-
PDF
- Split View
-
Views
-
Cite
Cite
Carmen Mingorance, Rosalia Rodriguez-Rodriguez, Maria Luisa Justo, Maria Dolores Herrera, Maria Alvarez de Sotomayor, Pharmacological effects and clinical applications of propionyl-L-carnitine, Nutrition Reviews, Volume 69, Issue 5, 1 May 2011, Pages 279–290, https://doi.org/10.1111/j.1753-4887.2011.00387.x
Close - Share Icon Share
Abstract
Propionyl-L-carnitine (PLC) is a naturally occurring derivative of carnitine that plays an important role in the metabolism of both carbohydrates and lipids, leading to an increase of ATP generation. PLC, however, is not only a metabolic drug; it is also a potent antiradical agent and thus may protect tissues from oxidative damage. PLC has been demonstrated to exert a protective effect in different models of both cardiac and endothelial dysfunction, to prevent the progression of atherosclerosis, and, more recently, to improve some of the cardiometabolic alterations that frequently accompany insulin resistance. As a result, most of the clinical trials conducted in humans highlight PLC as a potential treatment option in cardiovascular diseases such as peripheral arterial disease, chronic heart failure, or stable angina, especially when type 2 diabetes mellitus or hyperglycemia (i.e., patients on hemodialysis) are also present. The aim of this review is to summarize the pharmacological effects and possible therapeutic applications of PLC, including the most recent findings to date.
INTRODUCTION
L-carnitine is a naturally occurring essential cofactor of fatty acid metabolism that is synthesized endogenously or obtained from dietary sources. Along with L-carnitine, two short carnitine esters, acetyl-L-carnitine (ALC) and propionyl-L-carnitine (PLC), form the endogenous carnitine pools. It is well known that L-carnitine and its analogs are able to improve metabolic function, even under pathological conditions.1 Nevertheless, evidence suggests that the short-chain carnitine esters ALC and, especially, PLC appear to possess significant therapeutic advantages over L-carnitine itself.
ALC, with a structure similar to that of acetylcholine, penetrates the blood-brain barrier better than L-carnitine and is readily converted to carnitine as needed. ALC enhances energy production and protects mitochondria against oxidative stress, demonstrating neuroprotection in Parkinson's disease,2 Alzheimer's dementia,3 and neurotoxicity.4 Additionally, a recent study has demonstrated for the first time that oral ALC supplementation may have clinically relevant antihypertensive effects in patients at increased cardiovascular risk.5 The authors showed that ALC therapy reduced arterial blood pressure by ameliorating insulin resistance and increasing adiponectin bioavailability.
The PLC molecule has proved much more therapeutically beneficial than L-carnitine or ALC, possibly due to the pharmacokinetics of this carnitine ester and its high affinity for skeletal and cardiac muscle.6 Therefore, special attention has been focused on this short-chain carnitine derivative. When PLC is administered exogenously, it is rapidly converted into free L-carnitine and propionyl-coenzyme A (CoA), as a result of its high lipophilia and affinity for the acetylcarnitine transferase.7 In these two forms, PLC plays an important role in the metabolism of both carbohydrates and lipids, leading to an enhancement of ATP efflux. It has also been suggested that PLC may be used directly by cells before being hydrolyzed to propionate-CoA and L-carnitine.6 In fact, PLC has been demonstrated to protect tissues from oxidative damage8 and to stabilize biomembranes by affecting their molecular dynamics and turnover of phospholipids.9,–11
PLC does not enter uniformly into all tissues,6 but it is highly specific for skeletal and cardiac muscle.12 Consequently, most studies of the therapeutic uses of PLC are focused on the prevention and treatment of several cardiovascular diseases. More exactly, PLC has shown efficacy in the palliative treatment of ischemic heart disease, congestive heart failure, hypertrophic heart disease, and peripheral arterial disease,13 as described in the following sections. Interestingly, PLC offers some potentially advantageous properties over L-carnitine; namely, the ability to replenish intermediates of the tricarboxylic acid cycle by the propionyl-CoA moeity,14 a greater affinity for the sarcolemmal carrier,15 peripheral vasodilator activity, a greater positive inotropism, and a more rapid entry into the myocytes.15,16
This review describes the pharmacological effects and the possible therapeutic applications of PLC, including the most recent findings on this promising molecule.
PHARMACOLOGICAL EFFECTS OF PROPIONYL L-CARNITINE
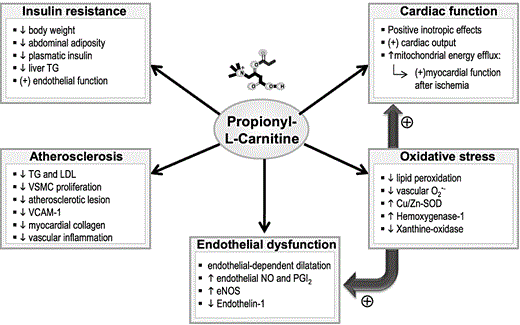
PLC activity has been classically related to the anaplerotic function of providing substrates for energy expenditure in ischemic tissues. Numerous studies have demonstrated the potential benefits of PLC in pathophysiological states, showing prevention of atherosclerosis and endothelial dysfunction and preservation of cardiac function (Figure 1). Nevertheless, the exact mechanisms through which PLC influences these pathological conditions remain largely under investigation.
