-
PDF
- Split View
-
Views
-
Cite
Cite
Alan Remde, Stephen N DeTurk, A Almardini, Lauren Steiner, Thomas Wojda, Plant-predominant eating patterns – how effective are they for treating obesity and related cardiometabolic health outcomes? – a systematic review, Nutrition Reviews, Volume 80, Issue 5, May 2022, Pages 1094–1104, https://doi.org/10.1093/nutrit/nuab060
Close - Share Icon Share
Abstract
The obesity epidemic is a main driver of the chronic disease epidemic; however, present treatment approaches have suboptimal efficacy.
To assess the efficacy of plant-predominant (vegan, vegetarian, plant-based whole foods [PBWFs]) diets in treating obesity and its main cardiometabolic sequelae: hyperlipidemia (HLD); indices of insulin resistance, glycemic control, and diabetes mellitus type 2 (DM2); and cardiovascular disease (CVD), including hypertension (HTN).
A systematic search of multiple databases was conducted for articles published between November 2019 and February 2020; databases searched included: PubMed, Medline (Ovid), Cochrane, CENTRAL, and CINAHL.
All interventional trials (randomized controlled trials [RCTs] and trials of non-randomized experimental design) that met the inclusion criteria (English language, duration of at least 4 weeks, primary end point congruent with above objectives, no major flaws in research design that would prevent interpretation) were included in the review. A total of 3135 articles were scanned and 84 were selected. The articles were collated and summarized in 2 evidence tables. Risk of bias for RCTs was assessed using the Cochrane Risk-of-Bias tool 2 as a guide. For non-randomized trials, higher risk of bias was assumed, and the JBI Critical Appraisal tool was used as a guide to determine inclusion.
Plant-based diets, in general, demonstrated improved weight control and cardiometabolic outcomes related to lipids, cardiovascular end points, blood pressure, insulin sensitivity, A1C, and fasting glucose, and a lower risk of diabetes compared with usual diets and in some cases standard health-oriented diets such as the American Heart Association (AHA), American Diabetic Association (ADA), and Mediterranean diets. Preliminary studies suggest plant-predominant diets practiced as part of healthy lifestyle interventions may stabilize or even reverse DM 2 and CVD. The acceptability and sustainability of plant-predominant diets where measured were generally similar to other health-oriented diets.
Plant-predominant diets can play a major role in reversing the obesity and chronic disease epidemics. In the setting of sustained lifestyle intervention programs, they may arrest or even reverse DM2 and CVD. Further higher-level RCTs are needed to confirm and expand on these findings.
INTRODUCTION
Chronic disease drives much of morbidity, mortality, and direct healthcare cost, and significantly impacts the economy in developed countries, including the USA.1 Obesity rates have risen markedly in the past few decades and are a major driver of cardiometabolic disorders (such as hyperlipidemia [HLD]), diabetes mellitus type 2 [DM2]), hypertension [HTN]), and cardiovascular disease [CVD]), and multiple other disorders (such as osteoarthritis [OA]) and mental illnesses [such as depression].2 A main driver of obesity is the consumption of high calorie/nutrient ratio foods.3 Such foods include high-fat animal products (such as most beef4) and highly processed plant-based foods containing added fat, salt, and sugars.3 Highly processed “junk food” has a higher profit margin than whole foods and is designed to maximize the consumer’s “bliss point”, leading to cravings, habituation, and overeating, thus increasing market share and corporate profits.5 This may be why the quantity of junk food advertising dwarfs that of whole foods.6,7
Modifying lifestyle with improved eating patterns, exercise, and fitness is first-line treatment for obesity and its cardiometabolic sequelae. However, most approaches for treating obesity have poor long-term efficacy.8 Factors driving poor long-term efficacy for weight loss and maintenance include the limiting of interventions to nutrition education only, with failure to address psychosocial factors (such as deeply ingrained family- and culturally derived eating habits, stress, and emotionally driven eating). Lack of ongoing support also leads to poorer long-term outcomes.3 Overreliance on calorie counting and portion control, with failure to lower the calorie:nutrient ratio of foods eaten, decreases long-term success.3 Given this poor track record in treating obesity and its sequelae, there has been renewed interest in plant-predominant diets to support healthy weight and the reduction of related cardiometabolic and other disorders. Healthy characteristics of these diets include the following:
low calorie:nutrient ratio
rich in micronutrients and phytonutrients
increased fiber to support adequate satiety and control of eating
freedom from the habituation tendencies of highly processed “pseudo-food” such as chips, pastries, and fast-food offerings such as French fries and pizza.
