-
PDF
- Split View
-
Views
-
Cite
Cite
Jeffrey S. Prince, Paul Micah Johnson, Ultrastructural comparison of Aplysia and Dolabrifera ink glands suggests cellular sites of anti-predator protein production and algal pigment processing, Journal of Molluscan Studies, Volume 72, Issue 4, November 2006, Pages 349–357, https://doi.org/10.1093/mollus/eyl017
Close - Share Icon Share
Abstract
Ultrastructural comparison between the ink gland of a sea hare species that produces copious purple ink (Aplysia californica) and one that produces none (Dolabrifera dolabrifera), suggests that the rough endoplasmic reticulum rich cell and not the ink vesicle cell is the site for synthesis of A. californica's anti-predator ink protein, escapin. Dolabrifera dolabrifera were found to have vestigial ink glands incapable of producing ink or its associated anti-predator proteins regardless of diet. This study also suggests that the granulate cells serve only as a storage site for excess ink pigment acquired during periods of luxury feeding on red algae. Slit dimensions in sieve areas of granulate cells are also significantly different between the two species. These slit sizes are larger than those of rhogocytes, a related cell type commonly found in connective tissue of gastropod molluscs. Several traits of granulate cells suggest that they are distinct from rhogocytes and are a special cell type in the ink gland of sea hares.
Introduction
The release of a bright purple ink from the ink glands of many species of sea hares (Gastropoda: Opisthobranchia: Anaspidea) is a well-known part of their defensive arsenal against predators (Kandel, 1979; Nolen, Johnson & Kicklighter, 1995; Johnson & Willows, 1999; Kicklighter et al., 2005). Though all sea hares possess ink glands in their mantle cavities, not all of these glands release purple ink and, in fact, some release no ink at all (Johnson & Willows, 1999). It is unclear whether those species that do not release ink simply lack the dietary precursors necessary for its production or have ink glands incapable of ink production regardless of diet.
Ink of the best studied ink-producing sea hare species, Aplysia californica Cooper, consists largely of two components; 65% is phycoerythrobilin (the red algal photosynthetic pigment r-phycoerythrin minus its low molecular mass protein component) and 35% is high molecular mass protein not obtained from the red algal pigment (Troxler, Offner & Capo, 1981; Yamazaki et al., 1986, 1989; MacColl et al., 1990; Prince, Nolen & Coelho, 1998; Johnson, 2002). This 60 kDa ink protein of A. californica was isolated, cloned, sequenced and named ‘escapin’ for its potential anti-predator properties (Johnson, 2002; Yang et al., 2005). Several other proteins have been isolated from the ink of related sea hare species, including aplysianin-P from A. kurodai (Yamazaki et al., 1986, 1989), julianin-S from A. juliana (Kamiya et al., 1989), dactylomelin-P from A. dactylomela (Melo et al., 1998, 2000), bursatellanin-P from Bursatella leachii (Rajaganapathi & Kathiresan, 2002) and Aplysia punctata ink toxin from A. punctata (Butzke et al., 2004) which, like escapin, has also been cloned and sequenced. All of these proteins, including escapin, are bioactive and most are known to be capable of both antimicrobial and anti-tumour activity (see references above).
Where does synthesis of escapin take place in the ink gland? The only ultrastructural study of the ink gland of a sea hare (Prince et al., 1998) found that it is characterized in A. californica by two prominent cell types, rough endoplasmic reticulum rich (RER) cells and granulate cells, and three types of vesicles (red-purple, amber and clear). Prince et al. (1998) suggested that ink protein is produced in the RER cells; they characteristically have numerous vacuoles that consist of distended cisternae of the rough endoplasmic reticulum surrounding material of low electron density. The ink vesicle cell itself, however, might also be involved in synthesis of the ink protein since this cell is very metabolically active as indicated by the abundance of small vacuoles, numerous mitochondria and the large nucleus with numerous nuclear pores (Prince et al., 1998).
In order to localize the site of escapin production in the ink gland of A. californica we compare its ultrastructure to that of a species of sea hare, Dolabrifera dolabrifera (Cuvier), which has an ink gland, but is not known to produce any ink (Marcus, 1972; Gosliner, 1994). In addition, we examine the ultrastructure of the granulate cell in both genera of sea hares to determine (1) whether this cell type is a required link for the movement of ink pigment from haemolymph to red-purple vesicles as proposed by Coelho, Prince & Nolen (1998); (2) its ultrastructural relationship to another common cell type found in the connective tissue of gastropod molluscs, the rhogocyte, which has a similar modification of the cell membrane.
