-
PDF
- Split View
-
Views
-
Cite
Cite
Brian Patrick O'Neill, Steven Vernino, Ahmet Dogan, Caterina Giannini, EBV-associated lymphoproliferative disorder of CNS associated with the use of mycophenolate mofetil, Neuro-Oncology, Volume 9, Issue 3, July 2007, Pages 364–369, https://doi.org/10.1215/15228517-2007-004
Close - Share Icon Share
Abstract
Epstein-Barr virus (EBV)-associated lymphoid proliferations are a well-recognized complication of congenital or acquired systemic immunosuppression. The CNS is a frequent site for development of such lymphoid proliferations. We describe the clinical, imaging, and pathologic observations of a CNS disorder histologically similar to posttransplantation lymphoproliferative disorder that occurred in four patients with autoimmune disease treated with mycophenolate mofetil (MM). Two patients had polymorphous lymphoplasmacytic infiltration of brain parenchyma, and two had monomorphous infiltrations consistent with diffuse large B-cell lymphoma. In situ hybridization for EBV-encoded RNA was positive in all four patients. All patients improved after MM withdrawal and the use of rituximab. Because of a favorable toxicity profile, MM is now being used as steroid-sparing immunomodulatory therapy in autoimmune disorders. Based on our experience presented herein, we recommend caution in patient selection for MM and strict surveillance of those patients with autoimmune disorders who receive MM.
Epstein-Barr virus (EBV)-associated lymphoid proliferations are a well-recognized complication of congenital or acquired systemic immunosuppression and vary from an infectious mononucleosis-type lesion to frank lymphomas.1 Clinically, the most common associations are with iatrogenic immunosuppression following organ transplantation (posttransplantation lymphoproliferative disorders [PTLD])2,3 and with the acquired immunodeficiency of human immunodeficiency virus (HIV) infection.4
Although such proliferations can be seen in any anatomical site, they have a predilection for extranodal sites and particularly immune-privileged sites such as the CNS.5 To that point, the CNS version of PTLD (CNS-PTLD) accounted for 24% of all extranodal PTLD cases at one institution.6 Since then, we and others have documented an increased overall number of PTLD cases, and an increased percentage of CNS-PTLD cases, seemingly paralleling the use of the newer immunosuppressants such as cyclosporine and muromonab-CD3.7-9
We report clinical, imaging, and pathologic observations of four patients with EBV-mediated B-cell proliferations that were histologically similar to CNS-PTLD but occurred without organ transplantation. These proliferations occurred in patients with autoimmune disorders treated with mycophenolate mofetil (MM). All were from our routine clinical practice. In situ hybridization for EBV-encoded RNA (EBER) was positive in all patients. Two of the four patients had frank diffuse large B-cell lymphoma (DLBCL) consistent with primary CNS lymphoma (PCNSL). All patients improved after MM withdrawal and the introduction of rituximab.
Case Reports and Description of Findings
Patient characteristics are summarized in Table 1.
