-
PDF
- Split View
-
Views
-
Cite
Cite
Paula Noonan, Siobhan Prout, Virginia Hayssen, Pteronura brasiliensis (Carnivora: Mustelidae), Mammalian Species, Volume 49, Issue 953, 8 September 2017, Pages 97–108, https://doi.org/10.1093/mspecies/sex012
Close - Share Icon Share
Abstract
Pteronura brasiliensis (Zimmermann, 1780), the giant otter, is the largest freshwater otter. Found in South America, it inhabits slow-moving rivers and creeks and feeds predominantly on fish. Extinct in the southern portions of its former range, P. brasiliensis is listed as “Endangered” by the International Union for Conservation of Nature and Natural Resources. Threats to P. brasiliensis include habitat destruction, illegal hunting, and disease.
Pteronura Gray, 1837
Lutra Zimmermann, 1777:485. Type species Lutra brasiliensis Ray, 1693.
Saricoviene Zimmermann, 1777:485. Type species Lutra brasiliensis Ray, 1693.
Mustela Gmelin, 1788:93. Type species Mustela lutris brasiliensis (Ray, 1693).
Saricovienne Schinz, 1821:213. Type species Lutra brasiliensis Ray, 1693.
Pteronùra Gray, 1837:580. Type species Pteronùra sambachii.
Pterura Wiegmann, 1839a:285. Type species Pteronùra [sambachii] Gray, 1837.
Pteronurus Lesson, 1842:72. Unjustified emendation of Pteronura Gray, 1837.
Saricovia Lesson, 1842:72. Type species Lutra brasiliensis Ray, 1693.
Lontra Gray, 1843:70. Type species Lutra brasiliensis Ray, 1693.
Pteroneura Sanderson, 1949:774. Unjustified emendation of Pteronura Gray, 1837.
Context and Content. Order Carnivora, family Mustelidae, subfamily Lutrinae. Pteronura is monotypic.
Pteronura brasiliensis (Zimmermann, 1780)
Giant Otter
Lutra brasiliensis Ray, 1693:189. No type locality given; stated as “in fluviis americae meridionalis” by Gmelin (1788:93); and subsequently restricted to “río São Franciso, en la orilla correspondiente al estado de Alagoas” Brazil by Cabrera (1958:274).
Lutra nigricans Barrère, 1749:155. No type locality; refers to a composite specimen.
Lutra atri coloris Brisson, 1762:278. Type locality “Brésil.”
Lutra (brasiliensis) Zimmermann, 1780:316. First legitimate use after 1758. Parentheses in original.
[Mustela lutris] brasiliensis: Gmelin, 1788:93. Name combination.
Lutra brasiliana Shaw 1800:446. Unjustified emendation of brasiliensis Ray, 1693.
Lutra nitens Olfers, 1818:233. Type locality “Brasilien.”
Lutra lupina Schinz, 1821:213. No type locality.
Lutra paraguaensis Schinz, 1821:213. No type locality.
Lutra paranensis Rengger, 1830:128. Type locality “Paraguay.”
Pteronùra sambàchii Gray, 1837:580. Type locality “Demerara.”
Pteronura sanbachii Gray, 1839:Plate XIV. Unjustified emendation of sambachii Gray, 1837.
Pteronurus sandbackii Lesson, 1842:72. Unjustified emendation of sambachii Gray 1837.
Lutra sandbackii: Lesson, 1842:72. Name combination.
Saricovia brasiliensis: Lesson, 1842:72. Name combination.
Mustela brasiliensis: Lesson, 1842:72. Name combination.
Lontra brasiliensis: Gray, 1843:70. Name combination.
Pterura sandbachii: Gray, 1869:114. Name combination attributed to Wiegmann, 1839b:392.
Pteronura sandbachii kappleri Gray, 1869:114. Type locality “Surinam.”
Pteronura brasiliensis: Thomas, 1908:390. First use of current name combination.
[Pteronura] sambacchii Pohle 1919 (1920):115. Unjustified emendation of sambachii Gray, 1837.
P[teronura]. b[rasiliensis]. lupina: Pohle 1919 (1920):117. Name combination.
P[teronura]. b[rasiliensis]. paranensis: Pohle 1919 (1920):117. Name combination.
Pteroneura braziliensis Sanderson, 1949:774. Unjustified emendation of brasiliensis Ray, 1693.
Context and Content. Context as for genus. Historically a northern and southern subspecies have been named but genetic data do not support this distinction (Garcia et al. 2007). Thus, the species is monotypic.
Nomenclatural Notes. Lutra brasiliensisRay, 1693 and Lutra nigricansBarrère, 1749 predate the formal start of binomial nomenclature. In addition, Barrère’s description refers to a composite of P. brasiliensis and Eira barbara identified as Carigueya brasilienebus by Marcgraf 1648:222. Saricoviene was used by Zimmermann (1777:485) in reference to the giant otter but Saricovienne was used by Shaw (1800:447) for a cat-sized otter and thus not Pteronura. Gray (1869:114) attributes Pterura sandbachii to “Wiegmann’s Arch. iv. p392, 1838 (published 1839)” but Wiegmann refers only to the genus and does not give a species name. Confusion regarding nomenclature arose from early descriptions confounding the sea otter (Enhydra lutris) and Pteronura brasiliensis (extensive details in Harris 1968; Husson 1978).
Common names include hwana-dagu (Sanderson 1949), lontra (Gray 1843), margin-tailed otter (Thomas 1908), and Surinam otter (Gray 1868). Other common names are Guiana flat-tailed otter, winged-tailed otter, grote waterhond, grote visotter, platstaart otter, and watradogoe (Husson 1978), as well as ariranha, lobito de cola ancha, lobo del río, lobo corbata, londra, lobo del río grande, lontra gigante, onça-d’água, and perro de água (Carter and Rosas 1997; Rodrigues et al. 2013). Local names in Argentina include lobo gargantilla, arirai, and nutria gigante (Chehèbar 1991); in Guyana, water dog, turara, saaru, eniabu peru, and turáclá (van der Waal 2012).