Pharmacological effects attributed to propionyl-L-carnitine (PLC). Abbreviations: eNOS, endothelial nitric oxide synthase; LDL, low-density lipoprotein; NO, nitric oxide; PGI2, prostacyclin; SOD, superoxide dismutase; TG, triglycerides; VCAM-1, vascular cell adhesion molecule-1; VSMC, vascular smooth muscle cells.
PLC and endothelial dysfunction
The vascular endothelium plays a crucial role in numerous vascular homeostatic functions, including preservation of antithrombotic and antiadhesive properties, prevention of inflammatory processes, and regulation of vascular tone. Upsetting the balance between the production of vasodilator (e.g., nitric oxide [NO]) and vasoconstrictor (e.g., endothelin-1) substances in response to physical or chemical stimuli leads to endothelial dysfunction, which is considered one of the initial steps in atherogenesis.17 Moreover, endothelial dysfunction occurs in physiological processes such as aging18 and in association with cardiovascular risk factors such as arterial hypertension, dyslipidemia, diabetes, and obesity.19,20 This alteration in endothelial cells is characterized by an increased response to specific vasoconstrictor agents and a pronounced attenuation of endothelium-dependent vasorelaxation, mainly due to a decreased bioavailability of NO.19 Substances able to prevent damage to endothelial cells or to restore endothelial function may have important clinical implications. PLC, therefore, is a viable candidate for improving endothelial and vascular function because of its pharmacological profile.
The first evidence of the vasoactive properties of PLC was found in a model of tail thrombosis. More exactly, PLC was able to counteract the vasoconstrictor activity of endothelin-1,21 having an effect similar to that of the vasodilator prostacyclin.22 However, other factors in addition to prostanoids seemed to be involved because inhibition of prostanoid synthesis was unable to attenuate the protective activity of PLC on endothelin-induced thrombosis.23
In fact, recent investigations have shown how PLC promotes endothelium-dependent dilatation in arteries from hypertensive rats. Endothelial NO seemed to be the main mediator of vasodilatation.24 In accordance with these findings, it was also reported that chronic treatment with either L-carnitine or PLC reverses the endothelial dysfunction in spontaneously hypertensive rats (SHR) with no modifications in blood pressure.25 The major mechanism underlying this restoration involves an enhanced endothelial NO bioavailability in PLC-treated rats.25 On the other hand, Cipolla et al.26 demonstrated the participation of prostacyclin, instead of NO, in the vasodilatation induced by PLC in human arteries.
It is well known that an increased production of reactive oxygen species (ROS), particularly superoxide anions (O2-), contributes to endothelial dysfunction and vascular remodeling through oxidative damage and an impaired endothelium-dependent vasodilatation because of a reduction in NO bioavailability.27 In this regard, PLC has been shown to decrease lipid peroxidation and xanthine oxidase activity28,29 and to stimulate the gene expression of some antioxidative markers, such as heme oxygenase-1 and endothelial nitric oxide synthase in human endothelial cells.30 Data have also revealed that the beneficial effect of PLC on hypertension-related endothelial dysfunction is strongly linked to its antioxidant actions, since long-term administration of PLC to SHR decreased arterial production of O2-, which likely involves higher aortic expression of both copper/zinc superoxide dismutase and endothelial nitric oxide synthase.31,32
PLC and atherosclerosis
The initial alteration preceding the atherosclerotic process is endothelial dysfunction. In addition to this, hyperlipidemia and proliferation/migration of vascular smooth muscle cells are considered major events in the development of atherosclerosis. Vascular inflammation also plays a critical role in atherosclerosis, since monocytes and macrophages promote oxidation of low-density lipoprotein, release of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), and expression of adhesion molecules.33 Therefore, every molecule or factor able to reduce the described events is a candidate to attenuate the progression of atherosclerosis. The beneficial effect of PLC on atherogenesis was first reported by Spagnoli et al.34 in aged rabbits with diet-induced hyperlipidemia. They found that long-term oral administration of PLC reduced plasma triglycerides, low-density lipoprotein, and very-low-density lipoprotein and was associated with a decrease in cell proliferation and severity of aortic atherosclerosis. Administration of PLC also reduced carotid intimal hyperplasia after balloon injury in normocholesterolemic rabbits. This effect was related to the enhancement of vascular smooth muscle cell apoptosis and the antiproliferative activity of PLC.35 Orlandi et al.36 recently showed that the mechanisms underlying PLC-induced growth inhibition and increases in apoptosis involve inhibition of NF-κβ activation and regulation of Iκβ-α inhibitory protein expression. In addition, chronic treatment of aged rabbits with PLC notably decreased age-related myocardial interstitial collagen accumulation and reduced expression of vascular cell adhesion molecule 1.37 These findings highlight the role of PLC in the prevention of age-related myocardial interstitial remodeling and lend support for a cardioprotective activity of PLC.