Other reasons advocated for plant-based eating patterns outside of the scope of this review include environmental sustainability (since eating “low on the food chain” is much less demanding of environmental resources) and avoiding the ethical concerns associated with the treatment of animals.
Most of the observational studies (such as the cohort and cross-sectional studies) suggest plant-predominant diets are associated with lower rates of obesity (see, eg, References9–14), but this appears to depend on the quality of the diet, with poorer quality (eg, higher-fat) vegetarian diets sometimes being associated with higher BMI (body–mass index).15 Observational studies on related cardiometabolic disorders show associations between plant-predominant diets and improved HLD.9,16–23 Plant-predominant diets are associated with lower risk of DM2, better glycemic control,9,11,15,18,24–28 and less risk of CVD and HTN.14,23,25–27,29–35 For example, in the Cornell China study, Campbell et al36 noted that rural Chinese consumed about 1/10 the animal protein and less than half the fat compared with their US counterparts, and male and female rural Chinese had rates of heart disease 1/16 of their male and 1/5 of their female US counterparts, respectively. They had an average total cholesterol (TC) of 127 mg/dL. Rates of heart disease were inversely proportional to green plant intake, with no threshold for further benefit. A systematic review and meta-analysis of observational studies noted less ischemic heart disease and cancer with plant-predominant diets.37 Given these extensive associative findings, it was considered important to systematically review interventional studies on the use of plant-predominant diets to treat obesity and its major cardiometabolic sequelae (see Table S1 in the Supporting Information online).
METHODS
Search criteria
A systematic search of multiple databases was conducted between November 2019 and February 2020; databases searched included: PubMed, Medline (Ovid), Cochrane, CENTRAL, and CINAHL. Searches were developed to use Mesh terms if available, or key words if there was no suitable Mesh term. The search terms focused on diets of interest: (plant based OR whole food* OR “diet, vegetarian” [Mesh] OR “diet, vegan” [Mesh]), combined with disease states of interest: (“cardiovascular diseases/diet therapy”[Mesh] OR “cardiovascular diseases/prevention and control” [Mesh] OR “overweight/diet therapy” [Mesh] OR “overweight/prevention and control” [Mesh] OR “obesity/diet therapy” [Mesh] OR “obesity/prevention and control” [Mesh] OR “diabetes mellitus/diet therapy” [Mesh] OR “diabetes mellitus/prevention and control” [Mesh] OR “dyslipidemias/diet therapy” [Mesh] OR “dyslipidemias/prevention and control” [Mesh] OR “metabolic syndrome/diet therapy” [Mesh] OR “metabolic yyndrome/prevention and control” [Mesh] OR “body weight changes” [Mesh]).
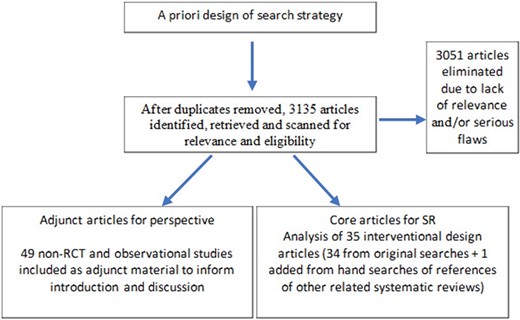
Results were filtered to include only articles in the English language and involving human subjects (Fig. 1). The types of article were not filtered in order to avoid missing articles that were not indexed by article type correctly. After removal of duplicates, 3135 unique papers were identified for possible inclusion. Publication titles and abstracts were screened by 5 reviewers for relevance. All primary interventional research study types were considered for inclusion, including RCTs and quasi-experimental designs. The core interventional articles were screened for relevant obesity-related cardiometabolic diseases and/or outcomes related to plant-predominant diet type. Studies were screened that reported markers of chronic disease (eg, weight changes, A1C or glucose changes, lipid changes, and blood pressure changes) or prevalence of chronic disease (eg, obesity, diabetes, hyperlipidemia, hypertension, and cardiovascular disease). After screening, 34 articles were included from the systematic search. The references from other relevant systematic reviews were also checked for additional resources, yielding an additional 1 article. (Non-interventional studies such as observational cohort and cross-sectional designs were not included in the main analysis and were not systematically analyzed, but were collected separately to give perspective to the introduction and discussion part of this article).
