-
PDF
- Split View
-
Views
-
Cite
Cite
Rebecca Wall, R Paul Ross, Gerald F Fitzgerald, Catherine Stanton, Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids, Nutrition Reviews, Volume 68, Issue 5, 1 May 2010, Pages 280–289, https://doi.org/10.1111/j.1753-4887.2010.00287.x
Close - Share Icon Share
Abstract
Omega-6 (n-6) and omega-3 (n-3) polyunsaturated fatty acids (PUFA) are precursors of potent lipid mediators, termed eicosanoids, which play an important role in the regulation of inflammation. Eicosanoids derived from n-6 PUFAs (e.g., arachidonic acid) have proinflammatory and immunoactive functions, whereas eicosanoids derived from n-3 PUFAs [e.g., eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] have anti-inflammatory properties, traditionally attributed to their ability to inhibit the formation of n-6 PUFA-derived eicosanoids. While the typical Western diet has a much greater ratio of n-6 PUFAs compared with n-3 PUFAs, research has shown that by increasing the ratio of n-3 to n-6 fatty acids in the diet, and consequently favoring the production of EPA in the body, or by increasing the dietary intake of EPA and DHA through consumption of fatty fish or fish-oil supplements, reductions may be achieved in the incidence of many chronic diseases that involve inflammatory processes; most notably, these include cardiovascular diseases, inflammatory bowel disease (IBD), cancer, and rheumatoid arthritis, but psychiatric and neurodegenerative illnesses are other examples.
INTRODUCTION
Polyunsaturated fatty acids (PUFAs) contain two or more double bonds that are classified as omega-3 (n-3) and omega-6 (n-6) based on the location of the last double bond relative to the terminal methyl end of the molecule. PUFA constitute an important component of all cell membranes and influence membrane fluidity and the behavior of membrane-bound enzymes and receptors. PUFAs regulate a wide range of functions in the body, including blood pressure, blood clotting, and correct development and functioning of the brain and nervous systems.1 Furthermore, PUFAs have a role in regulating inflammatory responses through the production of inflammatory mediators termed eicosanoids.1,2 The human body can produce all but two of the fatty acids it requires. Thus, linoleic acid (LA, C18:2n-6) (precursor of the n-6 series of fatty acids) and α-linolenic acid (ALA, C18:3n-3) (precursor of the n-3 series of fatty acids) are essential in the human diet; a dietary (n-6):(n-3) ratio of 4:1 is recommended as optimal. However, actual dietary intakes of these fatty acids are in excess of 15–16:1, particularly in Western countries, due to increased consumption of LA-rich vegetable oils.3 As an example, in Europe, consumption of LA has increased by 50% during the last two decades.4 In parallel with increased LA intakes, increased rates of many diseases that involve inflammatory processes have occurred, most notably cardiovascular diseases, inflammatory diseases, obesity, cancer, and certain psychiatric diseases such as depression.5 This increasing inflammatory disease incidence is associated with excessive production of the proinflammatory eicosanoids, prostaglandin E2 (PGE2) and leukotriene B4 (LTB4), which are derived from the n-6 fatty acid arachidonic acid (C20:4n-6) that is maintained at high cellular concentrations by the high n-6 and low n-3 PUFA content of the modern Western diet.6 However, this imbalance can be corrected by dietary supplementation with n-3 fatty acids such as EPA (C20:5n-3) and DHA (C22:6n-3) or by eating fish rich in EPA and DHA. EPA and DHA in the diet partially replace arachidonic acid as an eicosanoid substrate in cell membranes; this probably occurs in all cells, but it is especially true in the membranes of erythrocytes, neutrophils, monocytes, and liver cells and thus suppresses the production of n-6 proinflammatory eicosanoids.7 Marine fish are the principal sources of EPA and DHA.8 However, the content of marine n-3 fatty acids varies greatly according to the species of fish. For example, fish such as salmon, trout, and herring are higher in EPA and DHA than others (e.g., cod, haddock, and catfish). It is acknowledged that EPA and DHA can prevent the development of inflammatory diseases by directly or indirectly affecting different stages of the immune response. In addition, EPA and DHA can alleviate inflammatory processes that already exist, thus highlighting the therapeutic importance of these fatty acids.