PLC and cardioprotection
Myocardial ischemia occurs in several pathological conditions, such as chronic heart failure (CHF), effort angina, or after myocardial infarct. During and after ischemia, mitochondrial activity and energy utilization capacity are deteriorated.38 Additionally, under these conditions, an important increase in oxidative stress and a decrease in L-carnitine levels in myocardial tissues can be observed. This situation contributes to the alteration of heart contractility and to the establishment of heart hypertrophy. Experimental evidence suggests that PLC exerts a protective effect in in vitro as well as in vivo models of heart ischemia and hypertrophy.
The effect of millimolar concentrations of PLC on the working heart was first evaluated in isolated hearts from healthy rats. Under these conditions, PLC decreased the release of purines and, thus, the imbalance between ATP production and utilization, leading to an improvement in cardiac output and double product (product of heart rate and aortic systolic pressure).39 In contrast, Ferrari et al.40,41 showed that the same concentrations of PLC did not exert any effect when PLC was delivered directly into the perfusate, but chronic intraperitoneal administration of PLC led to positive inotropy. Chronic administration of PLC also produced positive inotropy in the hearts of diabetic rats with experimentally induced cardiomyopathy,42 in an in vivo animal model of anesthetized dogs,43 and in a model of hereditary dilated cardiomyopathy.44,45
Cardioprotective effects of PLC become more remarkable when they are evaluated in the presence of ischemic injury. In fact, during postmyocardial infarction heart failure, chronic administration of PLC led to an amelioration of exercise intolerance,46 a reduction in the infarct size,47 and an improvement in cardiac function.47,48 Additionally, pretreatment of the isolated ischemic perfused hearts with PLC improved functional recovery of the myocardium,15,16 as shown by the enhanced left ventricular pressure observed in the PLC-treated hearts.49,–51 The first mechanism by which these effects of PLC may be explained is the correction of L-carnitine loss that occurs during ischemia.52,53 In fact, a significant lowering of the carnitine content in cardiac muscle makes the heart more vulnerable to the metabolic and contractile derangements in ischemia.54 Furthermore, these beneficial effects were partially explained by different antioxidant mechanisms in most of the cases.49,–55 However, the most important finding in the ischemic injury that was ameliorated by PLC was enhanced mitochondrial function,49,56 as shown by improved ATP replenishment and creatine phosphate myocardial levels during the reperfusion phase.49,–55 Fatty acids are the major oxidation fuel for the heart, but the actions of PLC cannot be explained as exclusively dependent on the stimulation of fatty acid oxidation but rather on a marked increase in glucose oxidation.57 Both the free L-carnitine and the propionyl-CoA moieties of PLC have been demonstrated to cause the metabolic effects of PLC. Nevertheless, such effects were specific to PLC because the addition of L-carnitine or propionic acid did not modify myocardial damage.16 The ischemic heart tissue may also utilize the exogenous PLC to stimulate the tricarboxylic acid cycle via the transformation of the resultant propionate into succinate.14,–58 This anaplerotic effect of PLC has been well documented in ischemic hearts from diabetic rats after chronic oral administration of PLC.59,60
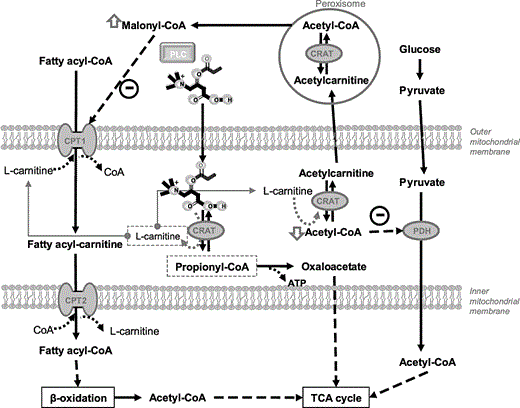
PLC may increase intramitochondrial free L-carnitine concentrations, decreasing the acetyl-CoA/CoA ratio inside the mitochondria by an efficient mass-action effect on the freely reversible reaction catalyzed by acylcarnitine transferase (CRAT) (Figure 2). The consequent decrease in intramitochondrial acetyl-CoA would be expected to translate into a less active pyruvate dehydrogenase kinase and, hence, a more active pyruvate dehydrogenase and an increased level of glucose oxidation.61 The decrease in the acetyl-CoA/CoA ratio may also lead to acetylcarnitine efflux from the matrix to the cytosol, followed by enhanced production of malonyl-CoA via peroxisome acetylcarnitine transferase, and, finally, to inhibition of the long-chain fatty acids transport into the mitochondrial matrix38 (Figure 2).
