-
PDF
- Split View
-
Views
-
Cite
Cite
James J. Vredenburgh, Annick Desjardins, David A. Reardon, Henry S. Friedman, Experience with irinotecan for the treatment of malignant glioma, Neuro-Oncology, Volume 11, Issue 1, February 2009, Pages 80–91, https://doi.org/10.1215/15228517-2008-075
Close - Share Icon Share
Abstract
Malignant glioma is the most commonly occurring primary malignant brain tumor. It is difficult to treat and is usually associated with an inexorable, rapidly fatal clinical course. Chemotherapy, radiotherapy, and surgical excision are core components in the management of malignant glioma. However, chemotherapy, even with the most active regimens currently available, achieves only modest improvement in overall survival. Novel agents and new approaches to therapy are required to improve clinical outcomes. Irinotecan, a first-line treatment for metastatic colorectal cancer and an agent with high activity against solid tumors of the gastrointestinal tract, is an inhibitor of topoisomerase I, a critical enzyme needed for DNA transcription. Irinotecan crosses the blood-brain barrier and, in preclinical investigations, has demonstrated cytotoxic activity against central nervous system tumor xenografts. Its antitumor activity has also been demonstrated against glioblastoma cells with multidrug resistance. Studies in adult and pediatric patients with recurrent, intractable malignant glioma have evaluated irinotecan as monotherapy and in combination with other agents, including temozolomide, carmustine, thalidomide, and bevacizumab. Studies of irinotecan in combination with other medications, particularly temozolomide and bevacizumab, have yielded promising results. Irinotecan monotherapy has demonstrated efficacy; however, its efficacy appears to be enhanced when used in combination with other chemotherapeutic agents. When administered concurrently with enzyme-inducing antiepileptic drugs, the dosage must be increased to compensate for enhanced cytochrome CY3A4/5 enzyme activity. Toxicities associated with irinotecan have been manageable; the most important dose-limiting toxicities are neutropenia and diarrhea. Irinotecan-based chemotherapy of malignant glioma merits further study.
Malignant primary tumors of the brain and CNS portend a grim clinical course and a poor prognosis. Although relatively uncommon compared with other solid tumors, their prevalence has increased dramatically in recent decades.1,2 In 2000, it was estimated that more than 81,000 Americans were living with malignant primary brain or CNS tumors, and an additional 10,000 with tumor behavior characterized as uncertain.3 The age-adjusted incidence rate for malignant brain or CNS tumors, using the 2000 U.S. standard population, was estimated at 7.4/100,000 person-years.3 More recently (2006), the American Cancer Society estimates for new CNS cancers and tumor deaths were 18,820 and 12,820, respectively.4
Malignant Glioma Treatment Considerations
Malignant glioma—predominately glioblastoma, the most common histologic subtype—accounts for nearly 80% of all malignant brain tumors.3 Malignant glioma presents severe management challenges: it is difficult to treat, devastating in its progressive and disabling manifestations, and highly lethal, with a median survival of 9–12 months.5 Fewer than 4% of patients with glioblastoma survive for ⩾5 years following diagnosis,3 and most deaths occur within 2 years.5
The therapeutic objective for patients with resectable high-grade glioma is excision to the greatest extent feasible. However, malignant glioma is surgically incurable in the majority of patients.6 Regardless of the degree of operability, combining radiotherapy (RT) with chemotherapy is highly recommended. This integrated approach to treatment has resulted in a significant survival benefit,7–11 and is the current standard of treatment.1,6
Temozolomide is an orally administered alkylating agent that crosses the blood-brain barrier and is distributed to the CNS. Several studies have shown efficacy with temozolomide in the treatment of high-grade glioma, particularly in combination with RT.1,11,12 However, the benefits associated with temozolomide are substantially less in patients with tumors that exhibit high endogenous activity of the DNA repair enzyme 06-methylguanine-DNA methyltransferase (MGMT), which leads to the emergence of an alkylating-tolerant, treatment-resistant phenotype.13–18 Tumor cells expressing MGMT are considerably more resistant to temozolomide, nitrosoureas (e.g., carmustine [BCNU]), and related compounds.13
Finally, most patients with malignant glioma eventually experience disease recurrence. Treatment decisions for these individuals are complicated because additional RT may pose a risk of cumulative toxicity, and options for chemotherapy may be limited by the development of resistance.6 To improve clinical outcomes in patients with malignant glioma, novel chemotherapeutic agents and effective new regimens are needed.
Methods
Information included in this review was obtained through online query of the National Library of Medicine MEDLINE and PubMed databases. The query was conducted from November 2006 through February 2008, covering the period from 1996, which coincides with the introduction of irinotecan, through February 2008. Search criteria included the keywords “irinotecan,” “glioma,” “glioblastoma,” and others. Keyword search strategies were structured to ensure a breadth of returned citations across clinical trials, review articles, commentaries, and practice guidelines. Only articles published in English were considered. Full articles were obtained; in the case of review articles, primary sources were obtained for corroboration. In addition, abstract collections from the most recent annual meetings of the Society for Neuro-Oncology were reviewed for relevant material.
Irinotecan: A New Therapeutic Option
Irinotecan is a camptothecin derivative that inhibits topoisomerase I, an essential nuclear enzyme required for relaxation of supercoiled DNA, which yields topologic changes that facilitate RNA transcription and DNA replication.19 Topoisomerase I and II activities are significantly enhanced in malignant gliomas following DNA damage.20 Chromatin-bound topoisomerase I and II levels correlate with the induction of apoptosis by DNA-damaging agents, and the induction of apoptosis is associated with a decline in Bcl-2.20
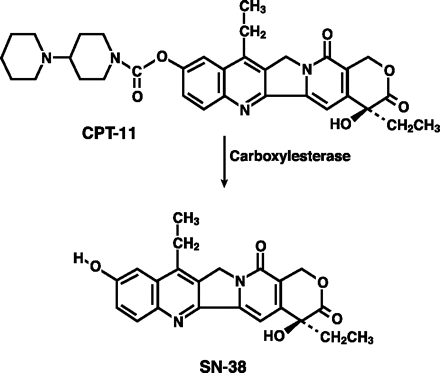
The active metabolite of irinotecan, 7-ethyl-10-hydroxycamptothecin (SN-38) (1),21 is approximately 100–1,000 times more potent than irinotecan as an inhibitor of topoisomerase.22,23 SN-38 is a product of the carboxylesterase-mediated breakdown of irinotecan.24 Glioma cells can convert irinotecan to SN-38 directly.25 Increased SN-38 concentrations induce cytotoxic changes morphologically, decrease proliferation, and increase cytotoxicity.25 The mechanism of cytotoxicity is apoptosis. In studies, SN-38 has led to decreased concentration of the antiapoptotic protein Bcl-2 and increased expression of the proapoptotic protein Bax.25–27 Resistance to irinotecan and SN-38 is likely mediated by production of IL-1 beta, with activation of NF-kB, a key transcriptional factor that inhibits the apoptotic response.28,29 SN-38 is further metabolized in the liver to an inactive metabolite, SN-38 glucuronide. Inactivation and metabolism of SN-38 require uridine diphosphateglucuronosyltransferase 1A1 (UGT1A1), but an inheritable polymorphism of the gene for UGT1A1 is associated with reduced expression and a heightened risk of treatment-related toxicity, particularly severe diarrhea and neutropenia.22,30,31
Irinotecan is currently approved for the treatment of metastatic colorectal cancer, for which it is a first-line therapeutic selection along with leucovorin (LV) and 5-fluorouracil (5-FU) and a recommended agent for recurrent and intractable disease that has progressed despite treatment with 5-FU.32,33 The recommended dosage for its use as a single agent is 125 mg/m2 i.v. every 4 weeks followed by a 2-week rest, or 350 mg/m2 i.v. every 3 weeks.34 When used in combination with leucovorin and 5-FU, the recommended regimens are irinotecan 125 mg/m2 i.v., LV 20 mg/m2 bolus, and 5-FU 500 mg/m2 bolus weekly for 4 weeks of a 6-week cycle and irinotecan 180 mg/m2 i.v. on days 1, 15, and 29, with LV 200 mg/m2 i.v., 5-FU 400 mg/m2 bolus, and 5-FU 600 mg/m2 i.v. on days 1, 2, 15, 16, 29, and 30 on a 6-week cycle.34
Metabolism of irinotecan (CPT-11) by carboxylesterase to its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38). Source: HS Friedman et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17:1516–1525. Reprinted with permission from the American Society of Clinical Oncology.21
Irinotecan, which crosses the blood-brain barrier, has shown activity against a range of CNS tumor xenografts in animal models.35–38 In addition, antitumor activity has been demonstrated against human glioblastoma cells with multidrug resistance.39 The persistently poor prognosis associated with standard treatments in people with primary malignant brain tumors has led to the clinical study of alternative agents with novel mechanisms of action. Irinotecan, administered as a single agent or in combination with other chemotherapy medications, has been extensively investigated for use against these aggressive and frequently fatal malignancies. The most important dose-limiting toxicities associated with irinotecan treatment are neutropenia and severe diarrhea; cumulative toxicity is less of a concern.40
Pharmacokinetic Profile
Following i.v. administration, irinotecan plasma concentrations decline, with a mean terminal elimination half-life (t½) of 6–12 hours; the mean t½ of SN-38 is approximately 10–20 hours. Maximum concentrations of SN-38 are achieved within 60 minutes of administration, and dose-normalized SN-38 area-under-the-curve (AUC) values are comparable between adults and children.34
A finding of great practical importance for irinotecan use in CNS tumor populations receiving concurrent anticonvulsant therapy is that, in addition to biotransformation to SN-38 via a pathway requiring UGT1A1, systemic clearance of irinotecan requires oxidation by cytochrome P450 enzymes (specifically CY3A4/5) to form a number of relatively inactive metabolites.41,42 Several antiepileptic medications have been shown to induce cytochrome P450 enzyme activity, and concurrent use increases irinotecan clearance while substantially reducing systemic exposure to the drug and its active metabolite.43 The appropriate starting dose of irinotecan for subjects taking stable doses of enzyme-inducing antiepileptic drugs (EIAEDs) has not been formally defined,34 but upward dosage adjustment (to 750 mg/m2) was recommended in a phase II study by Kuhn.42 A patient who discontinues EIAEDs during irinotecan treatment without dose modification may be at risk of irinotecan toxicity due to increased dosage, as well as uncontrolled seizure activity due to discontinuation of anticonvulsant medication. A period of at least 4 weeks should be allowed following discontinuation of an EIAED before the P450 enzyme system reaches a steady state. EIAEDs in common use include phenytoin, phenobarbital, primidone, and carbamazepine.44 Oxcarbazepine, a newer EIAED, is less likely to induce cytochrome P450 enzyme activity.45
Irinotecan Monotherapy: Phase II Studies
Irinotecan (125 mg/m2), administered as a 90-minute infusion once weekly for 4 weeks followed by a 2-week rest (1 cycle) for a total of 6 cycles, has demonstrated activity in adult patients with recurrent or progressive malignant glioma.21 Fifty-three patients (88%) had previously received RT, but only 6 (10%) had received temozolomide. Of 60 patients enrolled, 9 (15%; 95% CI, 6%–24%) demonstrated a partial response, defined as a reduction in tumor size of ⩾50% maintained for 4 weeks, stable or reduced corticosteroid dose, and neurologic stability or improvement (Table 1). The duration of responses ranged 12–42 weeks. Thirty-three patients (55%) achieved stable disease lasting at least 12 weeks (2 cycles). Toxicities were manageable in this study and were limited to infrequent neutropenia, nausea, vomiting, and diarrhea. Low plasma concentrations of irinotecan and SN-38 were consistently noted, however, and were attributable to concurrent therapy with EIAEDs. Table 2 summarizes trials employing irinotecan as monotherapy.