METABOLISM OF OMEGA-6 AND OMEGA-3 POLYUNSATURATED FATTY ACIDS IN HUMANS
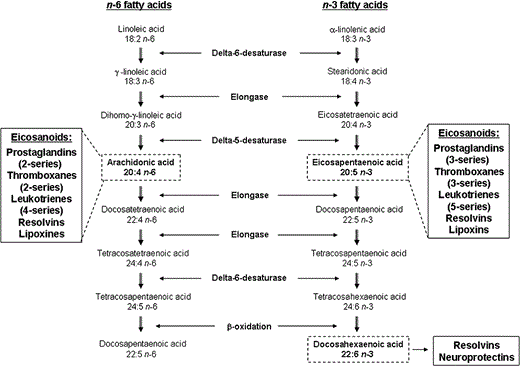
The main dietary sources of LA include plant oils, such as sunflower, safflower, and corn oils, cereals, animal fat, and wholegrain bread, while ALA is abundant in green leafy vegetables, flaxseed, and rapeseed oils. Although mammalian cells cannot synthesize LA and ALA, they can metabolize them into more physiologically active compounds by the introduction of further double bonds (desaturation) via Δ5 and Δ6 desaturases and by lengthening the acyl chain (elongation) via elongases. Consequently, LA is converted into γ-linolenic acid (C18:3n-6) via the action of Δ6 desaturase, and γ-linolenic acid is elongated via elongase to dihomo-γ-linolenic acid (C20:3n-6). Dihomo-γ-linolenic acid is further converted to arachidonic acid (C20:4n-6) via the action of Δ5 desaturase. Arachidonic acid is either metabolized to docosatetraenoic acid (C22:4n-6) via elongase or to eicosanoids via cyclooxygenase (COX) and lipoxygenase (LOX) (Figure 1).
Using the same series of enzymes as those used to metabolize n-6 PUFAs (elongases, Δ5, and Δ6 desaturases), ALA is converted to stearidonic acid (C18:4n-3) by Δ6 desaturase; stearidonic acid is then elongated to eicosatetraenoic acid (C20:4n-3), which is converted to EPA via Δ5 desaturase. EPA is either metabolized to DHA or to eicosanoids via COX and LOX. Conversion of EPA into DHA involves the following processes: the addition of two carbons via elongase to form docosapentaenoic acid (C22:5n-3), the addition of two more carbons via elongase to produce tetracosapentaenoic acid (C24:5n-3), desaturation using Δ6 desaturase to form tetracosahexaenoic acid (C24:6n-3), and removal of two carbons by limited β-oxidation to yield DHA (C22:6n-3)9 (Figure 1). In mammals, the pathway of desaturation and elongation of n-6 and n-3 fatty acids occurs mainly in the liver. Since LA and ALA are metabolized by the same set of enzymes, competition exists between these two fatty acids, with an excess of one causing a decrease in the metabolism of the other. Normally, Δ5 and Δ6 desaturases and elongases exhibit affinity to metabolize n-3 over n-6 PUFAs, provided that both exist in a physiological ratio of 1:1–4.1,3 However, the higher concentrations of LA typically found in the Western diet results in a greater conversion of LA to arachidonic acid.10,11 Nonetheless, increasing ALA intake, such that total intake exceeds 4.5 g/day, appears to result in significant enhancements in the EPA content of plasma phospholipids.12,–14 Furthermore, decreasing the LA content of the diet has been shown to result in increased metabolism of ALA to its longer chain derivatives. For example, a recent study showed that decreasing LA in the diet to an optimal LA : ALA ratio of 4:1 resulted in higher plasma phospholipid EPA and a 40% lower arachidonic acid : EPA ratio than a diet containing a LA : ALA ratio of 10:1.15 Approximately 8–20% of ALA is converted to EPA in humans, while conversion of ALA to DHA is less and estimated to be around 0.5–9%.16,17 Consequently, this route is unlikely to provide sufficient levels of EPA and DHA for optimal human health, which emphasizes the importance of dietary intake of EPA and DHA. Indeed, dietary recommendations now include not only LA and ALA but also EPA and DHA for optimal nutrition and for improving health outcomes. These recommendations are in the range of 0.5–1.8 g n-3 fatty acids per day (or the consumption of at least two servings of fish per week).18
