moderate to high bootstrap values (82%–100%; Fig. 3a). Three major clades can be recognized within M. incurvatum on either ITS1 or ITS-5.8S-ITS2 trees (Fig. 3a and 3b). The two phylogenies differed mostly on the position of the UK isolates (CBS 172.64 and CBS 173.64). These two UK isolates were the sister group to either of the other two clades on the two trees, but with low to moderate bootstrapping supports (61% and 76%). Nevertheless, the four Taiwan isolates were clearly separated into two distinct groups on both of the trees. Two of them (MCCF 1687 and MCCF 1752) grouped with Japan isolates, while the other two (MCCF 1733 and MCCF 108.07) grouped with the Sri Lanka and Brazil strains (Fig. 3a) or with the Brazil strain (Fig. 3b).
Neighbor-joining trees based on (a) ITS1, and (b) ITS1-5.8S-ITS2. Bootstrap values were shown at nodes. Trichophyton erinacei CBS 511.73 was used as outgroup. Names of East Asian countries are in red, while those of non-East Asian countries are in blue. This Figure is reproduced in color in the online version of Medical Mycology.
Taxonomy
Microsporum incurvatum (Stockdale) P.-L. Sun & Y.-M. Ju comb. nov.
Basionym. Nannizzia incurvata Stockdale, Sabouraudia 1: 46. 1962.
≡ Arthroderma incurvatum (Stockdale) Weitzman, McGinnis, A.A. Padhye & Ajello, Mycotaxon 25: 514. 1986.
Mycobank no. MB803967
Beginning in 2013, there was a dramatic change in fungal nomenclature, that is, one fungus can have only one name [16]. Therefore, the term Microsporum gypseum should be adopted instead of Arthroderma gypseum and other competing synonyms because the generic name Microsporum has priority over Arthroderma. Furthermore, M. gypseum is used more widely than A. gypseum in clinics. Previously, M. gypseum was used for the anamorphs of M. incurvatum and M. gypseum because their respective anamorphs are difficult to separate on the basis of morphologic features. Both our ITS1 tree (Fig. 3a) as well as the ITS1-5.8S-ITS2 tree (Fig. 3b) clearly showed that isolates of M. incurvatum and M. gypseum were separated into two clades and that M. gypseum was more closely related to M. fulvum than to M. incurvatum. To comply with the recent change in the International Code of Nomenclature for algae, fungi, and plants and to reflect the results from our phylogenetic analyses, we feel justified in combining the epithets of Nannizzia incurvata with the genus Microsporum to form Microsporum incurvatum.
Discussion
There are only a few reports of favus on cats in the literature. In 1879, Smith described five human favus cases, two of which may have been contracted from sick cats [8]. Later in 1957, Von Zezschwitz published a case of cat favus with scutulum on its right ear [9]. No attempt was made to culture the samples from these human and feline cases. During the climax of T. quinckeanum endemics in Europe, which lasted from the 1940s until the 1960s, numerous infected mice transmitted this pathogen to humans and domestic animals, including cats. Infections by this fungus can give rise to various clinical presentations [17]; the most well known is the favic type, commonly called “mouse favus” to describe such infections. Photo documentation of cat favus caused by T. quinckeanum were presented by Szathmary [3] and La Touche [10].
Microsporum gypseum was first described by Bodin in 1907. According to his record, this fungus was isolated from a favus lesion on the right cheek of a 40-year-old woman [18]. Later study showed that M. gypseum is a geophilic fungus that infects only humans and animals on rare occasions. Since 1907, more cases involving horses, monkeys, dogs, cats, tigers, and chickens have been reported [19]. Microsporum gypseum was a species complex composed of at least five organisms, M. gypseum, M. fulvum, Arthroderma gypseum, A. incurvatum, and A. fulvum [20]. The differentiation between M. gypseum and M. incurvatum on a morphological basis is usually quite difficult and unreliable because the two fungi are so similar. Mating tests that use tester strains is the most reliable way to separate them. The procedure, however, is time consuming and labor intensive and thus unsuitable for clinical applications. Therefore, these two fungi are usually collectively identified as M. gypseum in routine laboratory reports.
M. gypseum in a relatively common agent of infections, with various clinical presentations in humans [21]; favus and favus-like lesions have been reported on only 13 occasions [22–28]. Of these, 11 had lesions on scrotum; however, unlike the scalp favus caused by T. schoenleinii, no mousy odor was noted [25]. All cases responded to antifungal treatments. The reason for predilection of scrotal involvement is unknown, but high humidity and high temperature of the scrotal region have been postulated [24]. Host immune status may not be a predisposing factor because half of the cases were healthy. None of the isolates from these 13 cases have been further identified as M. gypseum or M. incurvatum.
