-
PDF
- Split View
-
Views
-
Cite
Cite
Lindsay J. Chesterton, Mhairi K. Sigrist, Terence Bennett, Maarten W. Taal, Christopher W. McIntyre, Reduced baroreflex sensitivity is associated with increased vascular calcification and arterial stiffness, Nephrology Dialysis Transplantation, Volume 20, Issue 6, June 2005, Pages 1140–1147, https://doi.org/10.1093/ndt/gfh808
Close - Share Icon Share
Abstract
Introduction. Vascular calcification is a critical determinant of cardiovascular morbidity and mortality in chronic haemodialysis (HD) patients. The pathophysiology underlying this observation remains obscure. Baroreceptor sensitivity (BRS) is important in the maintenance of an appropriate cardiovascular status both at rest and under the physiological stress of HD. BRS is determined by both the mechanical properties of the vascular wall, mediating the transfer of transmural pressure, and afferent and efferent autonomic function. We aimed to study the association between arterial structure, function and BRS in chronic HD patients.
Methods. We studied 40 chronic HD patients mean age 62±2 (26–86) years who had received HD for a mean 40±4 (9–101) months. Spontaneous BRS was assessed using software studying the relationship between inter-beat variability and beat to beat changes in systolic blood pressure. Functional characteristics of conduit arteries (pulse wave analysis) were studied with applanation tonometry at the radial artery. Arterial calcification was assessed in lower limbs using reconstructed multi-slice computed tomography and quantified with volume-corrected calcification scores within the superficial femoral artery.
Results. Mean BRS was 4.43±0.44 ms/mmHg, with a wide range from 1.0 to 11.5 ms/mmHg. This correlated with arterial stiffness as measured by time to shoulder calculated from the central pulse wave analysis (r = 0.4, P = 0.01). BRS was also associated with vascular calcification (P = 0.01) but not by other factors such as dialysis vintage, age or pre-dialysis systolic/diastolic blood pressure.
Conclusion. The reduction in BRS and the resulting aberrant blood pressure response to the physiological stress and volume changes of HD may be important in the further understanding of the pathophysiology of the increased mortality in HD patients with vascular calcification.
Introduction
Vascular calcification and arterial stiffness are well recognized as being associated with poor cardiovascular outcomes in chronic haemodialysis (HD) patients [1]. The structure and function of the arterial wall are altered by atherogenic/atherosclerotic factors and changes in the haemodynamic burden. The structural changes can include activation and proliferation of smooth muscle cells, and rearrangement of cellular elements and extracellular matrix of the vessel wall [2]. Autonomic function, a key component of cardiovascular control, is often impaired in chronic kidney disease (CKD) and is important in the development of intra-dialytic hypotension (IDH), which recently has been demonstrated to be associated with increased mortality in HD patients [3]. Few data are available concerning the natural history and progression of autonomic dysfunction. The baroreflex arc is under autonomic regulation and is integral to the short-term regulation of blood pressure (BP). A rise in BP results in an increase in transmural stretch within the aorta and the common carotid arteries. This causes activation of the baroreceptors located within the arterial wall. Increased firing from these receptors is transmitted via the afferent nerves (vagus and glossopharyngeal) to the nucleus tractus solitarius within the midbrain. Sympathetic and parasympathetic systems influencing vessel tone and heart rate constitute the efferent response. A rise in BP results in an increase in the R–R interval and vasodilatation, restoring BP to normal limits. The regression of the pulse interval against systolic BP represents an index of baroreflex sensitivity (BRS) [4]. BRS is well recognized as a composite marker of the overall integrity of the autonomic nervous system [5].
BRS is therefore determined by the mechanical properties of the arterial wall, and the parasympathetic and sympathetic nervous system. A link between arterial structure, function and autonomic mechanisms has not been described until now. We aimed to investigate this relationship by studying the association between the presence of vascular calcification, arterial stiffness and BRS in chronic HD patients.
Subjects and methods
Patient demographics and experimental schedule
We studied 40 chronic HD patients, mean age 62±2 (26–86) years, mean length of time on HD 40±4 (9–101) months. All received 4 h of HD thrice weekly, dialysing with Hospal Integra® (Dasco, Italy) monitors. Dialysate consisted of 134 mmol/l sodium and 1.25 mmol/l calcium, with bicarbonate buffering. Dry weight was set prior to the study using a combination of clinical assessment and relative blood volume monitoring.
The patient characteristics are listed in Table 1. Five patients (12.8%) had diabetes mellitus. A clinical diagnosis of ischaemic heart disease was present in eight (20.5%). Smoking, either currently or prior to the study, was common (50%). The majority of patients had reached CKD stage 5 due to glomerulonephritis, macroscopic atherosclerotic renovascular disease or because of unknown aetiology (Table 2). Exclusion criteria included previous renal transplantation, age <18 years, and arrhythmias which would preclude assessment with applanation tonometry.