Production of n-3 PUFAs in marine bacteria and enrichment in fish
PUFAs were once thought to be absent in bacterial membranes,19 but numerous bacterial species of marine origin, such as Shewanella spp. and Photobacterium, have now been shown to produce long-chain n-3 PUFAs such as EPA and DHA. Such isolates inhabit relatively unusual environments including low-temperature deep-sea environments and the intestines of sea fish.20,–22 The enrichment of PUFA-producing strains from these environments has led to speculation that PUFA synthesis is an important adaptation for countering the effects of elevated hydrostatic pressure and low temperature on membrane fluidity. However, it is interesting that the PUFAs detected in these bacteria are mostly EPA and DHA and not C18-PUFAs such as LA and ALA, which are most common in animals, plants, fungi, and cyanobacteria.
In marine bacteria, EPA and DHA are synthesized de novo by polyunsaturated fatty acid synthase pfa genes following the polyketide biosynthesis pathway that forms PUFAs from C2-compounds (possibly acetyl-CoA) rather than by chain elongation and oxygen-dependent desaturation of existing fatty acids.23,–25 Various techniques have recently been presented in order to metabolically enhance the production of EPA and DHA by bacteria that inherently synthesize them26,27 or by host bacteria transformed with pfa genes.28,29 Expressing EPA and DHA biosynthesis gene clusters in various host organisms is an alternative way of providing these beneficial fatty acids, especially since the contamination of fish due to pollution has received much attention in recent years.
The content of marine n-3 fatty acids varies greatly according to the species of fish, the total fat content of the fish, and the geographical location of waters they inhabit.30 However, as a general rule, deep water fish such as tuna, salmon, mackerel, herring, and sardines (“oily” fish) from colder temperatures have the highest content of EPA and DHA (Table 1) since they store lipids in the flesh, whereas lean fish that store lipids in the liver (e.g., cod) contain less EPA and DHA. As an example, one portion of cod provides approximately 0.3 g of EPA and DHA, one portion of salmon provides approximately 1.5 g of EPA and DHA, whereas one portion of mackerel provides approximately 3 g of these fatty acids.31 The oil obtained from the flesh of oily fish or livers of lean fish is rich in EPA and DHA, and one fish oil capsule from these sources consists of approximately 30% of these fatty acids. Thus, consumption of a typical 1 g fish oil capsule provides approximately 300 mg of EPA and DHA, which is equivalent to the consumption of one portion of cod. However, the intake of n-3 fatty acids in the absence of oily fish or fish oil supplements is likely to be <100 mg/day.32,33 Thus, an individual who consumes little or no fish could increase their daily intake of n-3 fatty acids 5-fold (or more) by taking a single standard fish oil capsule per day.34
EPA and DHA content of fish and the amount of dietary fish required to provide approximately 1 g of EPA and DHA per day.
Based on data from the USDA Nutrient Data Laboratory, adapted from Kris-Etherton117. (The intakes of fish provided are rough estimates because oil content can vary markedly depending on species, season, diet, and packaging and cooking methods).
EPA and DHA content of fish and the amount of dietary fish required to provide approximately 1 g of EPA and DHA per day.