Cohort and results
| Characteristic . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age (years) | 88 | 58 | 65 | 57 |
| Gender | F | F | M | F |
| AID | Myasthenia gravis | CNS vasculitis | Relapsing polychondritis | Dermatomyositis |
| Duration (years) | 5 | 9 | 5 | 6 |
| MM dose | 1,000 mg b.i.d. | 1,000 mg b.i.d. | 1,000 mg b.i.d. | 1,000 mg b.i.d. |
| Duration (months) | 37 | 46 | 11 | 8 |
| Syndrome | Hemispheric | Posterior fossa | Hemispheric | Encephalopathy |
| Duration (months) | 2 | 2 | 3 | 1 |
| Imaging | Single, r/e | Multiple, c/e | Multiple, r/e | Multiple, irregular c/e |
| Pathology | DLBCL | DLBCL | PLPD | PLPD |
| EBV | Positive | Positive | Positive | Positive |
| Treatment | DMX, RTX | DMX, RTX | DMX, RTX | DMX, RTX |
| Response | CR | CR | PROG | CR |
| Characteristic . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age (years) | 88 | 58 | 65 | 57 |
| Gender | F | F | M | F |
| AID | Myasthenia gravis | CNS vasculitis | Relapsing polychondritis | Dermatomyositis |
| Duration (years) | 5 | 9 | 5 | 6 |
| MM dose | 1,000 mg b.i.d. | 1,000 mg b.i.d. | 1,000 mg b.i.d. | 1,000 mg b.i.d. |
| Duration (months) | 37 | 46 | 11 | 8 |
| Syndrome | Hemispheric | Posterior fossa | Hemispheric | Encephalopathy |
| Duration (months) | 2 | 2 | 3 | 1 |
| Imaging | Single, r/e | Multiple, c/e | Multiple, r/e | Multiple, irregular c/e |
| Pathology | DLBCL | DLBCL | PLPD | PLPD |
| EBV | Positive | Positive | Positive | Positive |
| Treatment | DMX, RTX | DMX, RTX | DMX, RTX | DMX, RTX |
| Response | CR | CR | PROG | CR |
Abbreviations: AID, autoimmune disorder; MM, mycophenolate mofetil; b.i.d., twice daily; r/e, ring enhancing; c/e, contrast enhancing; DLBCL, diffuse large B-cell lymphoma; PLPD, polymorphous B-cell lymphoproliferative disorder; EBV, Epstein-Barr virus; DMX, dexamethasone; RTX, rituximab; CR, complete response; PROG, progression.
Cohort and results
| Characteristic . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age (years) | 88 | 58 | 65 | 57 |
| Gender | F | F | M | F |
| AID | Myasthenia gravis | CNS vasculitis | Relapsing polychondritis | Dermatomyositis |
| Duration (years) | 5 | 9 | 5 | 6 |
| MM dose | 1,000 mg b.i.d. | 1,000 mg b.i.d. | 1,000 mg b.i.d. | 1,000 mg b.i.d. |
| Duration (months) | 37 | 46 | 11 | 8 |
| Syndrome | Hemispheric | Posterior fossa | Hemispheric | Encephalopathy |
| Duration (months) | 2 | 2 | 3 | 1 |
| Imaging | Single, r/e | Multiple, c/e | Multiple, r/e | Multiple, irregular c/e |
| Pathology | DLBCL | DLBCL | PLPD | PLPD |
| EBV | Positive | Positive | Positive | Positive |
| Treatment | DMX, RTX | DMX, RTX | DMX, RTX | DMX, RTX |
| Response | CR | CR | PROG | CR |
| Characteristic . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age (years) | 88 | 58 | 65 | 57 |
| Gender | F | F | M | F |
| AID | Myasthenia gravis | CNS vasculitis | Relapsing polychondritis | Dermatomyositis |
| Duration (years) | 5 | 9 | 5 | 6 |
| MM dose | 1,000 mg b.i.d. | 1,000 mg b.i.d. | 1,000 mg b.i.d. | 1,000 mg b.i.d. |
| Duration (months) | 37 | 46 | 11 | 8 |
| Syndrome | Hemispheric | Posterior fossa | Hemispheric | Encephalopathy |
| Duration (months) | 2 | 2 | 3 | 1 |
| Imaging | Single, r/e | Multiple, c/e | Multiple, r/e | Multiple, irregular c/e |
| Pathology | DLBCL | DLBCL | PLPD | PLPD |
| EBV | Positive | Positive | Positive | Positive |
| Treatment | DMX, RTX | DMX, RTX | DMX, RTX | DMX, RTX |
| Response | CR | CR | PROG | CR |
Abbreviations: AID, autoimmune disorder; MM, mycophenolate mofetil; b.i.d., twice daily; r/e, ring enhancing; c/e, contrast enhancing; DLBCL, diffuse large B-cell lymphoma; PLPD, polymorphous B-cell lymphoproliferative disorder; EBV, Epstein-Barr virus; DMX, dexamethasone; RTX, rituximab; CR, complete response; PROG, progression.