Flow chart demonstrating the method of literature search
Abbreviations: RCT, randomized controlled trial; SR, Systematic Review
Inclusion criteria for systematic review (SR) : We included RCTs and quasi-experimental studies that did not have serious risk of bias/conflict of interest issues related to their key outcomes or conclusions; English-language articles; studies that included adults; studies with a duration of a minimum of 4 weeks; studies with a primary or secondary end point including weight or change in weight/body–mass index (BMI)/waist:hip ratio/body composition (related to proportion of lean and fat); studies with parameters related to common cardiometabolic sequelae of obesity: DM, fasting glucose, A1c, insulin resistance, change in relevant medications, change in lipids: (total cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein (LDL), TC/HDL ratio, triglyceride (TG), change in relevant medications, change in HTN (change in systolic BP, diastolic BP, or change in relevant medications), CVD outcomes (rates of MI, heart failure, etc.), and measures of coronary disease (angiography results, etc.).
Exclusion criteria: Exclusion criteria included non-interventional studies, studies exclusively on children, non-English articles, studies that did not include weight or related measures of obesity. Studies in which the focus was on other less common cardiometabolic issues, study populations limited to special populations such as rheumatoid arthritis, chronic kidney disease, etc., and studies that did not have plant-predominant diet changes as a main focus of the intervention were excluded.
Diet types
This primary care–relevant systematic review of plant-predominant eating patterns discusses 3 main eating patterns – plant-based whole foods (PBWFs), vegan, and vegetarian – and their effectiveness in preventing and treating obesity and cardiometabolic sequelae. For the purpose of this review, these 3 patterns are defined as follows:
vegan: exclusively plant based; no animal products
vegetarian: predominantly plant-based, with various forms, such as ovo- (OV – allows eggs), lacto- (LV – allows dairy), and lacto-ovo (LOV – allows eggs and dairy)
plant-based whole foods (PBWFs): exclusively plant based; no (or minimal) animal products (including dairy and eggs), but some allow small amounts of dairy in recipes); no (or minimal) processed foods, such as any food made out of white flour or oil (even extra-virgin olive oil, though sometimes that is allowed in small amounts).
Risk of bias assessment
RCTs were assessed for risk of bias using the Cochrane Risk of Bias 2 tool.38 Quasi-experimental (non-randomized) interventional designs were assumed to have inherently higher risk of bias. The Joanna Briggs Institute Critical Appraisal Tool for Quasi-Experimental Studies was used to ensure that the listed studies did not have any critical flaws that would preclude inclusion.39 A few studies with higher risk of bias but unique perspectives were included. In these cases, the higher risk of bias was clearly noted. See Tables S2 and S3 in the Supporting Information online, last column.
RESULTS
Vegan
In one non-randomized trial, hypertensive patients prescribed a vegan diet had significant weight loss after 1 year.40 Two RCTs comparing the outcomes for adoption of a vegan diet with those for no dietary changes reported significantly greater decrease in weight and BMI after adoption of the vegan diet,41,42 with decreased waist circumference (WC) and visceral fat volume being reported in the study by Kahleova et al.41
In a study of diabetics, Barnard et al.43 showed that a vegan diet resulted in a significant decrease in weight, BMI, WC, and waist:hip ratio after 22 weeks, but only the changes in WC and waist:hip ratio were significantly different compared with the American Diabetic Association (ADA) diet control group. When only considering the subgroup who did not alter their diabetic medications, the decrease in weight, BMI, and WC was significantly greater in those on the vegan diet. This study population continued to be followed for another year, and after 74 weeks both groups had a significant decrease in weight, BMI, and WC, but there was no difference between them.44 In a study comparing adoption of a vegan diet with adoption of a Korean diabetic diet,45 those in the vegan group had a significant decrease in BMI and WC, but those in the Korean diabetic group only had a significant decrease in WC, compared with the control (unchanged diet).
The outcomes for fat-limited diets and other diets recommended as being heart healthy were also compared with vegan diets. In one study, a low-fat vegan diet was associated with greater weight loss over time compared with an omnivore healthy diet. The two groups had no caloric restrictions overall, although the prepared vegan meals were lower in calories than the meals of the omnivore healthy diet control group.46 In a study comparing a vegan diet with a diet following the National Cholesterol Education Program (NCEP) guidelines, it was reported that those in the vegan group had significantly greater decrease in weight, BMI, and WC.47 In continued follow-up of this study population, the vegan group’s weight loss was still greater than that of the NCEP control group after 1 year and also after 2 years.48 In addition, this follow-up examined the effects of offering a group support meeting every 2 weeks to those who enrolled later in the study. In both dietary groups, those who were offered the support meetings lost more weight compared with those not offered the same support system.