Tumor response data by histologic diagnosis
| . | . | Response . | . | Stable Disease . | . | Progressive Disease . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Histology . | No. of Patients . | No. . | % . | No. . | % . | No. . | % . | |||
| GBM | 48 | 8 | 17 | 26a | 54 | 14 | 29 | |||
| AA | 10 | 1 | 10 | 6 | 60 | 3 | 30 | |||
| AO | 2 | 0 | 0 | 1 | 50 | 1 | 50 | |||
| Overall | 60 | 9b | 15 | 33 | 55 | 18 | 30 | |||
| . | . | Response . | . | Stable Disease . | . | Progressive Disease . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Histology . | No. of Patients . | No. . | % . | No. . | % . | No. . | % . | |||
| GBM | 48 | 8 | 17 | 26a | 54 | 14 | 29 | |||
| AA | 10 | 1 | 10 | 6 | 60 | 3 | 30 | |||
| AO | 2 | 0 | 0 | 1 | 50 | 1 | 50 | |||
| Overall | 60 | 9b | 15 | 33 | 55 | 18 | 30 | |||
Abbreviations: AA = anaplastic astrocytoma; AO = anaplastic oligodendroglioma; GBM = glioblastoma multiforme
Source: HS Friedman et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17(5):1516–1525. Reprinted with permission from the American Society of Clinical Oncology21
Includes 4 patients with minor responses
Current duration (weeks) of response (+ indicates censored value): 12+, 12, 22+, 25, 30, 30+, 42+, 42+; data unavailable for one patient
Tumor response data by histologic diagnosis
| . | . | Response . | . | Stable Disease . | . | Progressive Disease . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Histology . | No. of Patients . | No. . | % . | No. . | % . | No. . | % . | |||
| GBM | 48 | 8 | 17 | 26a | 54 | 14 | 29 | |||
| AA | 10 | 1 | 10 | 6 | 60 | 3 | 30 | |||
| AO | 2 | 0 | 0 | 1 | 50 | 1 | 50 | |||
| Overall | 60 | 9b | 15 | 33 | 55 | 18 | 30 | |||
| . | . | Response . | . | Stable Disease . | . | Progressive Disease . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Tumor Histology . | No. of Patients . | No. . | % . | No. . | % . | No. . | % . | |||
| GBM | 48 | 8 | 17 | 26a | 54 | 14 | 29 | |||
| AA | 10 | 1 | 10 | 6 | 60 | 3 | 30 | |||
| AO | 2 | 0 | 0 | 1 | 50 | 1 | 50 | |||
| Overall | 60 | 9b | 15 | 33 | 55 | 18 | 30 | |||
Abbreviations: AA = anaplastic astrocytoma; AO = anaplastic oligodendroglioma; GBM = glioblastoma multiforme
Source: HS Friedman et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17(5):1516–1525. Reprinted with permission from the American Society of Clinical Oncology21
Includes 4 patients with minor responses
Current duration (weeks) of response (+ indicates censored value): 12+, 12, 22+, 25, 30, 30+, 42+, 42+; data unavailable for one patient
Irinotecan monotherapy studies for malignant glioma
| Study . | n . | Patient Characteristics . | Irinotecan Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Friedman et 60 al., 199921 | 60 | ≥3 wks after resection; ≥6 wks after RT or CT; ≤1 prior chemotherapy regimen; ≥29 on EIAEDs | 125 mg/m2 weekly for 4/6 wks | 15% (95% CI, 6%–24%) | Median TTP, 12 wks (range, 6–68 wks) |
| Buckner et al., 200346 | 64 | ≥8 wks after RT; 44 on EIAEDs | Trial A (n = 32): 125 mg/m2 or 100 mg/m2 with prior nitrosourea for 4/6 wks | Trial A (30 evaluable): 7% | |
| Trial B (n = 32): 300 mg/m2 or 250 mg/m2 with prior nitrosourea q3wks | Trial B: 13%; 10% overall | ||||
| Raymond et al., 200347 | 52 | Chemotherapy-naive; Group A (n = 25): inoperable or incompletely resected RT-naive GBM | Group A: 3 cycles of 350 mg/m2 q21d; | 2.2% ORR (95% CI, 0.2%–6.5%); 46 evaluable | Group A (n = 22): median TTP, 9 wks (range, 3.6–53.1; 95% CI, 8.1–22.4) |
| Group B (n = 27): relapsed after RT; most (n = 40) on anticonvulsant therapy | Group B: ≤6 cycles | Group B (n = 24): median TTP 14.4 wks (range, 5.5–36.8; 95% CI 9.0–21.1) | |||
| Chamberlain, 200248 | 40 | Previously treated with surgery, RT, and ≥1 CT w/alkylating agent but no iri; 25/40 on EIAEDs | 400 mg/m2 with 500 mg/m2 3 wks later | None | Median OS, 4 mos (range, 3–8 mos) |
| Turner et al., 200249 | 22 | Pediatric, variety of recurrent tumors (n = 18), or newly diagnosed GBM (n = 4) | 125 mg/m2 weekly for 4/6 wks | 4/9 w/GBM or AA (44% [95% CI, 11%–82%]) | 2 recurrent GBM: CR, 9 and >48 mos; 1 newly diagnosed GBM: PR, 18 mos; 1 recurrent AA: PR, 11 mos |
| Cloughesy et al., 200250 | 14 | ≥4 wks prior RT or CT; ≥10 d prior surgical resection; no iri or topo; 13 on EIAEDs | 300 mg/m2 q3wks for 2 cycles, then increased to 350 mg/m2 if tolerated | 14% (95% CI, 2%–43%) | Median TTP, 6 wks; median survival, 24 wks |
| Cloughesy et al., 200351 | 35 | ≥4 wks prior RT or CT; ≥10 d prior surgical resection; no previous iri or topo; 29 on EIAEDs | 350–400 mg/m2 q3wks, increasing q cycle by 100 mg/m2 w/EIAEDs or 50 mg/m2 wo/EIAEDs | 9% | Median TTP, 2.1 mos; median OS, 8.5 mos |
| Batchelor et al., 200452 | 18 | Prior RT; 16 prior CT; 12 on EIAEDs | 411 mg/m2 weekly for 4/6 wks w EIAEDs, or 117 mg/m2 w/o EIAEDs | 6% CR | Median PFS, 7.3 mos; median OS, 10.4 mos |
| Gilbert et al., 200353 | 40 | ≥3 m prior RT; ≥3 wks prior CT, except ≥6 wks prior CENU; 31 on EIAEDs | 125 mg/m2 q4wks w/escalation based on modified continual reassessment | 4 on EIAEDs showed OR | Median OS, 7.4 mos |
| Prados et al., 200454 | 48 | ≤2 prior CT regimens; all on EIAEDs | 350 mg/m2 q3wks escalated by 50 mg/m2 to 800 mg/m2 | None (42 pts evaluable) | Median PFS, 6 wks |
| Prados et al., 200655 | 51 | ≤1 prior CT regimen; 29 on EIAEDs | 350 mg/m2 q3wks wo/EIAEDs; 750 mg/m2 w/EIAEDs | 5.8% PR; 17 SD | 6-mo PFS, 17.6% |
| Study . | n . | Patient Characteristics . | Irinotecan Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Friedman et 60 al., 199921 | 60 | ≥3 wks after resection; ≥6 wks after RT or CT; ≤1 prior chemotherapy regimen; ≥29 on EIAEDs | 125 mg/m2 weekly for 4/6 wks | 15% (95% CI, 6%–24%) | Median TTP, 12 wks (range, 6–68 wks) |
| Buckner et al., 200346 | 64 | ≥8 wks after RT; 44 on EIAEDs | Trial A (n = 32): 125 mg/m2 or 100 mg/m2 with prior nitrosourea for 4/6 wks | Trial A (30 evaluable): 7% | |
| Trial B (n = 32): 300 mg/m2 or 250 mg/m2 with prior nitrosourea q3wks | Trial B: 13%; 10% overall | ||||
| Raymond et al., 200347 | 52 | Chemotherapy-naive; Group A (n = 25): inoperable or incompletely resected RT-naive GBM | Group A: 3 cycles of 350 mg/m2 q21d; | 2.2% ORR (95% CI, 0.2%–6.5%); 46 evaluable | Group A (n = 22): median TTP, 9 wks (range, 3.6–53.1; 95% CI, 8.1–22.4) |
| Group B (n = 27): relapsed after RT; most (n = 40) on anticonvulsant therapy | Group B: ≤6 cycles | Group B (n = 24): median TTP 14.4 wks (range, 5.5–36.8; 95% CI 9.0–21.1) | |||
| Chamberlain, 200248 | 40 | Previously treated with surgery, RT, and ≥1 CT w/alkylating agent but no iri; 25/40 on EIAEDs | 400 mg/m2 with 500 mg/m2 3 wks later | None | Median OS, 4 mos (range, 3–8 mos) |
| Turner et al., 200249 | 22 | Pediatric, variety of recurrent tumors (n = 18), or newly diagnosed GBM (n = 4) | 125 mg/m2 weekly for 4/6 wks | 4/9 w/GBM or AA (44% [95% CI, 11%–82%]) | 2 recurrent GBM: CR, 9 and >48 mos; 1 newly diagnosed GBM: PR, 18 mos; 1 recurrent AA: PR, 11 mos |
| Cloughesy et al., 200250 | 14 | ≥4 wks prior RT or CT; ≥10 d prior surgical resection; no iri or topo; 13 on EIAEDs | 300 mg/m2 q3wks for 2 cycles, then increased to 350 mg/m2 if tolerated | 14% (95% CI, 2%–43%) | Median TTP, 6 wks; median survival, 24 wks |
| Cloughesy et al., 200351 | 35 | ≥4 wks prior RT or CT; ≥10 d prior surgical resection; no previous iri or topo; 29 on EIAEDs | 350–400 mg/m2 q3wks, increasing q cycle by 100 mg/m2 w/EIAEDs or 50 mg/m2 wo/EIAEDs | 9% | Median TTP, 2.1 mos; median OS, 8.5 mos |
| Batchelor et al., 200452 | 18 | Prior RT; 16 prior CT; 12 on EIAEDs | 411 mg/m2 weekly for 4/6 wks w EIAEDs, or 117 mg/m2 w/o EIAEDs | 6% CR | Median PFS, 7.3 mos; median OS, 10.4 mos |
| Gilbert et al., 200353 | 40 | ≥3 m prior RT; ≥3 wks prior CT, except ≥6 wks prior CENU; 31 on EIAEDs | 125 mg/m2 q4wks w/escalation based on modified continual reassessment | 4 on EIAEDs showed OR | Median OS, 7.4 mos |
| Prados et al., 200454 | 48 | ≤2 prior CT regimens; all on EIAEDs | 350 mg/m2 q3wks escalated by 50 mg/m2 to 800 mg/m2 | None (42 pts evaluable) | Median PFS, 6 wks |
| Prados et al., 200655 | 51 | ≤1 prior CT regimen; 29 on EIAEDs | 350 mg/m2 q3wks wo/EIAEDs; 750 mg/m2 w/EIAEDs | 5.8% PR; 17 SD | 6-mo PFS, 17.6% |
AA = anaplastic astrocytoma; CENU = chloroethylnitrosourea regimen; CR = complete response; CT = chemotherapy; EIAED = enzyme-inducing antiepileptic drug; GBM = glioblastoma; iri = irinotecan; ORR = overall response rate; OS = overall survival; PR = partial response; PFS = progression-free survival; SD = stable disease; RT = radiotherapy; topo = topotecan; TTP = time to progression
Irinotecan monotherapy studies for malignant glioma
| Study . | n . | Patient Characteristics . | Irinotecan Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Friedman et 60 al., 199921 | 60 | ≥3 wks after resection; ≥6 wks after RT or CT; ≤1 prior chemotherapy regimen; ≥29 on EIAEDs | 125 mg/m2 weekly for 4/6 wks | 15% (95% CI, 6%–24%) | Median TTP, 12 wks (range, 6–68 wks) |