Characteristics of the study cohort
| Gender (male/female) | 28/12 |
| Age (years) | 62±2.1 |
| Duration of dialysis (months) | 40±3.9 |
| Diabetes mellitus, n (%) | 5 (12.8) |
| Smoking history, n (%) | 20 (50) |
| Ischaemic heart disease, n (%) | 8 (20.5) |
| Gender (male/female) | 28/12 |
| Age (years) | 62±2.1 |
| Duration of dialysis (months) | 40±3.9 |
| Diabetes mellitus, n (%) | 5 (12.8) |
| Smoking history, n (%) | 20 (50) |
| Ischaemic heart disease, n (%) | 8 (20.5) |
Characteristics of the study cohort
| Gender (male/female) | 28/12 |
| Age (years) | 62±2.1 |
| Duration of dialysis (months) | 40±3.9 |
| Diabetes mellitus, n (%) | 5 (12.8) |
| Smoking history, n (%) | 20 (50) |
| Ischaemic heart disease, n (%) | 8 (20.5) |
| Gender (male/female) | 28/12 |
| Age (years) | 62±2.1 |
| Duration of dialysis (months) | 40±3.9 |
| Diabetes mellitus, n (%) | 5 (12.8) |
| Smoking history, n (%) | 20 (50) |
| Ischaemic heart disease, n (%) | 8 (20.5) |
Cause of chronic kidney disease (CKD) in the study cohort
| Aetiology of renal failure . | n (%) . |
|---|---|
| Uncertain aetiology | 10 (25) |
| Glomerulonephritis | 9 (23) |
| Atherosclerotic renovascular disease | 8 (17) |
| Diabetic nephropathy | 5 (10) |
| Obstructive uropathy | 5 (10) |
| Tubulo-interstitial nephritis | 2 (4) |
| Adult polycystic kidney disease | 1 (2) |
| Aetiology of renal failure . | n (%) . |
|---|---|
| Uncertain aetiology | 10 (25) |
| Glomerulonephritis | 9 (23) |
| Atherosclerotic renovascular disease | 8 (17) |
| Diabetic nephropathy | 5 (10) |
| Obstructive uropathy | 5 (10) |
| Tubulo-interstitial nephritis | 2 (4) |
| Adult polycystic kidney disease | 1 (2) |
Cause of chronic kidney disease (CKD) in the study cohort
| Aetiology of renal failure . | n (%) . |
|---|---|
| Uncertain aetiology | 10 (25) |
| Glomerulonephritis | 9 (23) |
| Atherosclerotic renovascular disease | 8 (17) |
| Diabetic nephropathy | 5 (10) |
| Obstructive uropathy | 5 (10) |
| Tubulo-interstitial nephritis | 2 (4) |
| Adult polycystic kidney disease | 1 (2) |
| Aetiology of renal failure . | n (%) . |
|---|---|
| Uncertain aetiology | 10 (25) |
| Glomerulonephritis | 9 (23) |
| Atherosclerotic renovascular disease | 8 (17) |
| Diabetic nephropathy | 5 (10) |
| Obstructive uropathy | 5 (10) |
| Tubulo-interstitial nephritis | 2 (4) |
| Adult polycystic kidney disease | 1 (2) |
Each patient underwent Finometer® measurements and applanation tonometry (measuring BRS and arterial stiffness, respectively) immediately prior to their usual HD session. Computed tomography (CT) scanning to quantify calcification was performed within 6 weeks of the other measurements. BRS data were collected from all 40 HD patients. Applanation tonometry and CT data were collected from 39 patients. All patients were studied after the short interdialytic interval during the week. Each patient gave informed, written consent. The study was approved by the Local Research Ethics Committee.
Determination of BRS—Finometer®
Systemic haemodynamic function was assessed non-invasively using the Finometer® (TNO Instruments Amsterdam, The Netherlands). This allows assessment of cardiac output, stroke volume, peripheral vascular resistance, aortic impedance and peripheral arterial compliance. Cuffs attached to the Finometer® are placed on the middle finger and on the upper arm of the patient. All data subsequently are downloaded to a PC-based analysis program (Beatscope™), allowing averaging of results over defined time periods and display of variables as percentage change from baseline. All analyses are averaged over the period of at least 300 heartbeats. This technology provides unprecedented resolution of changes in the critical cardiovascular variables [6].
The Finometer® measures the continuous interbeat interval and beat to beat BP changes. BRS is a calculation of the regression slope between these two variables. Three consecutive changes in the R–R interval in the same direction are required before a phase shift calculation (incorporated into the software) is performed, and therefore BRS can be measured non-invasively with accuracy and precision [7]. These variables alter with time, and therefore a recording of at least 10 min taken at rest, prior to HD is used to assess resting BRS. BRS software is integrated into the device and has been made available to us as a stand-alone DOS-based offline analysis tool by Karl Wesseling (TNO Instruments, Amsterdam).