DIAGNOSIS
Pteronura brasiliensis is longer (maximum total length: up to 1,800 [intact]–2,440 mm [skin] versus 1,200 mm) and heavier (23–32 kg versus 5–15 kg) than the Neotropical otter, Lontra longicaudis (formerly, Lutra enudris—Harris 1968; Larivière 1999; Rosas et al. 2009a). The webbing on the feet of P. brasiliensis extends to the claws, but that of the Neotropical otter does not (Husson 1978). The tail of P. brasiliensis is flattened, while that of the Neotropical otter is cylindrical (Husson 1978). The Neotropical otter lacks the white patches on the throat (Duplaix 1980).
GENERAL CHARACTERS
Pteronura brasiliensis (Fig. 1) has fawn, reddish, dark grayish, or chestnut brown fur with white or cream patches on the throat (Harris 1968; Husson 1978). Individuals can be identified by their throat patterns (Duplaix 1980; Schenck and Staib 1998). Rarely, individuals (3 out of 294) have no throat marking (Groenendijk et al. 2014).
Adult Pteronura brasiliensis from Karanambu-Rupununi, Guyana (© Pete Oxford/naturepl.com).
For 11 captive males from the Amazon River basin, the maximum total length was 1,630 mm, and the maximum mass 22.5 kg; 4 females had a maximum length of 1,620 mm and mass of 28.8 kg (Rosas et al. 2009a). The mass–length relationship did not reveal significant sex differences and is expressed by W (kg) = 1.48 × 10−5L (cm)2.81 (Rosas et al. 2009a). Compiled from over 15 sources from 1817 to 1978 and using diverse techniques including measurements of skins, historical ranges for total length for males were 1,500–1,800 mm and for females 1,500–1,700 mm; the weight range for females (22–26 kg) was lower than that for males (26–32 kg—Duplaix 1980). External measurements (mm) for an adult male from Suriname were: head-body length, 1,050; tail length, 573; length of hind foot with claw, 175 (Sanderson 1949). Measurements (mm) for an adult male and female from the Araguaia River in Brazil were: head circumference, 325, 330; neck circumference, 325, 365; head length, 220, 190; head-body length: 1,070, 970; tail length, 650, 555; ear length, 22, 22; ear width, 20, 20; length of hind foot, 210, 190; height, 330, 280 (Silveira et al. 2011). Mean total length (mm) and mass (kg) of 6 captive males in Brazil, but of unknown origin, were: 1,556.67 and 21.25, respectively (de Oliveira et al. 2011). Mean measurements (mm; parenthetical n) for males and females, respectively, from unspecified locations were: head-body length, 948.33 (2), 1,045, (2); tail length, 492.67 (3), 555 (2); total length, 1,441 (3), 1,615 (3—Rengger 1830; Nehring 1899[1900]; Allen 1910; Vieira 1952). Mean lengths (mm; parenthetical n) of animals of unknown sex and location were: head-body length, 1,007.83 (6); tail length, 552.83 (6); total length, 1,480.1 (10—Schomburgk 1840; Gervais 1855; Gray 1869; Burmeister 1879; Waterton 1879; Quelch 1901; Fountain 1902; Santos 1945; Burton 1962). Masses (kg) for 3 animals of unknown sex were: 31.8, 34.2, 24.0 (Fountain 1902; Sanderson 1949). Measurements (mm) for a subadult female were: head-body length, 770; tail length, 500; and total length, 1,270 (Nehring 1899[1900]). The skeleton of a 4-month-old P. brasiliensis had a head-body length of 324 mm, tail length of 191 mm, and a total length of 515 mm (Rengger 1830). A 3- to 4-month-old male cub from Brazil weighed 4 kg; total length was 1,140 mm, tail length 330 mm, and hind foot length 130 mm (Lima and Marmontel 2011). Four (2 males and 2 females) 3-day-old cubs weighed 198, 177, 192, and 172 g, respectively; total lengths were 327, 325, 318, and 324 mm; tail lengths 121, 120, 118, and 123 mm; hind foot lengths 39, 37.5, 37.6, and 39 mm; and ear lengths 4.6, 4.5, 4.8, and 4.6 mm (Hantke and Kitchener 2015).