Patient 1
This 88-year-old woman with type II diabetes (previously reported in Vernino et al.10) presented with a five-year history of myasthenia gravis that for 37 months had been treated with MM (1,000 mg twice daily), and low-dose prednisone (5 mg every other day). She presented with mild headache and low-grade fever for two months, and then difficulty speaking (word-finding difficulty rather than dysarthria). She reported no systemic symptoms.
Examination demonstrated nonfluent speech, paraphasic errors, mild anomia, and right lower face and arm weakness. No fatigable weakness or peripheral lymphadenopathy was detected. Laboratory studies demonstrated marked lymphocytopenia, including a reduced CD4 count of 158/μl (reference range, 401-1,532 cells/μl). HIV testing was negative. MRI findings were consistent with PCNSL, glioma, or infection. A cerebrospinal fluid (CSF) examination was not performed. A week of dexamethasone (4 mg three times daily) did not alter the contrast enhancement.
A stereotactic biopsy led to diagnosis of EBV-associated DLBCL. The patient declined conventional chemotherapy and radiation. She was treated by a single dose of intravenous rituximab (375 mg/m2) and discontinuation of MM. Prednisone was increased to 60 mg daily for one week and then gradually tapered down to 20 mg every other day. Between August 2003 and May 2004, she improved to minimal right hemiparesis. MRI appearance of the tumor was improved. She subsequently received three additional weekly doses of intravenous rituximab. Follow-up MRI demonstrated complete resolution of PCNSL. She has remained clinically stable from the tumor for 3.5 years. Symptoms of myasthenia gravis returned and were managed with rest, low-dose prednisone, and pyridostigmine.
Patient 2
This 58-year-old woman presented with a two-month history of ataxia, diplopia, and dysarthria. CNS vasculitis was diagnosed nine years prior, and the patient was treated with high-dose corticosteroids and achieved complete remission. A year later, symptoms returned when corticosteroids were tapered. She was treated with azathioprine and then hydroxychloroquine sulfate. Three years later, MM replaced azathioprine.
She was clinically stable on a regimen of MM and hydroxychloroquine sulfate until the new symptoms began. MRI demonstrated new contrast-enhancing lesions in each middle cerebellar peduncle. The MM dose was increased from 2,000 mg daily to 2,500 mg daily, and 40 mg prednisone daily was added. Neurologic symptoms progressed, and a repeat MRI demonstrated a new contrast lesion in the corpus callosum and persistence of the prior lesions. Azathioprine, 50 mg three times daily, was added, but neurologic deterioration continued, and she was admitted to the hospital.
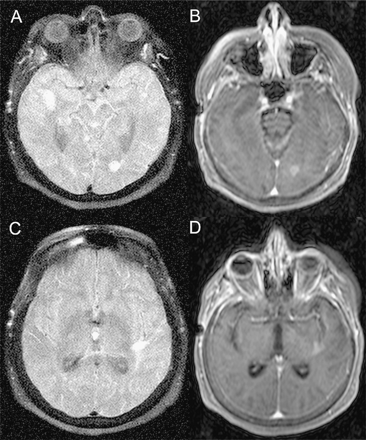
On general examination, she showed stigmata of prolonged corticosteroid usage such as proximal muscle weakness and a cushingoid appearance. Neurologic examination revealed gaze-evoked rotational nystagmus and marked bilateral dysmetria. The proximal muscle weakness precluded gait testing. Repeat MRI now showed progression of CNS lesions and new contrast-enhancing lesions in the right and left temporal lobes and in the left parietal and left occipital lobes (Fig. 1). Pertinent laboratory findings were confined to the CSF and included a lymphocytic pleocytosis of 80 mononuclear/μl (reference range, <5 mononuclear/μl), elevated protein of 114 mg/dl (reference range, 14-45 mg/dl), and six oligoclonal bands (reference range < four bands). Cytologic examination and flow cytometry were normal.