In a small pilot RCT of women with polycystic ovary syndrome, a low-fat vegan diet resulted in greater weight loss compared with a traditional low-calorie diet at 3 months, even though the vegan diet did not restrict calories. Both groups had a high attrition rate, and no difference was reported at 6 months.49
Two studies compared different types of plant-based diets. In a study comparing a low-carbohydrate vegan diet with a high-carbohydrate LOV diet, those in the vegan diet lost more weight and had a greater decrease in BMI.50 Another study compared a vegan diet with an omnivorous diet and multiple other plant-based diets (vegetarian, pesco-vegetarian, and semi-vegetarian).51 After 6 months, the weight loss of participants on the vegan diet was significantly greater than that of participants on the omnivorous diet, the semi-vegetarian diet, or the pesco-vegetarian diet.
Vegetarian
Weight/Obesity RCTS
The PREFER RCT compared the outcomes for two diets: a vegetarian diet, vs standard calorie & fat restricted diet. There were two arms for each of the 2 diets: choice of diet picked by patient vs diet type assigned, for a total of 4 study arms. In that study, the participants of all groups lost a significant amount of weight, but no difference in weight loss were found between the two diets taken as a whole (with the choice and assigned arms combined). Interestingly, however, those who were not allowed to choose their diet lost significantly more weight when compared with those who were given a choice.52
Two studies compared vegetarian diets with usual care control groups. In one study, participants on a LOV diet lost significantly more weight than those in the control group with no intervention.53 A significant decrease in skinfold thickness indicated that this difference was at least partially due to a difference in fat loss, but the participants on the LOV diet also lost a significant amount of muscle. In the Lifestyle Hearts Trial, a lifestyle intervention consisting of a 10% fat vegetarian diet, exercise, stress management, and smoking cessation resulted in a significant and sustained weight loss over 5 years.54
Kahleova et al compared the effects of a vegetarian diet and an isocaloric diabetic diet on weight, as well as on the distribution of adipose tissue. The vegetarian diet was associated with significantly greater weight loss and decrease in WC. In addition, MRI measurements that assessed changes in the fat and muscle volume showed a significantly greater decrease in abdominal subcutaneous and visceral fat in participants on the vegetarian diet55; imaging of the thigh muscles of participants showed that this group’s volume of adipose tissue had significantly decreased in multiple areas: subfascial, subcutaneous, and intramuscular. They also had a significant decrease in muscle volume, which was partially reversed after an exercise program was started.56
In a study comparing the outcomes for participants on vegetarian and Mediterranean diets, the vegetarian group lost a significant amount of weight and fat, but the changes were not significantly different from the Mediterranean diet group.57
Weight/Obesity non-RCTs
In 3 pre/post interventional trials, a vegetarian diet was shown to cause significant weight loss in as little as 3 weeks, which was sustained over a period of 2 years.58–60 A case–control matched study also demonstrated that a vegetarian diet resulted in significant weight loss.61
Plant-based whole foods
PBWFs RCTs
Two studies used a PBWFs diet in both treatment groups.62,63 One study showed that decreasing total dietary fat from 20% to 15% (based on serum cholesterol response) did not result in further weight loss. There was significant weight loss overall.62 In the other RCT,63 a PBWFs diet with and without a support system was compared. In that study, diet had no effect on BMI, WC, or hip circumference, and a support system only had an effect on hip circumference.
In two RCTs, the outcomes for a PBWFs diet group and a conventional diet control group were compared; in both, the PBWFs group lost significantly more weight than the group on the conventional diet. When the outcomes for a PBWFs diet group and an AHA diet group were compared in a small study, both groups were found to have had significant weight loss, but there was no significant difference between the two groups.64
PBWFs non-RCTs
Multiple single-arm intervention studies have shown that a PBWFs diet intervention results in significant weight loss.65–70
Diabetes
Of 21 interventional studies that scrutinized the relationships between diabetes and plant-predominant diets, 17 were RCTs and 4 were non-randomized interventional trials.