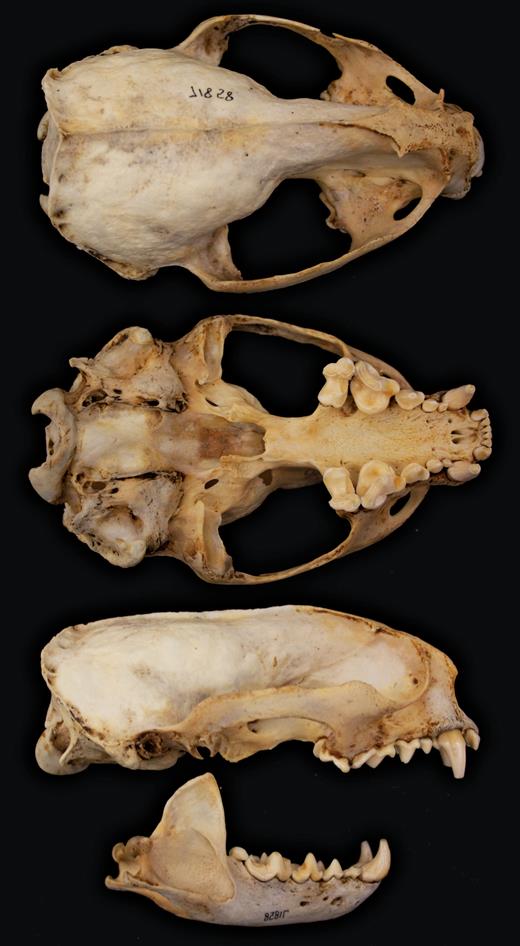
Skull (Fig. 2) measurements (mm) for 1 female and 4 males, respectively, from Suriname were: condylobasal length: 153.6, 143.1, 145.8, 155.8, 154.9; palatal length: 76.3, 71.5, 75.0, 77.8, 76.0; zygomatic breadth: 92.0, 85.0, 94.3, 94.9, 97.2; interorbital constriction: 17.1, 17.5, 15.8, 16.7, 17.4; postorbital constriction: 17.6, 18.1, 16.1, 16.7, 17.3; length of postorbital constriction: 35, 27, 32, 41, 35; mastoid breadth: 82.4, 76.0, 79.4, 87.4, 81.1; length of upper toothrow C–M1: 48.0, 45.8, 47.6, 50.0, 49.6; length of upper carnassial: 18.3, 16.8, 17.3, 17.8, 17.4; greatest diameter of upper molar: 16.7, 15.5, 15.5. 15.4, 16.1; width across canines: 35.0, 31.8, 31.8, 31.8, 34.0; length of mandible: 99.5, 95.9, 100.1, 104.1, 103.9; length of lower carnassial: 19.6, 19.0, 18.0, 18.7, 19.5 (Husson 1978). Skull measurements (mm) for 3 females were: condylobasal length: 148.0, 154.0, 164.0; basal length: 135.0, 142.0, —; width across postorbital processes: 31.0, 24.6, —; interorbital breadth: 19.3, 18.4, —; intertemporal length: 37.0, 34.0, —; mastoid breadth: 97.7, 85.2, —; zygomatic width: 97.4, 97.9; 98.0; length of mandible: —, —, 105.0; total length: —, —, 165.0 (Pohle 1919 (1920); Vieira 1952). Skull measurements (mm) for 2 males were: condylobasal length: —, 149.0; basilar length: 142.0, —; zygomatic width: 99.0, 98.0; width of mastoid process: 88.0, —; length of mandible: —, 105.0; height of forehead (including lower jaw): 74.0, —; total length: 157.0, 150.0 (Nehring 1899[1900]; Vieira 1952). Skull measurements (mm) for 2 adults of unknown sex were: condylobasal length: 147.1, 147.5; basal length: 136.5, 135.? [sic]; interorbital breadth: 18.6, 16.2; width across postorbital processes: 23.8, 19.2; intertemporal length: 32.0, 27.0; mastoid breadth: 75.3, 77.3; zygomatic width: 93.7, 92.1 (Pohle 1919 (1920)).
Dorsal, ventral, and lateral views of skull and lateral view of mandible of an adult male specimen AMNH (American Museum of Natural History) 71858. Greatest length of skull is 147.25 mm.
Limb measurements (mm) from 1 specimen (AMNH [American Museum of Natural History] 30190) were: scapula—maximum length of head, 22.7, maximum width of head, 14.2, length of neck, 20.2, width of neck, 9.6; humerus—length, 113.6, proximal width, 26.3, distal width, 33.3; radius—length, 74.9, proximal width, 14.6, proximal depth, 11.3, distal width, 20.5, distal depth, 14.8; scapholunar—length, 9.6, width, 9.4, depth, 12.5; cuneiform—length, 5.4, width, 9.4, depth, 12.2; metacarpal—length, II, 35.6, III, 40.7; femur—length, 102.0, proximal width, 33.3, midshaft width, 13.9, midshaft depth, 10.7, distal width, 30.1, distal depth, 25.6; tibia—length, 116.2, proximal width, 29.9, distal width, 23.2, distal depth, 15.2; astragalus—length, 26.7, width, 17.5; calcaneum—length, 40.9, width, 21.3; navicular—width, 20.0, depth, 10.7; cuboid—length, 14.6, width, 11.6; ectocuneiform—length 10.2, width, 10.0, depth, 16.2; metatarsal—length I, 32.4, II, 47.2, III, 56.9, IV, 60.1 (Bjork 1970).
The medullary cone apex was close to the L4 vertebra on 2 specimens and between the L3 and L4 vertebrae on a 3rd specimen (Machado et al. 2009). The medullary cone length was 55 mm for all 3 (Machado et al. 2009).
DISTRIBUTION
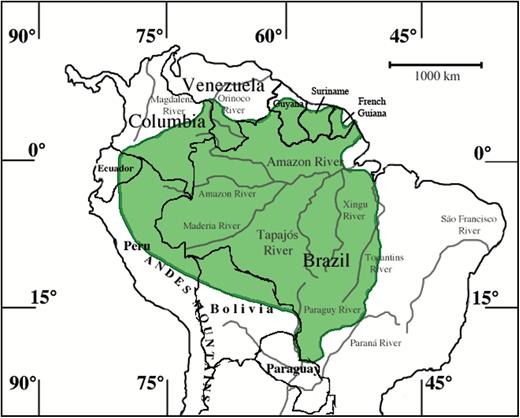
Pteronura brasiliensis (Fig. 3) lives on major river systems in South America from the Guianas to Uruguay (Harris 1968) up to elevations of about 1,000 m (Groenendijk et al. 2015). In Bolivia, the minimum population estimate is 350 individuals, mostly within national parks (van Damme et al. 2002). It has disappeared from the southern portion of Brazil, except for isolated populations in the Amazon basin (Chehébar 1990); 80 otters were sited near the Jauaperi River (Evangelista and Rosas 2011b). Colombia, Guyana, and Suriname have the strongest populations (Chehébar 1990; Barnett et al. 2000); in the Orinoquia region of Colombia, a group of 8 individuals was observed (Díaz and Sánchez 2002). In Ecuador, P. brasiliensis was found in isolated areas (Chehébar 1990). In French Guiana, it may live in the interior (Chehébar 1990). In Bolivia, small populations of P. brasiliensis are present in national parks (Carter and Rosas 1997). In Ecuador and Peru, P. brasiliensis lives east of the Andes, with populations in isolated Amazon tributaries (Carter and Rosas 1997); 8 otters were sited near the Tapiche River (Tramm 2014). In Paraguay, populations lived on the Paraguay and Prana rivers, with remnant populations elsewhere (Carter and Rosas 1997). P. brasiliensis is probably extinct in Uruguay (Carter and Rosas 1997) and Argentina (Parera 1992). In Venezuela, small populations exist in protected areas (Chehébar 1990). A decades old skull was recovered from Corrientes Province, Argentina (Beccaceci and Waller 2000).