Effects of propionyl-L-carnitine (PLC) on energy metabolism. Abbreviations: CACT, carnitine-acylcarnitine translocase; CoA, coenzyme A; CPT, carnitine palmitoyl transferase; CRAT, acylcarnitine transferase; PDH, pyruvate dehydrogenase; PLC, phospholipase C; TCA, tricarboxylic acid.
Moreover, in volume-overloaded hearts, long-term administration of PLC improved the kinetics of mitochondrial ATP production and normalized the degree of pyridine nucleotide reduction.62 In contrast to that described above, when cardiac hypertrophy was induced by pressure overload, the amelioration of cardiac dysfunction and hypertrophy by PLC was related to increased oxidation of the energetic substrate by both β-oxidation63,64 and the tricarboxylic acid cycle.65,–67
PLC and insulin resistance
The state of insulin resistance is defined as a condition in which body cells become less sensitive to the effects of insulin. As a consequence, normal blood levels of insulin become inadequate to keep blood glucose within a normal range. Insulin resistance is also characterized by the development of dyslipidemia, hepatic steatosis, endothelial dysfunction, and hypertension. The role of PLC in the energy flux suggests a potential effect of this carnitine derivate in the treatment of these metabolic disorders. In fact, the effect of a 20-week treatment with PLC was recently tested in an animal model of obesity and insulin resistance. The results showed a reduction in body weight, abdominal adiposity, plasmatic insulin, and liver triglyceride content. Moreover, chronic administration of PLC led to an amelioration of the endothelial dysfunction developed by the fatty animals.68 These beneficial effects of PLC in the insulin resistance state may indicate a need for clinical trials investigating the effect of PLC supplements on metabolic disorders.
Other effects related to the antioxidant activity of PLC
As previously mentioned, the antioxidant properties of PLC may account for its potential therapeutic usefulness. One of the major causes of delayed graft function after organ transplantation is ischemia-reperfusion syndrome, which is caused primarily by an increased production of ROS. Recent investigations have shown that PLC prevents structural injury and deterioration of renal function induced by ischemia-reperfusion in rat kidneys suitable for transplantation. This action of PLC was attributed mainly to a reduction in both lipid peroxidation and free radical generation,69 a decreased expression of inducible NO synthase and protein nitration, and inhibition of tubular necrosis and neutrophil infiltration in transplanted kidneys.70 The efficacy of PLC to modulate ischemia-reperfusion injury during kidney transplantation suggests its use in human transplantation is worth testing.
Increased ROS production is also relevant in hypertension, since the antioxidant action of chronic treatment with PLC was also confirmed in SHR.71 This study showed that impaired levels of glutathione peroxidase and superoxide dismutase in the liver and heart of SHR were significantly restored by PLC administration. In addition, lipid peroxidation was reduced in PLC-treated SHR.71
Other investigations highlight the potential benefits of PLC supplementation in cisplatin-treated cancer patients; the hope is that PLC may aid in reducing cisplatin-associated cardiomyopathy, hepatotoxicity, and kidney injury. The toxicity resulting from cisplatin therapy is mediated mainly by the increased generation of ROS and endogenous carnitine depletion. To date, some studies in rats have shown that intraperitoneal administration of PLC can prevent the development of cisplatin-induced cardiomyopathy,72 liver injury,73 and nephrotoxicity.74 The beneficial effects of PLC on cisplatin-associated toxicity are related partly to the ability of this molecule to increase intracellular carnitine content. This is followed by improved mitochondrial oxidative phosphorylation and energy production as well as by reduced oxidative stress.
CLINICAL EVIDENCE OF THE THERAPEUTIC VALUE OF PLC
The pharmacological actions of PLC described above make this molecule potentially beneficial for the treatment of a wide variety of diseases. The clinical data supporting the therapeutic use of PLC are reviewed below.
PLC and peripheral arterial disease
Peripheral arterial disease (PAD) results from decreased blood flow and the consequent reduction of oxygen supply to the lower limb muscles during exercise. PAD is caused by atherosclerosis of limb arteries and is usually complicated by vascular accidents occurring not only in peripheral circulation but also in coronary and cerebral arteries.75 When evaluated by flow-mediated dilatation, patients with PAD exhibit impaired endothelial function, which is exacerbated by acute exercise.76 Moreover, such patients exhibit an increase in plasma markers of inflammation77 together with enhanced oxidative stress78 and reduced NO concentrations.79
Several clinical trials have shown the beneficial effects of PLC on PAD. In most of them, PLC was found to enhance the ankle/brachial index and improve maximal walking distance and walking distance onset of claudication80,–83 (Table 1). Moreover, these improvements attributed to PLC contributed to an enhanced quality of life in PAD patients.81,82
Summary of propionyl-L-carnitine effects in patients with peripheral arterial disease.