Material and Methods
Animal collection
Aplysia californica, 100–200 g wet mass, were obtained from the NCRR Aplysia Resource Facility, University of Miami, where under hatchery conditions they are fed the red alga, Gracilaria tikvahiae, every 3 days; the food is totally consumed within the first day (T. Capo, personal communication). Data collected for another study (Coelho et al., 1998) provided information on ad libitum feeding on ink gland ultrastructure. In this latter study, 0.5 g individuals of A. californica were grown in culture with unlimited food until reaching the adult size of at least 5 g. Seven adult individuals of the relatively rare sea hare species, Dolabrifera dolabrifera (all between 5 and 7 cm in length), were collected from the bottom of two large outdoor tanks at the University of Guam Marine Laboratory where they were feeding on Enteromorpha clathrata.
Dolabrifera feeding experiments
Dolabrifera dolabrifera are known to feed on green algae such as Enteromorpha clathrata (Willan & Morton, 1984) and, though they have an ink gland, they are not known to produce any ink (Marcus, 1972; Gosliner, 1994). We conducted a limited dietary experiment to determine if this lack of ink production is due to a functional inability of the gland to process algal pigments into ink or if it is simply the result of a diet lacking in the necessary algal precursors. Two of seven individuals were maintained on an exclusive diet of the cyanobacterium, Lyngbya majuscula, the preferred diet of a sympatric, ink-producing sea hare from Guam, Stylocheilus striatus (Quoy & Gaimard). Two more D. dolabrifera individuals were provided with another cyanobacterium, Hormothamnion enteromorphoides, which when eaten by S. striatus causes this sea hare to produce blue, rather than purple, ink. The remaining three individuals were provided with their standard diet of E. clathrata. All individuals were isolated and maintained on these diets, with daily changes of seawater, until either death or the conclusion of the experiment after 4 weeks. Each was dissected and its ink gland examined for signs of ink pigment and protein production. The stomach contents of individuals that died prematurely, without clear signs of feeding, were also examined for signs of algae. Ink glands of three individual D. dolabrifera, two Lyngbya-fed and one Enteromorpha-fed, were analysed for protein content using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gels to determine if escapin-like proteins were present in the glands.
Ultrastructure
Tissue isolation for electron microscopy in all cases was done as described by Prince (2003). Thin sections were stained with lead citrate and uranyl acetate and viewed with a Philips 300 electron microscope at 60 kV. Spacing between adjacent fingers of cytoplasm in sieve areas of granulate cells were measured directly from negatives using an ocular micrometer. The number of caveolae per linear length of RER cell membrane (in microns) was determined by digitizing 7–11 photomicrograph negatives per species according to the methods of Prince et al. (1998) and then counting the number of caveolae with a dissection microscope at 20× magnification. Thick sections (1 µm) stained with toluidine blue provided granule number and size, and nuclear and cell characteristics for various cell types.
Statistical comparisons of the number of caveolae per length of cell membrane, the number of dense granules per granulate cell, and the size of slits in sieve areas were made using either the Mann–Whitney U-test or Student's t-test (Sokal & Rohlf, 1981).