Quantification of calcification
Vascular calcification has been assessed using 16 segments of multi-slice spiral CT (GE Medical Systems lightspeed16®). A standardized section of the superficial femoral artery (SFA), 20 cm above the tibial plateau and 6 cm in length, is imaged in 2.5 mm slices. Care is taken to ensure none of the slices overlap. This image allows accurate, reproducible quantification of calcium load in this section of artery and will allow comparison of change over time. Scoring was undertaken using GE Medical Systems® Advantage Workstation software and carried out as described by Agatston et al. [8]. The presence of calcification is only considered if the area is ≥1 mm2 and the density is >130 Hounsfield units. This eliminates single pixels, which could be present due to artefact. For each individual area of calcification in the SFA, a ‘region of interest’ is drawn. The software then calculates the area and density of any lesion ≥130 HU. A score for each lesion is then calculated by multiplying the density score by the area. A total calcium score for each image is then calculated by adding up all the scores from each of the 20 slices acquired. The reproducibility of this method was found to be excellent by Agatston et al. [8]. Inter-observer variability has shown a mean error of 2.5% (SD 5%).
This technique allows imaging of a representative conduit artery and is ideal for the quantification of both intimal and medial calcification (although it is unable to differentiate). Other advantages are speed of scanning and low radiation exposure (108 mGy compared with 120 mGy for a chest radiograph).
Arterial stiffness
The SphygmoCor® system (AtCor Medical Pty Ltd, New South Wales, Australia), a well-established, non-invasive technique utilizing applanation tonometry, was used to obtain pulse wave analysis. This technique has been well validated against direct invasive measurements obtained during cardiac catheterization [9]. In essence, peripheral pressure waveforms were recorded from the radial artery at the wrist, using applanation tonometry with a high-fidelity micromanometer. After 20 sequential waveforms had been acquired, a validated transfer function was used to generate the central aortic pressure waveform. The central aortic pressure wave is composed of a forward-travelling wave generated by left ventricular ejection and a wave that is reflected back from the periphery. If arterial stiffness is present, transmission of the forward and returning waves increases, resulting in augmentation of the central aortic waveform in late systole. Consequently, the time from the beginning of the waveform to the first systolic inflection point, otherwise known as time to shoulder (TTS), can be taken to represent the pulse wave velocity (PWV) (increased PWV will lead to a shorter TTS), and thus provides an indirect measure of arterial stiffness. Measurements were taken by a single operator (reproducibility studies r = 0.91).
Power and statistical method
All data were entered into Excel spread sheets. Statistical analysis was performed using the Prism v3.0 software package. Values are expressed as mean±SEM, unless otherwise stated. P-values of <0.05 were considered to be significant. The Mann–Whitney test was used to compare BRS values between groups of patients with and without vascular calcification. Regression analysis was used to determine the relationship between BRS, vascular calcification and arterial stiffness.
Results
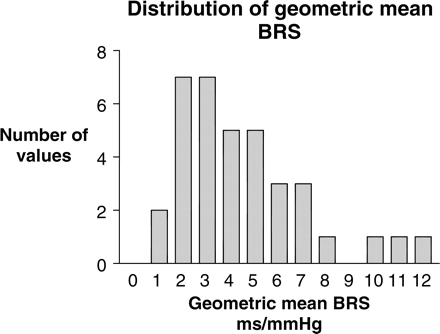
Mean pre-dialysis systolic BP was 155±4.4 mmHg, mean diastolic BP was 81±1.9 mmHg and mean pulse pressure was 73±3.9 mmHg. Mean resting pre-dialysis geometric mean BRS for the cohort was 4.43±0.44 ms/mmHg, with a wide range from 1 to 11.5 ms/mmHg (Table 3). BRS values were not normally distributed, demonstrating skewing towards lower values for BRS (Figure 1).