| Buckner et al., 200346 | 64 | ≥8 wks after RT; 44 on EIAEDs | Trial A (n = 32): 125 mg/m2 or 100 mg/m2 with prior nitrosourea for 4/6 wks | Trial A (30 evaluable): 7% | |
| Trial B (n = 32): 300 mg/m2 or 250 mg/m2 with prior nitrosourea q3wks | Trial B: 13%; 10% overall | ||||
| Raymond et al., 200347 | 52 | Chemotherapy-naive; Group A (n = 25): inoperable or incompletely resected RT-naive GBM | Group A: 3 cycles of 350 mg/m2 q21d; | 2.2% ORR (95% CI, 0.2%–6.5%); 46 evaluable | Group A (n = 22): median TTP, 9 wks (range, 3.6–53.1; 95% CI, 8.1–22.4) |
| Group B (n = 27): relapsed after RT; most (n = 40) on anticonvulsant therapy | Group B: ≤6 cycles | Group B (n = 24): median TTP 14.4 wks (range, 5.5–36.8; 95% CI 9.0–21.1) | |||
| Chamberlain, 200248 | 40 | Previously treated with surgery, RT, and ≥1 CT w/alkylating agent but no iri; 25/40 on EIAEDs | 400 mg/m2 with 500 mg/m2 3 wks later | None | Median OS, 4 mos (range, 3–8 mos) |
| Turner et al., 200249 | 22 | Pediatric, variety of recurrent tumors (n = 18), or newly diagnosed GBM (n = 4) | 125 mg/m2 weekly for 4/6 wks | 4/9 w/GBM or AA (44% [95% CI, 11%–82%]) | 2 recurrent GBM: CR, 9 and >48 mos; 1 newly diagnosed GBM: PR, 18 mos; 1 recurrent AA: PR, 11 mos |
| Cloughesy et al., 200250 | 14 | ≥4 wks prior RT or CT; ≥10 d prior surgical resection; no iri or topo; 13 on EIAEDs | 300 mg/m2 q3wks for 2 cycles, then increased to 350 mg/m2 if tolerated | 14% (95% CI, 2%–43%) | Median TTP, 6 wks; median survival, 24 wks |
| Cloughesy et al., 200351 | 35 | ≥4 wks prior RT or CT; ≥10 d prior surgical resection; no previous iri or topo; 29 on EIAEDs | 350–400 mg/m2 q3wks, increasing q cycle by 100 mg/m2 w/EIAEDs or 50 mg/m2 wo/EIAEDs | 9% | Median TTP, 2.1 mos; median OS, 8.5 mos |
| Batchelor et al., 200452 | 18 | Prior RT; 16 prior CT; 12 on EIAEDs | 411 mg/m2 weekly for 4/6 wks w EIAEDs, or 117 mg/m2 w/o EIAEDs | 6% CR | Median PFS, 7.3 mos; median OS, 10.4 mos |
| Gilbert et al., 200353 | 40 | ≥3 m prior RT; ≥3 wks prior CT, except ≥6 wks prior CENU; 31 on EIAEDs | 125 mg/m2 q4wks w/escalation based on modified continual reassessment | 4 on EIAEDs showed OR | Median OS, 7.4 mos |
| Prados et al., 200454 | 48 | ≤2 prior CT regimens; all on EIAEDs | 350 mg/m2 q3wks escalated by 50 mg/m2 to 800 mg/m2 | None (42 pts evaluable) | Median PFS, 6 wks |
| Prados et al., 200655 | 51 | ≤1 prior CT regimen; 29 on EIAEDs | 350 mg/m2 q3wks wo/EIAEDs; 750 mg/m2 w/EIAEDs | 5.8% PR; 17 SD | 6-mo PFS, 17.6% |
| Study . | n . | Patient Characteristics . | Irinotecan Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Friedman et 60 al., 199921 | 60 | ≥3 wks after resection; ≥6 wks after RT or CT; ≤1 prior chemotherapy regimen; ≥29 on EIAEDs | 125 mg/m2 weekly for 4/6 wks | 15% (95% CI, 6%–24%) | Median TTP, 12 wks (range, 6–68 wks) |
| Buckner et al., 200346 | 64 | ≥8 wks after RT; 44 on EIAEDs | Trial A (n = 32): 125 mg/m2 or 100 mg/m2 with prior nitrosourea for 4/6 wks | Trial A (30 evaluable): 7% | |
| Trial B (n = 32): 300 mg/m2 or 250 mg/m2 with prior nitrosourea q3wks | Trial B: 13%; 10% overall | ||||
| Raymond et al., 200347 | 52 | Chemotherapy-naive; Group A (n = 25): inoperable or incompletely resected RT-naive GBM | Group A: 3 cycles of 350 mg/m2 q21d; | 2.2% ORR (95% CI, 0.2%–6.5%); 46 evaluable | Group A (n = 22): median TTP, 9 wks (range, 3.6–53.1; 95% CI, 8.1–22.4) |
| Group B (n = 27): relapsed after RT; most (n = 40) on anticonvulsant therapy | Group B: ≤6 cycles | Group B (n = 24): median TTP 14.4 wks (range, 5.5–36.8; 95% CI 9.0–21.1) | |||
| Chamberlain, 200248 | 40 | Previously treated with surgery, RT, and ≥1 CT w/alkylating agent but no iri; 25/40 on EIAEDs | 400 mg/m2 with 500 mg/m2 3 wks later | None | Median OS, 4 mos (range, 3–8 mos) |
| Turner et al., 200249 | 22 | Pediatric, variety of recurrent tumors (n = 18), or newly diagnosed GBM (n = 4) | 125 mg/m2 weekly for 4/6 wks | 4/9 w/GBM or AA (44% [95% CI, 11%–82%]) | 2 recurrent GBM: CR, 9 and >48 mos; 1 newly diagnosed GBM: PR, 18 mos; 1 recurrent AA: PR, 11 mos |
| Cloughesy et al., 200250 | 14 | ≥4 wks prior RT or CT; ≥10 d prior surgical resection; no iri or topo; 13 on EIAEDs | 300 mg/m2 q3wks for 2 cycles, then increased to 350 mg/m2 if tolerated | 14% (95% CI, 2%–43%) | Median TTP, 6 wks; median survival, 24 wks |
| Cloughesy et al., 200351 | 35 | ≥4 wks prior RT or CT; ≥10 d prior surgical resection; no previous iri or topo; 29 on EIAEDs | 350–400 mg/m2 q3wks, increasing q cycle by 100 mg/m2 w/EIAEDs or 50 mg/m2 wo/EIAEDs | 9% | Median TTP, 2.1 mos; median OS, 8.5 mos |
| Batchelor et al., 200452 | 18 | Prior RT; 16 prior CT; 12 on EIAEDs | 411 mg/m2 weekly for 4/6 wks w EIAEDs, or 117 mg/m2 w/o EIAEDs | 6% CR | Median PFS, 7.3 mos; median OS, 10.4 mos |
| Gilbert et al., 200353 | 40 | ≥3 m prior RT; ≥3 wks prior CT, except ≥6 wks prior CENU; 31 on EIAEDs | 125 mg/m2 q4wks w/escalation based on modified continual reassessment | 4 on EIAEDs showed OR | Median OS, 7.4 mos |
| Prados et al., 200454 | 48 | ≤2 prior CT regimens; all on EIAEDs | 350 mg/m2 q3wks escalated by 50 mg/m2 to 800 mg/m2 | None (42 pts evaluable) | Median PFS, 6 wks |
| Prados et al., 200655 | 51 | ≤1 prior CT regimen; 29 on EIAEDs | 350 mg/m2 q3wks wo/EIAEDs; 750 mg/m2 w/EIAEDs | 5.8% PR; 17 SD | 6-mo PFS, 17.6% |
AA = anaplastic astrocytoma; CENU = chloroethylnitrosourea regimen; CR = complete response; CT = chemotherapy; EIAED = enzyme-inducing antiepileptic drug; GBM = glioblastoma; iri = irinotecan; ORR = overall response rate; OS = overall survival; PR = partial response; PFS = progression-free survival; SD = stable disease; RT = radiotherapy; topo = topotecan; TTP = time to progression
The North Central Cancer Treatment Group46 has reported on two sequential trials (A and B) enrolling a total of 64 patients. Although the authors did not specify how many patients had received prior RT, it can be assumed that the majority of patients had received it. However, since temozolomide was approved by the FDA in August 1999, it is unlikely that many patients had received prior temozolomide because the accrual period for these trials extended from May 1998 to May 1999. In trial A (n = 32), the efficacy of weekly irinotecan, administered in 6-week cycles at a dosage of 100 or 125 mg/m2, was evaluated; patients with prior exposure to nitrosoureas received the lower dose. At study conclusion, 2 of 30 evaluable patients experienced tumor regression, for an overall response rate of 7%. In trial B (n = 32), irinotecan was administered as a single triweekly dose of 250 or 300 mg/m2, depending on prior chemotherapy exposure. Objective responses were noted in 4 of 32 subjects (13%). Thus, an overall response rate of 10% was demonstrated in 62 patients treated with irinotecan either weekly or triweekly. Median leukocyte nadirs were similar in both treatment groups, and gastrointestinal toxicities (predominately mild diarrhea) developed with both schedules. A majority of patients in each group received concurrent anticonvulsant therapy. Multivariate regression analysis suggested that concurrent administration of phenytoin, phenobarbital, and carbamazepine was associated with increased irinotecan clearance.
In a large phase II trial, 52 chemotherapy-naive patients received irinotecan triweekly at a dose of 350 mg/m2. Patients who had not yet received RT (n = 25) were administered 3 cycles of irinotecan (with consideration for RT subsequently based on clinical response), while patients who had received RT (n = 27) but had relapsed were scheduled for up to 6 cycles of irinotecan based on investigator assessment. Irinotecan demonstrated limited clinical activity in this study, achieving an overall objective response rate of only 2.2%. However, the 6-month progression-free survival (PFS) rate was 43% in patients with recurrent disease.47
A study of the effects of a single cycle of a high-dose irinotecan regimen (consisting of one dose of 400 mg/m2 with a second dose of 500 mg/m2 administered 3 weeks later)48 demonstrated no effect on disease progression in 40 patients previously treated with RT and an alkylating agent (carmustine [BCNU] in 20, vincristine [PCV] in 18, procarbazine in 2). Treatment-related diarrhea was observed in 16 patients (40%), thrombocytopenia in 9 patients (23%), and neutropenia in 6 patients (15%). There was no evidence of grade 3/4 myelosuppression or gastrointestinal toxicity, and no patient required transfusion or treatment for neutropenic fever. Results of this study may be consistent with a suboptimal dosage and treatment schedule. All subjects had undergone prior chemotherapy, indicating an opportunity for acquired resistance. In addition, results may have reflected the preponderance of patients receiving EIAEDs (25/40: phenytoin, 15; carbamazepine, 10) and dexamethasone (26/40).