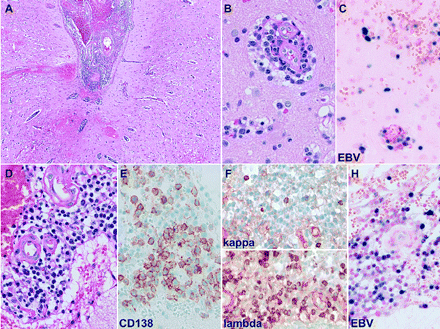
A five-day treatment region of methylprednisolone, 1 g daily, failed to improve her clinical status. A stereotactic biopsy of the right temporal lobe lesion led to the diagnosis of EBV-associated polymorphous B-cell lymphoproliferative disorder. EBV in situ hybridization showed the presence of EBV-positive populations of lymphocytes and plasma cells. The kappa/lambda light chain stains displayed an excess of lambda light-chain-expressing plasma cells (Fig. 2).
Distinctive MRI of patient 2. The initial scan after the onset of new symptoms showed contrast-enhancing lesions in each middle cerebellar peduncle. The prebiopsy scan demonstrates new contrast-enhancing lesions in the right and left temporal lobes, the left parietal, and the left occipital lobes. The figure displays the left occipital lobe lesion on fluid-attenuated inversion recovery (FLAIR) (A) and postgadolinium (B) images; and the left temporal lobe lesion on FLAIR (C) and postgadolinium (D) images.Note homogeneous and dense contrast enhancement. A scan 11 months after diagnosis and treatment with rituximab and cyclophosphamide showed only scattered T2 hyperintensities without contrast enhancement.
Characteristic features of Epstein-Barr virus (EBV)-associated polymorphous B-cell lymphoproliferative disorder observed in patient 2. There is evidence of a polymorphous infiltrate involving both leptomeninges (A, D) and parenchyma (B), with perivascular distribution. The infiltrate is composed predominantly of lymphocytes and plasma cells, as confirmed by the plasma cell marker CD138 (E). The kappa/lambda light chain stains displayed an excess of lambda light-chain-expressing plasma cells (F). The EBV in situ hybridization showed the presence of EBV-positive populations of lymphocytes and plasma cells both in parenchyma (C) and leptomeninges (H).
MM was discontinued, and the patient commenced a regimen of rituximab and intravenous cyclophosphamide. After 3 months cyclophosphamide was discontinued, and the patient continued to receive monthly rituximab. Eleven months later, the patient was living at home, independent in all daily activities, and walking without assistance. The most recent MRI demonstrated only scattered T2 hyperintensities without contrast enhancement.
Patient 3
This 65-year-old man presented with a three-month history of headaches followed by right-side weakness and sensory difficulties. His neurologic syndrome quickly followed installation of a pacemaker. He was initially evaluated at an outside hospital where CT imaging of the head (MRI contraindicated because of pacemaker) demonstrated numerous areas of low attenuation both supratentorially and infratentorially. The lesions had a ring-enhancing appearance with a minimal degree of mass effect associated with them.
The patient had a five-year history of relapsing polychondritis in the setting of long-standing hypothyroidism and psoriasis. He was initially managed with prednisone; azathioprine was added a year later to allow steroid tapering. Inadequate control of disease activity, however, prompted the introduction of MM one year later. At the time of referral, his immunosuppressant medication was MM 1,000 mg twice a day, azathioprine 50 mg three times day, and prednisone 2.5 mg daily.
Neurologic examination demonstrated a right-side hemiparesis and a mild degree of dysarthria but no language disturbance. Serum lactate dehydrogenase (LDH) was normal. A peripheral blood smear showed unusual red blood cell morphology such as toxic neutrophils, and spiculated cells. A CT scan done for surgical planning demonstrated numerous areas of low-attenuation change near the gray-white junction throughout the cerebrum and cerebellum; there was minimal, if any, mass effect. These lesions had relatively uniform ring enhancement; a small lesion adjacent to the atrium of the right lateral ventricle demonstrated an incomplete ring of enhancement. There were also enhancing lesions involving the left cerebral peduncle, upper left pons, and the ventral medulla at the cervicomedullary junction. A fluorodeoxyglucose-positron emission tomography study co-registered with CT demonstrated intense isotope uptake through both cerebellar hemispheres, moderate to intense homogeneous uptake corresponding to the right cerebral and left peduncle lesions, and moderate nonhomogeneous uptake throughout both cerebral and cerebellar hemispheres.