Pre-dialysis measurements of BP, BRS, augmentation index, TTS and vascular calcification scores highlighting the presence of autonomic dysfunction, increased arterial stiffness and vascular calcification in the study cohort
| Measurement . | Mean . | Range . |
|---|---|---|
| Mean systolic BP (mmHg) | 155±4.4 | 84–250 |
| Mean diastolic BP (mmHg) | 81±1.9 | 48–114 |
| Pulse pressure (mmHg) | 73±3.9 | 10–154 |
| Geometric mean BRS (ms/mmHg) | 4.43±0.44 | 1.0–11.5 |
| Augmentation index (P2/P1 %) | 140.6±3.6 | 98–199 |
| Time to shoulder (ms) | 106±2.9 | 87–221 |
| Calcification score | 465.9±89.2 | 0–2279 |
| Measurement . | Mean . | Range . |
|---|---|---|
| Mean systolic BP (mmHg) | 155±4.4 | 84–250 |
| Mean diastolic BP (mmHg) | 81±1.9 | 48–114 |
| Pulse pressure (mmHg) | 73±3.9 | 10–154 |
| Geometric mean BRS (ms/mmHg) | 4.43±0.44 | 1.0–11.5 |
| Augmentation index (P2/P1 %) | 140.6±3.6 | 98–199 |
| Time to shoulder (ms) | 106±2.9 | 87–221 |
| Calcification score | 465.9±89.2 | 0–2279 |
Pre-dialysis measurements of BP, BRS, augmentation index, TTS and vascular calcification scores highlighting the presence of autonomic dysfunction, increased arterial stiffness and vascular calcification in the study cohort
| Measurement . | Mean . | Range . |
|---|---|---|
| Mean systolic BP (mmHg) | 155±4.4 | 84–250 |
| Mean diastolic BP (mmHg) | 81±1.9 | 48–114 |
| Pulse pressure (mmHg) | 73±3.9 | 10–154 |
| Geometric mean BRS (ms/mmHg) | 4.43±0.44 | 1.0–11.5 |
| Augmentation index (P2/P1 %) | 140.6±3.6 | 98–199 |
| Time to shoulder (ms) | 106±2.9 | 87–221 |
| Calcification score | 465.9±89.2 | 0–2279 |
| Measurement . | Mean . | Range . |
|---|---|---|
| Mean systolic BP (mmHg) | 155±4.4 | 84–250 |
| Mean diastolic BP (mmHg) | 81±1.9 | 48–114 |
| Pulse pressure (mmHg) | 73±3.9 | 10–154 |
| Geometric mean BRS (ms/mmHg) | 4.43±0.44 | 1.0–11.5 |
| Augmentation index (P2/P1 %) | 140.6±3.6 | 98–199 |
| Time to shoulder (ms) | 106±2.9 | 87–221 |
| Calcification score | 465.9±89.2 | 0–2279 |
Histogram of geometric mean BRS measurements illustrating the non-Gaussian distribution in this HD study cohort and the skewing of BRS measurements towards values of severely impaired autonomic function.
A considerable range in augmentation index and TTS was demonstrated in the entire cohort. Mean augmentation index was 140.6±3.6 (98–199), and mean TTS was 106±2.9 (87–221) ms (Table 3).
The presence of calcification within the SFA varied widely as indicated by the range of calcification scores from 0 to 2279 (mean calcification score 465.9) (Table 3). Scores >11 were taken to represent significant calcification (associated with visible vascular calcification on plain radiography) and were present in 24 out of 39 patients (64%). Calcification scores for the whole population did not correlate with age or length of time on dialysis (Table 5).
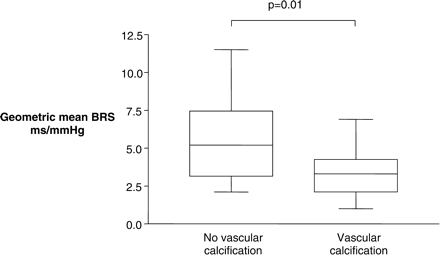
Differences in mean BP, arterial stiffness (augmentation index and TTS) and geometric mean BRS in those HD patients with and without significant vascular calcification
| . | HD patients with no vascular calcification (n = 15) . | . | HD patients with vascular calcification (n = 24) . | . | P-value . | ||
|---|---|---|---|---|---|---|---|
| . | Mean . | Range . | Mean . | Range . | . | ||
| Systolic BP (mmHg) | 141.1±6.9 | 84–194 | 162.4±5.2 | 99–250 | 0.02 | ||
| Diastolic BP (mmHg) | 83.8±3.9 | 48–111 | 79.5±2.0 | 61–114 | 0.17 | ||
| Augmentation index (P2/P1)(%) | 140.2±8.0 | 98.0–199.0 | 140.8±3.7 | 99.0–198.0 | 0.75 | ||
| TTS (ms) | 108.9±4.1 | 91.0–146.0 | 102.2±1.4 | 87.0–114.0 | 0.50 | ||
| Geometric mean BRS (ms/mmHg) | 5.67±0.76 | 2.1–11.5 | 3.43±0.38 | 1.0–6.9 | 0.01 | ||
| . | HD patients with no vascular calcification (n = 15) . | . | HD patients with vascular calcification (n = 24) . | . | P-value . | ||
|---|---|---|---|---|---|---|---|
| . | Mean . | Range . | Mean . | Range . | . | ||
| Systolic BP (mmHg) | 141.1±6.9 | 84–194 | 162.4±5.2 | 99–250 | 0.02 | ||
