-
PDF
- Split View
-
Views
-
Annotate
-
Cite
Cite
Jianni Liu, Rudy Lerosey-Aubril, Michael Steiner, Jason A Dunlop, Degan Shu, John R Paterson, Origin of raptorial feeding in juvenile euarthropods revealed by a Cambrian radiodontan, National Science Review, Volume 5, Issue 6, November 2018, Pages 863–869, https://doi.org/10.1093/nsr/nwy057
Close - Share Icon Share
Abstract
The rapid rise of arthropods during the Cambrian quickly established some clades, such as the euarthropod stem-group called Radiodonta, as the dominant and most diverse predators in marine ecosystems. Recent discoveries have shown that the size and dietary ecology of radiodontans are far more diverse than previously thought, but little is known about the feeding habits of juveniles. Here, we document a very small (∼18-mm-long), near-complete specimen of the radiodontan Lyrarapax unguispinus from the early Cambrian Chengjiang Biota of China. This specimen is the smallest radiodontan individual known, representing a juvenile instar. Its adult-like morphology—especially the fully developed spinose frontal appendages and tetraradial oral cone—indicates that L. unguispinus was a well-equipped predator at an early developmental stage, similar to modern raptorial euarthropods, such as mantises, mantis shrimps and arachnids. This evidence, coupled with the basal phylogenetic position of radiodontans, confirms that raptorial feeding habits in juvenile euarthropods appeared early in the evolutionary history of the group.
INTRODUCTION
Many Cambrian members of the iconic euarthropod stem-group known as radiodontans (Anomalocaris and kin) have been viewed as giant apex predators ever since their true body plan was revealed over 30 years ago [1–3]. However, more recent findings have shown that the body size of some early Palaeozoic radiodontans can range from 4 cm to over 200 cm in length [4–6] and that the highly variable frontal appendage morphologies are suggestive of a range of feeding modes, from shell-crushing to filter-feeding [6–11]. Lyrarapax from the early Cambrian Chengjiang Biota of China represents the smallest radiodontan taxon, with previously reported body sizes ranging from 4 to 8 cm in length [4,5]. Until now, the frontal appendage morphology of the type species, Lyrarapax unguispinus was only known from a single, incomplete appendage [4,5], but the preserved details imply a predatory function. The juvenile specimen of L. unguispinus described here not only provides novel information on the frontal appendages and feeding mode in this taxon, but sheds new light on the morphology of the mouth apparatus and the phylogenetic concept of the Radiodonta.
RESULTS AND DISCUSSION
General morphology
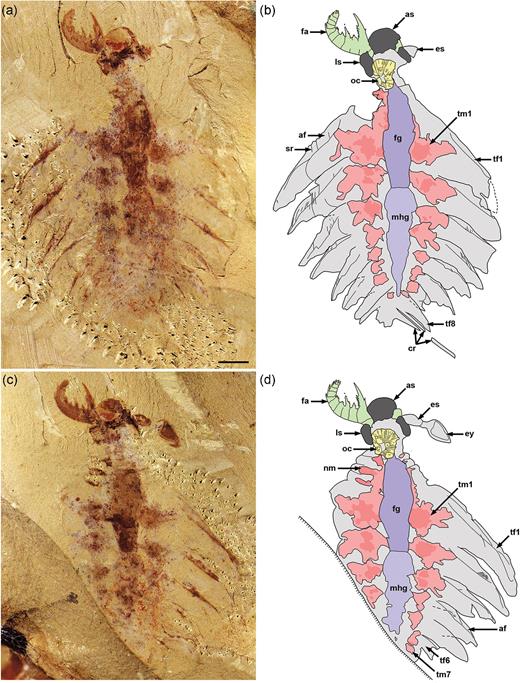
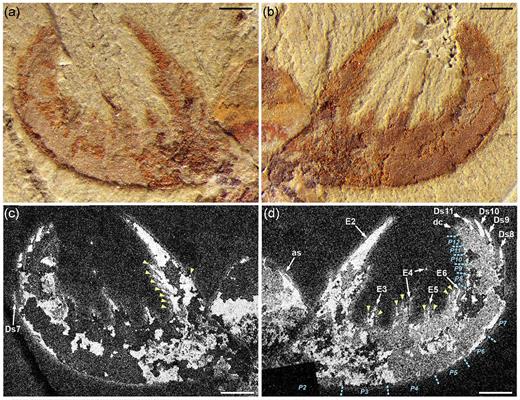