In Latin favus means “honeycomb” [29], being originally adopted for the clinical appearance of cup-shaped scutula that are configured like a honeycomb. The lesions on the four kittens presented here are single or multiple yellow, thick, waxy scutula similar to those of cat and mouse favus cases recorded previously [3,9,10]. Unlike the favus lesions caused by T. schoenleinii and T. quinckeanum, the lesions described here were caused by M. incurvatum and lacked typical cup-shaped scutula. However, the yellow color and fungal mycelial nature of the scutula are of great diagnostic value for favus. The species that compose the M. gypseum complex can form typical scutula in humans [23,25,26] but rarely in cats. The only two documented cases were from Japan. The first was a 1-month-old domestic kitten with scutula on his pads. The pathogen was identified as Nannizia incruvata using the mating test [11]. The second case was a 1- to 2-month-old female crossbreed kitten with scutula on her tail. The pathogen was identified as Arthroderma gypseum (≡Nannizia gypsea) by random amplification of the polymorphic DNA and by the mating test [12]. In these cases and the four cases reported here, we found that all the patients were young kittens and most lesions were found on the head. This may be related to skin integrity of young animals, which is less resistant to fungal pathogens, and the cleansing behavior of kittens.
Favus on the ears of a kitten should be differentiated from proliferative and necrotizing otitis externa, which is a rare disease characterized by rapid-growing well-defined erythematous plaques with thick keratinous debris on the concave surface of pinnae. The lesions develop rapidly and usually regress spontaneously and slowly within 12 to 24 months [30,31]. It was thought to be a disease exclusively in kittens, but recently adult cases have been reported [31]. The diagnosis is based on skin biopsy. The histopathological characteristics include prominent acanthosis of epidermis and external root sheath of hair follicles, eosinophilic shrunken keratinocytes, and marked luminal folliculitis [31]. Kitten favus can be differentiated from this disease when fungal elements from the crust are found under a microscope.
The histopathology of cases 3 and 4 elucidate the development of favic lesions. The fungus gained entry to the skin from the stratum corneum of the epidermis and then invaded horizontally and downward into hair follicles. The hair shafts within the follicles were also infected. Hyphae then proliferated very quickly between the stratum corneum and Malpighian layer, forming the main body of scutula. The hyphae that grew upward between the two tissue layers and became fragmented into arthroconidia. Histological staining revealed alternate horizontal dense and loose zones of fungal growth. The exact mechanism of the alternate zone formation remains unknown. It should be noted that similar features have been seen in cases of favus caused by T. quinckeanum [32]. The underlying Malpighian layer adjacent to fungal hyphae was destroyed by fungi and became necrotic. This explains why favic scutula were easily removed to reveal the eroded dermal base underneath. The dermal invasion in case 4 was not obvious despite infiltration of dense inflammatory cells. Favus differs from tinea primarily because its fungal growth forms a thallus that is visible to naked eyes rather than microscopic sparse hyphae. Furthermore, the favus strains seem toxic to keratinocytes, a phenomenon rarely seen in tinea.
The mating test with tester strains is a standard for differentiating M. gypseum and M. incurvatum. This method, however, suffers from a major drawback: it is time consuming. Also, the likelihood of losing fertility in tested strains and/or testers may result in failure of teleomorphic formation between compatible strains. Today, molecular identification, which is achieved by sequencing certain DNA regions, is a powerful tool that can be used to identify fungus quickly and possibly suggest taxonomy. Using ITS1, Iwasawa et al. [33] successfully differentiated M. incurvatum from M. gypseum and found that M. incurvatum isolates could be segregated into two geographical strains: the East Asian strain and non–East Asian strain. Interestingly, all four cat favus isolates were isolated from Taiwan. However, in the phylogenetic tree that is based on ITS1, two isolates belonged to the East Asian strain and the other two belonged to the non–East Asian strain (Fig. 3a). In the phylogenetic tree based on ITS1-5.8S-ITS2, the four Taiwan isolates remained segregated into two groups, even though the phylogenetic tree's topography was somewhat different from that based on ITS1 (Fig. 3b). Our results indicate that two cat favus strains of M. incurvatum exist in Taiwan, corresponding with the two strains identified by Iwasawa et al. [33]. However, the two strains are sympatric in Taiwan and cannot be delineated on the basis of geographic distribution. More isolates and other DNA loci should be analyzed to further assess the phylogeography of M. incurvatum.
Regarding favus caused by M. incurvatum, several questions remain unanswered: (1) Is favus formation determined by hosts, by fungal pathogens, or by both? (2) How many strains of favus-forming M. gypseum species complex are there worldwide? (3) What is the exact pathogenesis of favus by M. gypseum species complex? (4) What is the mechanism of formation of alternate zonations in scutula histopathologically? Animal models and more clinical cases are needed to elucidate this unique clinical presentation of dermatophyte infection. Favus is a highly contagious disease that can cause a substantial public health problem in both small animals and humans. Once diagnosed, patients should be treated as early as possible because the fungal pathogens develop favus quickly, within days or weeks after infection.
This work was supported by research grants from the National Science Council, Taiwan (100-2314-B-195-008) and Mackay Memorial Hospital, Taiwan (MMH-10119) to P.-L.S.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.