| Diastolic BP (mmHg) | 83.8±3.9 | 48–111 | 79.5±2.0 | 61–114 | 0.17 | ||
| Augmentation index (P2/P1)(%) | 140.2±8.0 | 98.0–199.0 | 140.8±3.7 | 99.0–198.0 | 0.75 | ||
| TTS (ms) | 108.9±4.1 | 91.0–146.0 | 102.2±1.4 | 87.0–114.0 | 0.50 | ||
| Geometric mean BRS (ms/mmHg) | 5.67±0.76 | 2.1–11.5 | 3.43±0.38 | 1.0–6.9 | 0.01 | ||
Differences in mean BP, arterial stiffness (augmentation index and TTS) and geometric mean BRS in those HD patients with and without significant vascular calcification
| . | HD patients with no vascular calcification (n = 15) . | . | HD patients with vascular calcification (n = 24) . | . | P-value . | ||
|---|---|---|---|---|---|---|---|
| . | Mean . | Range . | Mean . | Range . | . | ||
| Systolic BP (mmHg) | 141.1±6.9 | 84–194 | 162.4±5.2 | 99–250 | 0.02 | ||
| Diastolic BP (mmHg) | 83.8±3.9 | 48–111 | 79.5±2.0 | 61–114 | 0.17 | ||
| Augmentation index (P2/P1)(%) | 140.2±8.0 | 98.0–199.0 | 140.8±3.7 | 99.0–198.0 | 0.75 | ||
| TTS (ms) | 108.9±4.1 | 91.0–146.0 | 102.2±1.4 | 87.0–114.0 | 0.50 | ||
| Geometric mean BRS (ms/mmHg) | 5.67±0.76 | 2.1–11.5 | 3.43±0.38 | 1.0–6.9 | 0.01 | ||
| . | HD patients with no vascular calcification (n = 15) . | . | HD patients with vascular calcification (n = 24) . | . | P-value . | ||
|---|---|---|---|---|---|---|---|
| . | Mean . | Range . | Mean . | Range . | . | ||
| Systolic BP (mmHg) | 141.1±6.9 | 84–194 | 162.4±5.2 | 99–250 | 0.02 | ||
| Diastolic BP (mmHg) | 83.8±3.9 | 48–111 | 79.5±2.0 | 61–114 | 0.17 | ||
| Augmentation index (P2/P1)(%) | 140.2±8.0 | 98.0–199.0 | 140.8±3.7 | 99.0–198.0 | 0.75 | ||
| TTS (ms) | 108.9±4.1 | 91.0–146.0 | 102.2±1.4 | 87.0–114.0 | 0.50 | ||
| Geometric mean BRS (ms/mmHg) | 5.67±0.76 | 2.1–11.5 | 3.43±0.38 | 1.0–6.9 | 0.01 | ||
Geometric mean BRS directly correlates with TTS as a marker of arterial stiffness but does not correlate with conventional features such as age, length of time on dialysis, pulse pressure or augmentation index
| . | r . | P-value . |
|---|---|---|
| BRS and age | 0.07 | 0.66 |
| BRS and length of time on dialysis | 0.09 | 0.60 |
| BRS and pulse pressure | 0.05 | 0.81 |
| BRS and augmentation index | 0.24 | 0.14 |
| BRS and TTS | 0.41 | 0.01 |
| Calcification and age | 0.22 | 0.13 |
| Calcification and length of time on dialysis | 0.07 | 0.65 |
| . | r . | P-value . |
|---|---|---|
| BRS and age | 0.07 | 0.66 |
| BRS and length of time on dialysis | 0.09 | 0.60 |
| BRS and pulse pressure | 0.05 | 0.81 |
| BRS and augmentation index | 0.24 | 0.14 |
| BRS and TTS | 0.41 | 0.01 |
| Calcification and age | 0.22 | 0.13 |
| Calcification and length of time on dialysis | 0.07 | 0.65 |
Vascular calcification does not directly correlate with age or dialysis vintage in this study group.
Geometric mean BRS directly correlates with TTS as a marker of arterial stiffness but does not correlate with conventional features such as age, length of time on dialysis, pulse pressure or augmentation index
| . | r . | P-value . |
|---|---|---|
| BRS and age | 0.07 | 0.66 |
| BRS and length of time on dialysis | 0.09 | 0.60 |
| BRS and pulse pressure | 0.05 | 0.81 |
| BRS and augmentation index | 0.24 | 0.14 |
| BRS and TTS | 0.41 | 0.01 |
| Calcification and age | 0.22 | 0.13 |
| Calcification and length of time on dialysis | 0.07 | 0.65 |
| . | r . | P-value . |
|---|---|---|
| BRS and age | 0.07 | 0.66 |
| BRS and length of time on dialysis | 0.09 | 0.60 |
| BRS and pulse pressure | 0.05 | 0.81 |
| BRS and augmentation index | 0.24 | 0.14 |
| BRS and TTS | 0.41 | 0.01 |
| Calcification and age | 0.22 | 0.13 |
| Calcification and length of time on dialysis | 0.07 | 0.65 |
Vascular calcification does not directly correlate with age or dialysis vintage in this study group.
Patients with significant degrees of vascular calcification exhibited reduced BRS [geometric mean BRS 3.43±0.38 (1.0–6.9) ms/mmHg in patients with significant vascular calcification, vs 5.67±0.76 (2.1–11.5) ms/mmHg in those without (P = 0.01) (Table 4)].