Vegan: Participants in RCTs have shown favorable declines in A1C on a vegan diet compared with those on a conventional ADA diet, when controlling for medications,43–45 with greater compliance being associated with more marked decline.45 A small RCT comparing outcomes for participants on a low-fat vegan diet with those for participants on the NCEP diet showed that both diets improved the glycemic control indices; there was a trend towards improved fasting glucose in the low-fat vegan group, but no other significant differences were observed in glycemic control markers. An 18-week intervention using a low-fat vegan diet statistically and clinically improved the glycemic control of diabetics compared with their usual diet, in a corporate work environment.42 A small pilot study comparing outcomes for DM patients on a low-fat vegan diet with those for DM patients on a relatively healthy conventional diet found that the former included statistically significant reductions in serum glucose (note, the diets were not isocaloric, and exercise was not recommended with either diet).46 Shah et al71 did not find differences in glycemic control between a vegan group and an AHA diet group in coronary artery disease (CAD) patients, approximately 30% of whom had DM. Similarly, Sucher et al72 showed in a 6-week RCT that diets supplemented with high plant vs high animal protein had similar glycemic control.
Vegetarian: A RCT of a calorie-restricted vegetarian diet increased insulin sensitivity more than an isocaloric conventional diabetic diet in patients with DM2 over a period of 6 months.55 The advantageous effects of a vegetarian diet may be partly explained by the association with weight loss, especially loss of visceral fat, and a consequent increase in insulin sensitivity. An RCT of a LOV diet vs a standard low-calorie, low-fat diet showed reduction in fasting insulin levels, but no significant effects on blood glucose levels.52 A RCT testing a low-carbohydrate vegan (“Eco-Atkins”) diet vs a high-carbohydrate LOV diet found similar reductions in glycemic indices between diets.50
PBWFs: A 16-week RCT demonstrated superior outcomes for a high-carbohydrate, low-fat vegan diet compared with a control “usual” diet in terms of reduction in weight and visceral fat volume, and increase in insulin sensitivity; higher consumption of high-quality carbohydrates and dietary fiber was correlated with these improvements.41 Plant dietary protein was also found to be correlated with reductions in body weight and visceral fat volume, and increase in insulin sensitivity in this study population.73 Importantly, a PBWFs diet has been shown to be associated with a statistical and clinically significant decrease in A1C in medically underserved areas.63 This underscores the importance that a culturally appropriate lifestyle intervention may have on vulnerable populations. A RCT testing a non-energy-restricted PBWFs diet vs usual care showed a statistically and clinically significant decrease in A1C over a period of 1 year.74 This demonstrates that, even without a formal exercise regimen or focus on reduction in calories, a PBWFs diet may improve diabetes management. Non-randomized trials also have shown a benefit in markers of glycemic control,60,66,67 although evidence was more mixed in another study.59
Lipids/hyperlipidemia
Twenty-nine interventional studies included data on the effect of plant-predominant diets on lipids; of these, 16 were RCTs and 13 were non-randomized intervention studies. The majority of studies showed a statistically significant association between plant-predominant diets with decreased lipids, compared with various controls, such as usual diet, or other health-oriented diets (eg, AHA, ADA, NCEP diets). These studies included several RCTs and pre/post diet interventions in which the patients served as their own controls (ie, there was a quasi-experimental design), as follows:
Vegan. Two related RCTs in the same population by Barnard et al,43,44 treating DM2 with a low-fat vegan diet compared with a standard ADA diet, demonstrated more reduction in some lipids in the vegan group in diabetics on stable lipid medication doses. In another study, the low-carbohydrate vegan (“Eco-Atkins”) diet lowered most lipids more than did the comparison LOV diet.50 A multicenter RCT testing a low-fat vegan diet (in a corporate health intervention setting) lowered TC and LDLs, moderately raised TGs, and slightly lowered HDLs compared with usual diet.42 The results of a 12-week RCT intervention with a brown rice–based vegan diet showed no difference in lipid levels compared with a standard Korean diabetic diet.45 A small pilot study testing a low-fat vegan diet vs a health-promoting conventional diet in DM patients was underpowered to find differences in lipid levels, but did note a decrease in HDLs.46 Shah et al71 did not note a significant change in lipid levels compared with an AHA diet, but did show improvement in high sensitivity C-reactive protein. Lindahl et al,40 in a pre/post vegan diet study, noted improved lipid levels.