Geographic distribution of Pteronura brasiliensis. Modified from the International Union for Conservation of Nature and Natural Resources Red List of Threatened Species (2015).
FOSSIL RECORD
The extinct Pliocene Satherium piscinaria may be an ancestor to Pteronura brasiliensis, based on comparisons of skull, dental, and skeletal measures (Bjork 1970). A fossil specimen of P. brasiliensis, the first to be found in Argentina, was also determined to be the oldest of existing records, at 125,000–130,000 years ago (Prevosti and Ferrero 2008). The body was larger than current specimens and showed minor differences in the skull and dentition (Prevosti and Ferrero 2008). Analysis of 21 P. brasiliensis skins from the upper Amazon and the Orinoco basin determined that 2 clades diverged about 37,000 years ago, and divergence within those clades was between 10,000 and 20,000 years ago (de Thoisy et al. 2013).
FORM AND FUNCTION
Form
The iris is pale green-yellow ochre (Sanderson 1949). The nose is hairy (Gray 1868). Pteronura brasiliensis has long mysticial, supraciliary, and gular virbrissae (Duplaix 1980). Outside each ankle is a large tuft of hair (Gray 1868). Anal glands are 40 mm with 5-mm diameter, black openings, on either side of the anus (Sanderson 1949). The secretions of the anal gland are a cream color, can be forcibly ejected, and have a fishy smell (Sanderson 1949). The tail is furred, thick, and rounded with a ridge on each side (Gray 1868). The plantigrade feet have 5 webbed digits with claws (Duplaix 1980).
The pars intermedia of the hair shafts exhibit an irregular mosaic pattern (Kuhn and Meyer 2010). The primary hairs (mm, mean ± SD) are short and parallel, 11 ± 0.6; the secondary hairs are 5 ± 0.2 (Kuhn and Meyer 2010). The width of the primary hairs is 0.097 ± 0.0099 (Kuhn and Meyer 2010).
Function
Hemoglobin values (mean ± SD) of 6 captive individuals (3 male, 3 female) were 16.36 ± 2.81 g/dl; mean packed cell volume was 50.66 ± 7.77% (Rosas et al. 2008a). Red blood cell counts were 6.82 ± 1.64 106/mm3 for females, 5.860 ± 0.59 106/mm3 for males. White blood cell counts were 6.325 ± 1.52 103/mm3 for females, 4.77 ± 1.66 103/mm3 for males (Rosas et al. 2008a). Other hematology values (not separated by sex) were: mean corpuscular value, 74.63 fl; mean corpuscular hemoglobin, 24.58 pg; mean corpuscular hemoglobin concentration 33.05 g/dl; red cell distribution width, 14.54%; platelets, 401.81 103/mm3; platelet distribution width, 14.28%; basophils, 0.30%; eosinophils, 0.87%; segmented neutrophils, 80.03%; lymphocytes, 16.24%; monocytes, 1.10%. Mean biochemistry values were: glucose, 100.67 mg/dl; uric acid, 1.33 mg/dl; urea, 221.50 mg/dl; creatinine, 1.80 mg/dl; cholesterol, 314.33 mg/dl; triglycerides, 19.50 mg/dl; aspartate aminotransferase, 183.67 U/l; alanine aminotransferase, 114.33 U/l; total bilirubin, 0.20 mg/dl; alkaline phosphates, 53.33 U/l; lactic dehydrogenase, 1226.33 mg/dl; gamma GT, 17.67 U/l; amylase, 9.33 U/l; calcium 9.57 mg/dl; phosphorus, 5.80 mg/dl; magnesium, 3.73 mg/dl; total protein, 7.00 g/dl; albumin, 3.60 g/dl; globulin, 3.70 g/dl (Rosas et al. 2008a).
Dentition is i 3/3, c 1/1, p 4/3, m 1/2, total 36, with the lower premolar sometimes absent (Husson 1978; Duplaix 1980). Infrared thermography shows heat dissipation from the entire body and tail due to its relatively short and thin hair, although the coldest parts of the body are the feet and tail (Kuhn and Meyer 2009, 2010).
ONTOGENY AND REPRODUCTION
Gestation is 52–70 days (Hayssen et al. 1993; Carter and Rosas 1997). In a captive population of Pteronura brasiliensis, the mean interval between births was 180–214 days (n = 3—Londoño and Muñoz 2006). Litter size is 1–5 young with an average of 2 (Hayssen et al. 1993; Carter and Rosas 1997; Groenendijk et al. 2014). Pseudopregnancies may last as long as a normal pregnancy (Londoño and Muñoz 2006).
