-
PDF
- Split View
-
Views
-
Cite
Cite
Marion Haubitz, Reinhard Brunkhorst, C‐reactive protein and chronic Chlamydia pneumoniae infection—long‐term predictors for cardiovascular disease and survival in patients on peritoneal dialysis, Nephrology Dialysis Transplantation, Volume 16, Issue 4, April 2001, Pages 809–815, https://doi.org/10.1093/ndt/16.4.809
Close - Share Icon Share
Abstract
Background. Accelerated arteriosclerosis with cardiovascular disease is the main cause of death in end‐stage renal disease patients. Increased, levels of C‐reactive protein (CRP) and evidence of chronic Chlamydia pneumoniae infection have been identified as risk factors for cardiovascular disease in the general population. We tested the hypothesis that elevation of CRP, indicating chronic inflammation, and positive serum antibody titres for C. pneumoniae are associated with an increased cardiovascular mortality in patients on chronic peritoneal dialysis.
Methods. We measured CRP and antibodies to C. pneumoniae in 34 patients on peritoneal dialysis. CRP was measured by a sensitive ELISA and C. pneumoniae antibodies by microimmunofluorescence. In addition, risk factors such as lipids, smoking status and hypertension were assessed. Coronary artery disease (CAD) was defined by cardiac stress testing and/or angiography. Patients showing clinical evidence of systemic or peritoneal dialysis‐associated infection during the investigation period of 6 months (between 1990 and 1991) were excluded.
Results. The incidence of CAD was significantly increased in patients with CRP values >1.5 mg/l (odds ratio 7.0, P<0.022) during 72 months of follow‐up. In addition, in patients seropositive for IgA C. pneumoniae antibodies, the incidence of CAD was significantly increased (odds ratio 7.2, P<0.014). These findings resulted in an increased risk of death in patients with mean CRP values >1.5 mg/l at the start of the study (odds ratio 20.0, P<0.001). Furthermore, in patients seropositive for IgA C. pneumoniae antibodies, the risk of death (odds ratio 10.2, P<0.005) was significantly increased. There was a highly significant correlation between CRP and seropositivity for IgA C. pneumoniae antibodies (r=0.445, P<0.01).
Conclusions. Increased circulating CRP and seropositivity for C. pneumoniae in patients on chronic peritoneal dialysis are associated with reduced survival due to cardiovascular complications. CRP and C. pneumoniae antibodies may indicate a chronic inflammatory process as an underlying cause and/or result of arteriosclerosis.
Introduction
Chronic uraemic patients, either dialysed or on conservative treatment, exhibit an increased incidence of occlusive arterial disease with coronary and cerebrovascular accidents [1,2]. Several risk factors have been thought to be involved in the development of this accelerated arteriosclerosis. Classic factors like hypertension, smoking or lipid disorders are frequently observed in patients with chronic renal insufficiency, but studies have demonstrated that the excess of atherothrombotic cardiovascular disease in end‐stage renal disease (ESRD) patients is not adequately explained by traditional risk factor indices [3–5]. In the last decade it has been emphasized that inflammation is an important feature of atheroma, which is characterized (at an early stage) by activation and proliferation of macrophages, endothelial cells and smooth muscle cells. It has been suggested that chronic infection with Chlamydia pneumoniae, Helicobacter pylori and Cytomegalovirus are involved in the pathogenesis of coronary heart disease [6–11]. Overall inflammatory activity is indicated by an increase in circulating concentrations of acute phase reactants. Higher serum levels of C‐reactive protein (CRP), the most sensitive acute phase protein in human beings, have been shown to predict or to be directly correlated with the presence and severity of coronary, cerebral and peripheral arterial atherosclerosis in patients without renal impairment [12,13]. Raised circulating concentrations of CRP have been shown to predict coronary events in patients with stable or unstable angina [14].
Patients with ESRD have increased CRP levels compared with normal controls [15,16]. In patients on long‐term haemodialysis therapy, these elevated levels, among other factors, are probably caused by the induction of the inflammatory activity by the dialyser membrane, the dialysate buffer or contamination of the dialysate with bacterial fragments [17]. An increase in CRP concentration has been demonstrated 24 h after dialysis treatment [18]. In patients treated with long‐term peritoneal dialysis (PD), CRP values were significantly lower compared with patients on long‐term haemodialysis therapy [15], but still significantly increased compared with normal controls.
In this study we investigated the relationship of serially measured CRP and a seropositivity of chronic C. pneumoniae infection indicated by elevated antibody titre on the incidence of coronary artery disease (CAD) and survival of patients on long‐term PD.