| Reference . | Treatment duration . | Dose . | Result . | Mechanism . |
|---|---|---|---|---|
| Brevetti et al. (1995)79 | 24 weeks | Oral PLC: 1 g/day (weeks 1–8) 2 g/day (weeks 9–16) 3 g/day (weeks 17–24) | ↑ MWD | |
| Bolognesi et al. (1995)87 | 8 days | Oral PLC: 1 g/day | No effect on muscular or subcutaneous blood flow | No direct vasoactive action |
| Taylor et al. (1996)96 | 12 weeks | Oral PLC: 2 g/day | ↑ MWD | Lessens decrease in PC |
| Brevetti et al. (1997)80 | 2 days | Intravenous PLC: 1.5 g + Infusion of PLC (30 min): 1 mg/kg/min | ↑ Glycogen muscle content | ↑ Muscle carnitine content Anaplerotic activity |
| Cittanti et al. (1997)88 | 12 weeks | Oral PLC: 3 g/day | No effect on blood flow ↑ Mitochondrial viability | |
| Brevetti et al. (1999)81 | 1 year | Oral PLC: 1 g/day | ↑ MWD (when initial MWD ≤250 m) | |
| Dal Lago et al. (1999)89 | 90 days | Oral PLC: 3 g/day | ↑ Claudication distance ↑ Blood flow velocity ↑ PAI-1 activity | |
| Hiatt et al. (2001)82 | 24 weeks | Oral PLC: 2 g/day | ↑ Peak walking time ↑ MWD ↑ Maximal walking speed ↓ Bodily pain | |
| Signorelli et al. (2006)90 | 1 year | Intravenous PLC: 1.8 g/week | ↑ ABI | ↓ Plasma MDA and 4-HNE ↓ Plasma NO2/NO3 ratio ↓ Plasma ET-1 ↓ Plasma homocysteine |
| Santo et al. (2006)83 | 1 year | Oral PLC: 2 g/day | ↑ ABI ↑ Distance of pain-free walking | ↓ Plasma MDA and 4-HNE ↑ Plasma NO2/NO3 ratio ↑ Oxidation time of LDL |
| Loffredo et al. (2006)92 | 7 days | Intravenous PLC: 6 g/day | ↑ MWD ↑ Flow-mediated dilatation | ↓ Plasma 8-OHdG ↑ Plasma NO2/NO3 ratio |
| Milio et al. (2009)86 | 20 days | Intravenous PLC: 1–2 g/day + Intravenous PGE-1: 60 mg/day | ↑ Maximal walking capacity ↓ Bodily pain ↓ Ulcerative lesion size |
| Reference . | Treatment duration . | Dose . | Result . | Mechanism . |
|---|---|---|---|---|
| Brevetti et al. (1995)79 | 24 weeks | Oral PLC: 1 g/day (weeks 1–8) 2 g/day (weeks 9–16) 3 g/day (weeks 17–24) | ↑ MWD | |
| Bolognesi et al. (1995)87 | 8 days | Oral PLC: 1 g/day | No effect on muscular or subcutaneous blood flow | No direct vasoactive action |
| Taylor et al. (1996)96 | 12 weeks | Oral PLC: 2 g/day | ↑ MWD | Lessens decrease in PC |
| Brevetti et al. (1997)80 | 2 days | Intravenous PLC: 1.5 g + Infusion of PLC (30 min): 1 mg/kg/min | ↑ Glycogen muscle content | ↑ Muscle carnitine content Anaplerotic activity |
| Cittanti et al. (1997)88 | 12 weeks | Oral PLC: 3 g/day | No effect on blood flow ↑ Mitochondrial viability | |
| Brevetti et al. (1999)81 | 1 year | Oral PLC: 1 g/day | ↑ MWD (when initial MWD ≤250 m) | |
| Dal Lago et al. (1999)89 | 90 days | Oral PLC: 3 g/day | ↑ Claudication distance ↑ Blood flow velocity ↑ PAI-1 activity | |
| Hiatt et al. (2001)82 | 24 weeks | Oral PLC: 2 g/day | ↑ Peak walking time ↑ MWD ↑ Maximal walking speed ↓ Bodily pain | |
| Signorelli et al. (2006)90 | 1 year | Intravenous PLC: 1.8 g/week | ↑ ABI | ↓ Plasma MDA and 4-HNE ↓ Plasma NO2/NO3 ratio ↓ Plasma ET-1 ↓ Plasma homocysteine |
| Santo et al. (2006)83 | 1 year | Oral PLC: 2 g/day | ↑ ABI ↑ Distance of pain-free walking | ↓ Plasma MDA and 4-HNE ↑ Plasma NO2/NO3 ratio ↑ Oxidation time of LDL |
| Loffredo et al. (2006)92 | 7 days | Intravenous PLC: 6 g/day | ↑ MWD ↑ Flow-mediated dilatation | ↓ Plasma 8-OHdG ↑ Plasma NO2/NO3 ratio |
| Milio et al. (2009)86 | 20 days | Intravenous PLC: 1–2 g/day + Intravenous PGE-1: 60 mg/day | ↑ Maximal walking capacity ↓ Bodily pain ↓ Ulcerative lesion size |
Abbreviations: 8-OHdG, 8-hydroxy-2-deoxy-2-deoxyguanosine; 4-HNE, 4-hydroxynonenal; ABI, ankle/brachial index; ET-1, endothelin-1; Hyc, homocysteine; LDL, low-density lipoprotein; MDA, malondialdehyde; MWD, maximal walking distance; PAI-1, plasminogen activator inhibitor 1; PC, phosphocreatine; PGE-1,prostaglandin E-1.