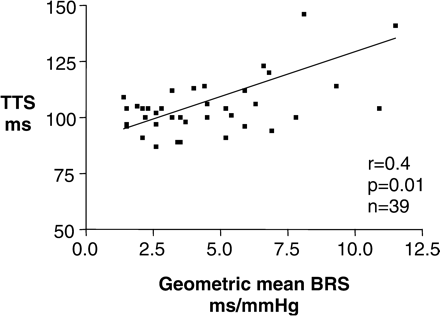
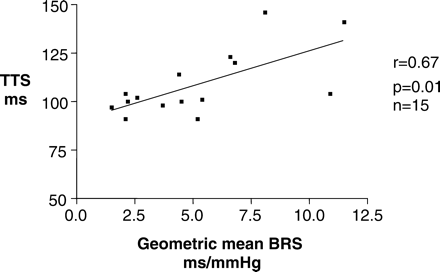
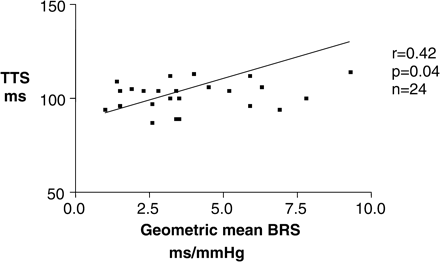
TTS measurements correlated with geometric mean BRS within the whole population (Figure 2, r = 0.4, P = 0.01), and further analysis revealed that this relationship existed in patients with and without vascular calcification [(Figure 4, r = 0.42, P = 0.04); (Figure 3, r = 0.67, P = 0.01) respectively].
Autonomic dysfunction represented by reduced geometric mean BRS measurements is associated with shorter TTS measurements representing arterial stiffness in the whole study cohort, illustrating the links between arterial structure and function.
The relationship between reduced autonomic dysfunction represented by geometric mean BRS and arterial stiffness represented by reduced TTS measurements is present even in the absence of vascular calcification.
The relationship between reduced autonomic dysfunction represented by geometric mean BRS and arterial stiffness represented by reduced TTS measurements is present in the presence of calcification.
The presence of significant vascular calcification did not alter the augmentation index [mean augmentation index 140.2±8.0 (98–199) in patients without vascular calcification vs 140.8±3.7 (99–198) in those with vascular calcification (P = 0.75) (Table 4)]. TTS values were not associated with the degree of vascular calcification [mean TTS 102.2±1.4 (87.0–114.0) ms in those with vascular calcification vs 108.9±4.1 (91.0–146.0) ms in those without (P = 0.50) (Table 4)]. Reduced BRS was associated with vascular calcification (Figure 5, P = 0.01) but not with other factors such as length of time on dialysis, age, pulse pressure or augmentation index (Table 5).
Vascular calcification is associated with reduced geometric mean BRS, highlighting the relationship between structure of the arterial wall and autonomic function.
Discussion
Our study has demonstrated significant associations between arterial functional characteristics, peripheral vascular calcification and integral autonomic mechanisms.
Arterial stiffness has been found to be an independent predictor of all-cause mortality in the chronic dialysis population [1]. Ueda and colleagues have published evidence that the inflection point of ascending aortic waveforms is also a predictive factor for all-cause and cardiovascular mortality in HD patients [10]. Furthermore, they comment that as the reflection waveform in the ascending aortic pressure reflects large artery function and systemic arterial stiffness, then TTS is related to the degree of arteriosclerosis. The Moens–Korteweg equation, based upon the theory that the square of the PWV through the artery is directly related to its stiffness, does not, however, provide information as to where a locus of arteriosclerosis may lie [11]. Interestingly, there is much debate as to the most representative site to derive markers of arterial stiffness. Studies of arterial stiffness in the past 12 months have utilized cardiac catheterization [10], the carotid and femoral artery [12], and measurements from the heart to the posterior tibial artery [11]. Our study used the radial artery to measure markers of arterial stiffness. Justification for the use of the radial artery (in association with a validated transfer function to derive the central aortic waveform) includes the similarities of upper limb vasculature in the general population, calibration of the radial artery waveform to the brachial from cuff values, and that upper limb velocity changes little with age, elevation of arterial pressure or with types of vasoactive therapy used in clinical practice. In contrast, carotid calibration relies on indirect determination, whether through the use of the transfer function from the radial artery to the ascending aorta, then from the ascending aorta to the carotid or through assuming that the mean and diastolic pressure are the same in the carotid as well as in the brachial and radial artery [9].
PWV may be thought to be a more accurate marker of arterial stiffness than TTS, but PWV measurements also have disadvantages. Takenaka et al. [11] comment that with a reduction in the ankle–brachial pressure index, the PWV becomes inaccurately low, and indeed they had to withdraw all smokers from their study of HD patients on this basis. We therefore feel that the use of the radial artery has been well validated as a non-invasive technique, has a justifiable use, and conclude that TTS may give better representation of the overall arterial tree especially in light of the other peripheral measurements in our study.