Stereotactic biopsy of the right frontal lesion demonstrated EBV-associated DLBCL. CSF analysis showed seven oligoclonal bands, an elevated CSF index of 1.67 (reference range, 0-0.85), and pleocytosis consisting of 98 white blood cells/μl (reference range, <5/μl). EBER was positive in CSF. Flow cytometric immunophenotyping showed a population of CD19-positive B-cells with a monotypic staining pattern for lambda surface immunoglobulin light chains, consistent with involvement of large B-cell lymphoma. Staging tests revealed no evidence of occult systemic lymphoma.
Following discontinuation of MM and azathioprine, the patient was started on a regimen of dexamethasone and rituximab. He received a total of four doses of rituximab with modest improvement of imaging but without a clinical response. He was transferred to his home community for supportive care.
Patient 4
This 57-year-old woman presented with a cognitive impairment and hallucinations. She had a six-year history of biopsy-proven dermatomyositis treated with a variety of immunosuppressants, including high-dose corticosteroids and azathioprine. Approximately eight months prior to admission, MM was started at a dose of 1,000 mg twice daily. Up to the onset of her neurologic symptoms, she had been well and functioning normally.
Neurologic examination was normal with the exception of word-finding difficulties and a decrease in intermediate memory function. MRI demonstrated three enhancing lesions. The largest was in the mesial left temporal lobe with irregular contrast enhancement and a small zone of surrounding T2 signal hyperintensity. There was a smaller right temporal lobe contrast-enhancing lesion and a subtle focus of contrast enhancement in the right operculum. These two latter lesions had subtle high signal and fluid-attenuated inversion recovery (FLAIR) sequences. None demonstrated mass effect. Initial laboratory studies including LDH were normal. CSF exam was normal except for a lymphocytic pleocytosis of 19 cells/μl; cytologic examination was negative.
Stereotactic biopsy of the left temporal lobe lesion demonstrated an EBV-associated polymorphous B-cell lymphoproliferative disorder composed of a mixture of small B-cells, large transformed immunoblasts, and plasma cells. In situ hybridization for EBER was positive in lymphoid cells. JC virus in situ hybridization studies were negative. MM was discontinued, and the patient commenced dexamethasone and rituximab treatment. Three months after diagnosis, the patient had no imaging evidence of active disease and had improved neurologic function.
Discussion
Extrapolating from PTLD, we assume the lymphoproliferation and subsequent lymphoma were associated in some way with immune deficiency, whether by drugs, underlying medical condition, or other means. In EBV-associated lymphoproliferation, the specific immune deficiency appears to be inadequate cytotoxic T-cell activity.11 After EBV infection in immune-competent individuals, the virus remains present in vivo in lymphocytes as a life-long latent infection under strict EBV-specific T-cell control. If this control is removed (or probably even if it is modified), the oncogenic properties of the virus may become apparent.12 This is assumed to be one of the mechanisms of PTLD development. However, even with robust immunosuppression, PTLD occurs infrequently,13 and severe T-cell immunodeficiency alone does not invariably result in lymphomatous conditions.14 Furthermore, athymic mice (T-cell immunodeficient) can still reject EBV-immortalized cells.15 We are unable to comment on the role of cytotoxic T-cells in this cohort.
The incidence of lymphoma in patients with congenital and acquired immunodeficiency is elevated, and some forms resemble PTLD by histologic appearance and by being associated with EBV.16 However, the genetic lesion responsible for the immunodeficiency itself may predispose to tumorigenesis, as in ataxia telangiectasia.17 Similarly, there is an excess risk of developing lymphoma in patients with autoimmune disorders such as rheumatoid arthritis, some of which are EBV negative.18 Although standard treatments such as methotrexate or cyclophosphamide are potentially mutagenic, cases have been reported where no immunosuppressive agent was ever used.18 In our cohort, each had an underlying autoimmune disorder, so MM may have been unrelated or no more than a contributing factor to the development of lymphoma.