Vegetarian. Vegetarian diets also decreased TC55 and LDL35,55,57,59,75 in interventional trials, including when compared with the ADA diet in some studies. Burke et al52 demonstrated a vegetarian diet being associated with lower LDLs at interim times, but not at the end of the study, compared with a calorie- and fat-restricted omnivore diet. Ornish et al54 demonstrated lower LDLs without medications over a 5-year intensive lifestyle program that included a low-fat vegetarian diet in CAD patients, reaching levels similar to those of controls, 60% of whom took lipid-lowering medications ; TGs and HDLs did not differ. The randomized crossover study by Sofi et al57 compared a Mediterranean diet vs LOV diet and found both significantly decreased TC. The Mediterranean diet lowered TGs more and the LOV diet lowered LDLs when comparing the two diets. Mild decreases in HDL were found in a few studies.59,61 One study compared a vegetarian diet including soy, a vegetarian diet including casein supplementation, and a control group, with no significant changes in LDL, HDL , TGs, or TC being reported.76 A number of pre/post vegetarian diet intervention studies have shown improvement in lipid levels.58,60
PBWFs. Two RCTs showed low-fat PBWFs diets decreased TC and LDL, and there was often some lowering of HDL, compared with conventional77 or AHA diets.64 Decreases in TG levels were found in one study.62 The intensive community-based Coronary Health Improvement Project lifestyle program, highlighting a PBWFs diet, showed reduced CVD risk factors, including improved lipid levels.67 In adherent patients, Wright et al74 noted lower TC in patients on PBWFs diet compared to conventional diet at 3 and 6 months, with lower HDL, with some confounding due to medication adjustments that would bias against the treatment effect. Koebnick et al, in a double-blind RCT, noted that decreasing total dietary fat from 20% to 15% (based on initial cholesterol response) did not further lower cholesterol levels.62 A number of pre/post PBWFs diet intervention studies have shown improvement in lipid levels, with most also slightly lowering HDLs.65–70
Cardiovascular disease/HTN
Twenty-eight interventional trials assessed the effect of plant-predominant diets on HTN and CVD; 14 were RCTs and 14 were non-randomized interventional trials.
Vegan. Barnard et al,43,44 in 2 related RCTs in the same population, noted that vegan and ADA diets lowered BP similarly. Medication adjustments may have confounded the findings. Jenkins et al50 noted similar BP lowering in study participants on a low-carbohydrate vegan (“Eco-Atkins”) and in those on a high-carbohydrate LOV diet. The RCT by Lee et al45 noted similar BPs in patients with DM2 on vegan and Korean ADA diets. Mishra et al’s42 RCT noted similar BP lowering in overweight and/or DM patients on a low-fat vegan diet compared with those on usual diet, as did Nicholson et al’s46 small pilot RCT of diabetic subjects placed on a low-fat vegan vs a healthy mixed diet. Lindahl et al’s40 non-RCT demonstrated reduced BP in hypertensive Swedes treated with a vegan diet. The RCT conducted by Shah et al71 testing a vegan diet noted a 32% lower concentration of high-sensitivity C-reactive protein in CAD patients compared with those on an AHA diet. In a pre/post low-fat, raw vegan diet intervention case series of 3 congestive heart failure patients, cardiac MRI showed a significant average improvement in cardiac output of 17%, stroke volume of 62%, increase in ejection fraction of 92%, and decrease in left ventricle mass of 21%.78 Higher-level studies are needed to confirm these findings.
Vegetarian. The RCT by Margetts et al79 demonstrated that a LOV diet lowered BP in Australians with mild, untreated HTN compared with usual diet. Rouse et al’s80 RCT in normotensive subjects demonstrated that a LOV diet lowered BP compared with usual diet. Not surprisingly, most trials that did not focus on treating HTN patients showed either stable BP or lowering similar to that of various other healthy diet comparators.53,54,63,74
The RCT by Ornish et al54 demonstrated that an intensive lifestyle program featuring a vegetarian diet improved CVD parameters and outcomes, with evidence of reversal of CAD. Non-randomized interventional trials involving intensive lifestyle change featuring a vegetarian diet also demonstrated reduced CVD risk markers or improved outcomes.61 Artzenuis et al58 treated stable angina patients with a pre/post vegetarian diet for 2 years and noted decreased systolic blood pressure (SBP) and halting of coronary lesions on angiograms in 18 of 39 patients whose TC/HDL was <6.9.