Results
Dolabrifera feeding experiment
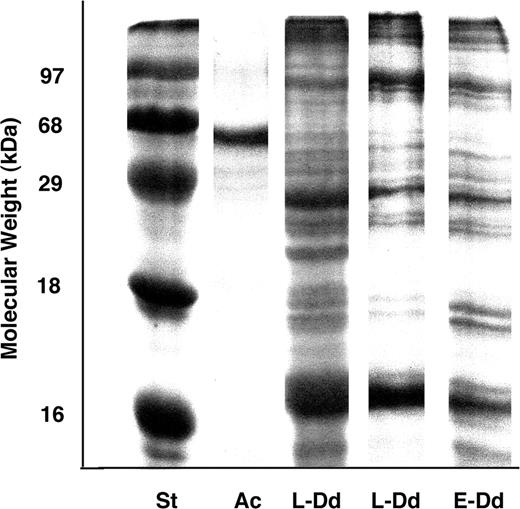
After 1 week, the two Dolabrifera dolabrifera individuals offered exclusively the pigmented cyanobacterium Hormothamnion enteromorphoides on which to feed, were dead. Dissected ink glands of both individuals examined under a dissecting microscope revealed only transparent vesicles, possibly mucus filled, with pores leading to the gland surface. One of the two animals had H. enteromorphoides in its stomach, the other did not. The individuals offered the other two diets were still alive after 4 weeks, during which time all consumed food and produced faeces pigmented like their food. These individuals were anesthetized and dissected. The ink glands of the two individuals grown on the pigmented cyanobacterium Lyngbya majuscula showed no observable differences under the dissecting microscope from the three animals grown on their standard diet of the green alga, Enteromorpha clathrata, and no difference from those grown on H. enteromorphoides; the ink glands of individuals of all groups contained only transparent vesicles and were devoid of any red-purple, blue or amber pigmented vesicles (Fig. 1). The stomach contents of the L. majuscula- and E. clathrata-fed individuals were not examined, as 4 weeks of faecal production and disappearance of food showed that all had been eating. No dominant proteins in the range 60–70 kDa were found by 12% SDS PAGE in the ink glands of any of the three D. dolabrifera individuals tested (Fig. 2); such proteins have been found in all ink-producing sea hares examined (see Introduction).
Ink glands (neither fixed nor stained) of Aplysia californica (A) and Dolabrifera dolabrifera (B, C). Abbreviations: av, amber vesicle; cav, calcium carbonate containing vesicles [Prince, unpublished]; cv, clear vesicle; rpv, red-purple vesicle. Scale bars: A=0.1 mm; B=0.25 cm; C=0.6 mm.
Gel electrophoresis of the proteins isolated from the ink glands of Aplysia californica (Ac) and Dolabrifera dolabrifera fed Lyngbya (L-Dd) and Enteromorpha (E-Dd) compared to standard proteins (St).
Another pilot feeding experiment conducted in Japan [P.M. Johnson, unpublished data] examined the ink gland structure of six adult (all greater than 50 g) Aplysia juliana Quoy & Gaimard, a green-algal-feeding sea hare that produces white ink (Kamiya et al., 1989). In that case individuals provided only a diet of the red alga Porphyra sp. began producing purple ink vesicles in addition to the white ink vesicles usually found in this species.
Ultrastructure
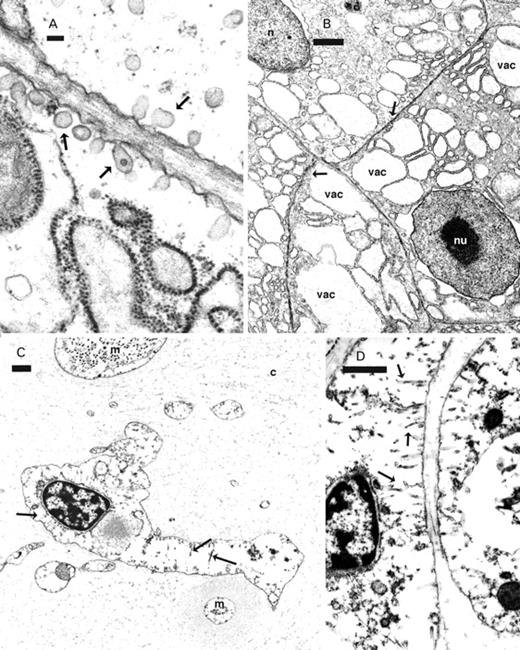
The ink gland of D. dolabrifera, unlike that described for A. californica (Prince et al., 1998), consisted of copious collagen, muscle, vesicles and a greatly reduced number of RER and granulate cells (Figs 3C, 4A, C, 5A; cf. Prince et al., 1998: Figs 3B, 8A). The nucleus of RER cells appeared to lack a nucleolus (Fig. 3C, D) and the cell membrane gave rise to numerous (mean±SD=2.26±0.44 per micron of cell membrane, n=11) elongate caveolae that were also frequently branched (Figs 3C, D, 6A). Vacuoles composed of distended cisternae of rough endoplasmic reticulum were few (Figs 3C, 4A), while the vacuoles that were present consisted primarily of membrane-bound sacs containing electron dense debris.