Findings in Pediatric Patients
Irinotecan (125 mg/m2 i.v. weekly for 4 weeks + 2 weeks' rest) showed activity in pediatric patients (n = 22) with a variety of malignant brain tumors, including recurrent (n = 18) and newly diagnosed (n = 4) high-risk lesions.49 Recurrent tumors included glioblastoma multiforme (GBM), anaplastic astrocytoma (AA), ependymoma, medulloblastoma/primitive neuroectodermal tumor, and diffuse pontine glioma; all four newly diagnosed lesions were GBM. All patients with recurrent tumors had progressed after RT. No patients had received prior temozolomide. A median of two courses of treatment (range, 1–16) was administered to the 22 patients. Those with recurrent tumors received therapy until disease progression or development of unacceptable toxicity. Patients with newly diagnosed tumors received three cycles of irinotecan prior to RT, with responders continuing irinotecan treatment after radiation until disease progression or unacceptable toxicity. Responses were seen in 4/9 patients with GBM or AA (44%; 95% CI, 11%–82%). These included 2 complete responses among 4 patients with recurrent GBM (50%), with durations of 9 and >48 months. Partial responses were achieved in one of four patients with newly diagnosed GBM (25%), in the one patient with recurrent AA (100%), and in one of five patients with recurrent ependymoma (20%). Disease stabilized in two of three patients with medulloblastoma/primitive neuroectodermal tumor (at 9 and 13 months). None of the five patients with recurrent diffuse pontine glioma responded to treatment. Overall tox icity was acceptable, with 12 of 22 patients experiencing neutropenia of grade 2 or higher; 7 of these required dose modification.
Monotherapy in the Presence of EIAEDs: Dosage Heterogeneity
Results from phase II studies evaluating irinotecan monotherapy in patients receiving concurrent EIAEDs have employed a variety of dosages. In two studies, irinotecan, at initial doses of 300 to 400 mg/m2, was administered once every 3 weeks. Although this regimen was shown to be feasible, results suggested that the maximum tolerated dose had not yet been identified in patients receiving concurrent anticonvulsants. In one study, 14 patients with progressive or recurrent malignant gliomas received irinotecan 300 mg/m2 once weekly every 3 weeks for two treatments, at which time the dose was increased to 350 mg/m2 in those who tolerated the therapy. The authors did not specify prior treatments, but all patients had progressed from prior RT or chemotherapy. The partial response rate was 14% (95% CI, 2%–43%), and median survival was 24 weeks. Toxicities were low, with grade 3/4 neutropenia observed in two patients (14%), grade 3/4 nausea and vomiting in one (7%), and no cases of late grade 3/4 diarrhea.50,51
The second study specifically addressed potential irinotecan underdosing. Patients with recurrent malignant glioma began treatment with irinotecan at 350 to 400 mg/m2 every 3 weeks. Dosages were increased by 100 mg/m2 at each subsequent treatment in patients receiving concurrent EIAEDs and by 50 mg/m2 in those not on such medications. Thirty-five patients (26 with GBM; 9 with AA) completed at least two cycles of chemotherapy. Dose-limiting toxicity was reached in 12 patients at doses ranging from 400 to 1700 mg/m2. Efficacy data showed that 3 patients achieved a partial response and 15 exhibited stable disease. Median time to tumor progression was 2.1 months, and median survival was 8.5 months. Patients not receiving anticonvulsants achieved higher peak concentrations of the active metabolite SN-38 than patients in the EIAED group.51
In a small study (n = 18) evaluating treatment for recurrent glioma, patients concurrently receiving EIAED (n = 12) received an irinotecan dose of 411 mg/m2, an amount calculated in an earlier phase I study as the maximum tolerated dose in patients on anticonvulsive therapy. Patients not on anticonvulsants (n = 6) received 117 mg/m2, similarly calculated to be appropriate for a non-EIAED population. Although these dosages differed by a factor of 3.5, prior study indicated that mean AUC values for the active metabolite SN-38 would be comparable in the two groups. All patients had received prior RT, 16 (89%) had received prior chemotherapy, and 9 had received prior temozolomide. Each cycle consisted of a 90-minute infusion once weekly for 4 weeks followed by a 2-week rest. Patients received a median of two cycles. Irinotecan demonstrated minimal efficacy in this study: one patient (6%) demonstrated complete radiographic resolution of tumor and five had temporary stabilization. There were no partial responses. Dose-limiting toxicities were observed in eight patients, and six were removed from the study because of toxicity.52,53
In the most recent study of irinotecan monotherapy employing dosage stratification based on the presence or absence of concurrent EIAED use, 51 adult patients with recurrent anaplastic glioma (AG) or GBM received either 350 mg/m2 (EIAED–) or 750 mg/m2 (EIAED+) every 3 weeks. The authors did not specify prior treatments, but given the eligibility requirement for progressive or recurrent malignant glioma, the majority of the patients had received RT and failed one prior chemotherapy regimen. These doses had been established in a previous phase I study in which the AUC for irinotecan and its active metabolite was characterized over an escalating range of 350 to 800 mg/m2. Enrolled patients had no more than one prior chemotherapy exposure. Six-month PFS was 15.7% (95% CI, 0.07%–0.31%) for the GBM patients and 23% (95% CI, 0.07%–0.52%) for AG patients, suggesting that little benefit was obtained from monotherapy at doses determined by pharmacokinetic profiling.54,55
Irinotecan Combination Therapies: Phase I/II Study
With Temozolomide
The combination of irinotecan plus temozolomide for chemotherapy of recurrent malignant glioma has produced consistently high response rates and encouraging 6-month PFS, despite the majority of patients having progressed following RT. In the phase II efficacy portion of the study,56 a regimen of irinotecan (200 mg/m2 every 2 weeks) plus temozolomide (150 mg/m2/day × 5 every 28 days) was administered to 30 patients (GBM, 23; non-GBM, 7) not on EIAEDs who presented with recurrent disease. Results indicated a 6-month PFS of 38% in GBM patients (95% CI, 22%–66%). Of 20 patients evaluable for response, 5 achieved a partial response (25%), 10 had no change in disease status (50%), and 5 experienced disease progression (25%). In phase I of the same study, 21 EIAED+ patients received the identical temozolomide dose in combination with an escalating irinotecan dose (350 mg/m2 in 6; 400 mg/m2 in 4; 450 mg/m2 in 3; 500 mg/m2 in 8). Responses in this group included one complete response, three partial responses, six stable disease, and seven tumor progressions. The maximum tolerated dose of irinotecan appeared to be 450 mg/m2 every 2 weeks for subjects on concurrent anticonvulsant therapy; diarrhea was the dose-limiting toxicity at 500 mg/m2 (Table 3).
Irinotecan combination therapy studies for malignant glioma
| Study . | n . | Patient Characteristics . | Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Gilbert et al., 200356 | 30 | No EIAEDs | 200 mg/m2 iri q2wks; 150 mg/m2/d × 5 + tem q28d | 25% PR (5/20 evaluable); 50% SD | 6-mo PFS for 23 GBM, 38% (95% CI, 22%-66%) |
| Gilbert et al., 200356 | 21 | All receiving EIAEDs | 350 mg/m2 iri escalating to 500 mg/m2 q2wks + 150 mg/m2/d × 5 tem q28d | N/A | 1 CR; 3 PR; 6 SD |
| Gruber et al., 200457 | 32 | No prior tem or iri; receiving anticonvulsants carbamazepine or levetiracetam | Schedule A: 200 mg/m2 tem daily for 5 d + 125 mg/m2 iri d 6, 13, 20 of 28-d cycle | 83% (15/18) GBM responded; 100% (14/14) AG responded | GBM: median duration of response 24 wks; 6-mo PFS, 39% (7/18); |
| Schedule B: 200 mg/m2 tem daily for 5 d + 350 mg/m2 irinotecan on d 6 | AG: median duration of response, 29 wks; 6-month PFS, 71% (10/14) | ||||
| Brandes et al., 200458 | 42 | ≥3 months prior surgery and RT; prior tem-based CT; all on EIAEDs | 100 mg/m2 BCNU d 1 + 175 mg/m2 iri wkly 4/6 wks; iri escalated to 200 mg/m2 if tolerated | 21% (9/42) PR | Median TTP, 17 wks (95% CI, 11.9-23.9); 6-mo PFS, 30.3% (95% CI, 18.5%-49.7%) |
| Reardon et al., 200459 | 76 | 37 newly diagnosed; 39 recurrent | 100 mg/m2 BCNU d 1 + 225 mg/m2 iri w/EIAEDs, or 125 mg/m2 iri wo/EIAEDs wkly | Newly diagnosed: 14%; | Newly diagnosed: median OS, 51.3 wks (95% CI, 32.1-62.6 wks); |
| Recurrent: 13% | Recurrent: median TTP, 11.4 wks (95% CI, 6.0-14.3 wks); median OS, 31.3 wks (95% CI, 25.7-45.6 wks) | ||||
| Puduvalli et al., 200661 | 32 | ≤2 relapses prior surgery and RT; no EIAEDs | 125 mg/m2 iri wkly 4/6 wks + 100 mg thalidomide daily increased as tolerated to 400 mg maximum | 1 CR; 1 PR; 19 SD | Median PFS, 13 wks (95% CI, 10-24 wks); median OS, 36 wks (95% CI, 24-56 wks) |
| Reardon et al., 200562 | 37 | 36, previous CT; 35, previous RT; 21, EIAEDs | 350 mg/m2 iri with or 125 mg/m2 w/o EIAEDs wks 1, 2, 4, 5 of 6-wk cycle; 400 mg celecoxib twice/d | 17% | Median PFS, 11 wks; median OS, 31.5 wks |
| Vredenburgh et al., 200764 | 32 | Prior surgery and RT w/concurrent tem | 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs or 340 mg/m2 iri w/EIAEDs q2wks | 63% (20/32) | Median PFS, 23 wks (95% CI, 15-30 wks); 6-mo OS, 72% (95% CI, 58-89%) |
| Goli et al., 200765 | 68 | Prior RT and tem | First 32: 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q other wk; | 59% (2 CR, 38 PR) | 35 grade 4 tumors: median PFS, 23 wks; median OS, 40 wks; 33 grade 3 tumors: median PFS, 42 wks; median OS, 60 wks |
| Last 36: 15 mg/kg bev d 1 and d 22 + 125 mg/m2 iri wo/EIAEDs, or 350 mg/m2 iri w/EIAEDs d 1, 8, 22, and 29 | |||||