An association between PWV and arterial medial calcification has been demonstrated in a study of 202 HD patients [13]. However, in a report of 110 patients, the same group were unable to demonstrate the same link between arterial calcification and other arterial parameters including PWV [1]. One study has demonstrated a linear relationship between the degree of coronary electron beam computed tomography (EBCT) calcification and aortic pulse wave velocity utilizing a carotid–femoral approach [12]. The sample included 55 HD patients and the correlation was relatively weak. Our study failed to reveal an association between calcification and TTS (as a measure of arterial stiffness). Therefore, we conclude that there may be inadequate patient numbers in this cohort with values across the whole calcification/arterial stiffness spectrum in order to demonstrate a direct relationship between calcification and TTS. Vascular calcification is clearly an additional factor in the complex process resulting in reducing arterial compliance, but we have been unable to demonstrate this ourselves. The augmentation index did not differ between the calcified and non-calcified groups in our study. The index is considered to be a valuable marker of arterial stiffening but it does depend on many factors such as PWV, body height, ejection duration and, importantly, the reflective properties of the arterial system [14]. Furthermore, O’Rourke and colleagues have not been able to gauge the severity of atherosclerosis on the basis of the arterial pressure wave alone [9]. Therefore, this may be evidence that stiffening cannot be excluded in small patient groups merely because the augmentation index is unchanged.
Historically, BRS (as a marker of the overall integrity of the autonomic nervous system) was measured with orthostatic techniques and clinical manoeuvres such as the Valsalva. With the advent of spectral analysis, cautious separation of the autonomic control of BP into sympathetic and parasympathetic divisions became a possibility. Studies in the non-renal population have increasingly returned to BRS as an overall marker of the autonomic nervous system. A study comparing spontaneous, Finapres-derived BRS (forerunner to the Finometer) with BRS measured by invasive pharmacological techniques contends that the central organization of the cardiac parasympathetic and sympathetic reflex is a ‘black box’, implying that it is the overall response to BP changes that is significant [15]. Therefore, we chose to concentrate on BRS as a composite value that reflects a physiological mechanism of direct relevance to HD patients (short-term regulation of BP in response to dialysis and volume removal). Finometer-derived BRS measurements may be a novel technique, but haemodynamic data generated with this method have already been well validated in the literature [6], and, pragmatically, this non-invasive technique is easily performed within the dialysis unit. Previous studies have reported that the presence of parasympathetic or sympathetic dysfunction is variable within renal populations [16]. Our results have also demonstrated considerable variation in the presence of autonomic dysfunction and consequently provide further evidence that impaired baroreflex sensitivity is not normally distributed within the chronic HD population.
Reduced BRS has been found to be associated with the presence of vascular calcification in this study, and this relationship has been reported previously [17]. We have also demonstrated that in chronic HD patients, reduced BRS is associated with reduced TTS, a marker of arterial stiffness, and that this relationship is present both with and without the presence of vascular calcification. Possible explanations include the direct effect of vascular calcification upon the compliance on the arterial wall, resulting in a reduction of transmission of the transmural stretch that is associated with changes in BP, leading to delayed activation of the arterial baroreceptors. There may also be paracrine effects, either directly from the calcification reducing endothelial function or from other processes within the arterial wall associated with calcification, on baroreceptor structure and performance. The effect of vascular calcification on BRS may also go beyond the reduction of arterial compliance. The mechanisms involved remain unclear, and further work is required to explore this relationship further.
While it is clear that vascular calcification may adversely affect BRS, it is interesting to note that the reduction in autonomic function might also contribute to the pathogenesis of vascular calcification. Earlier studies investigating the presence of vascular calcification after lumbar sympathectomy demonstrated that in those patients that had undergone bilateral sympathectomy, medial calcification was seen in both limbs. However, patients who had undergone unilateral sympathectomy demonstrated ipsilateral medial calcification on plain radiography of the lower limbs [18]. Autonomic dysfunction may be an additional factor influencing the response of arterial structure to the uraemic milieu, resulting in calcification. This remains conjecture, and further work is required to pursue the possibility that the presence of autonomic neuropathy is itself involved in the pathophysiology of accelerated vascular calcification.
One potential weakness of this study is the use of multiple arterial sites for the various measurements, namely the radial artery to derive arterial stiffness, the digital artery to identify the central haemodynamic variables including BRS, and the use of the SFA to derive a femoral calcification score. First, however, and as documented above, the Finometer derives central aortic waveforms from validated mathematical analysis of the digital artery. Secondly, markers of central arterial stiffness derived from the radial artery have been widely utilized. Thirdly, we justify the use of the SFA for the following reasons. Detection of lower limb vascular calcification (including the SFA) by plain radiography has been well described. Semi-quantitive analysis of vascular calcification (using plain film radiology and ultrasonography in the carotid artery, abdominal aorta, iliofemoral axis and femorotibial axis) concluded that the presence of multiple sites of vascular calcification could be included as an independent predictor of outcome [1]. They continue that this technique has its limitations, and more sensitive methods such as CT should be used in future. Atherosclerosis has a predilection for certain sites such as ostia and bifurcations. This is just one of the reasons that a site distal from bifurcation was chosen (20 cm above the tibial plateau). It follows that if vascular calcification can be detected at the femoral artery, then it may equal or even underestimate the quantity of coronary artery calcification. Anatomically, both radial and femoral arteries are muscular. Traditionally, it has been thought that radial arteries are not prone to atherosclerosis and therefore are likely to be physiologically distinct from femoral vessels. However, recent reports indicate that the incidence of intimal hyperplasia, medial calcification and arteriosclerosis in the radial artery is greater than has hitherto been recognized [19]. They also comment that factors associated with increased medial calcification in the radial artery included traditional cardiovascular risk factors and chronic renal failure. The two sites may therefore have greater similarities than previously thought. Certainly digital artery/small vessel calcification is a common, recognized finding in the renal population. Studies have utilized coronary EBCT to elucidate coronary calcification [12] and this has obvious advantages over our technique. There are no other reported cases of CT-derived calcification scores from the femoral artery, and this is a drawback. However, the use of spiral CT to quantify vascular calcification at the SFA avoids radiation-sensitive areas, is rapid, thereby minimizing radiation exposure, and is easily available. Moreover, because of the minimal radiation exposure, it is possible to justify repeated exposure to monitor progression of vascular calcification. To conclude, therefore, although the femoral site is novel as a method of quantifying calcification, there is no technique that can as yet both distinguish intimal from medial calcification and quantitatively analyse volumes or areas of calcification, and the femoral site does have advantages over coronary EBCT.