The small specimen (total body length: 18 mm) of L. unguispinus documented here (Figs 1–3 and Supplementary Fig. 1, available as Supplementary Data at NSR online), from the lower Cambrian (Series 2, Stage 3) Yu’anshan Member, Chiungchussu Formation of Yunnan Province in China, is relatively complete and shows features not seen in the type specimens [4,5], including a fully articulated frontal appendage (FA) and a sclerotized oral cone; see Supplementary Data at NSR online, for a detailed description and taxonomic discussion. The FA comprises 12 podomeres (P1–12): P2–6 bear complex endites of alternating sizes, but no dorsal spines; P7–11 are devoid of endites, but have stout dorsal spines; and P12 has a short, robust distal claw (Fig. 2 and Supplementary Fig. 1a, available as Supplementary Data at NSR online). Of note is the hypertrophied endite on P2 that hosts at least seven anterior auxiliary spines (Fig. 2c). The oral cone consists of many circumoral plates, including four large, perpendicularly arranged plates with surficial nodes (Fig. 3a and b). This tetraradial arrangement of large, node-bearing plates intercalated between a series of smaller plates is very similar to the Peytoia/Hurdia-like oral cone recently described from the early Cambrian Guanshan Lagerstätte of China [12].
Juvenile specimen of Lyrarapax unguispinus. (a) and (b) Part of a near-complete specimen (XDMU-133) and interpretative drawing. (c) and (d) Counterpart (mirrored) and interpretative drawing. Scale bar in (a) is 2 mm and applies to all images. af, anterior flange; as, anterior sclerite; cr, caudal rami; es, eye stalk; ey, eye; fa, frontal appendage; fg, foregut; ls, lateral sclerite; mhg, undifferentiated midgut-hindgut; nm, neck muscle; oc, oral cone; sr, strengthening rays; tf, trunk flap; tm, trunk muscle. Colours: dark grey, cephalic sclerites; dark purple, foregut; green, frontal appendages; light purple, undifferentiated midgut-hindgut; pink, muscle blocks; yellow, oral cone.
Frontal appendage of Lyrarapax unguispinus. (a) and (b) Photographs of part and counterpart. (c) and (d) Back-scattered electron micrographs of part and counterpart, showing the anterior sclerite (as), auxiliary (arrowheads) and dorsal (Ds) spines, endites (E), distal claw (dc) and podomere (P) boundaries. All scale bars: 1 mm.
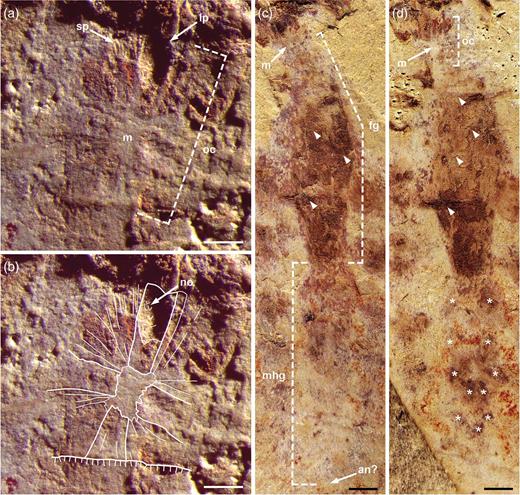
Mouth apparatus and gut of Lyrarapax unguispinus. (a) and (b) Oral cone showing tetraradial arrangement of large plates. (a) Detail showing clear impressions of large and small plates. (b) Outlines of preserved plates and nodes. (c) and (d) Complete gut with impressions of segment boundaries (arrowheads) in the foregut and possible midgut digestive glands (asterisks); (c) part and (d) counterpart (mirrored). All scale bars: 1 mm. an, anus; fg, foregut; lp, large plate; m, mouth; mhg, undifferentiated midgut-hindgut; no, node; oc, oral cone; sp, small plate.