Neonatal mass is 0.20 kg (Carter and Rosas 1997). The sex of cubs as young as 3 days old can be determined by comparing the distance between the genital and anal openings, with the distance for male cubs about 8 times longer than that of females (Hantke and Kitchener 2015). By 2 weeks, cubs can float and swim but with the tail in the air and superficial immersion (Evangelista and Rosas 2011a). Eyes open at 1 month (Carter and Rosas 1997). By 6–8 weeks, pups are able to swim independently (Londono and Munoz 2006; Evangelista and Rosas 2011a). Initial attempts at hunting begin around 2.5 months (Evangelista and Rosas 2011a). Weaning and autonomy occurs at 4–9 months (Hayssen et al. 1993; Carter and Rosas 1997; Evangelista and Rosas 2011a). Subadults usually swim in the middle of a group (Duplaix 1980). Growth layers in canines can be used to age P. brasiliensis (de Oliveira et al. 2007).
An analysis of 6 P. brasiliensis males that died in captivity in Brazil showed a ratio of testes mass to body mass of 0.046 ± 0.0071% (SD—de Oliveira et al. 2011). Mean diameter (μm ± SD) of the seminiferous tubules was 126.3 ± 13.37; for epididymal tubules, 198.8 ± 31.39 (de Oliveira et al. 2011). The length and width (cm ± SD) of the testes were 2.76 ± 0.15 and 1.46 ± 0.09, respectively (de Oliveira et al. 2011). The presence of spermatozoa indicated that 2-year-old P. brasiliensis are sexually mature (de Oliveira et al. 2011).
ECOLOGY
Population characteristics
Between 4,400 and 7,600 Pteronura brasiliensis are estimated to be in wild populations (Groenendijk et al. 2015). One male lived approximately 15 years in the wild (Davenport 2010). A long-term study in Peru recorded the oldest male at 15.5 years and oldest female at 13.5 years, although the average life expectancy was 4.5–5.5 years (Groenendijk et al. 2014). In captivity, maximum life span of P. brasiliensis is 17–20 years (Weigl 2005; de Oliveira et al. 2007). Two deaths in Brazil were described as the natural death of a young adult female due to emaciation, and the other the result of serious wounding to an adult (Rosas and de Mattos 2003).
Space use
Pteronura brasiliensis prefers slow-moving water with an abundance of food and sloping banks with protective cover (Duplaix 1980). Groups may remain in the same territory for 5 or more years (Schenck and Staib 1998), but may abandon territories during the flooding season (Leuchtenberger et al. 2013). The mean size of P. brasiliensis territories in the Panatanal of Brazil was 3.6–7.9 km2 in the wet season and 0.1–2.3 km2 in the dry season (Leuchtenberger et al. 2013). During the dry season in Xixuaú Reserve, Brazil, 4 territories ranged from 4.6 to 10.5 km; for 1 group, the range contracted when cubs were present (Evangelista and Rosas 2011b). The average distance between the centers of 2 group territories was 10.8 km (Leuchtenberger and Mourão 2008).
Campsites are areas along a riverbank that have been cleared of vegetation (Duplaix 1980). In Suriname, campsites were about 800 by 700 cm (Duplaix 1980). In Brazil, average campsite length (n = 193) was 312 cm and 270 cm wide (Lima et al. 2012). Average resting sites (n = 62), cleared but not associated with a latrine, were 408 cm long and 235 cm wide (Lima et al. 2012). In Brazil, mean den entrance width (n = 182) was 56 cm, with an average height of 41 cm (Lima et al. 2012). The average height of 49, oval, round or elongate den openings was 28.77 cm with a width of 56.11 cm (Rosas et al. 2007). Dens averaged 175 cm above water level with a slope of 29° (Lima et al. 2012). A den may have 1–3 chambers, as well as a backdoor tunnel (Duplaix 1980).
Spraints are found in communal latrines, where P. brasiliensis mixes the feces with mud (Duplaix 1980). The latrines are in the core of territories, but individual spraints may mark the edges of the territory (Duplaix 1980).
Diet
Pteronura brasiliensis is primarily piscivorous especially when dry conditions concentrate fish in small areas, but it will opportunistically add crustaceans, mollusks, and terrestrial vertebrates to its diet (Duplaix 1980; Estes 1989; Rosas et al. 1999; Staib 2005; Cabral et al. 2010; Silva et al. 2014). Studies of communal latrines on the Brazilian Amazon revealed Perciformes to be the most frequent prey, followed by Characiformes (Rosas et al. 1999; Cabral et al. 2010; Silva et al. 2014). P. brasiliensis feces at the Balbina hydroelectric reservoir in Brazil frequently contained remnants of pirhanas (Cabral et al. 2010). Drought conditions may have resulted in P. brasiliensis feeding on caimans in the Pantanal wetland (Ribas et al. 2012).
Pteronura brasiliensis immediately consumes what it catches; the prey is eaten headfirst (Duplaix 1980). Dives can last from 5 to 72 s (Duplaix 1980). Underwater, it turns over rocks, scrapes submerged wood, and digs in the substrate on the bottom (Costa-Pereira 2012).
Average daily food consumption was 2.29 kg for an adult female and 1.52 kg for a young male in Brazil (Carter et al. 1999). The mean digestive transit time for 3 otters was 3.13 h (SD = 0.52 h), as determined by particulate markers (Carter et al. 1999).
Diseases and parasites
Pteronura brasiliensis is highly susceptible to parvovirus and distemper, which may be transmitted by village dogs (Schenck and Staib 1998). Otters looking for mates are potential vectors for transmission of these diseases (Schenck and Staib 1998). One of 3 P. brasiliensis in a captive population tested positive for Salmonella aberdeen (Gopee et al. 2000).
Although 21 out of 26 carnivore species in Brazil had ticks (Amboyomma, Boophilus microplus, Dermacentor nitens, Ixodes aragaoi, and Rhipicephalus sanguineus), P. brasiliensis was 1 of the 5 species that did not (Labruna et al. 2005). One tick nymph (Amblyomma cajennense) was attached to the lower lip of a P. brasiliensis (Rosas et al. 2016). Endoparasites include Alaria clathrate, A. pseudoclathrata, Anchlostoma, Baschkirovitrema incrassatum, Cryptocotyle thapari, Cryptosporidium, Diphyllobothrium, Diplostomum alarioides, Dirofilaria, D. spectans, Galeiceps longispiculum, Molineus major, Paragonimus rudis, Strongyloides, Subulura amazonica, S. interrogans (Freitas and Lent 1941, 1949; Rosas et al. 2016).