Subjects and methods
Patients
Patients were recruited between 1990 and 1991, after informed consent had been obtained. All patients treated with PD in the PD outpatients unit of Hannover Medical School for at least 3 months, not suffering from congestive heart failure or autoimmune disease, and with a period for sample collection of 5–6 months were included. Patients with evidence of systemic infection (fever or leukocytosis) or a PD‐associated infection (exit‐site infection, tunnel infection or peritonitis) up to 2 months before or during the sample collection period were excluded (n=13; mean CRP in these patients, 12.970 mg/l). Thirty‐four ESRD patients (12 females, 22 males; average age 43±12 years, range 23–68 years) were eligible. Twenty‐seven patients were on automated peritoneal dialysis (APD), with six nightly exchanges using a total volume of 10–14 l dialysate (mean glucose concentration 2.4%), and seven patients were on continuous ambulatory peritoneal dialysis (CAPD), with four to five exchanges per 24 h and a total volume of 8 l.
Primary renal diseases were chronic glomerulonephritis (n=15), diabetic nephropathy (n=12), chronic interstitial nephritis (n=2), polycystic kidney disease (n=3), kidney hypoplasia (n=1) and haemolytic‐uraemic syndrome (n=1).
Diagnosis of arteriosclerotic disease
CAD was defined as a past history of cardiac ischaemic accident and/or angiographic documentation of stenosis of at least one major epicardial coronary vessel and/or an abnormal cardiac stress test. Cerebrovascular disease (CVD) was diagnosed in patients with a cerebral ischaemic accident, and the diagnosis was confirmed by computed tomography. Peripheral vascular disease (PVD) was diagnosed by a reduced walking capacity with claudication and the absence of appropriate pulses on clinical examination and/or a peripheral angiography. Moreover, the number of antihypertensive drugs (as an indicator of hypertension) and the smoking statuses of the patients were documented.
Follow‐up of the patients included assessment of survival, the development of arterial occlusive disease (CAD, CVD, PVD) and the incidence of PD‐associated infections (exit‐site infection, tunnel infection or peritonitis). The observation period ended on December 31, 1997.
Blood sampling
Blood samples without anticoagulant were taken in the morning, 2 h after the end of APD or during ongoing CAPD. Serum was separated without delay and stored at −20°C until analysis. For prospective CRP measurement over a period of 5–6 months, samples were taken every month and the results were averaged for each patient.
CRP determination
CRP values were measured using a sensitive enzyme‐linked immunosorbent assay (detection limit 200 pg/ml) as described previously [18]. The assay is based on a sandwich‐ELISA technique using an anti‐CRP antibody as a catching antibody and an identical biotinylated antibody as the detecting antibody.
Chlamydia pneumoniae antibody determination
Antibodies to C. pneumoniae (IgG and IgA) were investigated at the start of the study using the microimmunofluorescence method [19]. In this in‐house assay, elementary bodies of the TW 183 strain of C. pneumoniae (University of Washington Research Foundation, Seattle, Washington) were used. Fluorescein isothiocyanate (FITC)‐labelled anti‐human IgG or IgA antibodies (Bios, Gräfelfing, Germany) were used for the detection. IgG titres of ⩾64 and IgA titres of ⩾80 were considered positive. In 160 healthy staff members, IgG antibodies to C. pneumoniae were positive in 66% and IgA antibodies in 16%.
Lipid status
Serum cholesterol and triglyceride were collected retrospectively from the medical record (mean of three to four measurements between the start of the study and the following 12 months).
Statistical analysis
Data are given as means±standard deviations (SD). Univariate analyses were performed with Fisher's exact test. Continuous variables were analysed by the Mann–Whitney U‐test. Data on survival were compiled by life table methods (Kaplan–Meier) and analysed by log rank testing. Correlations were assessed by Spearman Rank Order. The data were analysed with the System Package for Statistical Software (SPSS).
Results
At the start of the study, three patients had evidence of CAD, three patients of CVD and nine of PVD. During the follow‐up, 10 further patients developed CAD, five CVD and four PVD.
The mean follow‐up period was 69±23 months (range 18–89 months). Over 6 years, 12 patients died (44±19 months, range 18–64). One patient died after 89 months. The causes of death were cardiac events in eight patients (infarction, cardiac arrhythmias), cerebral events in two patients, sepsis in one patient, and in two patients the cause of death remained unknown. There was no significant difference regarding the development of peritonitis or chronic exit‐site infections between patients who died and those who survived.
During the follow‐up, 10 patients received a successful renal transplant after 28±27 months. In 10 patients, long‐term renal replacement therapy was changed from PD to haemodialysis after 39±23 months.
Mean CRP serum levels were 1.480±1.444 mg/l. CRP values were significantly lower in the group of patients who did not develop CAD during follow‐up (0.945±0.819 mg/l) than in those patients who had or developed CAD (2.343±1.818 mg/l) (P<0.005). The risk of CAD was also significantly increased in patients with mean CRP values >1.5 mg/l versus patients with mean CRP values of ⩽1.5 mg/l (odds ratio 7.0, P<0.022) (Table 1). There was no significant difference regarding PVD or CVD.