The newer immunosuppressive regimens have a more selective and more profound effect on pathways of lymphocyte regulation than do earlier regimens that were more “global” in their effects. Azathioprine, the standard immunosuppressant in the early era of posttransplantation treatment, is a nonselective inhibitor of several enzymes involved in purine synthesis, a step critical to T- and B-cell division.19 In contrast, cyclosporine exerts its immunosuppressive action by inhibiting the enzyme calcineurin phosphatase and appears to more selectively affect T-lymphocytes by inhibiting the transcription of genes encoding interleukin-2 and other cytokines.20
The MM prodrug mycophenolic acid (MPA) is a selective inhibitor of inosine monophosphate dehydrogenase, the rate-limiting enzyme in de novo synthesis of guanosine nucleotides important in leukocyte production.21 MPA inhibits the subsequent proliferation of human T- and B-lymphocytes, but its effect appears to be more selective on T-lymphocytes.21 For example, MPA suppresses the recruitment of lymphocytes and monocytes into inflammatory sites and suppresses the elimination of allogeneic cells.22 It also suppresses T-lymphocyte-mediated immunopathogenetic events in rheumatoid arthritis, perhaps by inhibiting leukocyte attraction to activated endothelial cells.23
MM is widely used in adults and children as it improves the risk of rejection and permits a reduction or even withdrawal of corticosteroids without an increased overall risk for PTLD or solid cancers.24,25 Up to now, MM has not been considered to be mutagenic. However, at least contemporaneously, MM has been associated with an increase in CNS-PTLD at major transplantation centers. For example, nearly three-fourths of PTLD patients culled from the records of 11 French transplantation centers between 1976 and 1998 had their tumors develop in the late 1990s.9 In that cohort, 7 of 10 patients had their lymphoma occur after their long-term immunosuppression was switched from azathioprine to MM. In contrast, this type of “jump” has not been reported when prednisone and azathioprine was changed to muromonab-CD3.26
In support of our data regarding MM and PTLD-like disorders are two recent reports in addition to the report of Vernino et al.10 One described a 48-year-old woman who developed an EBV-associated B-cell lymphoma of the CNS one year after her immunosuppressive therapy for autoimmune skin disease was changed to MM. Discontinuation of her therapy led to a complete regression of the lymphoma.27 The second was a 58-year-old woman who developed a subacute mass lesion syndrome one year after MM was started for lupus nephritis.28 A brain biopsy revealed an EBV-positive DLBCL. Treatment consisted of chemotherapy and MM withdrawal. Clinical follow-up was not provided in the report.
Because of a favorable toxicity profile, MM is now being used as steroid-sparing immunomodulatory therapy in a variety of autoimmune disorders. MM may be associated with a higher incidence of PTLD and PTLD-like disorders than other immunosuppressive agents, but this requires further study. Until that time, based on our experience presented herein, we recommend caution in patient selection for MM and strict surveillance of those patients with autoimmune disorders who receive MM.
We acknowledge the financial support of this work by the University of Iowa-Mayo Clinic Specialized Program of Research Excellence (SPORE) in Lymphoma (P50 CA97274), by the Mayo Clinic SPORE in Brain Cancer (P50 CA108961; B.P.O.), and by “Steve's Run,” Dowagiac, Michigan. Core resources were supported by Cancer Center support grant P30 CA15083 to the Mayo Clinic Cancer Center, an NCI-designated Comprehensive Cancer Center. We thank L. Ottjes for superb secretarial and administrative help and R. Thompson for expert photographic assistance.
References
Worth A, Thrasher AJ, Gaspar HB. Autoimmune lymphoproliferative syndrome: molecular basis of disease and clinical phenotype.
Capello D, Rossi D, Gaidano G. Post-transplant lymphoproliferative disorders: molecular basis of disease histogenesis and pathogenesis.
Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders.