RER cells from the ink glands of Aplysia californica (A, B) and Dolabrifera dolabrifera (C, D). A, B. Small caveolae (arrows) emerge from the cell membrane of RER cells of Aplysia californica while elongate, branched caveolae (arrows) arise from the cell membrane of RER cells of Dolabrifera dolabrifera (C, D). B. Vacuoles (vac) in Aplysia californica contain low electron dense material while such vacuoles are absent in Dolabrifera dolabrifera (C). Abbreviations: c, collagen; m, muscle; n, nucleus; nu, nucleolus. Scale bars: A=100 nm; B–D=1 µm.
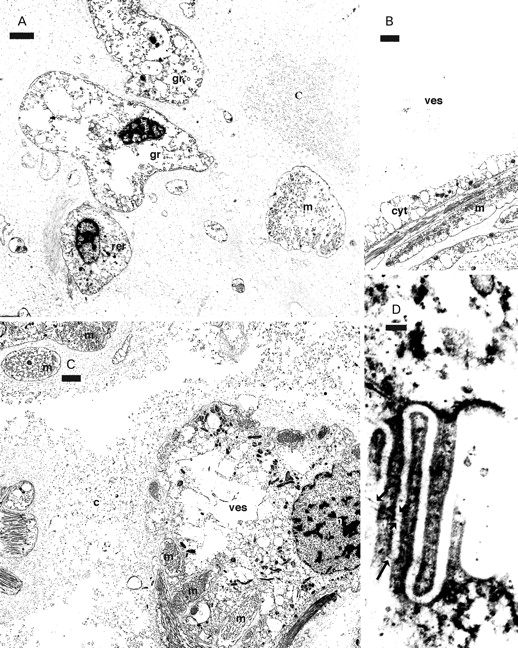
Granulate cells (A, D) and vesicle cells (B, C) from the ink gland of Dolabrifera dolabrifera. A. Granulate cells (gr) and RER (rer) cells among copious collagen and muscle. B. A mature vesicle showing an empty interior and a thin cytoplasmic layer (cyt) against the muscle wall. C. A young vesicle only partially enclosed by muscle. D. Tangential section through sieve area showing filaments (black arrows) joining cytoplasmic fingers. Abbreviations: c, collagen; cyt, cytoplasmic layer against muscle wall of vesicle; gr, granulate cell; m, muscle; n, nucleus; rer, RER cell; ves, vesicle. Scale bars: A–C=2 µm; D=100 nm.
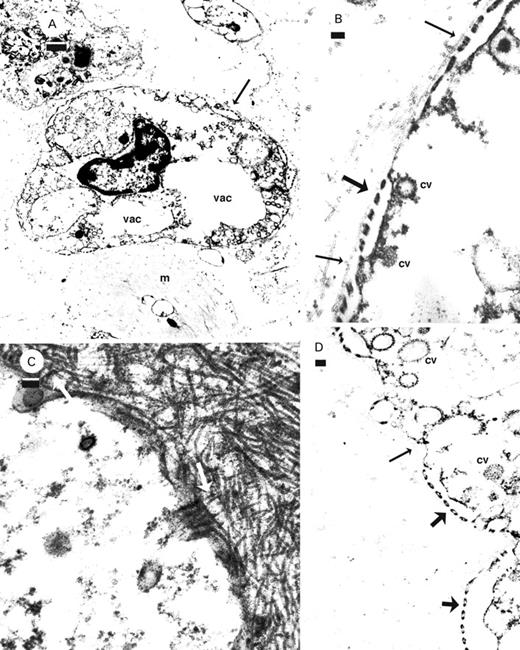
Granulate cells from the ink glands of Dolabrifera dolabrifera (A, B, D) and Aplysia californica (C). A. Sieve area (arrow) and empty vacuoles (vac) in a granulate cell among muscle and collagen. B, D. Cross section of sieve areas (thick arrows) showing coated vesicles associated with the membrane subtending the sieve area with the basal membrane (thin arrows) and collagen parallel to the cell surface. C. Collagen (white arrows) arising at right angles to the membrane. Abbreviations: cv, coated vesicle; m, muscle; vac, vacuole. Scale bars: A=1 µm; B–D=100 nm.