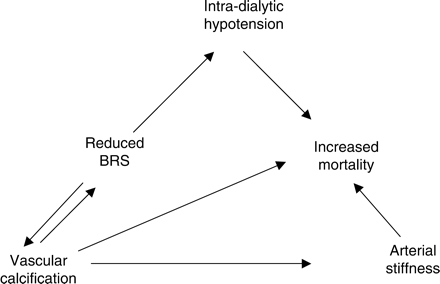
The clinical implication and consequences of autonomic dysfunction are widespread. In particular, chronic HD patients experience substantial changes in BP in response to volume removal from regular HD, and an ability to alter BP to compensate for these changes is essential in the maintenance of cardiovascular stability. The baroreceptors involved in the short-term regulation of BP are located in the arterial wall, and thus vascular calcification and arterial stiffness may determine BRS. Impaired sympathovagal balance has been linked to IDH, with previous suggestions that disturbed autonomic control in uraemic patients is linked to cardiac damage [20]. It is intradialytic haemodynamic instability (rather than pre-dialysis BP) that has been demonstrated to determine mortality in prospectively studied HD patients [3]. Relating arterial structure to function may contribute to the wider understanding of the relationship between vascular calcification, IDH and mortality in HD patients (summarized in Figure 6).
The proposed relationship between arterial structure, function, vascular calcification and increased cardiovascular mortality in chronic HD patients.
In conclusion, the changes in transmural pressure that are necessary to activate the arterial baroreceptors are likely to be affected by arterial stiffness. Medial calcification contributes to the burden of increasing arterial stiffness in chronic HD patients. Consequently, a reduction in BRS causing a reduction in the overall ability to regulate BP in the short term, resulting in aberrant cardiovascular responses to the physiological stress and volume changes of HD, may contribute to the pathophysiology of increased mortality in HD patients with vascular calcification.
Conflict of interest statement. None declared
References
Blacher J, Guerin AP, Pannier B et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease.
Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling.
Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients.
Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity.
Robinson TG, Carr SJ. Cardiovascular autonomic dysfunction in uremia.
Imholz BP, Wieling W, van Montfrans GA et al. Fifteen years experience with finger arterial pressure monitoring: assessment of the technology.
Gizdulich P, Imholz BP, van den Meiracker AH et al. Finapres tracking of systolic pressure and baroreflex sensitivity improved by waveform filtering.
Agatston AS, Janowitz WR, Hildner FJ et al. Quantification of coronary artery calcium using ultrafast computed tomography.
Ueda H, Hayashi T, Tsumura K et al. Inflection point of ascending aortic waveform is a predictive factor for all-cause and cardiovascular mortality in patients with chronic renal failure on hemodialysis.
Takenaka T, Kobayashi K, Suzuki H. Pulse wave velocity as an indicator of arteriosclerosis in hemodialysis patients.
Haydar AA, Covic A, Colhoun H et al. Coronary artery calcification and aortic pulse wave velocity in chronic kidney disease patients.
London GM, Guerin AP, Marchais SJ et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality.
Ferro C. Central aortic pressure augmentation in stable renal transplant recipients.
Parlow J, Viale JP, Annat G et al. Spontaneous cardiac baroreflex in humans. Comparison with drug-induced responses.
Jassal SV, Douglas JF, Stout RW. Prevalence of central autonomic neuropathy in elderly dialysis patients.
Goldsmith D, MacGinley R, Smith A, Covic A. How important and how treatable is vascular stiffness as a cardiovascular risk factor in renal failure?
Goebel FD, Fuessl HS. Monckeberg's sclerosis after sympathetic denervation in diabetic and non-diabetic subjects.
Chowdhury UK, Airan B, Mishra PK et al. Histopathology and morphometry of radial artery conduits: basic study and clinical application.
Author notes
1Department of Renal Medicine, Derby City General Hospital, Derby, 2Department of Biomedical Sciences, University of Nottingham, Queens Medical Centre and 3Centre For Systems Integrated Biology and Medicine (Nottingham University), Nottingham, UK





Comments