-
PDF
- Split View
-
Views
-
Cite
Cite
Zaccaria Ricci, Stefano Romagnoli, Claudio Ronco, Acute kidney injury: to dialyse or to filter?, Nephrology Dialysis Transplantation, Volume 35, Issue 1, January 2020, Pages 44–46, https://doi.org/10.1093/ndt/gfz022
Close - Share Icon Share
Dialysis derives from the Greek term  , which means ‘to lose through, to separate’. Even if the generic term ‘dialysis’ currently defines any form of blood purification, solute transport across semipermeable membranes like dialysis membranes may occur by diffusion or convection processes that are at the base of dialysis and haemofiltration modalities, respectively. The first is a process governed by a gradient of concentration of the solute between the two compartments (blood and dialysate) and statistically tends to reach an equilibrium of concentration in both compartments (full saturation). The velocity of this process depends directly on temperature, area of the membrane and concentration gradient, while it is inversely related to the distance between the two compartments that might be assimilated to the thickness of the membrane. The saturation of the dialysate is also a function of the respective blood and dialysate flows, being 100% only in case of proportionally high blood and low dialysate flow (Figure 1). Convection is a different process based on the solvent drag phenomenon. Part of the solvent is filtered through the membrane in response to a transmembrane pressure gradient. Based on the hydraulic permeability of the membrane, a given quantity of solvent will be transferred on the other side of the membrane. In this movement, the filtered solvent (plasma water in the case of dialysis) will drag with it some solutes. Depending on membrane sieving properties, the solutes in the ultrafiltrate (so called because it contains crystalloids but no colloids or cells) will have the same concentration as in plasma water (sieving = 1) or no concentration at all (sieving = 0) (Figure 1). Pure convection occurs in the absence of a gradient for diffusion and therefore in the absence of dialysate flow (haemofiltration). In the presence of dialysate, diffusion and convection will interfere with each other and we call this technique haemodiafiltration, where it is impossible to separate the specific contribution of each transport modality.
, which means ‘to lose through, to separate’. Even if the generic term ‘dialysis’ currently defines any form of blood purification, solute transport across semipermeable membranes like dialysis membranes may occur by diffusion or convection processes that are at the base of dialysis and haemofiltration modalities, respectively. The first is a process governed by a gradient of concentration of the solute between the two compartments (blood and dialysate) and statistically tends to reach an equilibrium of concentration in both compartments (full saturation). The velocity of this process depends directly on temperature, area of the membrane and concentration gradient, while it is inversely related to the distance between the two compartments that might be assimilated to the thickness of the membrane. The saturation of the dialysate is also a function of the respective blood and dialysate flows, being 100% only in case of proportionally high blood and low dialysate flow (Figure 1). Convection is a different process based on the solvent drag phenomenon. Part of the solvent is filtered through the membrane in response to a transmembrane pressure gradient. Based on the hydraulic permeability of the membrane, a given quantity of solvent will be transferred on the other side of the membrane. In this movement, the filtered solvent (plasma water in the case of dialysis) will drag with it some solutes. Depending on membrane sieving properties, the solutes in the ultrafiltrate (so called because it contains crystalloids but no colloids or cells) will have the same concentration as in plasma water (sieving = 1) or no concentration at all (sieving = 0) (Figure 1). Pure convection occurs in the absence of a gradient for diffusion and therefore in the absence of dialysate flow (haemofiltration). In the presence of dialysate, diffusion and convection will interfere with each other and we call this technique haemodiafiltration, where it is impossible to separate the specific contribution of each transport modality.
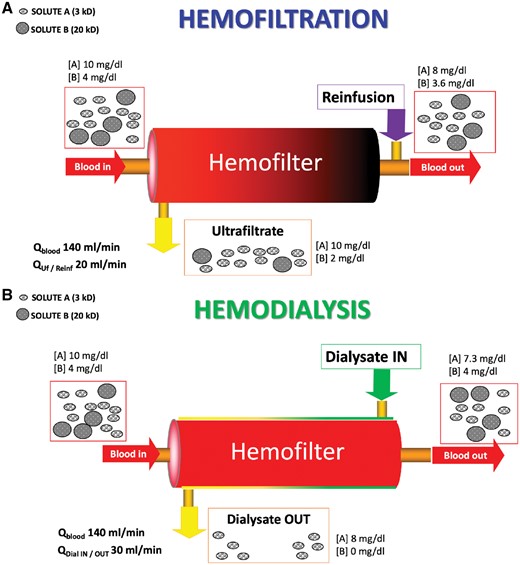
Schematic representation of mechanisms of solute transport during a purely convective treatment (haemofiltration) or during a purely diffusive treatment (haemodialysis). (A) During haemofiltration, if blood flow (Qblood) is set at 140 mL/min and plasma filtration fraction is 20%, ultrafiltration flow (QUf) will be 20 mL/min or 1200 mL/h, considering a haematocrit of 30%. When a re-infusion of 1200 mL/h (Qreinf) is delivered post-dilution, the clearance of the system will allow the removal of 1/5 of the small solute A since its sieving coefficient is 1. Clearance of solute B, whose molecular weight is close to standard haemofilters nominal cut-off, will be significantly lower than solute A, strictly depending on sieving coefficient and filter fouling. The colour gradient of the haemofilter represents the progressive haemoconcentration occurring inside the filter during (post-dilution) haemofiltration. (B) During haemodialysis, setting a similar Qblood, it will be possible to set dialysate flow (QdialIN) at 30 mL/min (1800 mL/h) since there is not a filtration fraction in this case. If this setting warrants a saturation of dialysate of 80%, then solute A will have a proportional concentration in the effluent but a slightly higher clearance compared with haemofiltration, considering the 50% increase in effluent flow. However, solute B will not be consistently removed during haemodialysis. In this case, there is no colour gradient inside the filter; however, a gradient occurs in countercurrent dialysate, flowing outside the hollow fibres, which is progressively saturated with waste solutes.