Based on data from the USDA Nutrient Data Laboratory, adapted from Kris-Etherton117. (The intakes of fish provided are rough estimates because oil content can vary markedly depending on species, season, diet, and packaging and cooking methods).
POLYUNSATURATED FATTY ACIDS AND EICOSANOID PRODUCTION
Eicosanoids, including prostaglandins (PGs), thromboxanes (TXs), leukotrienes (LTs), and hydroxyeicosatetraenoic acids (HETEs), are derived from 20-carbon PUFAs, mainly arachidonic acid and EPA, and are key mediators and regulators of inflammation.35 These mediators are involved in modulating the intensity and duration of inflammatory responses.35,36 However, the overall physiological outcome of this response depends upon the cells present, the nature of the stimulus, the timing of eicosanoid generation, the concentrations of different eicosanoids generated, and the sensitivity of target cells and tissues to the eicosanoids generated.37 Competition between n-6 and n-3 fatty acids occurs in the production of eicosanoids by COX and LOX enzymes. Since inflammatory cells typically contain a high proportion of arachidonic acid and low proportions of EPA, arachidonic acid is usually the major substrate for eicosanoid synthesis.38,39 Arachidonic acid in the cell membrane is released by phospholipases, most notably phospholipase A2, and the free acid subsequently acts as a substrate for COX and LOX. Metabolism of arachidonic acid by COX gives rise to the 2-series PGs and the 2-series TXs. Monocytes and macrophages produce large amounts of prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α), neutrophils produce moderate amounts of PGE2, and mast cells produce prostaglandin D2 (PGD2). Metabolism of arachidonic acid by the 5-lipoxygenase (5-LOX) pathway gives rise to hydroxy and hydroperoxy derivatives, such as 5-HETE and 5-hydroperoxyeicosatetraenoic acid (5-HPETE), and the 4-series LTs; leukotriene A4 (LTA4), leukotriene B4 (LTB4), leukotriene C4 (LTC4), leukotriene D4 (LTD4), and leukotriene E4 (LTE4). Neutrophils, monocytes and macrophages produce LTB4, while LTC4, LTD4, and LTE4 are produced by mast cells, basophils, and eosinophils. The eicosanoids derived from arachidonic acid are generally ascribed to be proinflammatory.40 For example, PGE2 induces production of the proinflammatory cytokine interleukin (IL)-6 (IL-6) in macrophages and causes pain and vasodilation.35,40 LTB4 is a potent chemotactic agent for leukocytes and an activator of neutrophils. It also leads to the production of inflammatory cytokines such as tumor necrosis factor alpha (TNFα), IL-1 beta (IL-1β), and IL-6 by macrophages.35 Although the arachidonic acid-derived eicosanoids are generally categorized as proinflammatory mediators, they play an important modulatory role in the immune response through complex interactions with leukocytes; they also have a crucial role in the early phase of inflammation.35,39 However, in excessive concentrations, they can cause damage to host tissues and contribute to the formation of thrombi and the development of inflammatory disorders.7 Indeed, a common characteristic of chronic inflammatory diseases such as inflammatory bowel disease (IBD) and rheumatoid arthritis is excessive production of arachidonic acid-derived eicosanoids.38,–42
EPA acts as a substrate for COX and LOX enzymes and gives rise to a different family of eicosanoids, the 3-series PGs and TXs, the 5-series LTs, and the hydroxy-EPAs. The eicosanoids derived from EPA are considered to be less inflammatory or even anti-inflammatory, compared to eicosanoids derived from arachidonic acid.40,43 The best example of differential inflammatory potencies of eicosanoids produced from arachidonic acid and EPA is that of LTB4 versus LTB5. LTB5, derived from EPA, is 10- to 100-fold less potent as a neutrophil chemotactic agent than LTB4, derived from arachidonic acid, and thus a much weaker inducer of inflammation.44,45 Moreover, Bagga et al. 40 demonstrated that PGE3 was a less potent inducer of IL-6 production by macrophages than PGE2.