Interspecific interactions
In Guyana, predators of Pteronura brasiliensis are reported as jaguars (Panthera onca), anacondas (Eunectes), and caimans (Shackley 1998) but without direct evidence. Felids (e.g., puma—Puma concolor), birds of prey (e.g., harpy eagle—Harpia harpyja), and large snakes (e.g., bushmaster—Lachesis muta) are potential predators. One cub was preyed on or scavenged by a lizard in Brazil (Rosas et al. 2008b). Also in Brazil, a solitary, sleeping adult female P. brasiliensis was killed by a jaguar (Ramalheira et al. 2015). In contrast, P. brasiliensis groups can mob jaguars and force them to leave the area (Leuchtenberger et al. 2016).
In Suriname, P. brasiliensis and the Neotropical otter are sympatric seasonally and perhaps year-round on several river systems (Duplaix 1980). However, their diets differ in composition and size of prey (Duplaix 1980; Silva et al. 2014). P. brasiliensis consumes larger fish, but the Neotropical otter eats a wider variety of prey, including a rodent (Silva et al. 2014). Also in Brazil, a study showed that the Neotropical otter has a large ecological space that encompasses a smaller P. brasiliensis space, with the Neotropical otter associated with deep, wide waterways and P. brasiliensis with creeks (Muanis and Oliveira 2011).
A close association has been observed between P. brasiliensis and Inia geoffrensis, the freshwater dolphin, in Colombia (Defler 1983). P. brasiliensis fishes close to riverbanks while dolphins fish 1–5 m away, and the 2 species occasionally swim upriver together, a short distance apart (Defler 1983). In Brazil, small fishes have been observed emerging to feed after P. brasiliensis disturbs the underwater sediment (Costa-Pereira 2012).
Miscellaneous
Radiotagging 2 Pteronura brasiliensis with 42-g transmitters implanted in the peritoneal cavity provided location data for up to 12 months (Silveira et al. 2011; Leuchtenberger et al. 2013). Biopsy darts were used to obtain tissue samples for genetic analysis of 45 P. brasiliensis (Ribas 2012).
HUSBANDRY
Two forms of anesthesia have been given intramuscularly (mean ± SD) in a captive population. The 1st form (n = 4) consisted of 5% ketamine hydrochloride (8.78 ± 0.91 mg/kg) + 2% xylazine (1.92 ± 0.15 mg/kg). The induction time was 7.75 ± 0.96 min (Rosas et al. 2008a). The 2nd form (n = 7) consisted of tiletamine hydrochloride/zolazepam hydrochloride (1.93 ± 0.57 mg/kg) followed by 1% atropine sulfate (0.09 ± 0.02 mg/kg). The induction time was 4.33 ± 0.58 min (Rosas et al. 2008a). Mean rectal temperature under anesthesia (n = 8) was 39.16 ± 0.59°C (Rosas et al. 2008a). A mixture of the 2 anesthesia protocols is also used (Leuchtenberger et al. 2013). Three males captured in Brazil were anesthetized for implantation of radiotransmitters using a combination of tiletamine and zolazepam (2.0 mg/kg) and a dosage of 1.5 mg/kg ketamine hydrochloride (10%) combined with 0.25 mg/kg midazolam (Leuchtenberger et al. 2013). The otters were also given 0.5 ml of penicillin (intramuscularly) and 2 mg/kg of an anti-inflammatory/analgesic (subcutaneously—Leuchtenberger et al. 2013).
Minor wounds and lacerations of young adult rehabilitated P. brasiliensis (15–22 kg) were treated with 250 mg of amoxicillin (15–20 mg/kg 2 to 3 times a day), 120 mg enrofloxacin (5–7.5 mg/kg once a day), and 325 mg aspirin (15 mg/kg once or twice a day) with the medications added to the fish diet (McTurk and Spelman 2005).
Young adult rehabilitated P. brasiliensis were monthly given the following on a rotating basis: pyrantel pamoate (10 mg/kg) for nematodes, ivermection (0.2 mg/kg) for parasites (including ectoparasites), and praziquantel (5 mg/kg) for cestodes (McTurk and Spelman 2005). Extensive husbandry information for captive-bred populations can be found in Sykes-Gatz (2004) and Smith et al. (2009). Captive breeding occurs internationally at several facilities in South America, Europe, North America, and Asia (Sykes-Gatz 2004; Wildlife Reserves Singapore 2013).
BEHAVIOR
Grouping behavior
Pteronura brasiliensis is diurnal, social, and territorial (Duplaix 1980; Carter and Rosas 1997; Ribas and Mourão 2004). In Suriname, groups were made up of an alpha pair and different generations of their offspring, up to 20 individuals total (Duplaix 1980). In Balbina hydroelectric lake in Brazil, 130 P. brasiliensis were observed in 29 groups, with a mean group size of 4.14 (the largest was 12—Rosas et al. 2007); in the Xixuaú Preserve, 80 otters were recorded as part of 15 groups that averaged 4.46 individuals (Evangelista and Rosas 2011b). In Argentina, P. brasiliensis individuals have been seen but not in groups (Chehébar 1990). Although social groups are generally assumed to be composed of a dominant male and female and their prior offspring, genetic analysis of 13 P. brasiliensis groups indicated variable relatedness among groups although on average the relatedness within groups was high (r = 0.23—Ribas et al. 2016). Groups of P. brasiliensis mark and defend their territories with scent marks and vocalizations (Duplaix 1980; Ribas and Mourão 2004; Leuchtenberger and Mourão 2009).