PBWFs. Macknin et al’s64 RCT noted similar BPs in participants on PBWFs and AHA diets. A PBWFs diet was associated with a significant decrease in CVD risk markers and/or adverse outcomes (stroke, MI, angina).61,64–69,81 BP decreases were noted when compared with conventional, low-carbohydrate or animal-based protein diets.66,68,70 A PBWFs diet–based lifestyle intervention demonstrated an improved ejection fraction in CAD patients at risk for heart failure.70
DISCUSSION
This review focuses on overall eating patterns as being more relevant than reductionistic, single-nutrient or even nutrient-category studies because of the irreducible complexity of nutrient interactions. Eating patterns also inextricably interact with exercise, stress level, mood, and cultural forces, and the conclusions here need to be placed in the setting of a total healthy lifestyle. Because of their inherent complexity, it is notoriously difficult to study nutritional outcomes with traditional scientific rigor, especially in free-living community subjects. Most RCTs had only partial blinding. Quasi-experimental (non-randomized) designs have an inherently higher risk of bias. The focus in this paper was on studies that did not demonstrate severe bias that would materially alter their conclusions. Despite these limitations, the great majority of the literature as noted in this systematic review gives a consistent message and suggests the following with reasonable certainty:
Plant-predominant diets are successful and often superior to standard health-oriented diets (such as AHA- and ADA-guided diets) in helping patients restore/maintain healthier weight and reduce cardiometabolic risk factors. They share core recommendations with the major associations’ guidelines of eating more whole foods, especially vegetables and fruit, and less processed food.
Plant-predominant diets, in the setting of a comprehensive and sustained lifestyle intervention and support, have potentially stabilized and even reversed coronary heart disease and DM2 in preliminary studies. Further high-quality RCTs are needed to confirm and expand upon this clinically important finding.
While there are differences in outcomes between the 3 types of diets reviewed (vegan, vegetarian, and PBWFs), these differences were in general less important than the differences between plant-predominant diets as a group and Western or conventional health-oriented diets, and there is some overlap in how authors define these plant-predominant diets. Bernard noted that the acceptability of a plant-predominant diet, namely a low-fat vegan diet, was similar to that of an ADA-guided health-oriented diet.44 While they limit animal products, well-balanced plant-predominant diets allow more liberal food intake, due to their low caloric density, and generally do not require portion control and calorie counting, which many patients find unsustainable.
This review had several strengths: the search strategy was designed a priori. An extensive literature search of multiple databases and manual searching of key recent articles increased the likelihood that major findings were represented. Differences regarding the inclusion and analysis of articles were resolved by consensus. Accuracy of results was checked by multiple authors.
This review also has several limitations. The medical literature reviewed was confined to the English language. The non-randomized studies cited have inherently higher risk of bias. Large RCTs, especially those with blinding, are relatively scarce, and there are few head-to-head trials testing the relative efficacy of common health-oriented diets. A formal publication bias assessment was not conducted. The results presented here are based on information in the journal articles themselves; there was not a systematic research of other sources of data, such as unpublished original study protocols or “grey literature”.
Theoretical considerations and opportunities for future research regarding plant-predominant diets include the following:
Human evolutionary experience is largely with omnivorous diets; however, the quality of wild animal meats/products is very different than that of “factory-farmed” livestock and other animal products, which are often abnormally high in total and saturated fat. The amount of animal products, if any, that is compatible with health, is unknown, as is the level of nutritional quality, especially the fatty acid profile, that would support health.
The health role of long-chain omega-3 fatty acids such as eicosapentaenoic acid and docosahexaenoic acid, which are decreased in exclusively plant-based diets, needs clarification, as does the role of the omega-3/omega-6 fatty acid balance.
Low-fat plant-predominant diets that lower CVD end points often also lower the surrogate marker HDLs, and concerns regarding the clinical significance of this have been raised. Lower HDLs may only be a risk factor for CVD in the setting of high TC and LDLs typical of Western diets and lifestyles. In the setting of very-low-fat plant-predominant diets, it may simply reflect less need for transport of lipids away from the periphery. It may be inappropriate to conclude that diet-induced decreases in HDLs are equivalent to low HDLs within a given established diet.82,83
Scientific validation of the habituating and even outright addictive qualities of many highly processed foods is ongoing but underappreciated. The public health consequences of heavily advertised and available empty-calorie nutrient-poor processed foods are likely profound but understudied. There is a need to better understand factors that increase susceptibility to processed food habituation, and how it alters brain and enteric nervous system functions and behavior. Anyone who has experienced overeating highly processed snack foods such as potato chips intuitively understands their habituating potential. More work is needed to understand how advertising of and habituation to processed foods contributes to the current culture of poor diet and the resultant obesity and chronic disease epidemics.