DHA can be metabolized to resolvins via LOX-initiated mechanisms.46 Resolvins are endogenous, local-acting mediators possessing potent anti-inflammatory and immunoregulatory properties.47,–49 At the cellular level, these include reducing neutrophil infiltration and regulating the cytokine-chemokine axis and reactive oxygen species, as well as lowering the magnitude of the inflammatory response.47,50 As an example, the DHA-derived resolvin E1 was shown to protect from experimental colitis in animal models.51
Increased consumption of n-3 fatty acids such as EPA and DHA results in increased proportions of these fatty acids in inflammatory cell phospholipids.52,53 The incorporation of EPA and DHA into human inflammatory cells occurs in a dose-response fashion54,55 and partly at the expense of arachidonic acid. Indeed, consumption of fish oil has been reported to increase the concentrations of EPA and DHA in inflammatory cells53,–56 and subsequently decrease the production of arachidonic acid-derived eicosanoids, such as PGE2,55,–58 thromboxane B2,57 LTB4,45,59 and LTE4.60
n-3 PUFAs and inflammatory gene expression
Another key anti-inflammatory effect of n-3 PUFAs is mediated at the level of altered inflammatory gene expression through their effects on transcription factors such as nuclear factor kappa B (NFκB) and peroxisome proliferator-activated receptors (PPARs).2,61 NFκB is a transcription factor that plays an important role in various inflammatory signaling pathways. It controls several cytokines (e.g., IL-1, IL-2, IL-6, IL-12, TNF-α), chemokines (e.g., IL-8, monocyte chemoattractant protein-1), adhesion molecules (intercellular adhesion molecule, vascular cell adhesion molecule, and E-selectin), and inducible effector enzymes (e.g., inducible nitric oxide synthase and COX-2).62 EPA has been shown to block the activity of NFκB through decreased degradation of the inhibitory subunit of NFκB, IκB, in cultured pancreatic cells and human monocytes.63,–65 This has also been supported by the finding that transgenic mice that endogenously biosynthesize n-3 PUFAs from n-6 PUFAs are protected from colitis through a decrease in NFκB activity.66 PPARs (α, β/δ, and γ) are ligand-activated nuclear transcription factors that play important roles in cellular differentiation, cancer, inflammation, insulin sensitization, atherosclerosis, and several metabolic diseases.67 Ligands for PPARs are PUFAs, especially those of the n-3 family and their eicosanoid derivatives.68 When activated, PPARs bind to the PPAR-response element and repress or induce the transcription of target genes. PPARs have been shown to inhibit NFκB and therefore play an important role in several inflammatory processes.69,70 As an example, both EPA and DHA downregulate lipopolysaccharide-induced activation of NFκB via a PPAR-γ-dependent pathway in human kidney-2 cells.71
OMEGA-3 PUFAS AND CHRONIC INFLAMMATORY DISORDERS
A large number of clinical investigations with long-chain n-3 PUFAs in chronic inflammatory disorders have been conducted, particularly in patients with rheumatoid arthritis (reviewed in Calder72) and IBD (reviewed in Calder37). In these studies, n-3 PUFAs have been demonstrated to exhibit immunomodulatory effects by changing the profiles of the eicosanoids produced and decreasing the levels of proinflammatory cytokines, via both lipid-mediator-related and nonlipid-mediator-related mechanisms.2,–73 The increased incidence of IBD in humans correlates with an increased dietary intake of n-6 fatty acids.74,75 Thus, increasing the ratio of n-3 to n-6 fatty acids in the diet may be of therapeutic importance for IBD. Indeed, it has been reported that n-3 PUFAs are incorporated into the gut mucosal tissue of patients with IBD who supplement their diet with fish oil, subsequently resulting in an anti-inflammatory effect with decreased production of LTB4 by neutrophils and colonic mucosa76,77 and decreased production of PGE2 and IFN-γ by blood mononuclear cells.78 A recent study using IL-10 knockout mice (mice that spontaneously develop colitis) demonstrated significantly reduced colonic inflammation of mice that were fed fish oil compared with mice that were fed n-6 PUFA-rich corn oil.79 Furthermore, in a 1-year study, patients with