A group of related P. brasiliensis may travel and hunt together and usually remain within calling distance of each other (Duplaix 1980). One or more females determine the group’s activities (Duplaix 1980). Two otters may touch nose to nose, even after a few minutes of separation (Duplaix 1980). When the mate of a breeding female disappears, her new mate adopts the cubs of the previous male (Evangelista 2004; Groenendijk et al. 2014).
In Brazil, a group was observed attacking an individual that had entered their territory; the 4 individuals swam at a high speed with their heads held above the surface for 15 s at a time (Ribas and Mourão 2004). This unusual swimming pattern was accompanied by vocalizations, and individuals took turns attacking the intruder (Ribas and Mourão 2004). Such attacks to the face, genitals, and forelimbs may result in death (Rosas and de Mattos 2003).
Transients are unmated individuals outside of a group (Duplaix 1980; Laidler 1986). In Guyana, transients were observed following a family group that contained cubs, perhaps in search of a mate (Laidler 1986). After the family left a site, the transients would mark it (Laidler 1986). Groups of 2–5 nonbreeding transients, either single-sex or mixed-sex individuals, have been observed (Groenendijk et al. 2014).
Reproductive behavior
A pair of Pteronura brasiliensis in captivity began copulating 3–10 days after pups were born (Londoño and Muñoz 2006). The male initiated copulation, which lasted 5–110 min (Londoño and Muñoz 2006). The male mounted the female while the pair was in shallow water (Londoño and Muñoz 2006).
In Brazil, births occurred August through December (n = 11) during periods of receding water or low water, though births may take place during the wet or dry seasons in other locations (Evangelista and Rosas 2011a). In Peru, births occurred year-round, but predominantly in the dry season (88.7%—Groenendijk et al. 2014). A 2nd litter may be born later in the season if the 1st litter does not survive (Duplaix 1980). One female in Peru produced 2 litters in a year (1 cub, followed by 5 cubs—Groenendijk et al. 2014). Lifetime reproductive success ranges from 0 to 25 cubs, with females averaging 6.9 and males 6.7 (Groenendijk et al. 2014). Litters are commonly raised in excavated dens but caves may also be used (Camilo-Alves and Desbiez 2005).
Pairs of male and female P. brasiliensis are monogamous but have help with parental care (Duplaix 1980; Davenport 2010). The 1 breeding female is dominant (Duplaix 1980). When the dominant female dies, a sister or daughter often takes her place; however, in 2 cases a sister and a daughter were observed displacing the dominant female (Groenendijk et al. 2014). The majority of care for young is alloparental and includes care by males (Duplaix 1980; Rosas et al. 2009b). Family members help raise the young by assisting with defense, grooming, and feeding (Davenport 2008, 2010; Evangelista and Rosas 2011b). In Brazil, cubs were left alone about one-half the time; otherwise, an adult babysitter (77% of occurrences) or parent and babysitter (23% of occurrences) remained with the cubs (Rosas et al. 2009b).
Only 65% of cubs survive; about 50% survive to the age of dispersal (Groenendijk et al. 2014). Young disperse at 1.5–4 years old (Duplaix 1980; Leuchtenberger and Mourão 2008; Davenport 2010). The higher presence of females in 1 group (19) compared to males (10) suggests that female offspring tend to be more philopatric (Leuchtenberger and Mourão 2008). No male philopatry was observed in a study of 294 individuals (Groenendijk et al. 2014).
For female P. brasiliensis, the earliest age of reproduction is 3.0 years, with average age of 4.4 years (Groenendijk et al. 2014). Females reproduce for an average of 3.2 years (Groenendijk et al. 2014). Males also reproduce as early as 3.0 years, with an average of 4.6 years (Groenendijk et al. 2014). They reproduce for an average of 3.1 years (Groenendijk et al. 2014).
Communication
Vocalizations include a loud bark reminiscent to that of a sea lion (Sanderson 1949). In Suriname, 9 vocalizations were described and sonograms recorded: a HAH! sound, a snort in reaction to alarm or danger, a warning scream, a growl, a hum, a coo, a whistle, newborn cub squeaks, and cub whines and wails (Duplaix 1980). Spectrograms of 5 vocalizations—snort, scream, purr, HAH! sound, and cub call—were recorded in Brazil (Bezerra et al. 2011). The loud, wavering scream signals distress and may cause other P. brasiliensis to offer assistance (Duplaix 1980; Davenport 2010). Recordings of wild P. brasiliensis in Peru and in captivity showed that individuals have distinct calls when trying to contact other otters (Mumm et al. 2014).
In Brazil, 4 types of scent marking were identified: stepping, with paws rubbed on the ground; forepaw rubbing, with paws rubbed on a vertical surface; body rubbing, with the body rubbed on the ground or other surface; and latrine use (feces and urine—Leuchtenberger and Mourão 2009). The alpha male marks his territory more often (62% of marking events) than the alpha female (17%—Leuchtenberger and Mourão 2008). Alpha males and females often mark over the scent marks of other group members (32 of 59 events and 5 of 16 events, respectively—Leuchtenberger and Mourão 2009). In Guyana, 1 marking site was over 4.6 m long (Laidler and Laidler 1995).
Miscellaneous behavior
Pteronura brasiliensis is diurnal, emerging at dawn and returning to its den at dusk (Duplaix 1980). However, nocturnal activity has been observed during the wet season in Brazil: 1 instance was due to the presence of a jaguar, others to nocturnal fishing and latrine use (Leuchtenberger et al. 2014). P. brasiliensis dries itself by rubbing on the ground and grooming as soon as it leaves the water (Duplaix 1980).