| Vredenburgh et al., 200766 | 35 | Prior RT and tem | First 23: 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q14d; | 57% (20/35) | Median PFS, 24 wks (95% CI, 18-36 wks); median OS, 42 wks (95% CI, 35-60 wks) |
| Last 12: 15 mg/kg bev q21d + 125 mg/m2 iri wo/EIAEDs, or 350 mg/m2 iri w/EIAEDs d 1, 8, 22, and 29 | |||||
| Raval et al., 200667 | 8 | ≥1 prior CT; all failed tem and RT; 4 prior iri | 5 mg/kg bev + 125 mg/m2 iri q2wks | 100%; 1 CR and 5 PR (6 evaluable) | |
| Bokstein et al., 200668 | 12 | ≥1 prior CT; EIAEDs replaced with non-EIAEDs | 5 mg/kg bev + 125 mg/m2 iri q2wks | 75% (8/12) | |
| Cloughesy T et al., 200769 | 167 | Prior tem | 85: 10 mg/m2 bev q2wks; | 38.8% OR (bev alone); | 6-mo PFS (bev alone), 44.7 weeks (95% CI, 33.9-55.6 wks); 6-mo PFS (bev + iri), 60.9 wks (95% CI, 49.5-72.3 wks) |
| 82: 10 mg/m2 bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q2wks | 46.3% OR (bev + iri) |
| Study . | n . | Patient Characteristics . | Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Gilbert et al., 200356 | 30 | No EIAEDs | 200 mg/m2 iri q2wks; 150 mg/m2/d × 5 + tem q28d | 25% PR (5/20 evaluable); 50% SD | 6-mo PFS for 23 GBM, 38% (95% CI, 22%-66%) |
| Gilbert et al., 200356 | 21 | All receiving EIAEDs | 350 mg/m2 iri escalating to 500 mg/m2 q2wks + 150 mg/m2/d × 5 tem q28d | N/A | 1 CR; 3 PR; 6 SD |
| Gruber et al., 200457 | 32 | No prior tem or iri; receiving anticonvulsants carbamazepine or levetiracetam | Schedule A: 200 mg/m2 tem daily for 5 d + 125 mg/m2 iri d 6, 13, 20 of 28-d cycle | 83% (15/18) GBM responded; 100% (14/14) AG responded | GBM: median duration of response 24 wks; 6-mo PFS, 39% (7/18); |
| Schedule B: 200 mg/m2 tem daily for 5 d + 350 mg/m2 irinotecan on d 6 | AG: median duration of response, 29 wks; 6-month PFS, 71% (10/14) | ||||
| Brandes et al., 200458 | 42 | ≥3 months prior surgery and RT; prior tem-based CT; all on EIAEDs | 100 mg/m2 BCNU d 1 + 175 mg/m2 iri wkly 4/6 wks; iri escalated to 200 mg/m2 if tolerated | 21% (9/42) PR | Median TTP, 17 wks (95% CI, 11.9-23.9); 6-mo PFS, 30.3% (95% CI, 18.5%-49.7%) |
| Reardon et al., 200459 | 76 | 37 newly diagnosed; 39 recurrent | 100 mg/m2 BCNU d 1 + 225 mg/m2 iri w/EIAEDs, or 125 mg/m2 iri wo/EIAEDs wkly | Newly diagnosed: 14%; | Newly diagnosed: median OS, 51.3 wks (95% CI, 32.1-62.6 wks); |
| Recurrent: 13% | Recurrent: median TTP, 11.4 wks (95% CI, 6.0-14.3 wks); median OS, 31.3 wks (95% CI, 25.7-45.6 wks) | ||||
| Puduvalli et al., 200661 | 32 | ≤2 relapses prior surgery and RT; no EIAEDs | 125 mg/m2 iri wkly 4/6 wks + 100 mg thalidomide daily increased as tolerated to 400 mg maximum | 1 CR; 1 PR; 19 SD | Median PFS, 13 wks (95% CI, 10-24 wks); median OS, 36 wks (95% CI, 24-56 wks) |
| Reardon et al., 200562 | 37 | 36, previous CT; 35, previous RT; 21, EIAEDs | 350 mg/m2 iri with or 125 mg/m2 w/o EIAEDs wks 1, 2, 4, 5 of 6-wk cycle; 400 mg celecoxib twice/d | 17% | Median PFS, 11 wks; median OS, 31.5 wks |
| Vredenburgh et al., 200764 | 32 | Prior surgery and RT w/concurrent tem | 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs or 340 mg/m2 iri w/EIAEDs q2wks | 63% (20/32) | Median PFS, 23 wks (95% CI, 15-30 wks); 6-mo OS, 72% (95% CI, 58-89%) |
| Goli et al., 200765 | 68 | Prior RT and tem | First 32: 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q other wk; | 59% (2 CR, 38 PR) | 35 grade 4 tumors: median PFS, 23 wks; median OS, 40 wks; 33 grade 3 tumors: median PFS, 42 wks; median OS, 60 wks |
| Last 36: 15 mg/kg bev d 1 and d 22 + 125 mg/m2 iri wo/EIAEDs, or 350 mg/m2 iri w/EIAEDs d 1, 8, 22, and 29 | |||||
| Vredenburgh et al., 200766 | 35 | Prior RT and tem | First 23: 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q14d; | 57% (20/35) | Median PFS, 24 wks (95% CI, 18-36 wks); median OS, 42 wks (95% CI, 35-60 wks) |
| Last 12: 15 mg/kg bev q21d + 125 mg/m2 iri wo/EIAEDs, or 350 mg/m2 iri w/EIAEDs d 1, 8, 22, and 29 | |||||
| Raval et al., 200667 | 8 | ≥1 prior CT; all failed tem and RT; 4 prior iri | 5 mg/kg bev + 125 mg/m2 iri q2wks | 100%; 1 CR and 5 PR (6 evaluable) | |
| Bokstein et al., 200668 | 12 | ≥1 prior CT; EIAEDs replaced with non-EIAEDs | 5 mg/kg bev + 125 mg/m2 iri q2wks | 75% (8/12) | |
| Cloughesy T et al., 200769 | 167 | Prior tem | 85: 10 mg/m2 bev q2wks; | 38.8% OR (bev alone); | 6-mo PFS (bev alone), 44.7 weeks (95% CI, 33.9-55.6 wks); 6-mo PFS (bev + iri), 60.9 wks (95% CI, 49.5-72.3 wks) |
| 82: 10 mg/m2 bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q2wks | 46.3% OR (bev + iri) |
AG = anaplastic glioma; BCNU = carmustine; bev = bevacizumab; CR = complete response; CT = chemotherapy; EIAED = enzyme-inducing antiepileptic drug; GBM = glioblastoma; iri = irinotecan; OR = objective response; ORR = overall response rate; OS = overall survival; PD = prior disease; PFS = progression-free survival; PR = partial response; RT = radiotherapy; SD = stable disease; tem = temozolide; TTP = time to progression
Irinotecan combination therapy studies for malignant glioma
| Study . | n . | Patient Characteristics . | Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Gilbert et al., 200356 | 30 | No EIAEDs | 200 mg/m2 iri q2wks; 150 mg/m2/d × 5 + tem q28d | 25% PR (5/20 evaluable); 50% SD | 6-mo PFS for 23 GBM, 38% (95% CI, 22%-66%) |
| Gilbert et al., 200356 | 21 | All receiving EIAEDs | 350 mg/m2 iri escalating to 500 mg/m2 q2wks + 150 mg/m2/d × 5 tem q28d | N/A | 1 CR; 3 PR; 6 SD |
| Gruber et al., 200457 | 32 | No prior tem or iri; receiving anticonvulsants carbamazepine or levetiracetam | Schedule A: 200 mg/m2 tem daily for 5 d + 125 mg/m2 iri d 6, 13, 20 of 28-d cycle | 83% (15/18) GBM responded; 100% (14/14) AG responded | GBM: median duration of response 24 wks; 6-mo PFS, 39% (7/18); |
| Schedule B: 200 mg/m2 tem daily for 5 d + 350 mg/m2 irinotecan on d 6 | AG: median duration of response, 29 wks; 6-month PFS, 71% (10/14) | ||||
| Brandes et al., 200458 | 42 | ≥3 months prior surgery and RT; prior tem-based CT; all on EIAEDs | 100 mg/m2 BCNU d 1 + 175 mg/m2 iri wkly 4/6 wks; iri escalated to 200 mg/m2 if tolerated | 21% (9/42) PR | Median TTP, 17 wks (95% CI, 11.9-23.9); 6-mo PFS, 30.3% (95% CI, 18.5%-49.7%) |
| Reardon et al., 200459 | 76 | 37 newly diagnosed; 39 recurrent | 100 mg/m2 BCNU d 1 + 225 mg/m2 iri w/EIAEDs, or 125 mg/m2 iri wo/EIAEDs wkly | Newly diagnosed: 14%; | Newly diagnosed: median OS, 51.3 wks (95% CI, 32.1-62.6 wks); |
| Recurrent: 13% | Recurrent: median TTP, 11.4 wks (95% CI, 6.0-14.3 wks); median OS, 31.3 wks (95% CI, 25.7-45.6 wks) | ||||
| Puduvalli et al., 200661 | 32 | ≤2 relapses prior surgery and RT; no EIAEDs | 125 mg/m2 iri wkly 4/6 wks + 100 mg thalidomide daily increased as tolerated to 400 mg maximum | 1 CR; 1 PR; 19 SD | Median PFS, 13 wks (95% CI, 10-24 wks); median OS, 36 wks (95% CI, 24-56 wks) |
| Reardon et al., 200562 | 37 | 36, previous CT; 35, previous RT; 21, EIAEDs | 350 mg/m2 iri with or 125 mg/m2 w/o EIAEDs wks 1, 2, 4, 5 of 6-wk cycle; 400 mg celecoxib twice/d | 17% | Median PFS, 11 wks; median OS, 31.5 wks |
| Vredenburgh et al., 200764 | 32 | Prior surgery and RT w/concurrent tem | 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs or 340 mg/m2 iri w/EIAEDs q2wks | 63% (20/32) | Median PFS, 23 wks (95% CI, 15-30 wks); 6-mo OS, 72% (95% CI, 58-89%) |
| Goli et al., 200765 | 68 | Prior RT and tem | First 32: 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q other wk; | 59% (2 CR, 38 PR) | 35 grade 4 tumors: median PFS, 23 wks; median OS, 40 wks; 33 grade 3 tumors: median PFS, 42 wks; median OS, 60 wks |
| Last 36: 15 mg/kg bev d 1 and d 22 + 125 mg/m2 iri wo/EIAEDs, or 350 mg/m2 iri w/EIAEDs d 1, 8, 22, and 29 | |||||
| Vredenburgh et al., 200766 | 35 | Prior RT and tem | First 23: 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q14d; | 57% (20/35) | Median PFS, 24 wks (95% CI, 18-36 wks); median OS, 42 wks (95% CI, 35-60 wks) |
| Last 12: 15 mg/kg bev q21d + 125 mg/m2 iri wo/EIAEDs, or 350 mg/m2 iri w/EIAEDs d 1, 8, 22, and 29 | |||||
| Raval et al., 200667 | 8 | ≥1 prior CT; all failed tem and RT; 4 prior iri | 5 mg/kg bev + 125 mg/m2 iri q2wks | 100%; 1 CR and 5 PR (6 evaluable) | |
| Bokstein et al., 200668 | 12 | ≥1 prior CT; EIAEDs replaced with non-EIAEDs | 5 mg/kg bev + 125 mg/m2 iri q2wks | 75% (8/12) | |
| Cloughesy T et al., 200769 | 167 | Prior tem | 85: 10 mg/m2 bev q2wks; | 38.8% OR (bev alone); | 6-mo PFS (bev alone), 44.7 weeks (95% CI, 33.9-55.6 wks); 6-mo PFS (bev + iri), 60.9 wks (95% CI, 49.5-72.3 wks) |
| 82: 10 mg/m2 bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q2wks | 46.3% OR (bev + iri) |
| Study . | n . | Patient Characteristics . | Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Gilbert et al., 200356 | 30 | No EIAEDs | 200 mg/m2 iri q2wks; 150 mg/m2/d × 5 + tem q28d | 25% PR (5/20 evaluable); 50% SD | 6-mo PFS for 23 GBM, 38% (95% CI, 22%-66%) |
| Gilbert et al., 200356 | 21 | All receiving EIAEDs | 350 mg/m2 iri escalating to 500 mg/m2 q2wks + 150 mg/m2/d × 5 tem q28d | N/A | 1 CR; 3 PR; 6 SD |
| Gruber et al., 200457 | 32 | No prior tem or iri; receiving anticonvulsants carbamazepine or levetiracetam | Schedule A: 200 mg/m2 tem daily for 5 d + 125 mg/m2 iri d 6, 13, 20 of 28-d cycle | 83% (15/18) GBM responded; 100% (14/14) AG responded | GBM: median duration of response 24 wks; 6-mo PFS, 39% (7/18); |
| Schedule B: 200 mg/m2 tem daily for 5 d + 350 mg/m2 irinotecan on d 6 | AG: median duration of response, 29 wks; 6-month PFS, 71% (10/14) | ||||
| Brandes et al., 200458 | 42 | ≥3 months prior surgery and RT; prior tem-based CT; all on EIAEDs | 100 mg/m2 BCNU d 1 + 175 mg/m2 iri wkly 4/6 wks; iri escalated to 200 mg/m2 if tolerated | 21% (9/42) PR | Median TTP, 17 wks (95% CI, 11.9-23.9); 6-mo PFS, 30.3% (95% CI, 18.5%-49.7%) |
| Reardon et al., 200459 | 76 | 37 newly diagnosed; 39 recurrent | 100 mg/m2 BCNU d 1 + 225 mg/m2 iri w/EIAEDs, or 125 mg/m2 iri wo/EIAEDs wkly | Newly diagnosed: 14%; | Newly diagnosed: median OS, 51.3 wks (95% CI, 32.1-62.6 wks); |