Summary of propionyl-L-carnitine effects in patients with peripheral arterial disease.
| Reference . | Treatment duration . | Dose . | Result . | Mechanism . |
|---|---|---|---|---|
| Brevetti et al. (1995)79 | 24 weeks | Oral PLC: 1 g/day (weeks 1–8) 2 g/day (weeks 9–16) 3 g/day (weeks 17–24) | ↑ MWD | |
| Bolognesi et al. (1995)87 | 8 days | Oral PLC: 1 g/day | No effect on muscular or subcutaneous blood flow | No direct vasoactive action |
| Taylor et al. (1996)96 | 12 weeks | Oral PLC: 2 g/day | ↑ MWD | Lessens decrease in PC |
| Brevetti et al. (1997)80 | 2 days | Intravenous PLC: 1.5 g + Infusion of PLC (30 min): 1 mg/kg/min | ↑ Glycogen muscle content | ↑ Muscle carnitine content Anaplerotic activity |
| Cittanti et al. (1997)88 | 12 weeks | Oral PLC: 3 g/day | No effect on blood flow ↑ Mitochondrial viability | |
| Brevetti et al. (1999)81 | 1 year | Oral PLC: 1 g/day | ↑ MWD (when initial MWD ≤250 m) | |
| Dal Lago et al. (1999)89 | 90 days | Oral PLC: 3 g/day | ↑ Claudication distance ↑ Blood flow velocity ↑ PAI-1 activity | |
| Hiatt et al. (2001)82 | 24 weeks | Oral PLC: 2 g/day | ↑ Peak walking time ↑ MWD ↑ Maximal walking speed ↓ Bodily pain | |
| Signorelli et al. (2006)90 | 1 year | Intravenous PLC: 1.8 g/week | ↑ ABI | ↓ Plasma MDA and 4-HNE ↓ Plasma NO2/NO3 ratio ↓ Plasma ET-1 ↓ Plasma homocysteine |
| Santo et al. (2006)83 | 1 year | Oral PLC: 2 g/day | ↑ ABI ↑ Distance of pain-free walking | ↓ Plasma MDA and 4-HNE ↑ Plasma NO2/NO3 ratio ↑ Oxidation time of LDL |
| Loffredo et al. (2006)92 | 7 days | Intravenous PLC: 6 g/day | ↑ MWD ↑ Flow-mediated dilatation | ↓ Plasma 8-OHdG ↑ Plasma NO2/NO3 ratio |
| Milio et al. (2009)86 | 20 days | Intravenous PLC: 1–2 g/day + Intravenous PGE-1: 60 mg/day | ↑ Maximal walking capacity ↓ Bodily pain ↓ Ulcerative lesion size |
| Reference . | Treatment duration . | Dose . | Result . | Mechanism . |
|---|---|---|---|---|
| Brevetti et al. (1995)79 | 24 weeks | Oral PLC: 1 g/day (weeks 1–8) 2 g/day (weeks 9–16) 3 g/day (weeks 17–24) | ↑ MWD | |
| Bolognesi et al. (1995)87 | 8 days | Oral PLC: 1 g/day | No effect on muscular or subcutaneous blood flow | No direct vasoactive action |
| Taylor et al. (1996)96 | 12 weeks | Oral PLC: 2 g/day | ↑ MWD | Lessens decrease in PC |
| Brevetti et al. (1997)80 | 2 days | Intravenous PLC: 1.5 g + Infusion of PLC (30 min): 1 mg/kg/min | ↑ Glycogen muscle content | ↑ Muscle carnitine content Anaplerotic activity |
| Cittanti et al. (1997)88 | 12 weeks | Oral PLC: 3 g/day | No effect on blood flow ↑ Mitochondrial viability | |
| Brevetti et al. (1999)81 | 1 year | Oral PLC: 1 g/day | ↑ MWD (when initial MWD ≤250 m) | |
| Dal Lago et al. (1999)89 | 90 days | Oral PLC: 3 g/day | ↑ Claudication distance ↑ Blood flow velocity ↑ PAI-1 activity | |
| Hiatt et al. (2001)82 | 24 weeks | Oral PLC: 2 g/day | ↑ Peak walking time ↑ MWD ↑ Maximal walking speed ↓ Bodily pain | |
| Signorelli et al. (2006)90 | 1 year | Intravenous PLC: 1.8 g/week | ↑ ABI | ↓ Plasma MDA and 4-HNE ↓ Plasma NO2/NO3 ratio ↓ Plasma ET-1 ↓ Plasma homocysteine |
| Santo et al. (2006)83 | 1 year | Oral PLC: 2 g/day | ↑ ABI ↑ Distance of pain-free walking | ↓ Plasma MDA and 4-HNE ↑ Plasma NO2/NO3 ratio ↑ Oxidation time of LDL |
| Loffredo et al. (2006)92 | 7 days | Intravenous PLC: 6 g/day | ↑ MWD ↑ Flow-mediated dilatation | ↓ Plasma 8-OHdG ↑ Plasma NO2/NO3 ratio |
| Milio et al. (2009)86 | 20 days | Intravenous PLC: 1–2 g/day + Intravenous PGE-1: 60 mg/day | ↑ Maximal walking capacity ↓ Bodily pain ↓ Ulcerative lesion size |
Abbreviations: 8-OHdG, 8-hydroxy-2-deoxy-2-deoxyguanosine; 4-HNE, 4-hydroxynonenal; ABI, ankle/brachial index; ET-1, endothelin-1; Hyc, homocysteine; LDL, low-density lipoprotein; MDA, malondialdehyde; MWD, maximal walking distance; PAI-1, plasminogen activator inhibitor 1; PC, phosphocreatine; PGE-1,prostaglandin E-1.