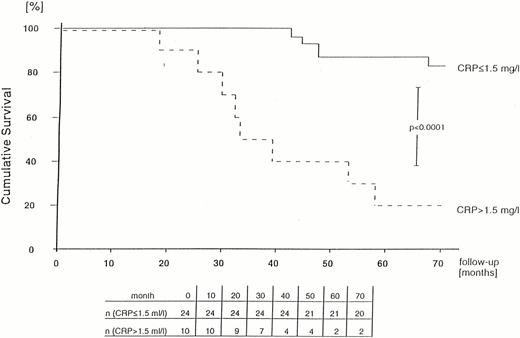
With respect to survival, CRP values in the group of patients who were still alive after 72 months were significantly lower (1.063±1.413 mg/l) than in those patients who had already died (2.245±1.205 mg/l) (P<0.001). The risk of death was significantly increased in patients with a mean CRP value >1.5 mg/l versus patients with mean CRP values of ⩽1.5 mg/l at the start of the study (odds ratio 20.0, P<0.001) (Table 2).
Regarding C. pneumoniae infection, 26 patients (76%) were positive for IgG antibodies and 14 (41%) for IgA antibodies. In the group of patients who had or developed CAD, 92% were positive for IgG and 69% for IgA, whereas in patients without CAD during follow‐up, only 67% were positive for IgG and 24% for IgA. The risk of CAD was significantly increased in patients with IgA antibodies (odds ratio 7.2, P<0.014) (Table 1). The difference did not reach significance for patients with IgG antibodies (odds ratio 6.0, P<0.116). There was no significant difference in patients who had or developed PVD or CVD.
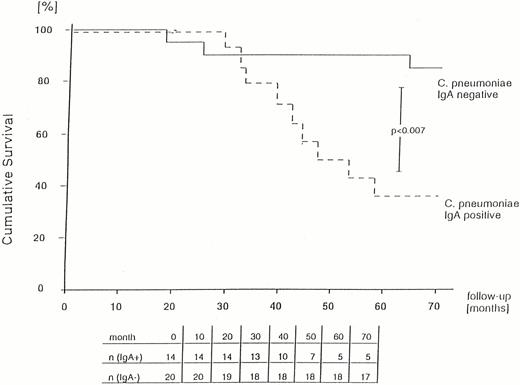
In the group of patients who died during the 72 months, 92% were positive for IgG and 75% for IgA antibodies, whereas of the patients still alive only 68% had IgG antibodies and 23% had IgA antibodies. The risk of death was significantly increased in patients with IgA antibodies (odds ratio 10.2, P<0.005; Table 2), whereas the difference was not significant for patients with IgG antibodies (odds ratio 5.1, P<0.21).
CRP levels and C. pneumoniae IgA and IgG antibodies were significantly correlated (IgA: r=0.445, P<0.01; IgG: r=0.368, P<0.05), as were CRP and age (r=0.507, P<0.01).
Gender and smoking habit were not associated with an increased risk of CAD or death, whereas diabetes as the cause of terminal renal failure is a signifcant risk factor. CAD, PVD or CVD before the study were significant risk factors for death (Table 2). Patients who developed CAD or died were significantly older (P<0.002) and tended to have higher cholesterol levels (P<0.082 and P<0.076, respectively). There was no significant difference regarding triglyceride levels, number of antihypertensive drugs or time passed since terminal renal failure.
Life table analysis revealed a significantly poorer survival of patients with mean serum CRP values >1.5 mg/l (P<0.0001) and for patients with IgA antibodies to C. pneumoniae (P<0.007) (Figures 1 and 2).
Probability of survival of PD patients in relation to mean serum CRP level (calculated using the Kaplan–Meier method).
Probability of survival of PD patients in relation to seropositivity for IgA to C. pneumoniae (calculated using the Kaplan–Meier method).