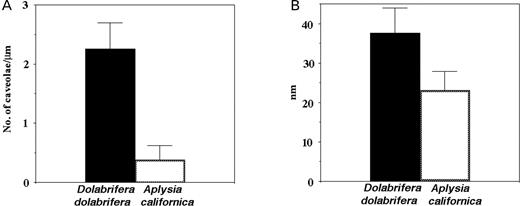
Characteristics of various cell types in the ink glands of Aplysia californica and Dolabrifera dolabrifera. A. The number (mean, SD) of caveolae per length (µm) of cell membrane in RER cells. B. Slit size (nm; mean, SD) in sieve areas of granulate cells.
Immature ink vesicle cells in D. dolabrifera, like those in A. californica, had a prominent nucleus and numerous mitochondria and vacuoles, and eventually became surrounded by muscle (Fig. 4B, C). The central portion of mature vesicles contained only a few scattered segments of cytoplasm, while a thin layer of highly vacuolate cytoplasm lined the muscle wall (Fig. 4B).
Granulate cells of D. dolabrifera often lacked detectable granules and any vacuoles appeared empty (Figs 4A, 5A). Slits between adjacent fingers of cytoplasm in grated areas were spaced 37±6.4 nm (mean±SD, n=43) apart (Figs 5B, D, 6B). Filaments crossing the slits in sieve areas (Fig. 4D) were spaced c. 48 nm apart (n=3); coated vesicles were associated with the membrane-subtending sieve areas (Fig. 5B, D). Nuclei of granulate cells lacked a nucleolus (Figs 4A, 5A) and a basal layer just external to the cell membrane was either absent or consisted of a single filamentous layer surrounding only part of the cell (Fig. 5B, D; thin arrows). Collagen filaments generally appeared to be arranged parallel to the cell surface (Fig. 5B), while in A. californica they periodically appeared to arise at right angles to the cell membrane (Fig. 5C).
The ink gland of A. californica had numerous RER cells with little space between adjacent cells (Fig. 3B; Prince et al., 1998: Fig. 8A). RER cells also contained numerous vacuoles consisting of distended cisternae of rough endoplasmic reticulum surrounding material of low electron density (Fig. 3B). The nucleus had a prominent nucleolus (Fig. 3B; Prince et al., 1998: Figs 6C, 8A) and the cell had few (mean±SD=0.38±0.24 per micron of cell membrane, n=7) short caveolae (Figs 3A, B, 6A). A Mann–Whitney test found a significantly greater number (U=0.00; P<0.0001) of caveolae per length of cell membrane in D. dolabrifera than in A. californica (Fig. 6A).
Aplysia californica fed red algae ad libitum had numerous (median=3.0, n=31) dense granules in each granulate cell (Prince et al., 1998: Fig. 8A) while hatchery-fed A. californica had significantly fewer (median=0, n=23; Mann–Whitney Test, U=93.000; P<0.0001). Vacuoles and coated vesicles adjacent to grated areas of the cell membrane were present in granulate cells of sea hares in both feeding regimens but, as mentioned above, vacuoles were generally empty in hatchery-grown A. californica. The spacing of adjacent fingers of cytoplasm in grated areas of granulate cells of A. californica (Fig. 6B; mean±SD=23.1±4.8 nm, n=32) was significantly less (Student's t-test, t=10.760, df=73; P<0.0001) than that for D. dolabrifera (see above). Thick sections showed nucleoli in RER cell nuclei, but not in granulate cell nuclei, regardless of feeding regimen.
Discussion
Inability of Dolabrifera dolabrifera to produce ink
Our results suggest that Dolabrifera dolabrifera may have lost the ability to produce ink. Even when the sea hares fed for several weeks on algae containing pigments necessary for ink production, no pigmentation ever appeared in the glands. This included not only the red-purple or blue pigmentation of ink vesicles, but also the amber pigmentation of vesicles known in Aplysia californica to contain the bioactive protein, escapin (Johnson, 2002). As the ink gland appears to be apomorphic in the Anaspidea (Medina & Walsh, 2000), it is likely that the gland in D. dolabrifera is now vestigial and incapable of producing either ink or its associated proteins regardless of diet. This contrasts with the pilot results for A. juliana, which found that a change in diet from green algae to red algae resulted concurrently in a change from the sole production of white ink vesicles to include the production of red-purple vesicles. These results make D. dolabrifera a potentially useful model for comparative studies with sea hare species that have functional ink glands, particularly if this rare species could be grown in culture like A. californica.