Significance of the oral cone
The presence of a tetraradial oral cone in L. unguispinus has important implications for radiodontan systematics. Previously documented specimens of Lyrarapax from the Chengjiang Biota do not display a plated mouth apparatus, but rather a series of concentric ridges and furrows [4,5]. Based on the occurrence of this feature in one specimen of each species (L. unguispinus and L. trilobus), a lack of circumoral plates was interpreted as a morphological characteristic of Lyrarapax, rather than a taphonomic artefact [5]. This prompted a revised generic diagnosis, and also challenged the original concept of the Radiodonta [3,5]. The oral cone in the new specimen of L. unguispinus (Fig. 3a and b) indicates that its absence in other specimens of Lyrarapax is indeed preservational. The displacement or lack of other external, sclerotized features (e.g. FAs) suggests that the previously illustrated Lyrarapax specimens represent carcasses that have suffered from some preferential decay of articulating membrane and post-mortem disturbance [13–15]; exuvia can be ruled out, as all specimens preserve internal labile tissues. Thus, an alternative interpretation of the oral structures previously described for L. unguispinus (Figs 1a–d, f in [4]) and L. trilobus (Figs 1.3, 1.5, 2.2, 2.3 in [5]) is that they represent soft tissues—possibly the pharynx and its musculature—internally located behind the oral cone; the ‘triangular areas’ seen in the holotype (Fig. 1f in [4]) may represent impressions of, or even attachment points for, the large plates of the oral cone. Moreover, isolated radiodontan oral cones have been found in most Cambrian Konservat-Lagerstätten [9,12,16], demonstrating that they were often disarticulated from the body during ecdysis or after death, and later subjected to biostratinomic sorting.
The discovery of a sclerotized oral cone in Lyrarapax demonstrates that the presence of radial circumoral plates remains a consistent trait of the Radiodonta (particularly as a single coherent unit) and indeed a characteristic feature of lower stem-group euarthropods [17,18] and other ecdysozoans [19]. Also, our phylogenetic analysis of radiodontans based on an updated version of a recent dataset [6] (available as Supplementary Data at NSR online) retrieves Lyrarapax nested within a monophyletic Amplectobeluidae (Supplementary Fig. 2, available as Supplementary Data at NSR online). The tetraradial arrangement appears to be plesiomorphic for Radiodonta, and the presence of node-bearing plates is a synapomorphy of Anomalocarididae + Amplectobeluidae [12,20].