Odds ratio for risk factors of CAD during a follow‐up of 72 months
| Patient characteristic | Number of patients | P‐value | Odds ratio (95% CI) | ||
| Without CAD (n=21) n (%) | With CAD (n=13) n (%) | ||||
| CRP>1.5 mg/l | 3 (14.3) | 7 (53.8) | 0.022 | 7.00 (1.36−36.01) | |
| IgG to C. pneumoniae | 14 (66.7) | 12 (92.3) | 0.116 | 6.00 (0.64−55.95) | |
| IgA to C. pneumoniae | 5 (23.8) | 9 (69.2) | 0.014 | 7.20 (1.53−33.85) | |
| Sex | 11 males | 11 males | 0.075 | 5.00 (0.88−28.30) | |
| Smoker | 4 (19.0) | 4 (30.8) | 0.680 | 1.89 (0.38−9.40) | |
| Diabetes | 2 (9.5) | 10 (76.9) | 0.0001 | 31.67 (4.52−221.72) | |
| Patient characteristic | Number of patients | P‐value | Odds ratio (95% CI) | ||
| Without CAD (n=21) n (%) | With CAD (n=13) n (%) | ||||
| CRP>1.5 mg/l | 3 (14.3) | 7 (53.8) | 0.022 | 7.00 (1.36−36.01) | |
| IgG to C. pneumoniae | 14 (66.7) | 12 (92.3) | 0.116 | 6.00 (0.64−55.95) | |
| IgA to C. pneumoniae | 5 (23.8) | 9 (69.2) | 0.014 | 7.20 (1.53−33.85) | |
| Sex | 11 males | 11 males | 0.075 | 5.00 (0.88−28.30) | |
| Smoker | 4 (19.0) | 4 (30.8) | 0.680 | 1.89 (0.38−9.40) | |
| Diabetes | 2 (9.5) | 10 (76.9) | 0.0001 | 31.67 (4.52−221.72) | |
| Patient characteristic | Number of patients | P‐value | ||
| Without CAD (n=21) (mean±SD) | With CAD (n=13) (mean±SD) | |||
| CRP (mg/l) | 0.95±0.82 | 2.34±1.82 | 0.005 | |
| Cholesterol (mg/dl) | 266±83 | 303±63 | 0.082 | |
| Triglyceride (mg/dl) | 231±98 | 294±145 | 0.100 | |
| Antihypertensive drugs | 1.3±1.1 | 1.46±0.9 | 0.708 | |
| Age (years) | 40±12 | 49±9 | 0.002 | |
| TRF for (months) | 14±18 | 12±12 | 0.804 | |
| PD for (months) | 6±5 | 5±3 | 0.944 | |
| Patient characteristic | Number of patients | P‐value | ||
| Without CAD (n=21) (mean±SD) | With CAD (n=13) (mean±SD) | |||
| CRP (mg/l) | 0.95±0.82 | 2.34±1.82 | 0.005 | |
| Cholesterol (mg/dl) | 266±83 | 303±63 | 0.082 | |
| Triglyceride (mg/dl) | 231±98 | 294±145 | 0.100 | |
| Antihypertensive drugs | 1.3±1.1 | 1.46±0.9 | 0.708 | |
| Age (years) | 40±12 | 49±9 | 0.002 | |
| TRF for (months) | 14±18 | 12±12 | 0.804 | |
| PD for (months) | 6±5 | 5±3 | 0.944 | |
TRF=terminal renal failure
Odds ratio for risk factors of CAD during a follow‐up of 72 months
| Patient characteristic | Number of patients | P‐value | Odds ratio (95% CI) | ||
| Without CAD (n=21) n (%) | With CAD (n=13) n (%) | ||||
| CRP>1.5 mg/l | 3 (14.3) | 7 (53.8) | 0.022 | 7.00 (1.36−36.01) | |
| IgG to C. pneumoniae | 14 (66.7) | 12 (92.3) | 0.116 | 6.00 (0.64−55.95) | |
| IgA to C. pneumoniae | 5 (23.8) | 9 (69.2) | 0.014 | 7.20 (1.53−33.85) | |
| Sex | 11 males | 11 males | 0.075 | 5.00 (0.88−28.30) | |
| Smoker | 4 (19.0) | 4 (30.8) | 0.680 | 1.89 (0.38−9.40) | |
| Diabetes | 2 (9.5) | 10 (76.9) | 0.0001 | 31.67 (4.52−221.72) | |
| Patient characteristic | Number of patients | P‐value | Odds ratio (95% CI) | ||
| Without CAD (n=21) n (%) | With CAD (n=13) n (%) | ||||
| CRP>1.5 mg/l | 3 (14.3) | 7 (53.8) | 0.022 | 7.00 (1.36−36.01) | |
| IgG to C. pneumoniae | 14 (66.7) | 12 (92.3) | 0.116 | 6.00 (0.64−55.95) | |
| IgA to C. pneumoniae | 5 (23.8) | 9 (69.2) | 0.014 | 7.20 (1.53−33.85) | |
| Sex | 11 males | 11 males | 0.075 | 5.00 (0.88−28.30) | |
| Smoker | 4 (19.0) | 4 (30.8) | 0.680 | 1.89 (0.38−9.40) | |
| Diabetes | 2 (9.5) | 10 (76.9) | 0.0001 | 31.67 (4.52−221.72) | |
| Patient characteristic | Number of patients | P‐value | ||
| Without CAD (n=21) (mean±SD) | With CAD (n=13) (mean±SD) | |||
| CRP (mg/l) | 0.95±0.82 | 2.34±1.82 | 0.005 | |
| Cholesterol (mg/dl) | 266±83 | 303±63 | 0.082 | |
| Triglyceride (mg/dl) | 231±98 | 294±145 | 0.100 | |
| Antihypertensive drugs | 1.3±1.1 | 1.46±0.9 | 0.708 | |
| Age (years) | 40±12 | 49±9 | 0.002 | |
| TRF for (months) | 14±18 | 12±12 | 0.804 | |