Site of ink protein synthesis
The 60 kDa protein, termed escapin in A. californica and comprising 35% of ink released by this sea hare, appears to be manufactured in the ink gland by A. californica and is not derived from the protein component of the red algal photosynthetic pigment (Troxler et al., 1981; Yamazaki et al., 1986, 1989; MacColl et al., 1990; Prince et al., 1998; Butzke et al., 2004; Yang et al., 2005). The present ultrastructural study of the vestigial ink gland of D. dolabrifera suggests that the RER cell, and not the ink vesicle, was the site for synthesis of this ink protein. The ink gland of D. dolabrifera compared to that of A. californica had few RER (or granulate) cells and the few that did occur were widely dispersed in copious collagen. In addition, RER cells of D. dolabrifera had few vacuoles of any type, in particular ones originating by expansion of rough endoplasmic reticulum. RER cells of this sea hare did have a high frequency of long, tubular caveolae, while the nucleus lacked a distinct nucleolus. All of these characteristics of the ink gland and RER cells of D. dolabrifera were in contrast to those in A. californica.
The ultrastructural development of ink vesicle cells was nevertheless similar in both genera of sea hares; however, mature vesicles of D. dolabrifera were empty, although their muscle wall was lined by a thin layer of vacuolated cytoplasm. RER cells and not the ink vesicle cells, therefore, appear to be site for the synthesis of ink protein based upon (1) the similarity in early development of the ink vesicle cell in both genera; (2) the presence of mature but empty vesicles in D. dolabrifera; (3) the ultrastructural dissimilarity between the RER cell types for the two genera; (4) the low frequency of the RER cells in D. dolabrifera.
Caveolae, a frequent feature of many cell types (Parton, 2001), constitute a stable membrane system with multiple functions (Pelkmans et al., 2004), involved with the transport of molecules into and out of the cell and possibly across the cell itself (Anderson, 1998; Anderson & Jacobson, 2002). Tubule-shaped caveolae appear to be part of a trans-cellular pathway and have been recorded for muscle, neutrophile and placental epithelia (Anderson, 1998; Anderson & Jacobson, 2002). Such tubular caveolae characterized the RER cells of D. dolabrifera, but not those in A. californica; this again suggests that the function of this cell type was different in these two genera of sea hares.
Transit of ink pigment from haemolymph to ink vesicle
The granulate cell in the ink gland of A. californica is proposed to be a necessary way station in the transit of ink pigment from haemolymph to the red-purple ink vesicles (Prince et al., 1998). The accumulation of ink pigment in several vacuoles in granulate cells (thereby becoming electron dense; see Fig. 8A, Prince et al., 1998) is one of the major traits that distinguishes this cell type from the other common cell type, the RER cell, in the ink gland of this sea hare. However, the presence of granules in granulate cells is also affected by ink pigment availability, a function of food type. Granulate cells of A. californica fed green algae or romaine lettuce lacked granules, even though the ultrastructural traits characteristic of granulate cells were still present (Prince et al., 1998).
The present study found that the abundance of red algal food also affected the presence of granules in granulate cells. Hatchery-reared A. californica (fed red algae every 3 days) had significantly fewer granules in granulate cells than those fed red algae ad libitum, but the ultrastructure of the granulate cells of the sea hares in both feeding regimens was similar. Hatchery-reared sea hares also grow more slowly by an order of magnitude; juvenile A. californica on an ad libitum diet grow at 0.24 g wet mass per day (Coelho et al., 1998), while those fed every 3 days grow at 0.01 g wet mass per day (Fieber, unpublished data). Under conditions of luxury feeding sea hares, therefore, appeared not only to grow rapidly, but appeared to consume more food and its pigment than could easily be handled by the digestive-haemolymph-ink gland system (Prince et al., 1998). Excess pigment then appeared to be shunted to granulate cells and stored as dense granules (Prince et al., 1998: Fig. 8A).