| Recurrent: 13% | Recurrent: median TTP, 11.4 wks (95% CI, 6.0-14.3 wks); median OS, 31.3 wks (95% CI, 25.7-45.6 wks) | ||||
| Puduvalli et al., 200661 | 32 | ≤2 relapses prior surgery and RT; no EIAEDs | 125 mg/m2 iri wkly 4/6 wks + 100 mg thalidomide daily increased as tolerated to 400 mg maximum | 1 CR; 1 PR; 19 SD | Median PFS, 13 wks (95% CI, 10-24 wks); median OS, 36 wks (95% CI, 24-56 wks) |
| Reardon et al., 200562 | 37 | 36, previous CT; 35, previous RT; 21, EIAEDs | 350 mg/m2 iri with or 125 mg/m2 w/o EIAEDs wks 1, 2, 4, 5 of 6-wk cycle; 400 mg celecoxib twice/d | 17% | Median PFS, 11 wks; median OS, 31.5 wks |
| Vredenburgh et al., 200764 | 32 | Prior surgery and RT w/concurrent tem | 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs or 340 mg/m2 iri w/EIAEDs q2wks | 63% (20/32) | Median PFS, 23 wks (95% CI, 15-30 wks); 6-mo OS, 72% (95% CI, 58-89%) |
| Goli et al., 200765 | 68 | Prior RT and tem | First 32: 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q other wk; | 59% (2 CR, 38 PR) | 35 grade 4 tumors: median PFS, 23 wks; median OS, 40 wks; 33 grade 3 tumors: median PFS, 42 wks; median OS, 60 wks |
| Last 36: 15 mg/kg bev d 1 and d 22 + 125 mg/m2 iri wo/EIAEDs, or 350 mg/m2 iri w/EIAEDs d 1, 8, 22, and 29 | |||||
| Vredenburgh et al., 200766 | 35 | Prior RT and tem | First 23: 10 mg/kg bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q14d; | 57% (20/35) | Median PFS, 24 wks (95% CI, 18-36 wks); median OS, 42 wks (95% CI, 35-60 wks) |
| Last 12: 15 mg/kg bev q21d + 125 mg/m2 iri wo/EIAEDs, or 350 mg/m2 iri w/EIAEDs d 1, 8, 22, and 29 | |||||
| Raval et al., 200667 | 8 | ≥1 prior CT; all failed tem and RT; 4 prior iri | 5 mg/kg bev + 125 mg/m2 iri q2wks | 100%; 1 CR and 5 PR (6 evaluable) | |
| Bokstein et al., 200668 | 12 | ≥1 prior CT; EIAEDs replaced with non-EIAEDs | 5 mg/kg bev + 125 mg/m2 iri q2wks | 75% (8/12) | |
| Cloughesy T et al., 200769 | 167 | Prior tem | 85: 10 mg/m2 bev q2wks; | 38.8% OR (bev alone); | 6-mo PFS (bev alone), 44.7 weeks (95% CI, 33.9-55.6 wks); 6-mo PFS (bev + iri), 60.9 wks (95% CI, 49.5-72.3 wks) |
| 82: 10 mg/m2 bev + 125 mg/m2 iri wo/EIAEDs, or 340 mg/m2 iri w/EIAEDs q2wks | 46.3% OR (bev + iri) |
AG = anaplastic glioma; BCNU = carmustine; bev = bevacizumab; CR = complete response; CT = chemotherapy; EIAED = enzyme-inducing antiepileptic drug; GBM = glioblastoma; iri = irinotecan; OR = objective response; ORR = overall response rate; OS = overall survival; PD = prior disease; PFS = progression-free survival; PR = partial response; RT = radiotherapy; SD = stable disease; tem = temozolide; TTP = time to progression
Thirty-two patients with recurrent malignant glioma participated in a study evaluating two treatment schedules of temozolomide plus irinotecan. Initial treatment (Schedule A) was temozolomide 200 mg/m2 daily for 5 days in combination with irinotecan 125 mg/m2 on days 6, 13, and 20; subsequent treatment (Schedule B) was temozolomide 200 mg/m2 daily for 5 days and irinotecan 350 mg/m2 on day 6. A minimum of two treatment cycles, each 28 days, was required for evaluable patients, and all responders received six cycles. Fifteen of 18 glioblastoma patients (83%) responded, with 2 demonstrating a complete response. Partial responses were noted in 3 patients and stable disease in 10. The median duration of response was 24 weeks, and the 6-month PFS was 39% (7/18). All patients with AG (14/14) responded or stabilized—three complete responses, two partial responses, and nine stable disease. The median duration of response in the AG group was 29 weeks, and the 6-month PFS was 71% (10/14). Only four patients in this study had deterioration in performance score, and all four had progressive disease at that time. Overall toxicity was low. Nonhematologic toxicity was typically gastrointestinal and mild. Grade 4 leukopenia occurred in one patient, and two required hospitalization for neutropenic fever.57
With Carmustine
In a study in which the primary end point was 6-month PFS,58 irinotecan plus BCNU demonstrated activity with manageable toxicity against malignant glioma recurring or progressing following initial therapy. All 42 patients in this study had received prior RT and temozolomide, and all were receiving concurrent EIAEDs. Regimens consisted of BCNU (100 mg/m2 on day 1) plus irinotecan (175 mg/m2 every week for 4 weeks) every 6 weeks for a maximum of eight cycles. The irinotecan dose was increased to 200 mg/m2 in the absence of grade 2 or higher toxicity. The 6-month PFS was 30.3% (95% CI, 18.5%–49.7%), and median time to progression was 17 weeks (95% CI, 11.9%–23.9%). Nine partial responses (21.4%; 95% CI, 9%–34%) were achieved. This regimen was not cross-resistant with temozolomide treatment and was associated with manageable toxicity. Of a total of 130 treatment cycles, dose reduction was necessary in 34 because of treatment-related toxicity. Sixteen cycles were delayed for a median of 1.4 weeks because of neutropenia (3 cycles) or diarrhea (13 cycles).
In another phase II trial,59 a combination of irinotecan with BCNU had activity against recurrent or newly diagnosed malignant glioma comparable to irinotecan alone but with apparent increased toxicity. In this study, BCNU (100 mg/m2) was administered on day 1 of each 6-week cycle. Irinotecan was administered weekly for 4 weeks at doses previously determined to be optimal for combination with BCNU and further stratified by EIAED status—225 mg/m2 for those receiving and 125 mg/m2 for those not receiving EIAEDs.60
The phase II trial of 76 patients treated with this combination (37 newly diagnosed; 39 recurrent disease) showed the following results: five newly diagnosed patients (14%) achieved a radiographic response, including one complete response and four partial responses (95% CI, 5%–29%); five with recurrent disease (13%) demonstrated a response, including one complete response (95% CI, 4%–27%); and stable disease was demonstrated in more than 40%.59 The median time to progression was 11.3 weeks for the recurrent GBM population and 16.9 weeks for patients with recurrent AA and anaplastic oligodendroglioma (AO). All except one patient with recurrent disease had prior RT, and the majority had received prior temozolomide. Four patients developed interstitial pneumonitis, and high-grade (⩾3) toxicities included infection (13%), thrombosis (12%), diarrhea (10%), and neutropenia (7%).
With Thalidomide
The combination of irinotecan and thalidomide demonstrated promising activity in patients with recurrent GBM.61 Thirty-two patients not on EIAEDs were treated in 6-week cycles with irinotecan (125 mg/m2 weekly for 4 weeks with 2 weeks off) and thalidomide (100 mg daily, initial dose, increased as tolerated to a maximum of 400 mg daily). The study was designed to detect an improvement in 6-month PFS compared with historical controls. Results demonstrated a median PFS of 13 weeks (95% CI, 10%–24%), with eight patients (25%) achieving PFS at 6 months (95% CI, 14%–46%). The range of responses included complete response in 1 patient, partial response in 1 patient, and stable disease in 19. Overall survival was 34% at 1 year (95% CI, 21%–56%), and median overall survival was 36 weeks (95% CI, 24–56).
With Celecoxib
Encouraging response rates were demonstrated in a study of irinotecan as the sole cytotoxic agent administered in conjunction with celecoxib. The investigation enrolled patients with recurrent GBM (n = 34) and recurrent AA (n = 3). Thirty-five patients (94%) had received prior RT, and 36 (97%) had received prior temozolomide. Patients received irinotecan as a 90-minute infusion on weeks 1, 2, 4, and 5 of each 6–week cycle plus twice-daily celecoxib 400 mg. Irinotecan was administered at 350 mg/m2 in patients taking EIAEDs and at 125 mg/m2 in those not taking these agents. Median follow-up was 76.9 weeks. The primary end point of radiographic response was achieved by six patients (16%), all of whom were diagnosed with recurrent GBM. An additional 13 patients (35%) achieved stable disease. Median PFS in this study was 11 weeks; 6-month PFS was 25.1%; and median overall survival was 31.5 weeks. Treatment was well tolerated. Grade 3 or higher hematologic toxicities occurred in 8.6% of treatment courses. Grade 3 diarrhea, the most commonly reported nonhematologic toxicity, occurred with equal frequency (8%) among patients regardless of EIAED status.62
With Bevacizumab
Antiangiogenic agents represent an important therapeutic advance for neuro-oncology.63 Targeted antiangiogenesis therapy with bevacizumab in combination with irinotecan has demonstrated promising activity against malignant glioma that is among the highest reported. A large phase II trial enrolled 68 patients with high-grade GBM, AA, and AO who had progressive disease after previous chemotherapy and RT.64,65 Patients were treated with two regimens, which were further stratified by EIAED use. One group (n = 32) was treated every other week with bevacizumab 10 mg/kg plus irinotecan 125 mg/m2 (EIAED–) or 340 mg/m2 (EIAED+). A second group (n = 36) was treated with irinotecan 125 mg/m2 (EIAED–) or 350 mg/m2 (EIAED+) on days 1, 8, 22, and 29, and bevacizumab 15 mg/kg on days 1 and 22. Although follow-up for the second cohort was shorter, efficacy was similar.66 Toxicity issues, however, were more apparent: nine patients in this group were removed from the study due to treatment-emergent toxicity. The response rate for the entire 68 patients enrolled in the study was an unprecedented 59% (36 partial and 4 complete responses). For the 35 GBM patients, the response rate was 57%; for grade III glioma patients, 61%. Six-month PFS for the GBM patients was 43%, and median survival was 40 weeks, both of which are significant improvements compared with historic controls. In the patients with grade III gliomas, 6-month PFS was 61%, and median survival was 60 weeks. The regimen was well tolerated.