The use of PLC in combination with physical training has been recommended to improve symptoms associated with PAD.84 In addition, some recent studies suggest that a cycle of PLC infusions could also be advised for patients with severe claudication who cannot be included in an exercise rehabilitation program.85 Moreover, PLC can reinforce the effect of prostaglandin E1 in patients with chronic critical limb ischemia.86
There may be two aspects of the mechanism underlying the effects of PLC: metabolic effects in skeletal muscle and restoration of endothelial function traditionally attributed to the antioxidant properties of PLC. Although some trials found no changes in blood flow in patients treated with PLC,87,88 others demonstrated an improvement in hemodynamic flow.78,–90 These contradictory findings might be explained by differences in study design, since trials that showed enhanced blood flow included higher doses of PLC. More recent trials also identified a reduction in oxidative stress markers78,–92 and an enhanced bioavailability of NO78,92 in PAD patients treated with PLC. In particular, a recent outstanding study by Stasi et al.93 showed that PLC injection accelerates rabbit hind-limb post-ischemic blood flow recovery and the restoration of vascular function by sustaining positive dilative arteriogenetic remodeling and by reducing oxidative-stress-induced impaired endothelial function. The latter involved mainly NADPH oxidase 4 (Nox4) activity and downstream pathways, including expression of intercellular adhesion molecule 1 and inducible NO synthase.93 The findings derived from this investigation better address PLC as a therapeutic strategy to counteract the manifestations of PAD and suggest additional pharmacological studies targeting impaired endothelial function that occurs in cardiovascular disease.93
As a result of ischemia, patients with severe PAD have markedly reduced levels of ATP in their skeletal musculature.94 Research carried out in a rat model of PAD confirmed that PLC restored normal levels of ATP and phosphocreatine in ischemic muscle, resulting in an increase in walking capacity.95 In patients with PAD, treatment with PLC increased maximum mitochondrial activity for ATP synthesis in calf muscle, a finding that correlated positively with improvements in maximal walking distance.96 Moreover, a significant increment in mitochondrial integrity in skeletal muscle of PAD patients was found after treatment with PLC.88
PLC and myocardial ischemia
Relative myocardial carnitine deficiency is observed during ischemia. Accordingly, many experimental data (see “PLC and cardioprotection” above) suggest some metabolic and biological effects of myocardial ischemia are ameliorated by PLC treatment.
One of the first observations in patients was published in 1992 by Bartels et al.,97 who observed an acute improvement in cardiac function in patients with coronary artery disease who received 15 mg/kg of PLC. The same authors confirmed that the same dose of PLC prevented ischemia-induced ventricular dysfunction in men with angina pectoris without affecting the myocardial oxygen supply-demand ratio.98 Nevertheless, the anti-ischemic effects of PLC are less pronounced than those of the calcium antagonist diltiazem: a comparative study showed that PLC reduced ST segment depression at maximal exercise but did not increase the time to onset of angina.99 A double-blind, placebo-controlled study of PLC conducted in patients with stable angina also confirmed the reduction of ST segment depression, together with an increased total work and prolonged exercise time and time to ischemic threshold.100
Intravenous PLC administered prior to coronary artery bypass grafting significantly improved early post-operative recovery in diabetic patients by increasing the cardiac index and reducing pulmonary artery pressure.101 PLC also reduced transcardiac endothelin concentrations and accelerated myocardial hypoxanthine washout. These data suggest an improved cardiac metabolism and a vascular mechanism underlying the beneficial effects of the drug.101 In conclusion, although PLC has interesting protective effects against myocardial ischemia, its therapeutic role in this pathology is not well established.