| PD for (months) | 6±5 | 5±3 | 0.944 | |
| Patient characteristic | Number of patients | P‐value | ||
| Without CAD (n=21) (mean±SD) | With CAD (n=13) (mean±SD) | |||
| CRP (mg/l) | 0.95±0.82 | 2.34±1.82 | 0.005 | |
| Cholesterol (mg/dl) | 266±83 | 303±63 | 0.082 | |
| Triglyceride (mg/dl) | 231±98 | 294±145 | 0.100 | |
| Antihypertensive drugs | 1.3±1.1 | 1.46±0.9 | 0.708 | |
| Age (years) | 40±12 | 49±9 | 0.002 | |
| TRF for (months) | 14±18 | 12±12 | 0.804 | |
| PD for (months) | 6±5 | 5±3 | 0.944 | |
TRF=terminal renal failure
Odds ratio for risk factors of death during a follow‐up of 72 months
| Number of patients | P‐value | Odds ratio (95% CI) | |||
| alive (n=22) n (%) | dead (n=12) n (%) | ||||
| CRP>1.5 mg/l | 2 (9.1) | 8 (66.7) | 0.001 | 20.00 (3.04−131.73) | |
| IgG to C. pneumoniae | 15 (68.2) | 11 (91.7) | 0.21 | 5.13 (0.55−47.98) | |
| IgA to C. pneumoniae | 5 (22.7) | 9 (75.0) | 0.005 | 10.20 (1.97−52.78) | |
| Sex | 12 males | 10 males | 0.140 | 4.17 (0.73−23.61) | |
| Smoker | 5 (22.7) | 3 (25.0) | 1.000 | 1.13 (0.22−5.86) | |
| CAD before study | 0 | 3 (25.0) | 0.037 | not applicable | |
| PVD before study | 0 | 9 (75.0) | 0.0001 | not applicable | |
| CVD before study | 0 | 3 (25.0) | 0.037 | not applicable | |
| Diabetes | 2 (9.1) | 10 (83.0) | 0.0001 | 50.00 (6.11−409.06) | |
| Number of patients | P‐value | Odds ratio (95% CI) | |||
| alive (n=22) n (%) | dead (n=12) n (%) | ||||
| CRP>1.5 mg/l | 2 (9.1) | 8 (66.7) | 0.001 | 20.00 (3.04−131.73) | |
| IgG to C. pneumoniae | 15 (68.2) | 11 (91.7) | 0.21 | 5.13 (0.55−47.98) | |
| IgA to C. pneumoniae | 5 (22.7) | 9 (75.0) | 0.005 | 10.20 (1.97−52.78) | |
| Sex | 12 males | 10 males | 0.140 | 4.17 (0.73−23.61) | |
| Smoker | 5 (22.7) | 3 (25.0) | 1.000 | 1.13 (0.22−5.86) | |
| CAD before study | 0 | 3 (25.0) | 0.037 | not applicable | |
| PVD before study | 0 | 9 (75.0) | 0.0001 | not applicable | |
| CVD before study | 0 | 3 (25.0) | 0.037 | not applicable | |
| Diabetes | 2 (9.1) | 10 (83.0) | 0.0001 | 50.00 (6.11−409.06) | |
| Number of patients | P‐value | |||
| alive (n=22)(mean±SD) | dead (n=12)(mean±SD) | |||
| CRP (mg/l) | 1.06±1.41 | 2.25±1.21 | 0.001 | |
| Cholesterol (mg/dl) | 261±67 | 312±86 | 0.076 | |
| Triglyceride (mg/dl) | 229±87 | 296±157 | 0.262 | |
| Antihypertensive drugs | 1.3±1.0 | 1.6±1.1 | 0.435 | |
| Age (years) | 39±10 | 52±10 | 0.002 | |
| TRF for (months) | 14±19 | 12±10 | 0.705 | |
| PD for (months) | 6±4 | 6±5 | 0.814 | |
| Number of patients | P‐value | |||
| alive (n=22)(mean±SD) | dead (n=12)(mean±SD) | |||
| CRP (mg/l) | 1.06±1.41 | 2.25±1.21 | 0.001 | |
| Cholesterol (mg/dl) | 261±67 | 312±86 | 0.076 | |
| Triglyceride (mg/dl) | 229±87 | 296±157 | 0.262 | |
| Antihypertensive drugs | 1.3±1.0 | 1.6±1.1 | 0.435 | |
| Age (years) | 39±10 | 52±10 | 0.002 | |
| TRF for (months) | 14±19 | 12±10 | 0.705 | |
| PD for (months) | 6±4 | 6±5 | 0.814 | |
Odds ratio for risk factors of death during a follow‐up of 72 months
| Number of patients | P‐value | Odds ratio (95% CI) | |||
| alive (n=22) n (%) | dead (n=12) n (%) | ||||
| CRP>1.5 mg/l | 2 (9.1) | 8 (66.7) | 0.001 | 20.00 (3.04−131.73) | |
| IgG to C. pneumoniae | 15 (68.2) | 11 (91.7) | 0.21 | 5.13 (0.55−47.98) | |
| IgA to C. pneumoniae | 5 (22.7) | 9 (75.0) | 0.005 | 10.20 (1.97−52.78) | |
| Sex | 12 males | 10 males | 0.140 | 4.17 (0.73−23.61) | |
| Smoker | 5 (22.7) | 3 (25.0) | 1.000 | 1.13 (0.22−5.86) | |
| CAD before study | 0 | 3 (25.0) | 0.037 | not applicable | |
| PVD before study | 0 | 9 (75.0) | 0.0001 | not applicable | |
| CVD before study | 0 | 3 (25.0) | 0.037 | not applicable | |
| Diabetes | 2 (9.1) | 10 (83.0) | 0.0001 | 50.00 (6.11−409.06) | |
| Number of patients | P‐value | Odds ratio (95% CI) | |||
| alive (n=22) n (%) | dead (n=12) n (%) | ||||