Raptorial feeding in adult and juvenile radiodontans
Of the diverse radiodontan FA morphologies [5,6,8,10], those of amplectobeluids appear the best suited for grasping and manipulating prey, characterized by a proximal hypertrophied endite and a series of robust dorsal spines distally [4,5,8,20–22] (Supplementary Fig. 3, available as Supplementary Data at NSR online). Lack of an articulation joint at the base of the hypertrophied endite indicates that it may have functioned as the rigid part of a ‘claw’, with the more flexible portion of the FA represented by the distal podomeres, thus permitting pincer-like capture of prey [2,8]. The stout, curved dorsal spines on the distal podomeres, when curled inwards, would have aided in securing the prey [8]. Also, the combination of a ‘dorsal kink’ in the proximal portion of the FAs (Fig. 4b and Supplementary Fig. 3, available as Supplementary Data at NSR online) and reduced head sclerites may have afforded the raptorial appendages a greater range of motion and degree of flexibility for striking and seizing prey [23]. As described above, the FA of L. unguispinus shows a pronounced morphological and therefore functional differentiation along its proximo–distal axis. The proximal podomeres are armed with complex endites, the smaller of which would have converged towards the serrated margin of the hypertrophied spine during flexion (Fig. 2 and Supplementary Fig. 1a, available as Supplementary Data at NSR online). This likely resulted in crushing or slicing of prey before ingestion; the similar FAs of Amplectobelua stephenensis may have had a comparable function [8]. In contrast, the endites of A. symbrachiata are more simple and slender and, while the FA of this species would have undoubtedly been used to grab prey, mastication was likely performed separately by gnathobase-like structures [20]. Amplectobeluid FAs may have been able to apply considerable force, as evidenced by the darker pigmentation of their spines in Chengjiang specimens (e.g. Supplementary Fig. 3a, available as Supplementary Data at NSR online; Figs 3.2, 3.4 in [5]; Fig. 1A in [20]). This darker colouration is often indicative of a local thickening of the cuticle, as recently demonstrated for the gnathobases of the Cambrian durophagous euarthropod Sidneyia inexpectans [24]. However, forceful capture may have caused occasional damage, as seen in A. symbrachiata FAs where the more delicate (non-hypertrophied) endites have broken off in some cases [20,21].
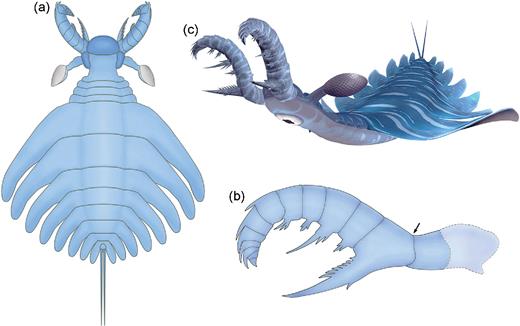
Reconstructions of Lyrarapax unguispinus. (a) Complete body in dorsal view. (b) Frontal appendage in lateral view, showing the spinosity pattern along the proximo–distal axis and dorsal kink (arrow). A more proximal, long podomere is suggested based on the morphology of Amplectobelua symbrachiata. (c) Artistic representation of the animal.
This raptorial feeding mode in Lyrarapax and Amplectobelua seems to extend to juveniles as well. Immature specimens of L. unguispinus (Figs 1 and 2) and A. symbrachiata (Fig. 3A in [2]; Fig. 125 in [25]; extended data Fig. 1b–d in [4]) show that the FAs have distinct adult-like morphologies. Also, the presence of large eyes, well-developed body flaps and an enlarged gut in juveniles [2,25] (Figs 1, 3c, 3d and Supplementary Fig. 1c–e, available as Supplementary Data at NSR online) suggests that Lyrarapax and Amplectobelua were already highly mobile visual predators during the early stages of post-embryonic development. It is possible that similar predatory modes occur among juveniles of some other radiodontans, such as Anomalocaris, given the comparable set of traits in adults [17,26,27], but this can only be confirmed by studying juvenile specimens.
Despite the lack of suitable modern analogues for reaffirming the proposed functional morphology and feeding ecology of radiodontans [28], there exist several predatory euarthropod groups that hunt using enlarged, spinose raptorial appendages. These include terrestrial clades such as the amblypygids (whip spiders) [29,30], uropygids (whip scorpions) [30] and mantodeans (mantises) [31], as well as the marine stomatopod crustaceans (mantis shrimps) [32–35] (Supplementary Figs 4 and 5, available as Supplementary Data at NSR online). Interestingly, the highly specialized morphology of grasping appendages develops very early during the ontogeny of these taxa.