Mazhar D, Stebbing J, Bower M. Non-Hodgkin's lymphoma and the CNS: prophylaxis and therapy in immunocompetent and HIV-positive individuals.
Schiff D, O'Neill BP. Post-transplant lymphoproliferative disorders of the central nervous system. In Roos K, ed.
Phan TG, Kurtin PJ, O'Neill BP. Posttransplant primary CNS lymphoma.
Webber SA, Naftel DC, Fricker FJ, et al. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Pediatric Heart Transplant Study.
Boubenider S, Hiesse C, Goupy C, Kriaa F, Marchand S, Charpentier B. Incidence and consequences of post-transplantation lymphoproliferative disorders.
Snanoudj R, Durrbach A, Leblond V, et al. Primary brain lymphomas after kidney transplantation: presentation and outcome.
Vernino SA, Salamao DR, Habermann TM, O'Neill BP. Primary CNS lymphoma complicating treatment of myasthenia gravis with mycophenolate mofetil.
Omiya R, Buteau C, Kobayashi H, Paya CV, Celis E. Inhibition of EBV-induced lymphoproliferation by CD4(+) T cells specific for an MHC class II promiscuous epitope.
Kelleher CA, Dreyfus DH, Jones JF, Gelfand EW. EBV infection of T cells: potential role in malignant transformation.
Libertiny G, Watson CJ, Gray DW, Welsh KI, Morris PJ. Rising incidence of post-transplant lymphoproliferative disease in kidney transplant recipients.
Daddona PE, Mitchell BS, Meuwissen HJ, Davidson BL, Wilson JM, Koller CA. Adenosine deaminase deficiency with normal immune function: an acidic enzyme mutation.
Tosato G, Sgadari C, Taga K, et al. Regression of experimental Burkitt's lymphoma induced by Epstein-Barr virus-immortalized human B cells.
Cohen PL. Autoimmunity and lymphoproliferation: two genes are worse than one.
Gumy-Pause F, Wacker P, Sappino AP. ATM gene and lymphoid malignancies.
Mellemkjaer L, Andersen V, Linet MS, Gridley G, Hoover R, Olsen J. Non-Hodgkin's lymphoma and other cancers among a cohort of patients with systemic lupus erythematosus.
Cattan S, Lemann M, Thuillier F, et al. 6-Mercaptopurine levels and study of blood lymphocyte subsets during azathioprine treatment of Crohn's disease.
Karamperis N, Koefoed-Nielsen PB, Brahe P, et al. Correlations between calcineurin phosphatase inhibition and cyclosporine metabolites concentrations in kidney transplant recipients: implications for immunoassays.
Allison AC, Eugui EM. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection.
Eugui EM, Mirkovich A, Allison AC. Lymphocyte-selective antiproliferative and immunosuppressive effects of mycophenolic acid in mice.
Laurent AF, Dumont S, Poindron P, Muller CD. Mycophenolic acid suppresses protein N-linked glycosylation in human monocytes and their adhesion to endothelial cells and to some substrates.
Dipchand AI, Benson L, McCrindle BW, Coles J, West L. Mycophenolate mofetil in pediatric heart transplant recipients: a single-center experience.
Gao SZ, Chaparro SV, Perloth M, et al. Post-transplantation lymphoproliferative disease in heart and heart-lung transplant recipients: 30-year experience at Stanford University.
Caillard S, Dharnidharka V, Agodoa L, Bohen N, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression.
Waldman MA, Callen JP. Self-resolution of Epstein-Barr virus-associated B-cell lymphoma in a patient with dermatomyositis following withdrawal of mycophenolate mofetil and methotrexate.
- mycophenolate mofetil
- herpesvirus 4, human
- in situ hybridization
- central nervous system dysfunction
- autoimmune diseases
- biological therapy
- diffuse large b-cell lymphoma
- lymphoproliferative disorders
- steroids
- therapeutic immunosuppression
- natural immunosuppression
- brain
- diagnostic imaging
- rituximab
- rna
- toxic effect
- surveillance, medical
- parenchyma