Granulate cells in other species of Aplysia collected from the field, A. juliana and A. parvula (unpublished data) as well as D. dolabrifera, either lacked or had few granules. However, granulate cells were still present in all of these species, based upon cells having the characteristic ultrastructural traits for this cell type (Prince et al., 1998). The large reduction in number of granules per granulate cell under conditions of restricted food availability (hatchery conditions), their reduction in number or complete absence from several different species of field-collected sea hares and their absence when sea hares are fed green algae or romaine lettuce (Prince et al., 1998) suggested that (1) granulate cells were a storage site for excess pigment and were not a necessary compartment in the movement of pigment from haemolymph to ink vesicle; (2) the presence or abundance of granules in granulate cells was based upon food abundance and type and could not, therefore, be used to distinguish different species of sea hares.
Granulate vs rhogocyte cell types
Ultrastructurally, the modification of the cell membrane into grated areas characterized granulate cells of both A. californica and D. dolabrifera (this study; Prince et al., 1998). A similar modification of the cell membrane into sieve-like structures containing a series of slits characterizes rhogocytes, a cell type frequently reported from connective tissue of gastropod molluscs (Haszprunar, 1996; Albrecht et al., 2001). Should granulate cells be considered an entirely different cell type or a rhogocyte, or a subtype of rhogocyte as Albrecht et al. (2001) suggest for cells involved in synthesis of the two haemocyanin isoforms in the keyhole limpet, Megathura sp.?
Granulate cells and rhogocytes share the following traits: localized modification of the cell membrane into grated (sieve) areas which are subtended by an enlarged cisternum of the cell membrane (this study; Simkiss & Mason, 1983; Haszprunar, 1996; Prince et al., 1998; Albrecht et al., 2001); slit (sieve) dimensions of c. 22–24 nm (Sundermann, 1980); filaments (diaphrams in Simkiss & Mason, 1983) crossing the slits every 15 nm (Prince et al., 1998; Albrecht et al., 2001) to 20 nm (Simkiss & Mason, 1983) providing an effective pore size of 15–24 nm; no junctional connections to adjacent cells; and the cell containing several vacuoles that are either filled with electron-dense material or empty (Haszprunar, 1996; Albrecht et al., 2001).
Traits unique to granulate cells include nuclei without a nucleolus (Prince et al., 1998). Rhogocyte nucleoli are either not mentioned (Martoja & Tue, 1980; Simkiss & Mason, 1983; Albrecht et al., 2001), present (Fioroni, Sundermann & Scheidegger, 1984) or both present and absent (Figs 3A, 5A respectively; Haszprunar, 1996). Fine filaments connect the external surface of the sieve area of the rhogocyte and the overlying basal layer (Haszprunar, 1996). Fine filaments were not observed to be associated with grated areas of granulate cells and, in addition, a basal layer was either thin or absent. Collagen fibres were observed to arise from the cell membrane of granulate cells of A. californica, but not in D. dolabrifera where they generally appeared parallel to the cell membrane. In addition, the cisternum beneath the sieve area of granulate cells frequently gave rise to numerous coated vesicles and became elaborated, apparently to provide a greater surface area for coated vesicle formation (Prince et al., 1998: Fig. 10). Under ad libitum feeding conditions the coated vesicles contain electron dense material similar to that within the vacuoles of the granulate cell and the purple ink vesicles themselves (Prince et al., 1998). Only Martoja & Tue (1980) and possibly Fioroni et al. (1984) show involvement of coated vesicles with this cisternum in rhogocytes. Simkiss & Mason (1983: Fig. 8), Haszprunar (1996: Fig. 5) and Albrecht et al. (2001: Fig. 2) show no involvement of vesicles with this sieve cisternum (in their artist reconstructions) but, rather, this membrane appears smooth. Haszprunar (1996) states, furthermore, that vesicle formation is rarely associated with the subsurface cisternum in rhogocytes. Coated vesicles were frequently seen associated with the subsurface membrane in the grated areas of granulate cells of D. dolabrifera, even though their vacuoles were generally empty of electron-dense material.