Perhaps the most striking resemblance to radiodontan FAs are the pedipalps of uropygids [30] (Supplementary Fig. 5, available as Supplementary Data at NSR online), which provide one of the best modern analogues for understanding the functional morphology of the FAs of Lyrarapax throughout ontogeny. Both adult and juvenile uropygids use their large, curved raptorial pedipalps to catch and hold prey, with the various stout spines on the tarsus, tibia and patella acting like prehensile pincers, while the opposing gnathobases on the proximal trochanters crush and masticate the victim [30]. The robust construction of the pedipalps affords uropygids a varied diet [30,36], but juveniles seem to avoid prey with hard exoskeletons [30]. This may have also been the case in Lyrarapax and Amplectobelua, although the reinforced spines in immature FAs may have allowed juveniles to manipulate hard (possibly even biomineralized) food items. So, while the FAs of amplectobeluid radiodontans may have functioned in a similar way to the pedipalps of uropygids, consumption of the prey was clearly different. Uropygids use their chelicerae to tear tissue from the prey before passing it to the preoral cavity, where it is liquefied by digestive fluids [30]. For radiodontans, there is still debate as to whether the oral cone performed an additional masticatory role or was used to ingest food via suction [16,28]; also, A. symbrachiata would have used its gnathobase-like structures to initially process food before passing it to the mouth opening [20]. However, given the variable morphologies of the oral cone (as corroborated by FAs), it is likely that the assorted radiodontan species used this structure in different ways [12,16].
The variety of radiodontan feeding structures clearly points to these stem-group euarthropods having played key, often high-tier, trophic roles within early Palaeozoic food webs, including the consumption of zooplankton, as well as nektonic and benthic fauna [6,8,10–12,16,20,37,38]. While certain taxa such as Anomalocaris [27] can still be considered giant apex predators of their time and capable of consuming large prey, the juveniles of some radiodontans like Lyrarapax (Fig. 4 and Supplementary Fig. 6, available as Supplementary Data at NSR online) demonstrate that predation in the water column was occurring on a variety of scales during the Cambrian [10,37,39–41]. On the smaller scale of prey, taxa such as Tamisiocaris were likely microphagous suspension feeders of plankton (≥0.5 mm in size) [10], whereas juvenile amplectobeluids were probably capable of feeding on very small (<5-mm) benthic and nektonic prey items, possibly including sclerotized taxa or biomineralized forms such as trilobites, molluscs and brachiopods. The consistent raptorial feeding habits of both juvenile and adult Lyrarapax contrast with the example of another early Cambrian euarthropod: the megacheiran Leanchoilia illecebrosa, where it is thought that juveniles and adults occupied separate ecological niches due to differing appendage morphologies [42]. Hence, the predatory lifestyles of certain radiodontan offspring adds further tiering complexity to Cambrian marine food webs, and would have likely placed extra selective pressures on animal communities, particularly small benthic and nektonic prey [28,37]. Intense predation occurring on all scales during the early phase of animal evolution was undoubtedly a critical driver behind the morphological and ecological innovations arising throughout the Cambrian [28].
ACKNOWLEDGEMENTS
Thanks to E. Baker, J. Haug, S. Huber, C. McLean, M. Seiter and J. Wolff for discussions and supplying images of modern raptorial euarthropods; J.-B. Caron and D. Huang for images of amplectobeluid frontal appendages; L. Yang for taking photos of A. symbrachiata specimens; K. Wang for the artistic 3D reconstructions; T. Brougham for conducting the phylogenetic analysis; and P. Van Roy and an anonymous referee for their helpful reviews. J.L. and D.S. designed the research. J.R.P., R.L.A. and J.L. wrote the text, and all authors discussed and approved the manuscript. J.L., R.L.A. and J.R.P. performed light photography, and M.S. acquired SEM images. J.L. produced the original camera lucida drawings for Fig. 1 and R.L.A. created the line drawings in Figs 4a and 4b. R.L.A. prepared all figures.
FUNDING
This research was supported by Project 973 of the Ministry of Science and Technology of China (2013837100), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB26010104), the National Natural Science Foundation of China (41222014, 41172023, 41621003, 41102012), the 111 Project and the Ministry of Education of China for Chengjiang Scholars to J.L., and an Australian Research Council Future Fellowship (FT120100770) to J.R.P.
Conflict of interest statement. None declared.
REFERENCES