PLC and chronic heart failure
It has been hypothesized that PLC could provide adjunct benefit over the standard therapy in CHF. Most of the facts supporting this hypothesis are related to the improvement of impaired metabolism of both skeletal and heart muscle. Presented here is a review of clinical outcomes in CHF patients treated with PLC.
Anand et al.102 studied the effect of acute and chronic PLC administration (1.5 g/day) on hemodynamics, hormonal levels, and oxygen consumption in patients with New York Heart Association (NYHA) class II or III CHF. Although no changes in hemodynamics or neurohormonal levels were found, a reduction in pulmonary artery pressure together with an increase in exercise capacity was observed, suggesting the improvement of peripheral muscle metabolism. Another study confirmed that PLC increased pyruvate influx into the Krebs cycle and decreased lactate production in the skeletal muscle of patients with idiopathic dilated cardiomyopathy.103 When examining the effects of the same dose of PLC in NYHA class IV CHF, an increase in exercise tolerance together with a reduction in lactate production was confirmed.104 This study as well as another trial105 found significant increases in left ventricular ejection fraction, stroke volume index, and cardiac index, whereas systemic vascular resistance decreased. Nevertheless, a large multicenter international study on the effects of PLC in CHF found only a slight nonsignificant difference in exercise test duration.106 The same study identified a subgroup of patients with an ejection fraction of 30–40% and relatively preserved myocardial function whose exercise test duration was significantly enhanced after PLC treatment.
PLC and erectile dysfunction
PLC has demonstrated usefulness in the treatment of erectile dysfunction (ED) of different causes. Clinical observations have proved that PLC enhances international index of erectile function (IIEF) scores in diabetic patients treated with the 5-phosphodiesterase inhibitor sildenafil107 and improves the drug's efficacy after retropubic prostatectomy.108 These reports suggest that combination therapy with PLC and sildenafil may be an alternative in patients with low response to sildenafil monotherapy. Furthermore, the combination of PLC plus ALC improved IIEF scores in aged men with partial androgen deficiency.109 A recent trial has also found that long-term administration of the combination of PLC, L-arginine, and nicotinic acid improves the efficacy of another 5-phosphodiesterase inhibitor, vardenafil, in the treatment of ED in aged diabetic patients.110
Finally, PLC plus verapamil has been shown to reduce plaques found in acute Peyronie's disease and to ameliorate IIEF score and resistivity index of cavernosal arteries.111 Conversely, PLC alone or in combination with vitamin C was not able to improve pain, penis curvatures, or plaque size in Peyronie's patients.112
ED is related to poor vascular supply to penile arteries, caused by low bioavailability of NO. The impaired blood supply to the corpus cavernosum limits the response to increased demand during sexual stimulation and causes fibrosis. The oxidative stress from a variety of offending conditions may contribute to endothelial dysfunction. The above-described antioxidant effects of PLC may prove beneficial in patients with ED. This hypothesis has been recently confirmed by Morano et al.,113 who found that PLC reduced endothelial dysfunction markers (i.e., serum intracellular adhesion molecule 1 and p-selectin) and ROS production by monocytes of diabetic patients with ED treated with sildenafil.
CONCLUSION
Experimental evidence supports the potential therapeutic use of supplementation with L-carnitine and its short-chain derivatives ALC and PLC. The latter two molecules have shown better bioavailability than carnitine, providing significant advantages over carnitine itself. The most remarkable benefits of ALC have been reported in the settings of neurotoxicity and neuronal pathology by decreasing the oxidative status and maintaining energy production in the mitochondria. The main focus of this review, however, was the value of PLC in preventing and/or ameliorating cardiovascular and metabolic disorders. Accordingly, PLC supplementation improves endothelial dysfunction associated with other cardiovascular risk factors such as hypertension by protecting the endothelium from oxidative stress and by increasing NO bioavailability. The ability of PLC to improve endothelial function and to exert antiproliferative activity on vascular smooth muscle cells makes it a useful anti-atherosclerotic substance. In addition, the role of PLC in the control of mitochondrial energy flux and lipid peroxidation provides notable cardioprotection as well as potential usefulness in the treatment of insulin resistance, obesity, and other metabolic alterations. The experimental findings have been widely supported by clinical studies that highlight the beneficial effect of PLC supplementation in peripheral arterial disease, myocardial ischemia, CHF, and ED. In these investigations, the antioxidant properties of PLC and its positive effects on energy expenditure may account for its potential as a therapeutic agent. Nevertheless, additional studies performed under well-controlled conditions are needed to further elucidate practical guidelines for the therapeutic use of PLC, especially with regard to optimal dosage, patient profile, and route of administration.
Acknowledgments
Declaration of interest
The authors have no relevant interests to declare.
REFERENCES