In another recent report,67 eight GBM patients who had failed temozolomide plus RT received 5 mg/kg bevacizumab plus 125 mg/m2 irinotecan infusion every 2 weeks until disease progression or development of unacceptable toxicity. Six patients were fully evaluable after a median of six cycles. Median length of follow-up was 31 months (range, 11–91 months). Responses in these six subjects included one complete and five partial responses; furthermore, improvements in cognitive function and performance status were observed. There were no grade 3/4 toxicities. The same regimen was evaluated in 12 patients (10 GBM, 1 AO, 1 anaplastic oligoastrocytoma), all of whom had been previously treated with at least one chemotherapy regimen. Enzyme-inducing antiepileptic drugs were replaced with alternative anti-convulsants. Eight of these patients responded to treatment, with two demonstrating complete response and six achieving considerable partial responses, characterized by a ⩾50% decrease in tumor volume growth. Treatment was well tolerated, with minimal toxicity and no adverse event higher than grade 2.68
A recent large, randomized phase II trial was conducted that investigated bevacizumab alone versus bevacizumab and irinotecan in 167 patients with recurrent GBM after prior temozolomide treatment.69 The addition of irinotecan to bevacizumab improved the response rate (21% for bevacizumab vs. 34% for the combination). More importantly, the 6-month PFS improved with the combination treatment (36% vs. 51%, respectively).
A very important issue in the use of antiangiogenesis agents, like bevacizumab, is the evaluation of response and determination of clinical utility. Gadolinium-contrast MRIs may give false responses, because antiangiogenic agents decrease extravasation of the gadolinium.70 A better clinical tool may be [18F] fluorothymidine (FLT) positron emission tomography (PET), which can be used to determine cell proliferation.71 In a report investigating FLT-PET in glioma patients, FLT tumor uptake correlated with the proliferation index Ki-67.72 Chen et al. used FLT-PET to predict tumor response in malignant glioma patients treated with irinotecan and bevacizumab.73 In the 19 patients assessable for metabolic response with FLT-PET, 9 demonstrated a response. FLT-PET metabolic response was a better predictor of overall survival than gadolinium-contrast MRI response.
As the previous studies show, irinotecan can contribute positively to the treatment of malignant glioma. In comparison, studies involving other topoisomerase 1 inhibitors and chemotherapeutic agents for recurrent glioma are detailed in Table 4.
Nonirinotecan therapy studies for malignant glioma
| Study . | n . | Patient Characteristics . | Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Balmaceda et al., 200874 | 120 | ≤Third recurrence of GBM, AA, AO | 200 mg/m2 tem followed by 9 doses 90 mg/m2 tem q12hrs q28d; 90 mg/m2 dose escalated to 100 mg/m2 if tolerated | GBM: 31% ORR | GBM: median PFS, 4.2 mos; median OS, 8.8 mos |
| Brandes et al., 200475 | 40 | ≥8 wks prior RT | 80 mg/m2 BCNU d 1 and 3 q8wks | 15% PR (6/40); 22.5% SD (9/40) | Median TTP, 13.3 wks (95% CI, 10.26–16.86 wks); median OS, 7.53 mos (95% CI, 4.64–11.47 mos) |
| Burch et al., 200076 | 33 | ≥1 mo prior RT; ≥6 wks prior ≤1 nitrosourea-based CT | 1.5 mg/m2 topo 5 consecutive d q3wks w/o prior CT or 1.25 mg/m2 w/prior CT | 3% ORR | Median TTP, 14.9 wks; median OS, 19.9 wks |
| Lesser et al., 200477 | 32 | ≤1 prior chemotherapy | 1.00 mg/m2 karenitecin 5 consecutive d q3wks, escalated through continual reassessment in EIAED cohort to maximum 2.1 mg/m2 and in non-EIAED cohort to 1.8 mg/m2 | None | Median OS, 6.5 mos (95% CI, 4.0–9.7 mos) |
| Macdonald et al., 199678 | 31 | ≥2 mos prior RT; ≥6 wks prior surgery or adjuvant CT | 1.5 mg/m2 topo 5 d q3wks | 6% (2/31); 68% SD (21/31) | Median duration of SD, 19 wks (range, 5–86+ wks) |
| Wagner et al., 200479 | 32 | Pediatric; prior RT and CT | 0.4 mg/m2 topo daily, escalated to maximum 2.0 mg/m2 | 1/14 CR; 2/14 PR; 7/14 SD | Median OS (n = 32), 3.6 mos (range, 0.4–24.0 mos); median OS for ≥4 wks of treatment (n = 14), 6.0 mos (range, 1.6–24 mos) |
| Wick et al., 200780 | 90 | Prior RT w/ or w/o ≥1 CT | 150 mg/m2 tem d 1 to 7 and 15 to 21 of 28-day cycle | 2% CR (1/45 w/measurable GBM); 13% PR (6/45) | 64 GBM: median PFS, 24 wks (range, 4–78 wks); median OS, 38 wks (range, 5–99 wks) |
| Study . | n . | Patient Characteristics . | Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Balmaceda et al., 200874 | 120 | ≤Third recurrence of GBM, AA, AO | 200 mg/m2 tem followed by 9 doses 90 mg/m2 tem q12hrs q28d; 90 mg/m2 dose escalated to 100 mg/m2 if tolerated | GBM: 31% ORR | GBM: median PFS, 4.2 mos; median OS, 8.8 mos |
| Brandes et al., 200475 | 40 | ≥8 wks prior RT | 80 mg/m2 BCNU d 1 and 3 q8wks | 15% PR (6/40); 22.5% SD (9/40) | Median TTP, 13.3 wks (95% CI, 10.26–16.86 wks); median OS, 7.53 mos (95% CI, 4.64–11.47 mos) |
| Burch et al., 200076 | 33 | ≥1 mo prior RT; ≥6 wks prior ≤1 nitrosourea-based CT | 1.5 mg/m2 topo 5 consecutive d q3wks w/o prior CT or 1.25 mg/m2 w/prior CT | 3% ORR | Median TTP, 14.9 wks; median OS, 19.9 wks |
| Lesser et al., 200477 | 32 | ≤1 prior chemotherapy | 1.00 mg/m2 karenitecin 5 consecutive d q3wks, escalated through continual reassessment in EIAED cohort to maximum 2.1 mg/m2 and in non-EIAED cohort to 1.8 mg/m2 | None | Median OS, 6.5 mos (95% CI, 4.0–9.7 mos) |
| Macdonald et al., 199678 | 31 | ≥2 mos prior RT; ≥6 wks prior surgery or adjuvant CT | 1.5 mg/m2 topo 5 d q3wks | 6% (2/31); 68% SD (21/31) | Median duration of SD, 19 wks (range, 5–86+ wks) |
| Wagner et al., 200479 | 32 | Pediatric; prior RT and CT | 0.4 mg/m2 topo daily, escalated to maximum 2.0 mg/m2 | 1/14 CR; 2/14 PR; 7/14 SD | Median OS (n = 32), 3.6 mos (range, 0.4–24.0 mos); median OS for ≥4 wks of treatment (n = 14), 6.0 mos (range, 1.6–24 mos) |
| Wick et al., 200780 | 90 | Prior RT w/ or w/o ≥1 CT | 150 mg/m2 tem d 1 to 7 and 15 to 21 of 28-day cycle | 2% CR (1/45 w/measurable GBM); 13% PR (6/45) | 64 GBM: median PFS, 24 wks (range, 4–78 wks); median OS, 38 wks (range, 5–99 wks) |
AA = anaplastic astrocytoma; AO = anaplastic oligodendroglioma; BCNU = carmustine; CR = complete response; CT = chemotherapy; EIAED = enzyme-inducing antiepileptic drug; GBM = glioblastoma; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; PR = partial response; RT = radiotherapy; SD = stable disease; tem = temozolide; topo = topotecan; TTP = time to progression
Nonirinotecan therapy studies for malignant glioma
| Study . | n . | Patient Characteristics . | Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Balmaceda et al., 200874 | 120 | ≤Third recurrence of GBM, AA, AO | 200 mg/m2 tem followed by 9 doses 90 mg/m2 tem q12hrs q28d; 90 mg/m2 dose escalated to 100 mg/m2 if tolerated | GBM: 31% ORR | GBM: median PFS, 4.2 mos; median OS, 8.8 mos |
| Brandes et al., 200475 | 40 | ≥8 wks prior RT | 80 mg/m2 BCNU d 1 and 3 q8wks | 15% PR (6/40); 22.5% SD (9/40) | Median TTP, 13.3 wks (95% CI, 10.26–16.86 wks); median OS, 7.53 mos (95% CI, 4.64–11.47 mos) |
| Burch et al., 200076 | 33 | ≥1 mo prior RT; ≥6 wks prior ≤1 nitrosourea-based CT | 1.5 mg/m2 topo 5 consecutive d q3wks w/o prior CT or 1.25 mg/m2 w/prior CT | 3% ORR | Median TTP, 14.9 wks; median OS, 19.9 wks |
| Lesser et al., 200477 | 32 | ≤1 prior chemotherapy | 1.00 mg/m2 karenitecin 5 consecutive d q3wks, escalated through continual reassessment in EIAED cohort to maximum 2.1 mg/m2 and in non-EIAED cohort to 1.8 mg/m2 | None | Median OS, 6.5 mos (95% CI, 4.0–9.7 mos) |
| Macdonald et al., 199678 | 31 | ≥2 mos prior RT; ≥6 wks prior surgery or adjuvant CT | 1.5 mg/m2 topo 5 d q3wks | 6% (2/31); 68% SD (21/31) | Median duration of SD, 19 wks (range, 5–86+ wks) |
| Wagner et al., 200479 | 32 | Pediatric; prior RT and CT | 0.4 mg/m2 topo daily, escalated to maximum 2.0 mg/m2 | 1/14 CR; 2/14 PR; 7/14 SD | Median OS (n = 32), 3.6 mos (range, 0.4–24.0 mos); median OS for ≥4 wks of treatment (n = 14), 6.0 mos (range, 1.6–24 mos) |
| Wick et al., 200780 | 90 | Prior RT w/ or w/o ≥1 CT | 150 mg/m2 tem d 1 to 7 and 15 to 21 of 28-day cycle | 2% CR (1/45 w/measurable GBM); 13% PR (6/45) | 64 GBM: median PFS, 24 wks (range, 4–78 wks); median OS, 38 wks (range, 5–99 wks) |
| Study . | n . | Patient Characteristics . | Treatment . | Response Rate . | Results . |
|---|---|---|---|---|---|
| Balmaceda et al., 200874 | 120 | ≤Third recurrence of GBM, AA, AO | 200 mg/m2 tem followed by 9 doses 90 mg/m2 tem q12hrs q28d; 90 mg/m2 dose escalated to 100 mg/m2 if tolerated | GBM: 31% ORR | GBM: median PFS, 4.2 mos; median OS, 8.8 mos |
| Brandes et al., 200475 | 40 | ≥8 wks prior RT | 80 mg/m2 BCNU d 1 and 3 q8wks | 15% PR (6/40); 22.5% SD (9/40) | Median TTP, 13.3 wks (95% CI, 10.26–16.86 wks); median OS, 7.53 mos (95% CI, 4.64–11.47 mos) |
| Burch et al., 200076 | 33 | ≥1 mo prior RT; ≥6 wks prior ≤1 nitrosourea-based CT | 1.5 mg/m2 topo 5 consecutive d q3wks w/o prior CT or 1.25 mg/m2 w/prior CT | 3% ORR | Median TTP, 14.9 wks; median OS, 19.9 wks |
| Lesser et al., 200477 | 32 | ≤1 prior chemotherapy | 1.00 mg/m2 karenitecin 5 consecutive d q3wks, escalated through continual reassessment in EIAED cohort to maximum 2.1 mg/m2 and in non-EIAED cohort to 1.8 mg/m2 | None | Median OS, 6.5 mos (95% CI, 4.0–9.7 mos) |
| Macdonald et al., 199678 | 31 | ≥2 mos prior RT; ≥6 wks prior surgery or adjuvant CT | 1.5 mg/m2 topo 5 d q3wks | 6% (2/31); 68% SD (21/31) | Median duration of SD, 19 wks (range, 5–86+ wks) |
| Wagner et al., 200479 | 32 | Pediatric; prior RT and CT | 0.4 mg/m2 topo daily, escalated to maximum 2.0 mg/m2 | 1/14 CR; 2/14 PR; 7/14 SD | Median OS (n = 32), 3.6 mos (range, 0.4–24.0 mos); median OS for ≥4 wks of treatment (n = 14), 6.0 mos (range, 1.6–24 mos) |
| Wick et al., 200780 | 90 | Prior RT w/ or w/o ≥1 CT | 150 mg/m2 tem d 1 to 7 and 15 to 21 of 28-day cycle | 2% CR (1/45 w/measurable GBM); 13% PR (6/45) | 64 GBM: median PFS, 24 wks (range, 4–78 wks); median OS, 38 wks (range, 5–99 wks) |
AA = anaplastic astrocytoma; AO = anaplastic oligodendroglioma; BCNU = carmustine; CR = complete response; CT = chemotherapy; EIAED = enzyme-inducing antiepileptic drug; GBM = glioblastoma; ORR = overall response rate; OS = overall survival; PFS = progression-free survival; PR = partial response; RT = radiotherapy; SD = stable disease; tem = temozolide; topo = topotecan; TTP = time to progression
Conclusions
The overall survival rate of patients with malignant glioma is disheartening. Novel agents and new approaches to therapy are needed to improve clinical outcomes. Irinotecan has been evaluated for chemotherapy of malignant glioma as monotherapy and in combination with other cytotoxic or targeted biological chemotherapy agents. Studies of irinotecan in combination with other medications, particularly temozolomide and bevacizumab, have yielded encouraging results with manageable toxicities. Irinotecan-based chemotherapy of malignant glioma merits further study as a core component of an integrated approach to treatment that also includes RT, surgical excision, and other cytotoxic agents.