Crohn's disease in remission were randomized to receive either placebo or 2.7 g of n-3 PUFAs (administered as fish oil capsules) per day. The primary outcome of the study was relapse. After 12 months, there was a significant difference in the proportion of patients who relapsed over this time: 11/39 (28%) in the fish-oil group versus 27/39 (69%) in the placebo group. There was also a significant difference in the proportion of patients who remained in remission at 12 months: 59% in the fish-oil group compared to 26% in the placebo group.80 Ferrucci et al. 81 examined the relationship between relative concentrations of fatty acids in fasting plasma and levels of inflammatory markers in 1,123 persons aged 20–98 years. The total concentrations of n-3 PUFAs were associated with lower levels of proinflammatory markers (IL-6, TNF-α, C-reactive protein) and higher concentrations of anti-inflammatory markers (IL-10, transforming growth factor-β). Thus, the authors concluded that n-3 fatty acids are beneficial in patients affected by diseases characterized by active inflammation.81
Uncontrolled inflammation also governs the pathogenesis of many prevalent diseases, such as cardiovascular disease, cancer, obesity, and Alzheimer's disease.82 For example, there is a strong association between systemic inflammation and coronary artery disease. This association is thought to be casual, i.e., inflammation increases the risk of the disease, rather than simply marking the presence of atherosclerosis, which is an inflammatory process.83 In the last decade it has been increasingly recognized that n-3 PUFAs can modulate the mechanisms of development and progression of atherosclerosis. Early studies in Greenland Eskimos, a population consuming a high-fat diet rich in n-3 PUFAs, demonstrated that the ingestion of EPA and DHA protected them from cardiovascular disease.84 Similarly, the Japanese population eats more fish than North Americans and presents a lower rate of acute myocardial infarction and atherosclerosis.85,86
Since the early observation in the Greenland Eskimos, many supplementation studies have been completed that have demonstrated associations between n-3 PUFAs and decreased risks of cardiovascular disease87,–89 and reduced mortality.90,91 However, some studies have shown no significant associations.92,93 Two major randomized trials have documented the effects of n-3 PUFAs in the prevention of cardiovascular disease.90,94 The GISSI-Prevenzione study randomized 11,323 patients with recent myocardial infarction to n-3 PUFAs (1 g/day), vitamin E (300 mg/day), both, or none for 3.5 years. Treatment with n-3 PUFAs significantly reduced cardiac death by 32% and sudden death by 45%, whereas vitamin E showed no significant benefit.94 In the more recent Japanese JELIS trial, 18,645 patients with hypercholesterolemia were randomized to receive statin alone or statin and highly purified EPA (1.8 g/day). At the end of the 5-year study, it was found that those patients who were randomized to EPA had a 19% significant reduction in major coronary events.90 Furthermore, Matsuzaki et al. 95 examined whether EPA is effective for the secondary prevention of coronary artery disease. Patients with established coronary artery disease were randomly assigned to receive either 1.8 g of EPA in combination with statin or statin alone. After 4.6 years, the incidence of major coronary events was significantly lower in the patients receiving EPA, suggesting that EPA is effective for secondary prevention of coronary artery disease.95
Although there are some inconsistent results between n-3 PUFA intake and cardiovascular health, which may be explained by variations in such factors as dosage, sample size, and follow-up period, the majority of experimental and observational studies have shown that intake of n-3 PUFAs is associated with reduced risk of cardiovascular diseases.96 Numerous organizations and health agencies are therefore recommending consumption of EPA and DHA for general cardiovascular health. It is not yet clear how much n-3 PUFA is required to significantly reduce the risk of cardiovascular diseases or what the optimal dose is for primary prevention. However, the typical recommendation for secondary prevention of myocardial infarction is a minimum intake of 1 g/day.97,98