A begging P. brasiliensis will remain near a family member with food and make consistent, loud vocalizations (Davenport 2010). The P. brasiliensis with food will growl defensively and avoid the beggar while it eats most of its prey (Davenport 2010). It will give the remainder of the food to the beggar without resistance (Davenport 2010). For juveniles that are no longer nursing, the main source of food is received by begging from their parents and siblings (Davenport 2010).
Family members were observed assisting an elderly, nonreproductive matriarch (Davenport 2010). If she lost the group during a hunt, the other P. brasiliensis would swim to her and bring her back to the other family members (Davenport 2010). She was no longer able to catch large prey, and she begged from her family members with a 26% success rate (Davenport 2010).
Pteronura brasiliensis does not normally engage in infanticide, but an adult male was observed preying on and eating a male cub that had been left unsupervised in its burrow (Mourão and Carvalho 2001).
GENETICS
Pteronura brasiliensis (n = 4) has diploid number (2n) of 38 chromosomes, including 2 sex chromosomes (fundamental number [FN] of 64—Franco-de-Sá 2007). Autosomal chromosomes include 14 metacentric, 8 submetacentric, 6 subtelocentric, and 8 acrocentric. Both sex chromosomes are submetacentric (Franco-de-Sá 2007).
Fecal sampling in Bolivia (n = 20) revealed 19 loci that could be amplified consistently; heterozygosity between 0.15 and 0.85 was observed (Pickles et al. 2009). Microsatellite loci for 14 P. brasiliensis from Brazil revealed 14 dinucleotide and trinucleotide polymorphic loci with 2–5 alleles each; heterozygosity was 0.577 (Ribas et al. 2011).
Cytochrome-b and cytochrome-c oxidase analysis did not support a subspecies division, although the populations may be geographically structured (Garcia et al. 2007). High genetic diversity exists across 4 lineages of P. brasiliensis in 4 geographic regions: Madre de Dios/Madeira, Itenez, Pantanal, and Amazon–Orinoco–Guianas (Pickles et al. 2011).
CONSERVATION
The International Union for the Conservation of Nature and Natural Resources lists the status of Pteronura brasiliensis as “Endangered,” with current trends unknown (Groenendijk et al. 2015). P. brasiliensis is listed under the Species Protection Regulations (1999) in Guyana (van der Waal 2012).
The greatest problems facing P. brasiliensis are habitat destruction, illegal hunting, heavy metal contamination, disease, overfishing by humans, and stress caused by tourism (Staib and Schenck 1994; Schenck and Staib 1998). Concerns exist regarding the contribution by the gold mining industry of mercury and methylmercury, which concentrates at higher trophic levels (Gutleb et al. 1997; Uryu et al. 2001; Roach et al. 2013). However, tissue samples from 2 specimens revealed levels below toxicity (Fonseca et al. 2005).
Pteronura brasiliensis populations thrive in uninhabited areas but are threatened in populated areas (McTurk and Spelman 2005). An investigation of 3 sites near the National Forest of Amapá in Brazil showed that the presence of P. brasiliensis decreased or disappeared as human habitation and boats increased, rarely occurring within 40 km of a town (de Oliveira et al. 2015).
Illegal traffic in skins remains a threat (Chehébar 1991). A hunter may receive $27–50 for a single pelt, with the skin going for 5 times that amount in the United States or Europe (Dourojeanni 1974; Ayres and Best 1979; Smith 1980–1981). Historic data show nearly 20,000 skins exported from the Brazilian Amazon between 1960 and 1969 and in the Peruvian Amazon, 24,000 skins (Smith 1980–1981). In 1989–1990, 240 P. brasiliensis pelts were found in a warehouse in Argentina (possibly from other countries—Chehébar 1991).
Pteronura brasiliensis is viewed as threatening because of its size or as competition for river fish and concern for damaged nets (Gómez and Jorgenson 1999; Gómez Serrano 2003; McTurk and Spelman 2005; Recharte et al. 2009, 2014; Michalski et al. 2012; Rosas-Ribeiro et al. 2012). Attempted attacks (no injuries sustained) on humans have been reported in Brazil (Lasmar et al. 2013). P. brasiliensis is captured and kept as pets in Colombia, but may become financial burdens on their host families (Gómez Serrano 2003). P. brasiliensis meat is occasionally used as bait for capturing tortoises (Uscamaita and Bodmer 2009). Because the Department of Agriculture of Peru banned the hunting of P. brasiliensis in 1973, sightings of individuals increased from 2 to 41 in 2004 in the Yvarí-Mirín and Yavarí rivers (Uscamaita and Bodmer 2009).
In Colombia, 2 P. brasiliensis cubs were successfully rehabilitated for 7 months and released into the wild, where 1 was adopted by a preexisting P. brasiliensis family, and the other became the matriarch of her own group (Gómez Serrano 2003). In Guyana, 18 of 34 orphaned P. brasiliensis were successfully rehabilitated and returned to the wild (McTurk and Spelman 2005). A juvenile, reintroduced to the Orinoco region of Colombia, was sited 17 months after release (Morales-Betancourt 2011). P. brasiliensis may return to disturbed areas (Calaça et al. 2015).
ACKNOWLEDGMENTS
Thanks to the staff of the American Museum of Natural History for their assistance with photography of the skull. Thanks also to D. Chen for photographic composition and map design. Dr. J. Quadros and an anonymous reviewer improved the content and quality of the manuscript.
LITERATURE CITED
Author notes
Synonymies completed 14 June 2017
Version of Record, first published online September 8, 2017, with fixed content and layout in compliance with Art. 8.1.3.2 ICZN.
Nomenclatural statement.—A life science identifier (LSID) number was obtained for this publication: urn:lsid:zoobank.org:pub: 202F2BF5-6E54-40CA-8A87-13AFA3D375FB
Associate Editor of this account was David Zegers. Editor was Meredith J. Hamilton.