Head-to-head RCTs with plant-predominant vs other health-oriented diets such as Mediterranean and DASH diets would clarify their respective health effects.
Further research is also needed on individualization of healthy diets to fit different genetic, epigenetic, and phenotypical subtypes.
Despite the areas of potential research listed above, some practical clinical guidance in treating obesity and its cardiometabolic sequelae can be made with a reasonable level of confidence:
Advise plant-predominant diets that focus mainly on whole foods. If patients decide to have some animal products, choose small amounts of high-quality forms such as cold-water fish or low-fat dairy.
Eliminate or at least minimize processed foods such as:
any food made of white flour (white bread, bagels, pastries, many commercial breakfast cereals, etc.)
junk/snack food with manipulated fat, salt, and sugar (such as chips and many children’s breakfast cereals)
conventional fast food, which is generally of poor nutritional quality. For patients who want to continue to eat fast food, consider newer healthy fast-food chains instead85
beverages with added sugars such as soda, energy, and sports drinks, and commercial juice drinks and ice teas
poor-quality animal products (such as factory-farmed beef and chicken, processed meats).
All motivated patients with DM2 and CVD should be informed of the potential reversibility or at least halting of progression of these illnesses with comprehensive lifestyle modification that includes plant-predominant eating patterns. More of these programs need to be made available to motivated patients, and insurance should cover their cost to reduce financial barriers.
While there are real differences in the recommendations from various organizations, and these need to be resolved by further research, most share the same core recommendation of eating more whole plant foods. Unfortunately, most Americans do not follow any of the established guidelines well. They eat too much empty-calorie nutrient-poor processed food, including excess poor-quality animal products. This, along with other lifestyle issues, such as sedentary habits and high stress, is likely the main driver of obesity and chronic disease. Educating patients on healthy nutrition, including plant-predominant diets that emphasize whole foods, is a necessary (but insufficient) first step and should never be viewed by itself as adequate to overcome the complex barriers needed to improve lifestyle. Long-term success in healthy lifestyle change likely requires ongoing support and guidance, eg, through group visits, health and nutrition classes, and other support programs.
CONCLUSIONS
Taken as a whole, the evidence suggests that plant-predominant eating patterns, especially those that emphasize whole foods and that are in settings that offer ongoing support for lifestyle modification, effectively treat obesity and related cardiometabolic health outcomes. They appear to be at least as effective and sometimes superior to standard recommended healthy eating patterns. Preliminary evidence suggests they may stabilize and even reverse DM2 and CAD. Higher-level RCTs are needed to confirm and expand on this important clinical finding.
Acknowledgments
Author contributions. A.R. oversaw the conceptualization and completion of this project. S.N.D.T. organized the search strategy, collated the articles, cited the references, and constructed the evidence tables. All researchers, in particular A.A.M., L.S., and T.W., participated in scanning and analysis of the articles (including risk of bias assessment), and writing of the main text. The researchers would like to acknowledge the generous support of St. Luke’s University Health System, especially Dr. Stan Stawicki and the Research Department, and Program directors Dr. Thomas McKinley and Dr. Nandhini Veeraraghavan. We are indebted to Dr. Kushee-Nidhi Kumar, Bradley Susskind, and Eman Shahzad for their diligent work in preparing the final manuscript. Finally, we would like to honor our families for their patience and good humor during the many late nights and weekends spent working on this project.
Funding. No external funding was received to support this work.
Declarations of interest. The authors have no relevant interests to declare.
Supporting information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Supplementary Table S1. PICOS criteria
Supplementary Table S2. Randomized control trials analyzed for risk-of-bias/conflict of interest
Supplementary Table S3. Non-randomized intervention analyzed using Joanne Briggs Institute appraisal checklist
Supplementary Appendix S1. PRISMA checklistS1
Supplementary Appendix S2. PRISMA checklist for abstractS1
Reference S1: Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed). 2021;372:n71. https://doi.org/10.1136/bmj.n71.
REFERENCES
Center for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) [updated March 25,
Center for Disease Control and Prevention. Adult Obesity Causes & Consequences [updated February 4, 2020]. Available at: https://www.cdc.gov/obesity/adult/causes.html. Accessed February 4, 2020.
et al.