| CRP>1.5 mg/l | 2 (9.1) | 8 (66.7) | 0.001 | 20.00 (3.04−131.73) | |
| IgG to C. pneumoniae | 15 (68.2) | 11 (91.7) | 0.21 | 5.13 (0.55−47.98) | |
| IgA to C. pneumoniae | 5 (22.7) | 9 (75.0) | 0.005 | 10.20 (1.97−52.78) | |
| Sex | 12 males | 10 males | 0.140 | 4.17 (0.73−23.61) | |
| Smoker | 5 (22.7) | 3 (25.0) | 1.000 | 1.13 (0.22−5.86) | |
| CAD before study | 0 | 3 (25.0) | 0.037 | not applicable | |
| PVD before study | 0 | 9 (75.0) | 0.0001 | not applicable | |
| CVD before study | 0 | 3 (25.0) | 0.037 | not applicable | |
| Diabetes | 2 (9.1) | 10 (83.0) | 0.0001 | 50.00 (6.11−409.06) | |
| Number of patients | P‐value | |||
| alive (n=22)(mean±SD) | dead (n=12)(mean±SD) | |||
| CRP (mg/l) | 1.06±1.41 | 2.25±1.21 | 0.001 | |
| Cholesterol (mg/dl) | 261±67 | 312±86 | 0.076 | |
| Triglyceride (mg/dl) | 229±87 | 296±157 | 0.262 | |
| Antihypertensive drugs | 1.3±1.0 | 1.6±1.1 | 0.435 | |
| Age (years) | 39±10 | 52±10 | 0.002 | |
| TRF for (months) | 14±19 | 12±10 | 0.705 | |
| PD for (months) | 6±4 | 6±5 | 0.814 | |
| Number of patients | P‐value | |||
| alive (n=22)(mean±SD) | dead (n=12)(mean±SD) | |||
| CRP (mg/l) | 1.06±1.41 | 2.25±1.21 | 0.001 | |
| Cholesterol (mg/dl) | 261±67 | 312±86 | 0.076 | |
| Triglyceride (mg/dl) | 229±87 | 296±157 | 0.262 | |
| Antihypertensive drugs | 1.3±1.0 | 1.6±1.1 | 0.435 | |
| Age (years) | 39±10 | 52±10 | 0.002 | |
| TRF for (months) | 14±19 | 12±10 | 0.705 | |
| PD for (months) | 6±4 | 6±5 | 0.814 | |
Discussion
In this study of ESRD patients on PD, an elevation of CRP serum levels was significantly associated with increased incidence of cardiovascular disease and poorer survival. Patients with CRP serum levels >1.5 mg/l (a limit well below the clinically used ‘normal range’ up to 5 mg/l) more frequently had, or developed, CAD. This risk of CAD and death were not significantly associated with factors known to enhance the development of cardiovascular morbidity, e.g. hyperlipidemia, blood pressure (indicated by the number of antihypertensive drugs) and smoking habit.
The relationship between CRP levels and CAD has been established in apparently healthy individuals [13]. Ridker and colleagues found that the base‐line plasma concentration of CRP predicts the risk of future myocardial infarction and stroke. In addition, reduction in the risk of myocardial infarction associated with the use of aspirin appears to be directly related to the level of CRP. Other study groups have shown comparable results in men recruited from the registers of general practitioners [20] and in smokers with CRP levels >2 mg/l [21]. In patients with angina, CRP concentrations >3 mg/l [22] or >3.6 mg/l [14] were associated with a significantly worse prognosis or a two‐fold increase in the risk of a coronary event, respectively.
In ESRD patients on chronic haemodialysis therapy, CRP levels exceeding the normal range (5 mg/l) have been described in about one‐ to two‐thirds of patients [15,16,23–29]. It has been reported that elevated CRP levels were associated with death during 1 and 3 years of follow‐up [28]. However, CRP values in this study were found to be in the pathological range (>5 mg/l) in most of the patients which, when associated with low albumin, might indicate severe non‐arteriosclerotic disease. Recently, in a large study including patients on chronic dialysis, CRP was a powerful predictor of not only mortality in general, but of cardiovascular death in particular [16]. However, in this study only a single CRP measurement was used and follow‐up ended after 24 months, thus limiting the strength of the study and stressing the need for a long‐term follow‐up and serial measurements of CRP over time, which the authors themselves suggested in their discussion.