Topoisomerase I inhibitors, for example, irinotecan, may hold importance for neuro-oncology for a number of reasons. Topoisomerase I inhibitors have a different mechanism of action than other glioma therapies, particularly alkylating agents. Preclinical and clinical data indicate that topoisomerase I inhibitors may be synergistic with alkylating agents. In addition, topoisomerase I inhibitors are in a small group of cytotoxic agents that readily cross the blood-brain barrier. Finally, the intriguing data for irinotecan and bevacizumab combination therapy suggest that there is a synergy between topoisomerase I inhibitors and anti-VEGF therapies, with acceptable toxicity. The inclusion of irinotecan and bevacizumab in the treatment of patients with newly diagnosed malignant glioma may significantly improve their survival.
Editorial support was provided by Adelphi Inc. and was funded by Pfizer Inc.
References
Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents.
Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000.
CBTRUS.
American Cancer Society.
Grossman SA, Batara JF. Current management of glioblastoma multiforme.
DeAngelis LM. Benefits of adjuvant chemotherapy in high-grade gliomas.
Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from twelve randomised trials.
DeAngelis LM, Burger PC, Green SB, Cairncross JG. Malignant glioma: who benefits from adjuvant chemotherapy?
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma.
van den Bent MJ, Hegi ME, Stupp R. Recent developments in the use of chemotherapy in brain tumours.
Liu L, Gerson SL. Targeted modulation of MGMT: clinical implications.
Belanich M, Pastor M, Randall T, et al. Retrospective study of the correlation between the DNA repair protein alkyltransferase and survival of brain tumor patients treated with carmustine.
Silber JR, Bobola MS, Ghatan S, Blank A, Kolstoe DD, Berger MS. O6–methylguanine-DNA methyltransferase activity in adult gliomas: relation to patient and tumor characteristics.
Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents.
Hegi ME, Diserens A, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma.
Donson AM, Addo-Yobo SO, Handler MH, Gore L, Foreman NK. MGMT promoter methylation correlates with survival benefit and sensitivity to temozolomide in pediatric glioblastoma.
Creemers GJ, Lund B, Verweij J. Topoisomerase I inhibitors: topotecan and irinotecan.
Singh S, Dwarakanath BS, Lazar MT. Role of topoisomerases in cytotoxicity induced by DNA ligand Hoechst-33342 and UV-C in a gliomas cell line.
Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma.
Innocenti F. UGT1A1 genotyping in patients undergoing treatment with irinotecan.
Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11.
Slatter JG, Su P, Sams JP, Schaaf LJ, Wienkers LC. Bioactivation of the anticancer agent CPT-11 to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactions.
Chen TC, Su S, Fry D, Liebes L. Combination therapy with irinotecan and protein kinase C inhibitors in malignant glioma.
Weller M, Winter S, Schmidt C, et al. Topoisomerase-1 inhibitors for human malignant gliomas: differential modulation of p53, p21, bax and bcl-2 expression and of CD95–mediated apoptosis by camptothecin and beta-lapachone.
Takeba Y, Sekine S, Kumai T, et al. Irinotecan-induced apoptosis is inhibited by increased P-glycoprotein expression and decreased p53 in human hepatocellular carcinoma cells.
Morandi E, Zingaretti C, Chiozzotto D, et al. A cDNA-microarray analysis of camptothecin resistance in glioblastoma cell lines.
Xu Y, Villalona-Calero MA. Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity.
Ando Y, Saka H, Ando M, et al. Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis.
Innocenti F, Undevia SD, Iyer L, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan.
NCCN.
Saunders M, Iveson T. Management of advanced colorectal cancer: state of the art.
Coggins CA, Elion GB, Houghton PJ, et al. Enhancement of irinotecan (CPT-11) activity against central nervous system tumor xenografts by alkylating agents.
Vassal G, Terrier-Lacombe MJ, Bissery MC, et al. Therapeutic activity of CPT-11, a DNA-topoisomerase I inhibitor, against peripheral primitive neuroectodermal tumour and neuroblastoma xenografts.
Blaney SM, Takimoto C, Murry DJ, et al. Plasma and cerebrospinal fluid pharmacokinetics of 9–aminocamptothecin (9–AC), irinotecan (CPT-11), and SN-38 in nonhuman primates.
Hare CB, Elion GB, Houghton PJ, et al. Therapeutic efficacy of the topoisomerase I inhibitor 7–ethyl-10–(4–[1–piperidino]-1–piperidino)-carbonyloxy-camptothecin against pediatric and adult central nervous system tumor xenografts.
Nakatsu S, Kondo S, Kondo Y, et al. Induction of apoptosis in multidrug resistant (MDR) human glioblastoma cells by SN-38, a metabolite of the camptothecin derivative CPT-11.
Fuchs C, Mitchell EP, Hoff PM. Irinotecan in the treatment of colorectal cancer.
Santos A, Zanetta S, Cresteil T, et al. Metabolism of irinotecan (CPT-11) by CYP3A4 and CYP3A5 in humans.
Kuhn JG. Influence of anticonvulsants on the metabolism and elimination of irinotecan. A North American Brain Tumor Consortium preliminary report.
Crews KR, Stewart CF, Jones-Wallace D, et al. Altered irinotecan pharmacokinetics in pediatric high-grade glioma patients receiving enzyme-inducing anticonvulsant therapy.
van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management.
Perucca E. Clinically relevant drug interactions with antiepileptic drugs.
Buckner JC, Reid JM, Wright K, et al. Irinotecan in the treatment of glioma patients: current and future studies of the North Central Cancer Treatment Group.
Raymond E, Fabbro M, Boige V, et al. Multicentre phase II study and pharmacokinetic analysis of irinotecan in chemotherapy-naive patients with glioblastoma.
Chamberlain MC. Salvage chemotherapy with CPT-11 for recurrent glioblastoma multiforme.
Turner CD, Gururangan S, Eastwood J, et al. Phase II study of irinotecan (CPT-11) in children with high-risk malignant brain tumors: the Duke experience.
Cloughesy TF, Filka E, Nelson G, et al. Irinotecan treatment for recurrent malignant glioma using an every-three-week regimen.
Cloughesy TF, Filka E, Kuhn J, et al. Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-three-week regimen.
Batchelor TT, Gilbert MR, Supko JG, et al. Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: final report of NABTT 97–11.
Gilbert MR, Supko JG, Batchelor T, et al. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma.
Prados MD, Yung WKA, Jaeckle KA, et al. Phase 1 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study.
Prados MD, Lamborn K, Yung WKA, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study.
Gilbert M, Wen P, Lieberman F, et al. Phase I/II study of combination temozolomide (TMZ) and irinotecan (CPT-11) for recurrent malignant gliomas: a North American Brain Tumor Consortium (NABTC) study [abstract].
Gruber ML, Buster WP. Temozolomide in combination with irinotecan for treatment of recurrent malignant glioma.
Brandes AA, Tosoni A, Basso U, et al. Second-line chemotherapy with irinotecan plus carmustine in glioblastoma recurrent or progressive after first-line temozolomide chemotherapy: a phase II study of the Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO).
Reardon DA, Quinn JA, Rich JN, et al. Phase 2 trial of BCNU plus irinotecan in adults with malignant glioma.
Quinn JA, Reardon DA, Friedman AH, et al. Phase 1 trial of irinotecan plus BCNU in patients with progressive or recurrent malignant glioma.
Puduvalli VK, Giglio P, Groves MD, et al.
Reardon DA, Quinn JA, Vredenburgh J, et al. Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma.
Purow B, Fine HA. Antiangiogenic therapy for primary and metastatic brain tumors.
Vredenburgh JJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant gliomas.
Goli KJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in the treatment of malignant gliomas [abstract].
Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme.
Raval SN, Rule A, Hwang SS.
Bokstein F, Blumenthal DT.
Cloughesy T, Prados M, Wen P, et al.
Olivero WC, Dulebohn SC, Lister JR. The use of PET in evaluating patients with primary brain tumors: is it useful?
Shields A, Grierson J, Dohmen B, et al. Imaging proliferation in vivo with F-18 FLT and positron emission tomography.
Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG.
Chen W, Delaloye S, Silverman DHS, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study.
Balmaceda C, Peereboom D, Pannullo S, et al. Multi-institutional phase II study of temozolomide administered twice daily in the treatment of recurrent high-grade gliomas.
Brandes AA, Tosoni A, Amistà P, et al. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial.
Burch PA, Bernath AM, Cascino TL, et al. A North Central Cancer Treatment Group phase II trial of topotecan in relapsed gliomas.
Lesser GJ, Grossman SA, Carson K, et al. Phase I study of karenitecin in the treatment of recurrent malignant gliomas (MG) [abstract].
Macdonald D, Cairncross G, Stewart D, et al. Phase II study of topotecan in patients with recurrent malignant glioma.
Wagner S, Erdlenbruch B, Längler A, et al. Oral topotecan in children with recurrent or progressive high-grade glioma: a phase I/II study by the German Society for Pediatric Oncology and Hematology.