Alzheimer's disease (AD) is a neurodegenerative disease that commonly affects the elderly. Currently, there is no known cure for AD. However, there is growing interest in the role of diet in the prevention of AD. DHA is one of the major fatty acids in the brain, representing between 12 and 16% of total fatty acids in grey-matter lipids.99 DHA is required for fetal brain development and is held to be critical for appropriate development and intelligence as well as the maintenance of cognitive capacity.100,101 Quite remarkably, the main source of DHA for the brain is diet. A number of animal studies have revealed that the dietary intake of DHA can significantly alter levels of DHA in the brain.102,–104 Two independent prospective studies have shown that lower plasma DHA levels are associated with increased risk of developing AD later in life.105,106 As an example, patients with blood DHA concentrations in the highest quartile had a lower risk of developing dementia compared with the lowest three quartiles during a follow-up period of 9 years.106 This suggests that dietary intake of DHA can alter the risk of developing AD over the long-term and that low blood DHA concentrations might be an important risk factor for AD. Studies have also shown that DHA provides support to learning and memory events in animal models of AD and protection against AD.107,–109 Moreover, several epidemiological studies have shown a protective effect associated with increased fish consumption and intake of unsaturated fats leading to low n-6/n-3 fatty acid ratios.110,–112 For example, van Gelder et al.113 studied the association between fish consumption and cognitive decline in patients aged 70–89 years. Fish consumers exhibited significantly less cognitive decline after 5 years compared with nonconsumers. Thus, n-3 fatty acids, particularly DHA, may have therapeutic utility for the prevention and treatment of AD.
Recent studies have also demonstrated that administration of n-3 fatty acids decreases the length of hospital stay in patients undergoing surgery by modulating the immune response.114 For example, parenteral infusion with a fish oil containing emulsion to patients following major abdominal surgery lead to a rapid incorporation of n-3 fatty acids (EPA and DHA) into plasma phospholipids of cell membranes of leukocytes and platelets and increased EPA : AA and LTB5 : LTB4 ratios.115 Moreover, the administration of n-3 fatty acid-supplemented oil to patients undergoing radical colorectal cancer resection decreased serum IL-6 concentrations and TNF-α concentrations and reduced the hospital stay for these patients.116 Thus, postoperative supplementation of n-3 PUFAs lower the magnitude of inflammatory responses and leads to shorter hospital and intensive care unit stays in surgical patients.
CONCLUSION
Increased consumption of fatty fish or fish oil supplements containing n-3 PUFAs increases the amount of these fatty acids and their metabolites in human immune cells and consequently changes the production of important mediators and regulators of inflammation and immune responses towards an anti-inflammatory profile. Since excessive intake of n-6 PUFAs, which is characteristic of Western diets, could potentiate inflammatory processes and consequently predispose to, or exacerbate, inflammatory diseases, increasing intake of fatty acids that elicit anti-inflammatory effects, such as n-3 PUFAs, could decrease the risk of many chronic diseases like arthritis, diabetes, obesity, inflammation, cancer, and cardiovascular disease as well as improve mental health. Based on the recognized health effects of n-3 PUFAs, recommendations have been made to increase dietary intake of these fatty acids; this can be achieved by increasing consumption of oily fish or by consuming fish oil supplements.
Acknowledgments
Funding
The authors are funded by Alimentary Pharmabiotic Centre through the Science Foundation Ireland (SFI), NUTRMARA, funded by the Marine Institute, Co. Galway, the Irish Ministry for Food and Agriculture, the Higher Education Authority, and the Health Research Board of Ireland.
Declaration of interest
The authors have no relevant interests to declare.
REFERENCES