In long‐term PD patients without intercurrent infectious episodes, very little data concerning CRP values are available [15,27]. However, the level of CRP was found to be within the normal range in most of these patients. Our study represents the first investigation using serial CRP measurements as a prognostic index for CAD and survival during long‐term follow‐up. Our results show the strong association between CRP and the risk of CAD and death in this patient group. However, a highly sensitive CRP assay, encompassing the ‘normal range’ of this acute phase protein is necessary, as the risk increases already with CRP values >1.5 mg/l. This critical CRP value in our study is in the same range as CRP serum levels indicating increased risk of coronary heart disease in patients with apparently normal renal function [12,13,21].
In our study, higher age and diabetes mellitus are also associated with an increasing incidence of CAD and mortality risk. This has been shown by other investigators [29]. CRP increases with age; however, the higher age in the group of patients who died cannot explain the clearly elevated CRP values, as it has been shown that CRP increases only by 2% a year [14]. With respect to diabetes as the underlying renal disease, other authors have shown that diabetes itself does not lead to increased CRP values [14,25]. Therefore, higher CRP values in diabetic patients seem to be independent of the underlying diabetes mellitus, but reflect the higher incidence of arteriosclerosis.
Whether CRP represents a marker for the development of arteriosclerosis or plays a part in its pathogenesis remains to be elucidated: CRP might simply indicate the subacute inflammation of the coronary artery system or other arteries as the lipid‐rich atheromatous material is itself a potent inducer of inflammation [30]. However, CRP might also point to an associated infection (C. pneumoniae, Cytomegalovirus, H. pylorii), which could play a major role in the pathogenesis of arteriosclerosis.
Our study shows that, in PD patients, the presence of elevated IgA antibody titres to C. pneumoniae is a risk factor for coronary heart disease and death. Recent evidence has suggested an association between elevated IgA titres and chronic bacterial infections, such as yersinial reactive arthritis and chronic pelvic inflammatory disease caused by Chlamydia trachomatis [7]. Elevated IgA titres to C. pneumoniae are considered to be a sign of a chronic and still active infectious process [7], whereas IgG might represent a marker of a past exposure as IgG antibody titres decrease slowly after an acute infection. Our results are in accordance with the study of Saikku and colleagues [7] in patients without renal disease. These authors showed that chronic C. pneumoniae infection is a significant and independent risk factor of the development of CAD. Moreover, it has been shown recently that C. pneumoniae IgA antibodies are associated with an increased mortality over a 13‐year period, which is mainly due to fatal CAD and largely independent of conventional cardiovascular risk factors [31]. In patients on chronic haemodialysis, a cross‐sectional investigation demonstrated an association between IgG antibodies to C. pneumoniae, CRP levels and atherosclerosis using the number of plaques in the carotic arteries [32]. In pre‐dialysis patients, this association was shown for IgA antibodies to C. pneumoniae and the calculated intima media area determined by ultrasonography [33].
Our results lead to the conclusion that a chronic C. pneumoniae infection might be an important risk factor in patients with ESRD. However, until now a causal association showing a direct role for C. pneumoniae infection in the pathogenesis of coronary sclerosis has not been demonstrated. The hypothesis of a chronic inflammation caused by C. pneumoniae is supported by the significant correlation between CRP level and seropositivity for C. pneumoniae antibodies in our study.
The link between the accelerated arteriosclerosis and the high incidence of chronic C. pneumoniae and elevated CRP levels in dialysis patients also remains hypothetical. However, the immune defect observed in dialysis patients [34] might predispose them to the persistence of infectious agents such as C. pneumoniae. Furthermore, the dialysis procedure is a continuous inflammatory stimulus [15–17].
In conclusion, CRP measurements over time may provide important information about subclinical arteriosclerosis and the risk of CAD and mortality in patients with ESRD on long‐term PD. As a chronic C. pneumoniae infection is suspected of being involved in low‐grade inflammation, clinical intervention trials, especially in patients with ESRD, are needed because the infection is common and amenable to treatment, and the proportion of patients on long‐term renal replacement therapy with clinical evidence of coronary artery disease is several times higher than the prevalence in subjects of similar age in the general population.
Correspondence and offprint requests to: Dr Marion Haubitz, Department of Nephrology, Medical School Hannover, D‐30623 Hannover, Germany.
We are grateful to Dr G. Tusch for his help with respect to statistical evaluation and to the Institute of Microbiology in Jena for the measurement of C. pneumoniae antibodies. The technical assistance of M. Flechsig is gratefully acknowledged. These findings were presented, in part, at the 31st Annual Meeting of the American Society of Nephrology 1998 and at the 19th Annual Conference on Peritoneal Dialysis 1999 (outstanding abstract award).