Vander’s Human Physiology [1], describing what happens in human glomeruli, the Bowman’s capsule and tubular epithelium, states that blood is filtered and the filtrate, except for larger proteins, contains all the substances, including some polypeptides, in virtually the same concentrations as in plasma. This cell-free filtrate, in which only low-molecular weight solutes appear, is called ultrafiltrate. Around 20% of the plasma reaching this ‘filtration unit’ is actually filtered, with the remaining being returned to the systemic circulation. About 99% of the filtered flow is eventually restored into the circulation and only the net water removal represents the fluid balance. A delicate equilibrium between hydrostatic and oncotic pressures regulates the forces involved in the filtration process. Hence, according to the current nomenclature of renal replacement therapy [2], nature has chosen continuous haemofiltration (CH), as the primary methodology of blood purification. The ‘dose’ of this ‘human’ blood purification (also known as creatinine clearance) in the healthy kidneys of a 70-kg man ranges from 50 to 100 mL/kg/h.
When the artificial replacement of renal function is applied in case of acute kidney injury, in order to reproduce the most ‘physiological’ mechanism of blood purification, CH should be selected. Certainly, intermittent forms of dialysis lead to significant shifts of solutes and volemia [3], whereas the optimal substitution of native kidneys should warrant a continuous function. Furthermore, filtration, different from diffusion, warrants that solutes in plasma having a diameter smaller than the glomerular/dialyser pores will appear with the same concentration in the ultrafiltrate: again, artificial substitution of renal function in terms of solute removal is theoretically optimized by convection.
As a matter of fact, human kidneys are much more complex than CH. For example, within the nephrons, the filtered plasma water is manipulated through readsorption mechanisms, aimed at minimizing the unwanted loss of non-waste solutes and at maximizing the excretion of waste products [1]. Excessive artificial clearance leads to uncontrolled loss of vital elements such as antibiotics, amino acids and electrolytes. This is one of the reasons why many studies that have attempted to approach the ‘physiological’ CH intensity of 40–60 mL/kg/h failed to show significant superiority versus lower prescriptions [4, 5]: the benefits provided by the increased clearance of harmful blood substances might be cancelled out by the drawbacks of excessive clearance of beneficial molecules. However, even if the perfectly set mechanism of healthy kidneys is never reproduced by any form of renal replacement therapy, it has been clearly shown that CH makes it possible to remove larger mediators compared with equivalent continuous dialysis (CD) intensity, since the mechanism of convective transport allows filter pores to be crossed by heavier molecules (e.g. β2 microglobulin) [6]. As a matter of fact, selected patients (e.g. in the septic shock phase of hypercytokinaemia) [7] or selected clinical conditions (e.g. metabolic acidosis) [8] may actually benefit from aggressive removal of specific solutes, likely better controlled by CH.
Few studies have attempted a direct comparison between CH and CD in order to show if mainly convective versus diffusive treatments could have a significant impact on clinical outcomes in critically ill patients. A meta-analysis pooling the results from randomized controlled trials did not find benefits in terms of improved mortality [9]. However, compared with CD, CH not only increased the clearance of medium to larger molecules, but also showed a decrease in average filter life (however, most of these studies were conducted in the absence of citrate anticoagulation) [9]. This meta-analysis concluded that a large definitive trial is currently lacking to show if CH compared with CD may significantly impact any valid clinical endpoint (e.g. use of vasopressors, improvement of haemodynamics, adjustment of perfusion indexes and modification of clinical course of selected patients).
As a practical approach, in order to achieve advantages from both techniques, the haemodiafiltration modality could be set. The prescription, ranging from 20 to 35 mL/kg/h of dialytic dose [10], should split the flows between haemofiltration and dialysis in order to balance prolonged circuit life and efficiency of blood purification. However, during haemodiafiltration, the highest possible convective dose should always be delivered by setting the haemofiltration rate to 20% of plasma flow, with the remaining prescription set as diffusion, just to reach the desired intensity target [11].
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES





Comments