Slit dimensions in sieve areas of granulate cells appeared, furthermore, to vary between different genera of sea hares; those of D. dolabrifera were significantly greater than those of A. californica. Additionally, filaments that crossed the slits also appeared more widely spaced in D. dolabrifera than in A. californica (15 nm, Prince et al., 1998). This provided an effective pore size of ca. 37 by 48 nm for D. dolabrifera. This is the first record of a significant difference in slit dimensions for sieve areas for either cell type. The slit dimension in D. dolabrifera was, therefore, larger than the size (ca. 35 nm) of the multidecamers of the respiratory pigment, haemocyanin, which is apparently synthesized in rhogocyte cells of the keyhole limpet (Albrecht et al., 2001). Albrecht et al. (2001) proposed holocrine secretion for the release of these respiratory pigments, since their large size precluded passage through the slits of the rhogocyte cell. If slit dimensions are, indeed, variable then this could provide an additional mechanism for release of large molecules by rhogocytes.
The biological role of rhogocytes is a matter of debate (for a review see Haszprunar, 1996; Albrecht et al., 2001), but in general it appears to be as a molecular sieve for the uptake of soluble material which is then either catabolically modified (Simkiss & Mason, 1983), detoxified (Martoja & Tue, 1980; Albrecht et al., 2001) or stored, frequently accumulating as electron dense vacuoles. However, haemocyanin is synthesized in rhogocytes (Albrecht et al., 2001), but questions remain about how it leaves the cell. Material uptake generally occurs by endocytosis (Simkiss & Mason, 1983; Haszprunar, 1996) but, as mentioned above, only Martoja & Tue (1980), Fioroni (1984) and Prince et al. (1998) show any involvement of vesicles with the sieve areas. Our studies (present report and Prince et al., 1998) suggest, furthermore, that granulate cells are a site for the storage of excess red algal pigment during episodes of luxury feeding. Coated vesicles that ‘bud off’ from the cisternum subtending the sieve area apparently take up the pigment and eventually fuse with vacuoles in the cell, the latter becoming electron dense. Ink pigment granules are apparently small enough to pass through the slits (Prince et al., 1998), while this seems not to be the case for respiratory pigments synthesized within rhogocytes (Simkiss & Mason, 1983; Albrecht et al., 2001).
Granulate cells in the ink glands of sea hares, therefore, share several characteristics with rhogocytes, in particular the modification of the cell membrane into sieve areas subtended by a cisternum of the cell membrane. Granulate cells, however, have several unique features and, furthermore, appear to function in the storage of excess pigment derived from their algal food and not synthesized by the cell itself. Movement of ink pigment to vacuoles appears, to be mediated by coated vesicles. These traits suggest that granulate cells are distinct from rhogocytes and should be considered as a special cell type found in the ink gland of sea hares. Our research also suggests that the site in A. californica for the synthesis of the 60–70 kDa, anti-predator protein component of released ink, escapin, is likely the RER cells and not the ink vesicles themselves. Current research using antibody labelling for the ink protein in A. californica will try to verify this hypothesis.
ACKNOWLEDGEMENTS
This research was supported in part by the Dauer Electron Microscopy Laboratory, Biology Department, University of Miami, the NCRR Aplysia Resource facility (1p40RR10294-06) and an NSF Graduate Research Fellowship to P.M. Johnson. We thank Drs Robert Rowan and Valerie Paul for help with the collection and maintenance of Dolabrifera dolabrifera.



![Ink glands (neither fixed nor stained) of Aplysia californica (A) and Dolabrifera dolabrifera (B, C). Abbreviations: av, amber vesicle; cav, calcium carbonate containing vesicles [Prince, unpublished]; cv, clear vesicle; rpv, red-purple vesicle. Scale bars: A=0.1 mm; B=0.25 cm; C=0.6 mm.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/mollus/72/4/10.1093_mollus_eyl017/2/m_eyl01701.jpeg?Expires=1716377298&Signature=DEcG~eQyyezyF6RlRPUwltyS2CG2IGW4oWnGczFaMruwXLFOYTcFayKmeS~zzmTBNgQXnwaCnBuWkOJnwYe-HnKOi5HV2sa37CiDyqOLG1e1khM-bCCu01Xd44H-1QyX6WqTLG1LY3Y-pdLFrPVrZ-mC3h2qLnj9zXT6HuB4LlNcpb~vtiVUMndmqinKl8hoPN2sW9SH1CphY9YaVTNIMJrIVg-Sirue1COeIoXSJvNx~1JFfVTgI7ITMhQKHoDFnhQkthgDSr4-ALxv8PozYa44tu9q~TI6ho1uWm1AcultDzdgkiHaa4xk5XkNoNqW7ag2PRvcJkR68WVnxjDcQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)