References
Lindner A, Charra B, Sherrard DJ, Scribner BH. Accelerated atherosclerosis in prolonged maintenance hemodialysis.
Ibels LS, Stewart JH, Mahony JF, Neale FC, Sheil AGR. Occlusive arterial disease in uraemic and haemodialysis patients and renal transplant recipients. A study of the incidence of arterial disease and of the prevalence of risk factors implicated in the pathogenesis of arteriosclerosis.
Ma KW, Greene EL, Raij L. Cardiovascular risk factors in chronic renal failure and hemodialysis populations.
Parfrey PS, Harnett JD. Long term cardiac morbidity and mortality during dialysis therapy.
Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Mäkelä PH, Huttunen JK,Valtonen V. Serological evidence of an association of a novel chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction.
Saikku P, Leinonen M, Tenkanen L, Linnanmäki E, Ekman MR, Manninen V, Mänttäri M, Frick MH, Huttunen JK. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study.
Thom DH, Grayston JT, Siscovick DS, Wang SP, Weiss NS, Daling JR. Association of prior infection with Chlamydia pneumoniae and angiographically demonstrated coronary artery disease.
Linnanmäki E, Leinonen M, Mattila K, Nieminen MS, Valtonen V, Saikku P. Chlamydia pneumoniae—specific circulating immune complexes in patients with chronic coronary heart disease.
Speir E, Modali R, Huang ES, Leon MB, Shawl F, Finkel T, Epstein SE. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis.
Patel P, Mendall MA, Carrington D et al. Association of Helicobacter pylori and Chlamydia pneumoniae infections with coronary heart disease and cardiovascular risk factors.
Heinrich J, Schulte H, Schönfeld R, Köhler E, Assmann G. Association of variables of coagulation, fibrinolysis and acute‐phase with atherosclerosis in coronary and peripheral arteries and these arteries supplying the brain.
Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin and the risk of cardiovascular disease in apparently healthy men.
Haverkate F, Thompson SG, Pyke SDM, Gallimore JR, Pepys MB. Production of C‐reactive protein and risk of coronary events in stable and unstable angina.
Haubitz M, Brunkhorst R, Wrenger E, Froese P, Schulze M, Koch KM. Chronic induction of C‐reactive protein by hemodialysis, but not by peritoneal dialysis therapy.
Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients.
Lonnemann G, Haubitz M, Schindler R. Hemodialysis‐associated induction of cytokines.
Haubitz M, Schulze M, Koch KM. Increase of C‐reactive protein serum values following haemodialysis.
Wang SP, Grayston JT. Human serology in Chlamydia trachomatis infection with microimmunofluorescence.
Mendall MA, Patel P, Ballam L, Strachan D, Northfield TC. C‐reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study.
Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C‐reactive protein and coronary heart disease in the MRFIT nested case‐control study.
Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C‐reactive protein and serum amyloid A protein in severe unstable angina.
Sethi D, Muller BR, Brown EA, Maini RN, Gower PE. C‐reactive protein in haemodialysis patients with dialysis arthropathy.
Owen WF, Lowrie EG. C‐reactive protein as an outcome predictor for maintenance hemodialysis patients.
Kaysen GA, Stevenson FT, Depner TA. Determinants of albumin concentration in hemodialysis patients.
Qureshi AR, Alvestrand A, Danielsson A, Divino‐Filho JC, Gutierrez A, Lindholm B, Bergström J. Factors predicting malnutrition in hemodialysis patients: a cross‐sectional study.
McIntyre C, Harper I, Macdougall IC, Raine AEG, Williams A, Baker LRI. Serum C‐reactive protein as a marker for infection and inflammation in regular dialysis patients.
Bergström J, Heimbürger O, Lindholm B, Qureshi AR. Elevated serum C‐reactive protein is a strong predictor of increased mortality and low serum albumin in hemodialysis patients (abstract).
Held PJ, Port FK, Webb RL et al. Excerpts from the United States renal data system 1996 annual report.
Bhakdi S, Dorweiler B, Kirchmann R, Torzewski J, Weise E, Tranum‐Jensen J, Walev I, Wieland E. On the pathogenesis of atherosclerosis: enzymatic transformation of human low density lipoprotein to an atherogenic moiety.
Strachan DP, Carrington D, Mendall MA, Ballam L, Morris J, Butland BK, Sweetnam PM, Elwood PC. Relation of Chlamydia pneumoniae serology to mortality and incidence of ischaemic heart disease over 13 years in the Caerphilly prospective heart disease study.
Zoccali C, Benedetto FA, Foca A et al. C‐reactive protein and atherosclerosis in dialysis patients.
Stenvinkel P, Heimbürger O, Jogestrand T, Kärnell A, Samuelsson A. Does persistent infection with Chlamydia pneumoniae increase the risk of atherosclerosis in chronic renal failure?





Comments